Development of a Rapid Gold Nanoparticle Immunochromatographic Strip Based on the Nanobody for Detecting 2,4-DichloRophenoxyacetic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Construction of Nanobody-Gold Nanoparticle
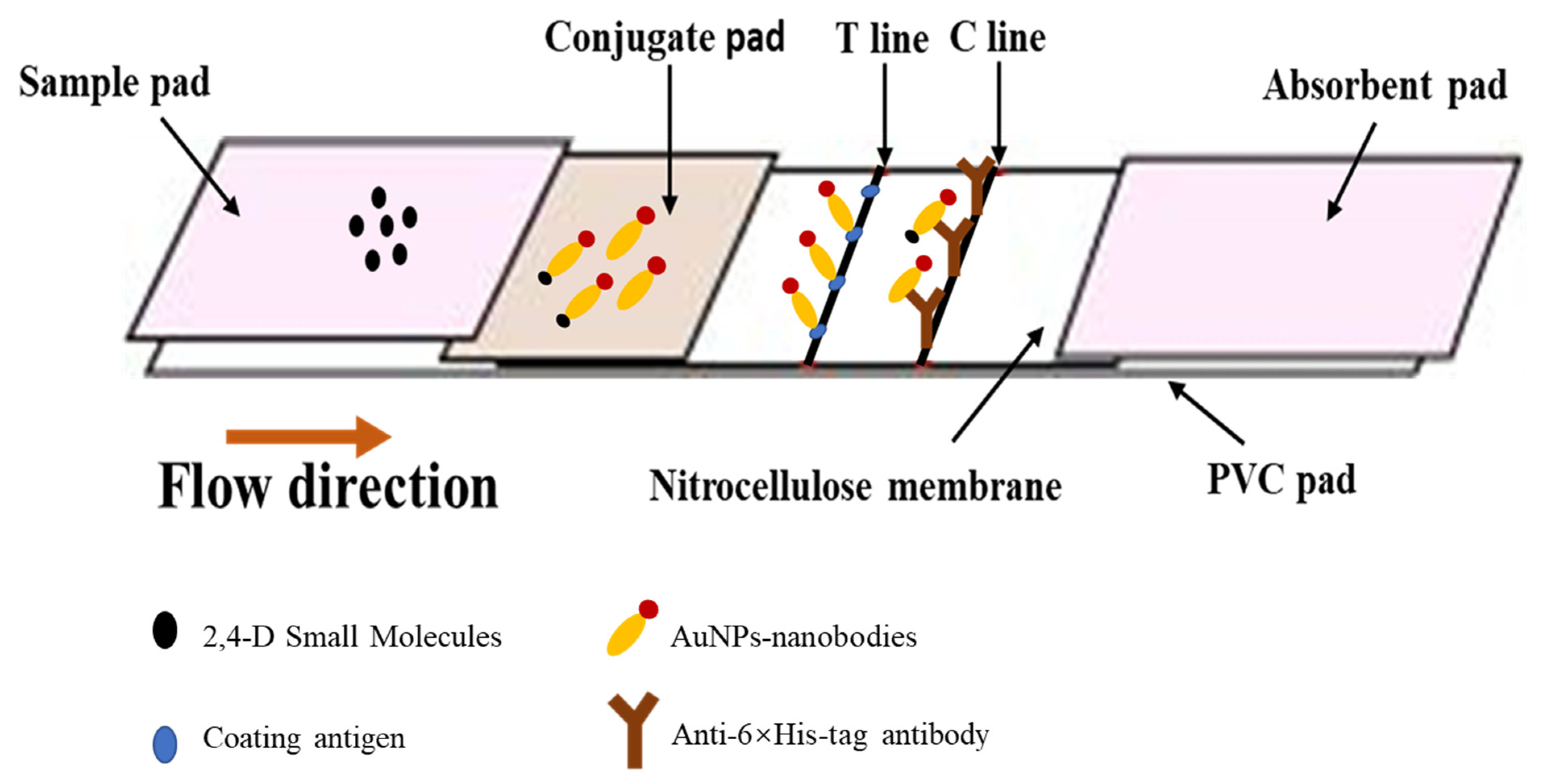
2.3. Optimization of the Combination of Antigen and Nanobody for Immunochromatographic Strip
2.4. Quantitative Detection of 2,4-D Based on the Color Snap APP
2.5. Cross-Reactivity
2.6. Stability of the Immunochromatographic Strip
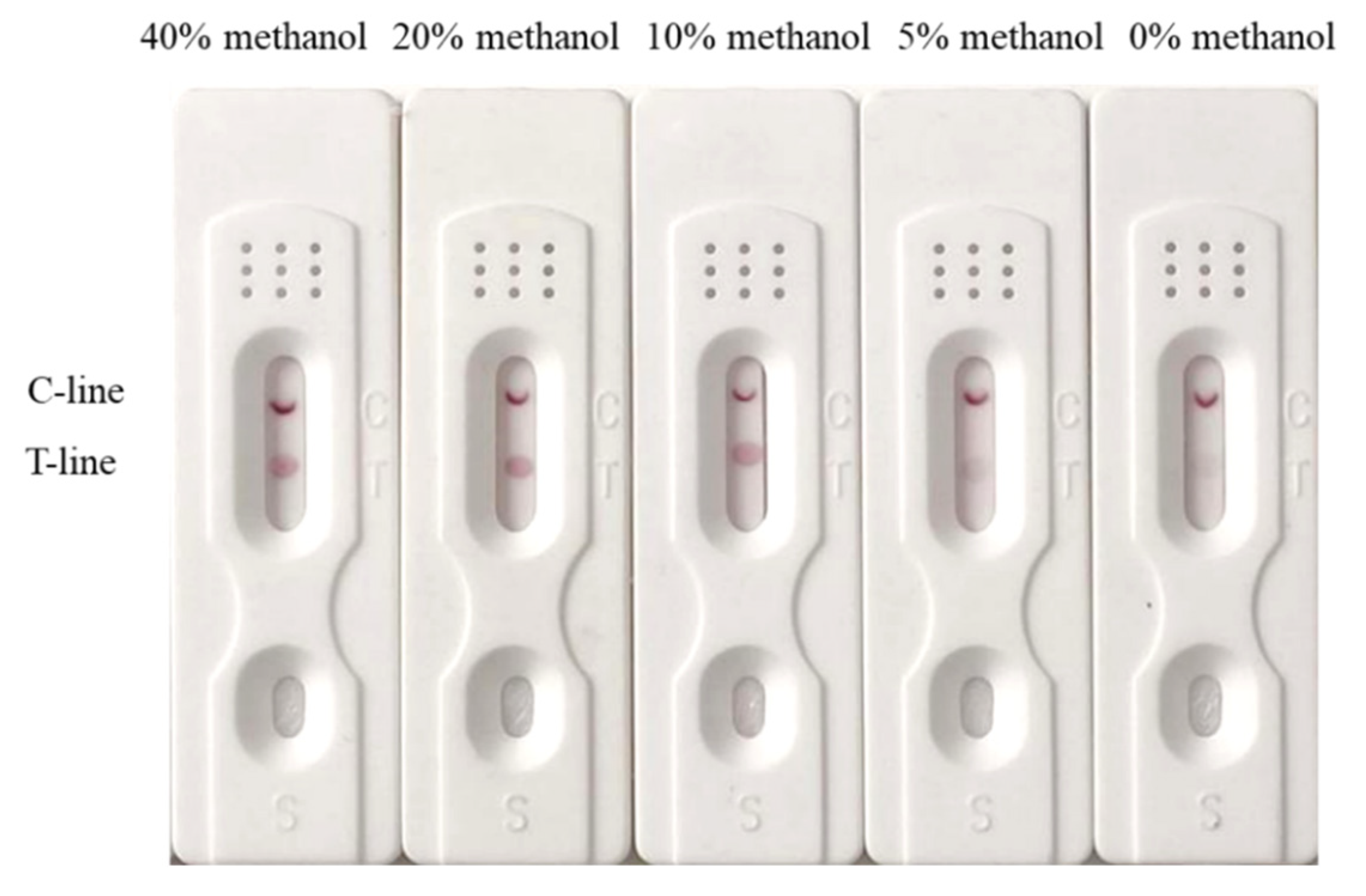
2.7. Matrix Effect and Sample Analysis
3. Results and Discussion
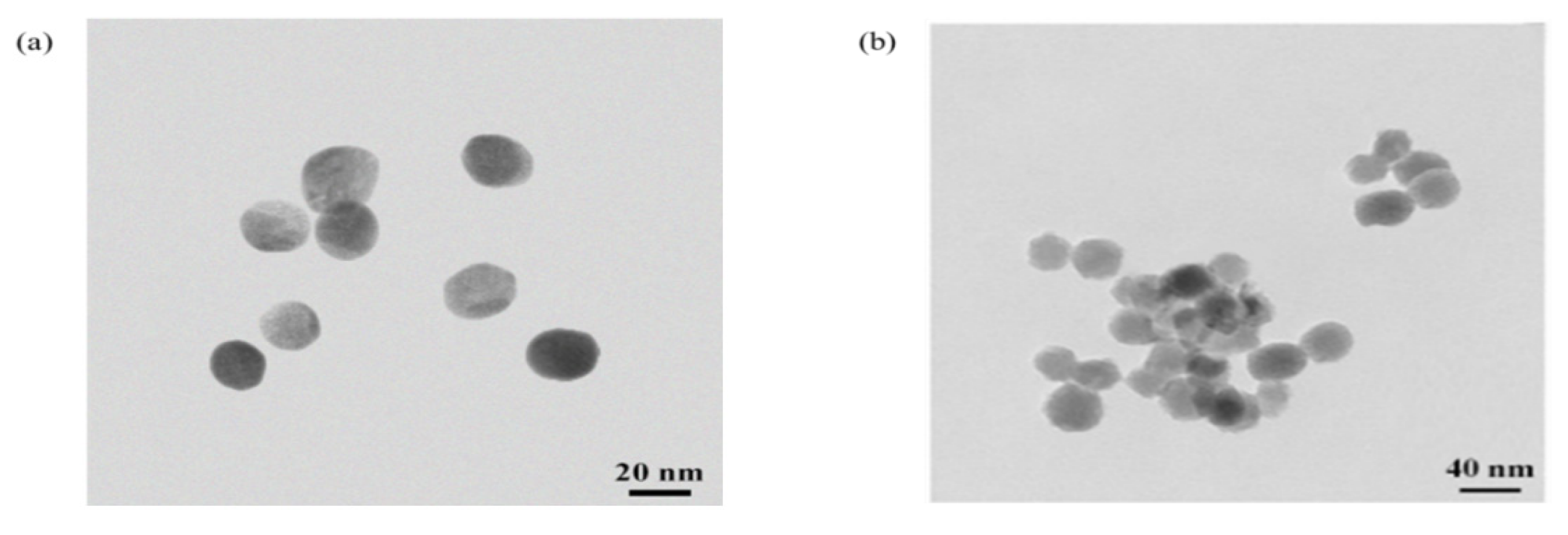
3.1. Characterization of Gold Nanoparticles
3.2. Construction of Nanobody-Gold Nanoparticle
3.3. Preparation and Optimization of Immunochromatographic Strip
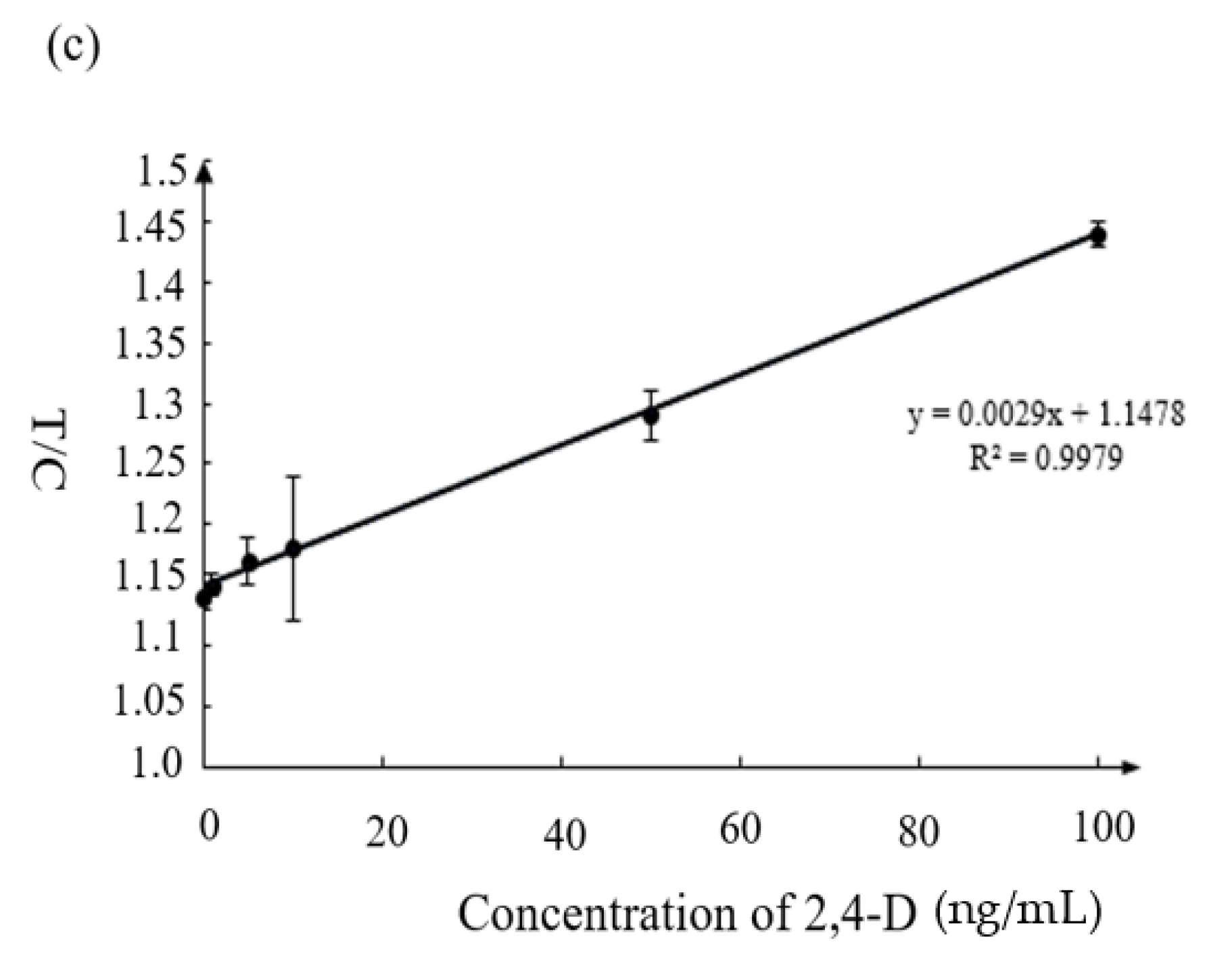
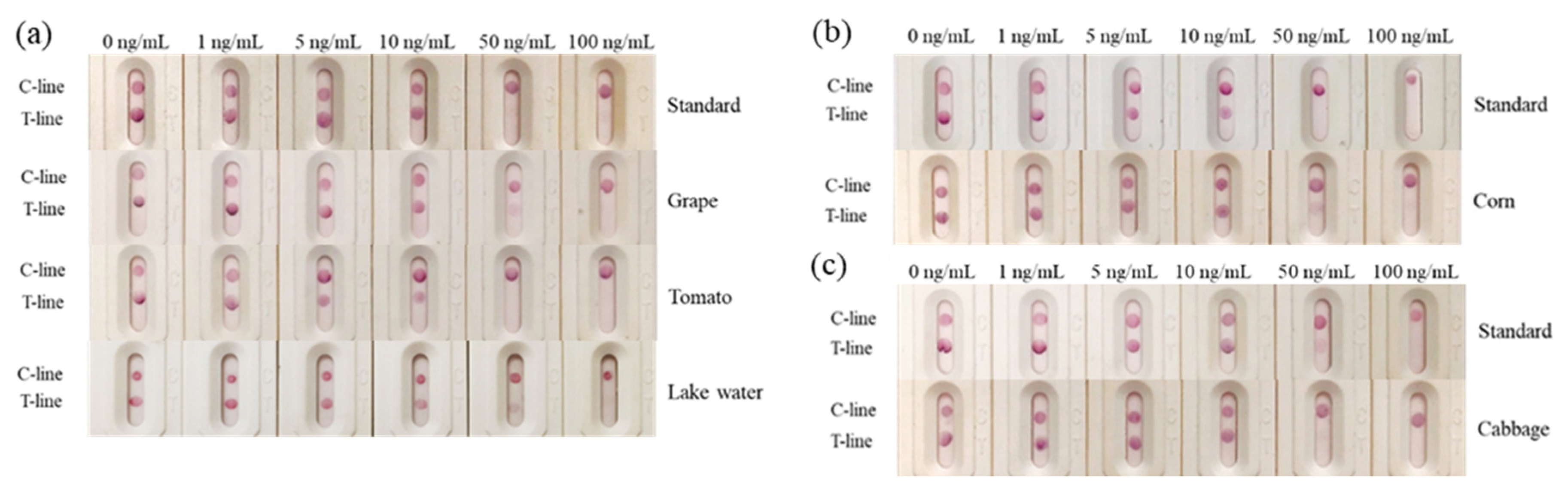
3.4. Sensitivity of Immunochromatographic Strip
3.5. Specificity of Immunochromatographic Strip
3.6. Stability of Immunochromatographic Strip
3.7. Analysis of the Spiked Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baharuddin, M.R.; Sahid, I.B.; Noor, M.A.; Sulaiman, N.; Othman, F. Pesticide risk assessment: A study on inhalation and dermal exposure to 2,4-D and paraquat among Malaysian paddy farmers. J. Environ. Sci. Health Part B 2011, 46, 600–607. [Google Scholar] [CrossRef]
- Tayeb, W.; Nakbi, A.; Trabelsi, M.; Miled, A.; Hammami, M. Biochemical and histological evaluation of kidney damage after sub-acute exposure to 2,4-dichlorophenoxyacetic herbicide in rats: Involvement of oxidative stress. Toxicol. Mech. Methods 2012, 22, 696–704. [Google Scholar] [CrossRef]
- Ueda, R.M.R.; Souza, V.M.D.; Magalhães, L.R.; Giuffrida, R.; Nai, G.A. Alteration of object recognition memory after chronic exposure to dichlorophenoxyacetic acid (2,4-D) in adult rats. Res. Soc. Dev. 2021, 10, e23310111695. [Google Scholar] [CrossRef]
- Obidike, I.R.; Aka, L.O.; Omoja, V.U.; Shoyinka, S.; Kamalu, T.N.; Anika, S.M. Testicular morphology and antispermatogenic effects of 2,4-dichlorophenoxyacetic acid (2,4-D) in male West African Dwarf (WAD) goats. Comp. Clin. Pathol. 2012, 21, 1457–1462. [Google Scholar] [CrossRef]
- Quan, X.; Shi, H.; Liu, H.; Lv, P.; Qian, Y. Enhancement of 2,4-dichlorophenol degradation in conventional activated sludge systems bioaugmented with mixed special culture. Water Res. 2004, 38, 245–253. [Google Scholar] [CrossRef]
- Islam, M.M. Movement and fate of 2,4-D in urban soils: A potential environmental health concern. ACS Omega 2020, 5, 13287–13295. [Google Scholar] [CrossRef]
- Qin, G.; Zou, K.; Tian, L.; Li, Y. Determination of five plant growth regulator containing carboxyl in bean sprouts based on chemical derivatization by GC-MS. Food Anal. Method. 2018, 11, 2628–2635. [Google Scholar] [CrossRef]
- Amani, V.; Roshan, S.; Asgharinezhad, A.A.; Najafi, E.; Abedi, H.; Tavassoli, N.; Zhad, Z.H.R.L. Determination of 2,4-D in environmental samples by three phases directly suspended LPME combined with HPLC-UV. Anal. Method. 2011, 3, 2261–2267. [Google Scholar] [CrossRef]
- Gökmen, V.; Acar, J. A study on the possibility of using HPLC for the determination of 2,4-D in tomatoes. J. Liq. Chromatogr. Relat. Technol. 2006, 19, 1917–1926. [Google Scholar] [CrossRef]
- Caldas, S.S.; Rombaldi, C.; Arias, J.; Marube, L.C.; Primel, E.G. Multi-residue method for determination of 58 pesticides, pharmaceuticals and personal care products in water using solvent demulsification dispersive liquid–liquid microextraction combined with liquid chromatography-tandem mass spectrometry. Talanta 2016, 146, 676–688. [Google Scholar] [CrossRef]
- Hong, M.; Kim, M.C.; Smith, A.E. Simultaneous determination of 2,4-D, dicamba, and mecoprop in soil leachates by gas chromatography with electron capture detection. J. AOAC Int. 1996, 79, 998–1004. [Google Scholar] [CrossRef][Green Version]
- Cai, J.; Zhou, M.; Yang, W.; Pan, Y.; Lu, X.; Serrano, K.G. Degradation and mechanism of 2,4-dichlorophenoxyacetic acid (2,4-D) by thermally activated persulfate oxidation. Chemosphere 2018, 212, 784–793. [Google Scholar] [CrossRef]
- Goulart, B.V.; Vizioli, B.D.C.; Espindola, E.L.G.; Montagner, C.C. Matrix effect challenges to quantify 2,4-D and fipronil in aquatic systems. Environ. Monit. Assess. 2020, 192, 797. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, X.; Li, B.W.; Zhao, J.; Qi, J.; Hao, G.Y.; Rong, J.H.; Yang, X.B. Fluorescence detection of 2, 4-dichlorophenoxyacetic acid by ratiometric fluorescence imaging on paper-based microfluidic chips. Analyst 2019, 145, 963–974. [Google Scholar] [CrossRef]
- Xu, Y.; Kutsanedzie, F.Y.H.; Hassan, M.; Zhu, J.J.; Ahmad, W.; Li, H.; Chen, Q.S. Mesoporous silica supported orderly-spaced gold nanoparticles SERS-based sensor for pesticides detection in food. Food Chem. 2020, 315, 126300. [Google Scholar] [CrossRef]
- Guo, L.L.; Wu, X.L.; Cui, G.; Song, S.S.; Kuang, H.; Xu, C.L. Colloidal gold immunochromatographic assay for rapid detection of carbadox and cyadox in chicken breast. ACS Omega 2020, 5, 1422–1429. [Google Scholar] [CrossRef]
- Chuang, J.C.; Emon, J.M.V.; Durnford, J.; Thomas, K. Development and evaluation of an enzyme-linked immunosorbent assay (ELISA) method for the measurement of 2,4-dichlorophenoxyacetic acid in human urine. Talanta 2005, 67, 658–666. [Google Scholar] [CrossRef]
- Vdovenko, M.M.; Stepanova, A.S.; Eremin, S.A.; Cuong, N.V.; Uskova, N.A.; Sakharov, I.Y. Quantification of 2,4-dichlorophenoxyacetic acid in oranges and mandarins by chemiluminescent ELISA. Food Chem. 2013, 141, 865–868. [Google Scholar] [CrossRef]
- Franek, M.; Kolar, V.; Granatova, M.; Nevorankova, Z. Monoclonal ELISA for 2, 4-dichlorophenoxyacetic acid: Characterization of antibodies and assay optimization. J. Agric. Food Chem. 1994, 42, 1369–1374. [Google Scholar] [CrossRef]
- Hall, J.C.; Deschamps, R.J.; Krieg, K.K. Immunoassays for the detection of 2, 4-D and picloram in river water and urine. J. Agric. Food Chem. 1989, 37, 981–984. [Google Scholar] [CrossRef]
- Li, Z.F.; Dong, J.X.; Vasylieva, N.; Cui, Y.L.; Wan, D.B.; Hua, X.D.; Huo, J.Q.; Yang, D.C.; Gee, S.J.; Hammock, B.D. Highly specific nanobody against herbicide 2,4-dichlorophenoxyacetic acid for monitoring of its contamination in environmental water. Sci. Total Environ. 2020, 753, 141950. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.W.; Yu, Y.A.; Liu, B.H.; Yu, F.Y. Development of a sensitive enzyme-linked immunosorbent assay and rapid gold nanoparticle immunochromatographic strip for detecting citrinin in monascus fermented food. Toxins 2018, 10, 354. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Huo, J.Q.; Li, Z.F.; He, C.; Li, D.Y.; Wang, Y.X.; Vasylieva, N.; Zhang, J.L.; Hammock, B.D. On-spot quantitative analysis of dicamba in field waters using a lateral flow immunochromatographic strip with smartphone imaging. Anal. Bioanal. Chem. 2020, 412, 6995–7006. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.Y.; Zhao, Y.J.; Wang, M.; Chen, X.J.; Wang, B.M.; Li, Q.X. Ultrasensitive quantitation of imidacloprid in vegetables by colloidal gold and time-resolved fluorescent nanobead traced lateral flow immunoassays. Food Chem. 2020, 311, 7. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Yusakul, G.; Nuntawong, P.; Kitisripanya, T.; Putalun, W.; Miyamoto, T.; Tanaka, H.; Morimoto, S. Development of an indirect competitive immunochromatographic strip test for rapid detection and determination of anticancer drug, harringtonine. J. Chromatogr. B 2017, 1048, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Braun, K.M. Color-to-grayscale conversion to maintain discriminability. Proc. SPIE 2003, 5293, 196–202. [Google Scholar] [CrossRef]
- Siangdee, N.; Youngvises, N. Smart sensor using cellulose-based material for TNT detection. Key Eng. Mater. 2019, 803, 124–128. [Google Scholar] [CrossRef]
- Yu, J.C.; Guo, T.T.; Zhang, W.; Zhu, Y.G.; Li, B.L. Simultaneous detection of 2, 4-dichlorophenoxyacetic acid and fenitrothion through lanthanide doped β-NaYF4 upconversion nanoparticles with different emitting light colors. Mater. Res. Bull. 2019, 111, 133–139. [Google Scholar] [CrossRef]
- Yu, J.C.; Guo, T.T.; Zhang, W.; Li, B.L.; Liu, L.T.; Hua, R.N. Green upconversion nanoparticles for 2, 4- dichlorophenoxyacetic acid and fenitrothion detection. J. Alloys Compd. 2019, 771, 187–194. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Yi, C.; Jiang, L.; Wu, J.; Chen, X. A smartphone-based quantitative detection device integrated with latex microsphere immunochromatography for on-site detection of zearalenone in cereals and feed. Sens. Actuator B-Chem. 2019, 290, 170–179. [Google Scholar] [CrossRef]



| Compound | Molecular Structures | Lateral-Flow Immunoassay | Cross Reactivity (%) 1 [21] |
|---|---|---|---|
| Negative control (0.01 M PBS (pH 7.4)) | - |  | - |
| 2,4-D |  |  | 100 |
| 2,4-Dichlorophenoxy butyric acid | 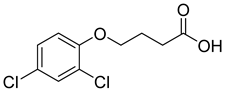 |  | 1.8 |
| 2-Methyl-4-chlorophenoxyacetic acid | 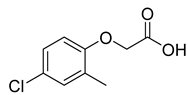 | 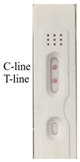 | 4.5 |
| 2-(4-Chloro-2-methylphenoxy)propanoic acid | 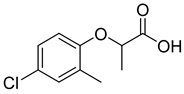 | 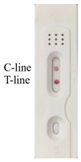 | 0.8 |
| 2,4-D methyl ester | 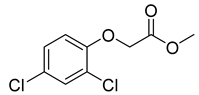 |  | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; He, C.; Li, Z.; Huo, J.; Xue, Y.; Xu, X.; Qi, M.; Chen, L.; Hammock, B.D.; Zhang, J. Development of a Rapid Gold Nanoparticle Immunochromatographic Strip Based on the Nanobody for Detecting 2,4-DichloRophenoxyacetic Acid. Biosensors 2022, 12, 84. https://doi.org/10.3390/bios12020084
Zhou H, He C, Li Z, Huo J, Xue Y, Xu X, Qi M, Chen L, Hammock BD, Zhang J. Development of a Rapid Gold Nanoparticle Immunochromatographic Strip Based on the Nanobody for Detecting 2,4-DichloRophenoxyacetic Acid. Biosensors. 2022; 12(2):84. https://doi.org/10.3390/bios12020084
Chicago/Turabian StyleZhou, Hui, Cong He, Zhenfeng Li, Jingqian Huo, Yu Xue, Xiaotong Xu, Meng Qi, Lai Chen, Bruce D. Hammock, and Jinlin Zhang. 2022. "Development of a Rapid Gold Nanoparticle Immunochromatographic Strip Based on the Nanobody for Detecting 2,4-DichloRophenoxyacetic Acid" Biosensors 12, no. 2: 84. https://doi.org/10.3390/bios12020084
APA StyleZhou, H., He, C., Li, Z., Huo, J., Xue, Y., Xu, X., Qi, M., Chen, L., Hammock, B. D., & Zhang, J. (2022). Development of a Rapid Gold Nanoparticle Immunochromatographic Strip Based on the Nanobody for Detecting 2,4-DichloRophenoxyacetic Acid. Biosensors, 12(2), 84. https://doi.org/10.3390/bios12020084





