High-Sensitive Detection and Quantitative Analysis of Thyroid-Stimulating Hormone Using Gold-Nanoshell-Based Lateral Flow Immunoassay Device
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Antibody Conjugation onto Gold Nanoshells
2.4. Antibody Conjugation onto Gold Nanoparticles
2.5. Fabrication of LFDs
2.6. Specimen Preparation
2.7. Assay Performance
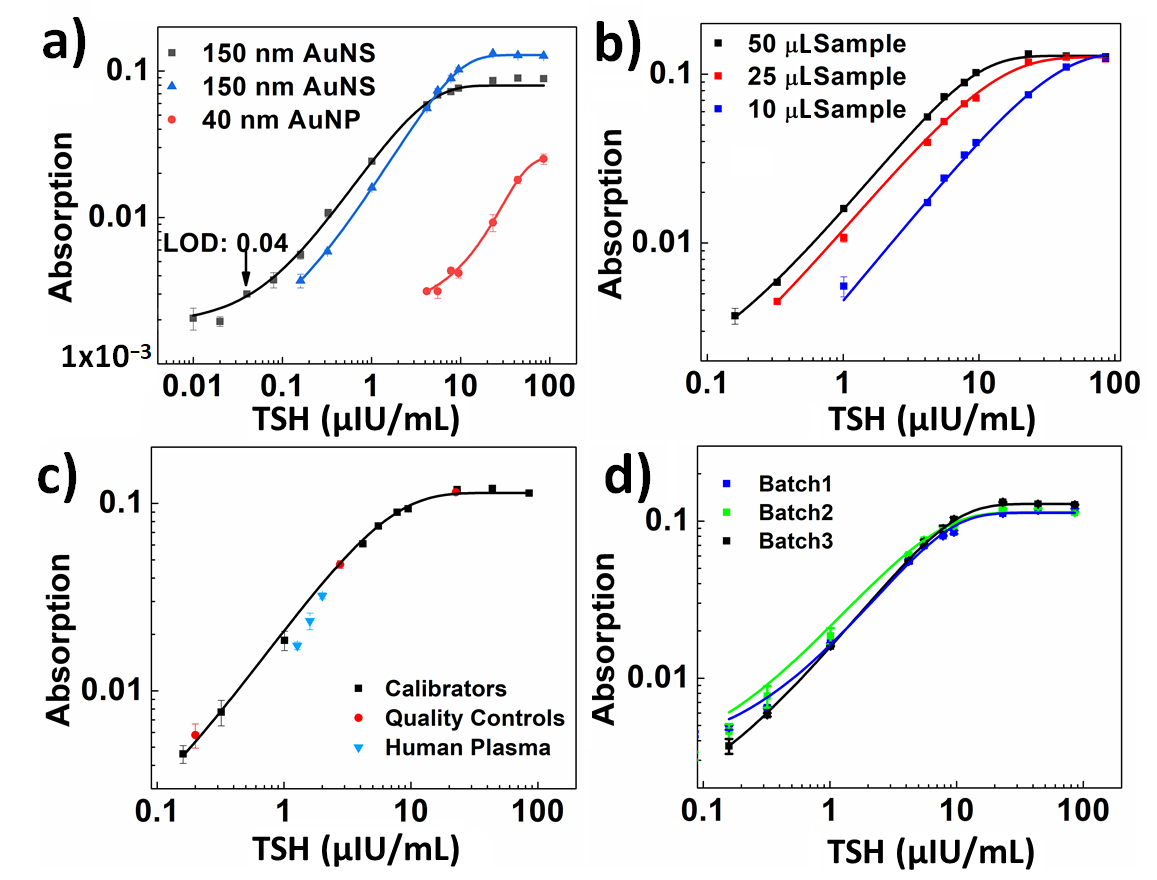
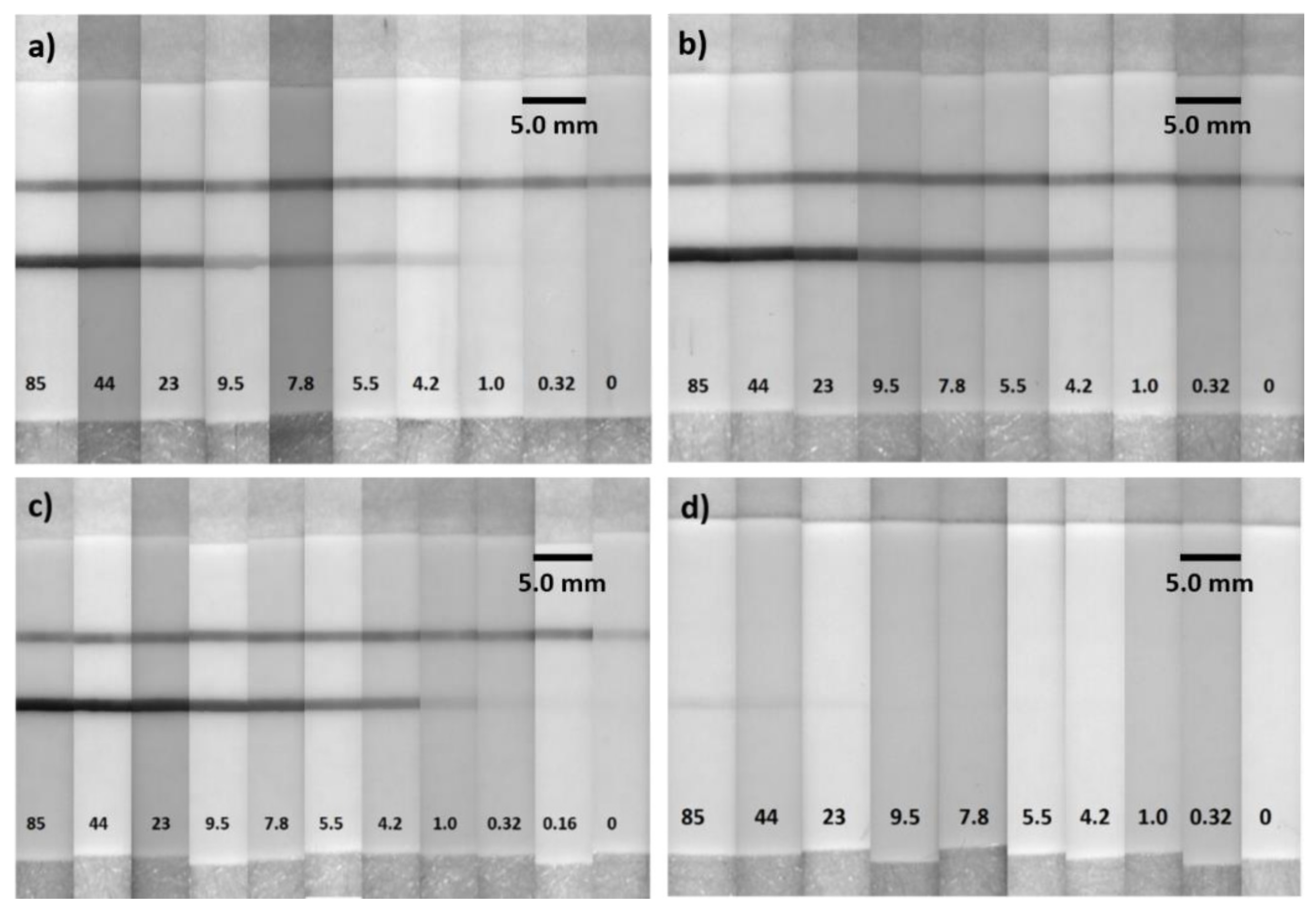
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, D.A. Physiological Variations in Thyroid Hormones: Physiological and Pathophysiological Considerations. Clin. Chem. 1996, 42, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wang, X.; Li, Z.; Ren, S.; Chen, G.; Ying, X.; Lin, J. Development of a Sensitive, Rapid, Biotin–streptavidin Based Chemiluminescent Enzyme Immunoassay for Human Thyroid Stimulating Hormone. Talanta 2008, 75, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Han, J.; Kai, J.; Lim, J.; Sul, D.; Ahn, C.H. An Innovative Sample-to-Answer Polymer Lab-on-a-Chip with on-Chip Reservoirs for the POCT of Thyroid Stimulating Hormone (TSH). Lab Chip 2013, 13, 4653–4662. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; de Lange, P.; Lombardi, A.; Silvestri, E.; Lanni, A.; Goglia, F. Metabolic Effects of Thyroid Hormone Derivatives. Thyroid 2008, 18, 239–253. [Google Scholar] [CrossRef]
- Scanlan, T.S.; Suchland, K.L.; Hart, M.E.; Chiellini, G.; Huang, Y.; Kruzich, P.J.; Frascarelli, S.; Crossley, D.A., II; Bunzow, J.R.; Ronca-Testoni, S.; et al. 3-Iodothyronamine is an Endogenous and Rapid-Acting Derivative of Thyroid Hormone. Nat. Med. 2004, 10, 638–642. [Google Scholar] [CrossRef]
- Weatherman, R.V. A Triple Play for Thyroid Hormone. ACS Chem. Biol. 2007, 2, 377–379. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Zhang, Q.; Wang, H.; Yuan, Y.; Chai, Y.; Yuan, R. An Electrochemiluminescence Immunosensor for Thyroid Stimulating Hormone Based on Polyamidoamine-Norfloxacin Functionalized Pd–Au Core–shell Hexoctahedrons as Signal Enhancers. Biosens. Bioelectron. 2015, 71, 164–170. [Google Scholar] [CrossRef]
- Leung, A.M. Subclinical Hyperthyroidism is Associated with Increased Risks of Hip Fractures, Fractures at any Site, Nonspine Fractures, and Clinical Spine Fractures in the Largest Meta-Analysis to Date. Clin. Thyroidol. 2015, 27, 174–176. [Google Scholar] [CrossRef]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global Epidemiology of Hyperthyroidism and Hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef]
- Garmendia Madariaga, A.; Santos Palacios, S.; Guillén-Grima, F.; Galofré, J.C. The Incidence and Prevalence of Thyroid Dysfunction in Europe: A Meta-Analysis. J. Clin. Endocrinol. Metab. 2014, 99, 923–931. [Google Scholar] [CrossRef]
- Vanderpump, M.P.J. The Epidemiology of Thyroid Disease. Br. Med. Bull. 2011, 99, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Hwang, J.; Lee, S.; Lim, D.W.; Joo, H.; Choo, J. Quantitative Analysis of Thyroid-Stimulating Hormone (TSH) using SERS-Based Lateral Flow Immunoassay. Sens. Actuators B Chem. 2017, 240, 358–364. [Google Scholar] [CrossRef]
- You, D.J.; Park, T.S.; Yoon, J. Cell-Phone-Based Measurement of TSH using Mie Scatter Optimized Lateral Flow Assays. Biosens. Bioelectron. 2013, 40, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, E.C.; McDermott, M.T. Subclinical Hypothyroidism Is Mild Thyroid Failure and Should Be Treated. J. Clin. Endocrinol. Metab. 2001, 86, 4585–4590. [Google Scholar]
- Beitollahi, H.; Ivari, S.G.; Torkzadeh-Mahani, M. Application of Antibody–Nanogold–Ionic Liquid–Carbon Paste Electrode for Sensitive Electrochemical Immunoassay of Thyroid-Stimulating Hormone. Biosens. Bioelectron. 2018, 110, 97–102. [Google Scholar] [CrossRef]
- Pomelova, V.G.; Osin, N.S.; Bychenkova, T.A.; Paramonov, D.V.; Kostryukova, T.S. Application of Eu(III) Nanoparticle Labels in Time-Resolved Phosphorescence Analysis for Detection of Thyroid Stimulating Hormone. Russ. J. Bioorg. Chem. 2017, 43, 377–385. [Google Scholar] [CrossRef]
- Owen, W.E.; Gantzer, M.L.; Lyons, J.M.; Rockwood, A.L.; Roberts, W.L. Functional Sensitivity of Seven Automated Thyroid Stimulating Hormone Immunoassays. Clin. Chim. Acta 2011, 412, 2336–2339. [Google Scholar] [CrossRef]
- Parolo, C.; Merkoçi, A. Paper-Based Nanobiosensors for Diagnostics. Chem. Soc. Rev. 2013, 42, 450–457. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Lateral Flow Assays: Principles, Designs and Labels. TrAC Trends Anal. Chem. 2016, 82, 286–306. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Carrilho, E.; Thomas, S.W.; Sindi, H.; Whitesides, G.M. Simple Telemedicine for Developing Regions: Camera Phones and Paper-Based Microfluidic Devices for Real-Time, Off-Site Diagnosis. Anal. Chem. 2008, 80, 3699–3707. [Google Scholar] [CrossRef]
- Brangel, P.; Sobarzo, A.; Parolo, C.; Miller, B.S.; Howes, P.D.; Gelkop, S.; Lutwama, J.J.; Dye, J.M.; McKendry, R.A.; Lobel, L.; et al. A Serological Point-of-Care Test for the Detection of IgG Antibodies Against Ebola Virus in Human Survivors. ACS Nano 2018, 12, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral Flow (Immuno)Assay: Its Strengths, Weaknesses, Opportunities and Threats. A Literature Survey. Anal. Bioanal. Chem. 2008, 393, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Qriouet, Z.; Cherrah, Y.; Sefrioui, H.; Qmichou, Z. Monoclonal Antibodies Application in Lateral Flow Immunochromatographic Assays for Drugs of Abuse Detection. Molecules 2021, 26, 1058. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, L.R.; et al. Advanced Methods of Plant Disease Detection. A Review. Agron. Sustain. Dev. 2015, 35, 1–25. [Google Scholar] [CrossRef]
- Ahmed, F.E. Detection of Genetically Modified Organisms in Foods. Trends Biotechnol. 2002, 20, 215–223. [Google Scholar] [CrossRef]
- Luo, K.; Kim, H.; Oh, M.; Kim, Y. Paper-Based Lateral Flow Strip Assay for the Detection of Foodborne Pathogens: Principles, Applications, Technological Challenges and Opportunities. Crit. Rev. Food Sci. Nutr. 2020, 60, 157–170. [Google Scholar] [CrossRef]
- Yao, L.; Xu, J.; Cheng, J.; Yao, B.; Zheng, L.; Liu, G.; Chen, W. Simultaneous and Accurate Screening of Multiple Genetically Modified Organism (GMO) Components in Food on the Same Test Line of SERS-Integrated Lateral Flow Strip. Food Chem. 2022, 366, 130595. [Google Scholar] [CrossRef]
- Bergua, J.F.; Hu, L.; Fuentes-Chust, C.; Álvarez-Diduk, R.; Hassan, A.H.A.; Parolo, C.; Merkoçi, A. Lateral Flow Device for Water Fecal Pollution Assessment: From Troubleshooting of its Microfluidics using Bioluminescence to Colorimetric Monitoring of Generic Escherichia coli. Lab Chip 2021, 21, 2417–2426. [Google Scholar] [CrossRef]
- Grubb, A.O.; Glad, U.C. Immunoassay with Test Strip Having Antibodies Bound Thereto. U.S. Patent No. 4,168,146, 18 September 1979. [Google Scholar]
- Gasperino, D.; Baughman, T.; Hsieh, H.V.; Bell, D.; Weigl, B.H. Improving Lateral Flow Assay Performance using Computational Modeling. Annu. Rev. Anal. Chem. 2018, 11, 219–244. [Google Scholar] [CrossRef]
- Zhou, W.; Gao, X.; Liu, D.; Chen, X. Gold Nanoparticles for In Vitro Diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold Nanoparticles: Optical Properties and Implementations in Cancer Diagnosis and Photothermal Therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Eustis, S.; El-Sayed, M. Why Gold Nanoparticles are More Precious than Pretty Gold: Noble Metal Surface Plasmon Resonance and its Enhancement of the Radiative and Nonradiative Properties of Nanocrystals of Different Shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef]
- Cao-Milán, R.; Liz-Marzán, L.M. Gold Nanoparticle Conjugates: Recent Advances toward Clinical Applications. Expert Opin. Drug Deliv. 2014, 11, 741–752. [Google Scholar] [CrossRef]
- Kumar, S.; Aaron, J.; Sokolov, K. Directional Conjugation of Antibodies to Nanoparticles for Synthesis of Multiplexed Optical Contrast Agents with both Delivery and Targeting Moieties. Nat. Protoc. 2008, 3, 314–320. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef]
- Wang, Y.; Rhéaume, É.; Lesage, F.; Kakkar, A. Synthetic Methodologies to Gold Nanoshells: An Overview. Molecules 2018, 23, 2851. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, L.; Qin, Z.; Sackrison, J.; Bischof, J.C. Ultrasensitive and Highly Specific Lateral Flow Assays for Point-of-Care Diagnosis. ACS Nano 2021, 15, 3593–3611. [Google Scholar] [CrossRef]
- Wu, L.; Qu, X. Cancer Biomarker Detection: Recent Achievements and Challenges. Chem. Soc. Rev. 2015, 44, 2963–2997. [Google Scholar] [CrossRef]
- Perfézou, M.; Turner, A.; Merkoçi, A. Cancer Detection using Nanoparticle-Based Sensors. Chem. Soc. Rev. 2012, 41, 2606–2622. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Chan, W.C.W.; Boulware, D.R.; Akkin, T.; Butler, E.K.; Bischof, J.C. Significantly Improved Analytical Sensitivity of Lateral Flow Immunoassays by using Thermal Contrast. Angew. Chem. Int. Ed. 2012, 51, 4358–4361. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ozsoz, M.; Liu, G. Gold Nanocage-Based Lateral Flow Immunoassay for Immunoglobulin G. Microchim. Acta 2017, 184, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Loynachan, C.N.; Thomas, M.R.; Gray, E.R.; Richards, D.A.; Kim, J.; Miller, B.S.; Brookes, J.C.; Agarwal, S.; Chudasama, V.; McKendry, R.A.; et al. Platinum Nanocatalyst Amplification: Redefining the Gold Standard for Lateral Flow Immunoassays with Ultrabroad Dynamic Range. ACS Nano 2018, 12, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Ye, H.; Tang, D.; Tao, J.; Habibi, S.; Minerick, A.; Tang, D.; Xia, X. Platinum-Decorated Gold Nanoparticles with Dual Functionalities for Ultrasensitive Colorimetric In Vitro Diagnostics. Nano Lett. 2017, 17, 5572–5579. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.; de Puig, H.; Tam, J.O.; Gómez-Márquez, J.; Bosch, I.; Hamad-Schifferli, K.; Gehrke, L. Multicolored Silver Nanoparticles for Multiplexed Disease Diagnostics: Distinguishing Dengue, Yellow Fever, and Ebola Viruses. Lab Chip 2015, 15, 1638–1641. [Google Scholar] [CrossRef] [PubMed]
- Rayev, M.; Shmagel, K. Carbon–Protein Covalent Conjugates in Non-Instrumental Immunodiagnostic Systems. J. Immunol. Methods 2008, 336, 9–15. [Google Scholar] [CrossRef]
- Linares, E.M.; Kubota, L.T.; Michaelis, J.; Thalhammer, S. Enhancement of the Detection Limit for Lateral Flow Immunoassays: Evaluation and Comparison of Bioconjugates. J. Immunol. Methods 2012, 375, 264–270. [Google Scholar] [CrossRef]
- Juntunen, E.; Myyryläinen, T.; Salminen, T.; Soukka, T.; Pettersson, K. Performance of Fluorescent Europium(III) Nanoparticles and Colloidal Gold Reporters in Lateral Flow Bioaffinity Assay. Anal. Biochem. 2012, 428, 31–38. [Google Scholar] [CrossRef]
- Wang, Y.; Fill, C.; Nugen, S.R. Development of Chemiluminescent Lateral Flow Assay for the Detection of Nucleic Acids. Biosensors 2012, 2, 32–42. [Google Scholar] [CrossRef]
- Sakurai, A.; Takayama, K.; Nomura, N.; Yamamoto, N.; Sakoda, Y.; Kobayashi, Y.; Kida, H.; Shibasaki, F. Multi-Colored Immunochromatography using Nanobeads for Rapid and Sensitive Typing of Seasonal Influenza Viruses. J. Virol. Methods 2014, 209, 62–68. [Google Scholar] [CrossRef]
- Badu-Tawiah, A.; Lathwal, S.; Kaastrup, K.; Al-Sayah, M.; Christodouleas, D.C.; Smith, B.S.; Whitesides, G.M.; Sikes, H.D. Polymerization-Based Signal Amplification for Paper-Based Immunoassays. Lab Chip 2015, 15, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.O.; de Puig, H.; Yen, C.; Bosch, I.; Gómez-Márquez, J.; Clavet, C.; Hamad-Schifferli, K.; Gehrke, L. A Comparison of Nanoparticle-Antibody Conjugation Strategies in Sandwich Immunoassays. J. Immunoass. Immunochem. 2017, 38, 355–377. [Google Scholar] [CrossRef]
- Tuersun, P.; Yusufu, T.; Yimiti, A.; Sidike, A. Refractive index sensitivity analysis of gold nanoparticles. Optik 2017, 149, 384–390. [Google Scholar] [CrossRef]
- Tuersun, P. Optimizing the figure of merit of gold nanoshell-based refractive index sensing. Optik 2015, 127, 250–253. [Google Scholar] [CrossRef]
- Omrani, M.; Mohammadi, H.; Fallah, H. Ultrahigh sensitive refractive index nanosensors based on nanoshells, nanocages and nanoframes: Efects of plasmon hybridization and restoring force. Sci. Rep. 2021, 11, 2045–2322. [Google Scholar] [CrossRef]
- Chen, H.; Kou, X.; Yang, Z.; Ni, W.; Wang, J. Shape- and size-dependent refractive index sensitivity of gold nanoparticles. Langmuir 2008, 24, 5233–5237. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bikkarolla, S.K.; McNamee, S.E.; Vance, P.; McLaughlin, J. High-Sensitive Detection and Quantitative Analysis of Thyroid-Stimulating Hormone Using Gold-Nanoshell-Based Lateral Flow Immunoassay Device. Biosensors 2022, 12, 182. https://doi.org/10.3390/bios12030182
Bikkarolla SK, McNamee SE, Vance P, McLaughlin J. High-Sensitive Detection and Quantitative Analysis of Thyroid-Stimulating Hormone Using Gold-Nanoshell-Based Lateral Flow Immunoassay Device. Biosensors. 2022; 12(3):182. https://doi.org/10.3390/bios12030182
Chicago/Turabian StyleBikkarolla, Santosh Kumar, Sara E. McNamee, Paul Vance, and James McLaughlin. 2022. "High-Sensitive Detection and Quantitative Analysis of Thyroid-Stimulating Hormone Using Gold-Nanoshell-Based Lateral Flow Immunoassay Device" Biosensors 12, no. 3: 182. https://doi.org/10.3390/bios12030182
APA StyleBikkarolla, S. K., McNamee, S. E., Vance, P., & McLaughlin, J. (2022). High-Sensitive Detection and Quantitative Analysis of Thyroid-Stimulating Hormone Using Gold-Nanoshell-Based Lateral Flow Immunoassay Device. Biosensors, 12(3), 182. https://doi.org/10.3390/bios12030182




