A Polydopamine-Coated Gold Nanoparticles Quenching Quantum Dots-Based Dual-Readout Lateral Flow Immunoassay for Sensitive Detection of Carbendazim in Agriproducts
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents and Instrumentals
2.2. Preparation of CBD-BSA
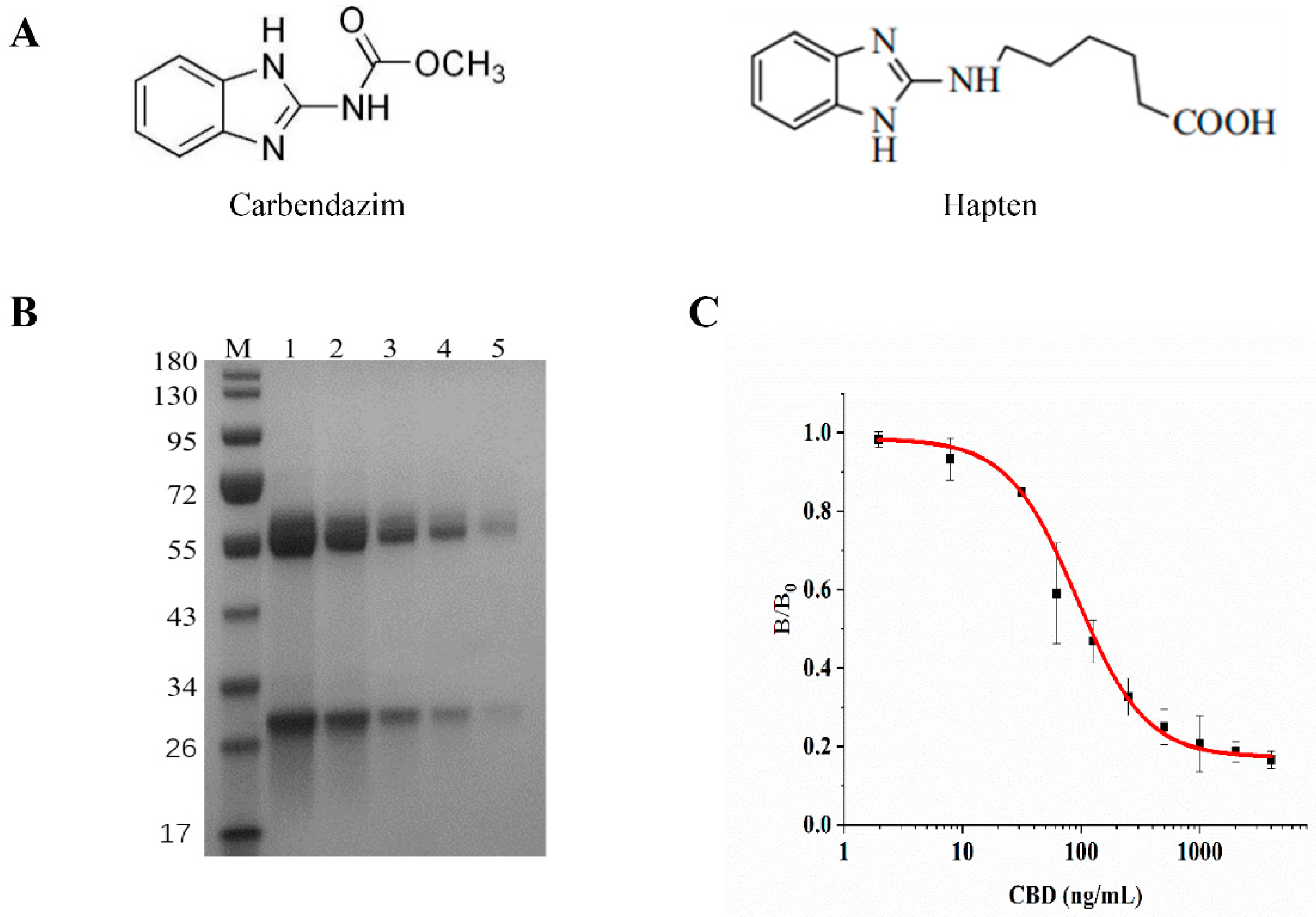
2.3. Production of Anti-CBD Monoclonal Antibody
2.4. Preparation of Colloidal Gold
2.5. Preparation of Au@PDA Nanoparticles
2.6. Conjugation of QDs-OVA
2.7. Preparation of Au@PDAs-mAb Probe
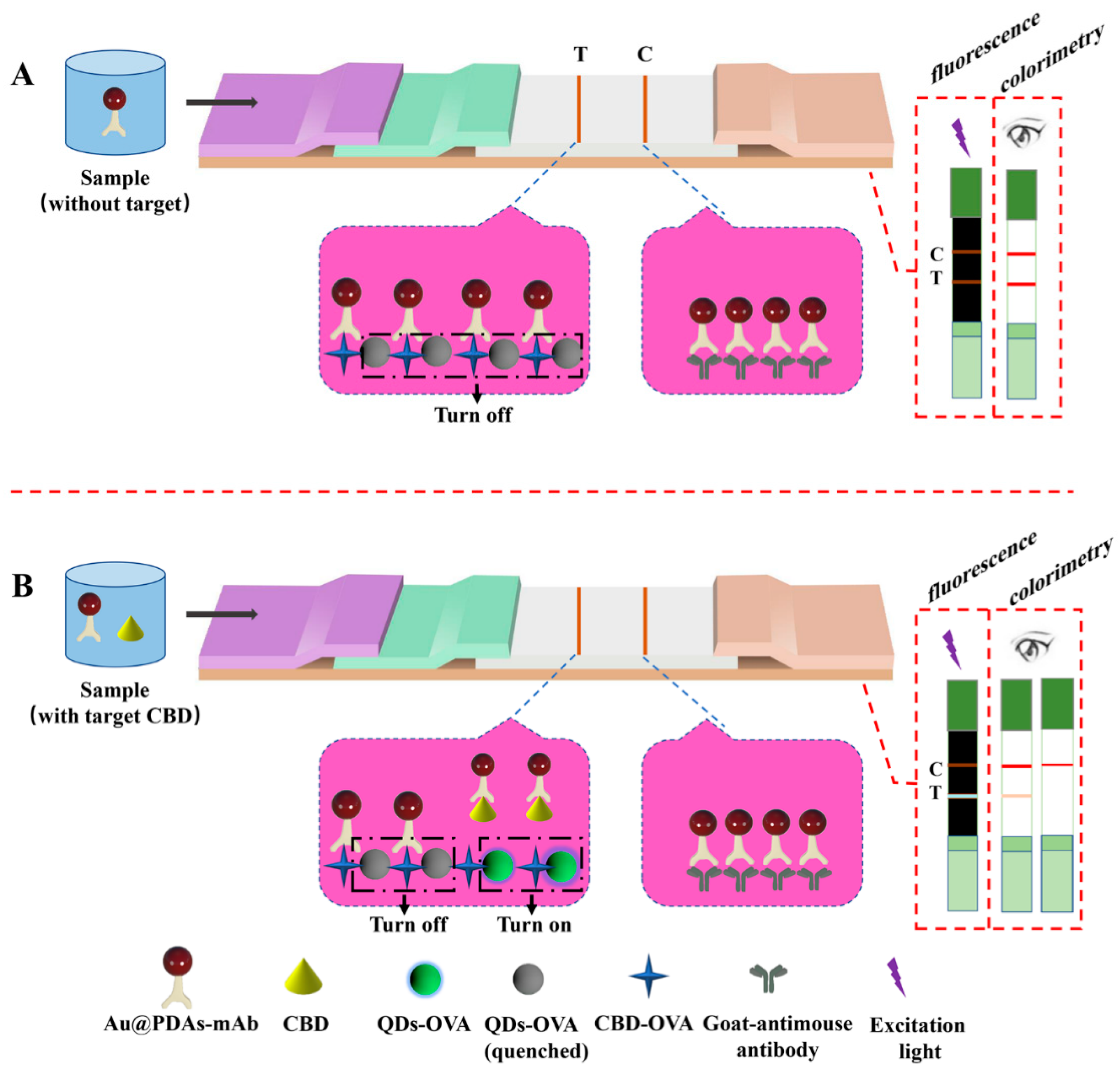
2.8. Fabrication of the Au@PDAs-QDs-LFIA Test Strips
2.9. Elimination of Sample Matrix Interference
2.10. Method Validation
2.11. Detection of Real Samples
3. Results and Discussion
3.1. Production of Monoclonal Antibody and the Preparation of Detection Probes
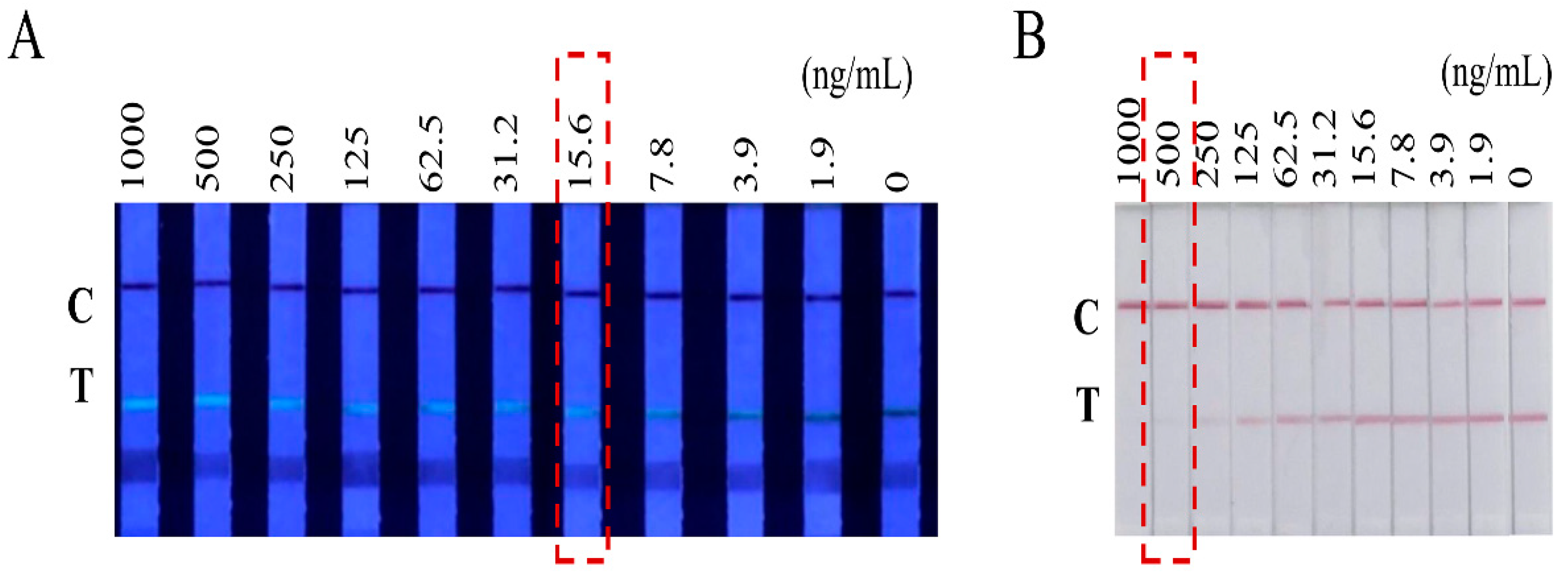
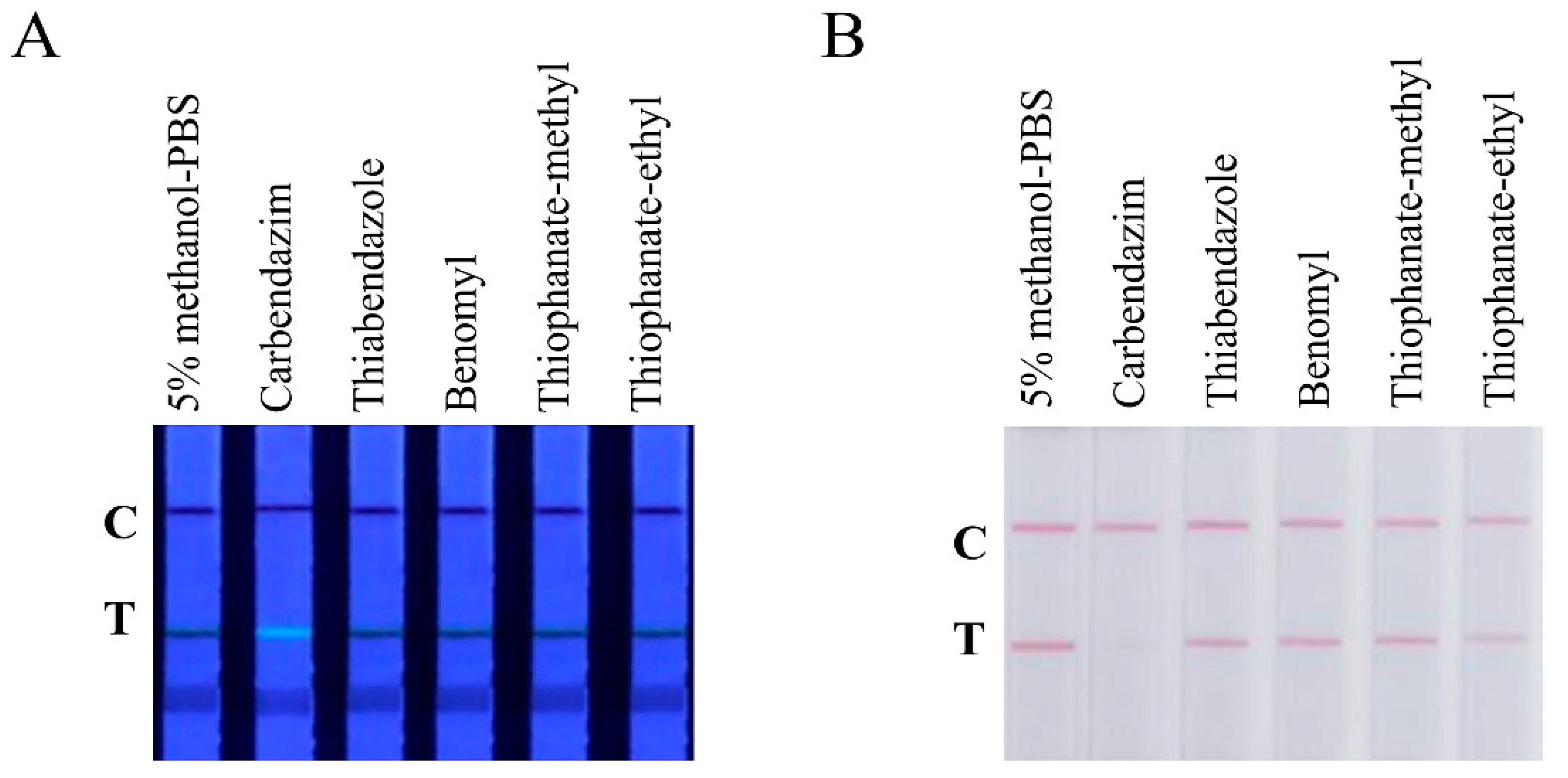
3.2. Assay Optimization
3.3. Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X. Determination of Carbendazim in Watermelon and Rice by HPLC, Digestion Dynamics and Final Residue Rule. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2014. [Google Scholar]
- Hao, L.L.; Jian, Q.; Liao, M.; Jiang, X.J. Determination of carbendazim and its main metabolites in tomato by LC-MS/MS. Chin. J. Pestic. Sci. 2012, 14, 429–434. [Google Scholar]
- Farajzadeh, M.A.; Nouri, N.; Khorram, P. Derivatization and microextraction methods for determination of organic compounds by gas chromatography. Trends Anal. Chem. 2014, 55, 14–23. [Google Scholar] [CrossRef]
- Xiao, W.H.; Yan, H.; Si, H.; Zheng, F.F.; Jing, J.L.; Ming, M.; Bo, C. Fluoro-functionalized paper-based solid-phase extraction for analysis of perfluorinated compounds by high-performance liquid chromatography coupled with electrospray ionization-tandem mass spectrometry. J. Chromatogr. A 2019, 1601, 79–85. [Google Scholar]
- Ho, P.L.; Wong, R.C.; Chow, K.H.; Que, T.L. Distribution of integron-associated trimethoprim-sulfamethoxazole resistance determinants among Escherichia coli from humans and food-producing animals. Lett. Appl. Microbiol. 2009, 49, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, P.; Ding, X.; Zhang, Q. Graphene oxide and carboxylated graphene oxide: Viable two-dimensional nanolabels for lateral flow immunoassays. Talanta 2017, 165, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Naik, L.; Sharma, R.; Mann, B.; Lata, K.; Rajput, Y.S.; Nath, B.S. Rapid screening test for detection of oxytetracycline residues in milk using lateral flow assay. Food Chem. 2017, 219, 85–92. [Google Scholar] [CrossRef]
- Zhen, H.; Zhi, J.X.; Yuan, C.; Song, H.; Lai, W.H. A Sensitive and matrix tolerant lateral flow immunoassay based on fluorescent magnetic nanobeads for the detection of clenbuterol in swine urine. J. Agric. Food Chem. 2019, 67, 3028–3036. [Google Scholar]
- Upadhyay, N.; Nara, S. Lateral flow assay for rapid detection of staphylococcus aureus enterotoxin A in milk. Microchem. J. 2018, 137, 435–442. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, R.; Wang, S.; Yang, X.; Bai, Z.; Li, X.; Rong, Z.; Shen, B.; Wang, S. Magnetic quantum dot based lateral flow assay biosensor for multiplex and sensitive detection of protein toxins in food samples. Biosens. Bioelectron. 2019, 146, 111754–111762. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Lateral flow assays: Principles, designs and labels. Trends Anal. Chem. 2016, 82, 286–306. [Google Scholar] [CrossRef]
- Sajid, M.; Kawde, A.N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015, 19, 689–705. [Google Scholar] [CrossRef] [Green Version]
- Hao, K.; Suryoprabowo, S.; Hong, T.; Song, S.; Liu, L.; Zheng, Q.; Kuang, H. Immunochromatographic strip for ultrasensitive detection of fumonisin B1. Food Agric. Immunol. 2018, 29, 434–444. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Zhou, X.; Zhang, Y.; Song, B.; Zhang, J.; Shi, H. Highly sensitive and simultaneous detection of melamine and aflatoxin M1 in milk products by multiplexed planar waveguide fluorescence immunosensor (MPWFI). Food Chem. 2016, 197, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Sobia, N.; Xiaole, W.; Imran, P.; Mahmood, K.I.; Sen, Z.; Muhammad, S.; Shijia, W.; Zhouping, W. A novel bioassay based on aptamer-functionalized magnetic nanoparticle for the detection of zearalenone using time resolved-fluorescence NaYF4: Ce/Tb nanoparticles as signal probe. Talanta 2018, 186, 97–103. [Google Scholar]
- Zhong, Y.; Chen, Y.; Yao, L.; Zhao, D.; Zheng, L.; Liu, G.; Ye, Y.; Chen, W. Gold nanoparticles based lateral flow immunoassay with largely amplified sensitivity for rapid melamine screening. Microchim. Acta 2016, 183, 1989–1994. [Google Scholar] [CrossRef]
- Bu, T.; Huang, Q.; Yan, L.; Huang, L.; Zhang, M.; Yang, Q.; Yang, B.; Wang, J.; Zhang, D. Ultra technically-simple and sensitive detection for Salmonella Enteritidis by immunochromato-graphic assay based on gold growth. Food Control 2018, 84, 536–543. [Google Scholar] [CrossRef]
- Chiu, R.Y.T.; Jue, E.; Yip, A.T.; Berg, A.R.; Wang, S.J.; Kivnick, A.R.; Kamei, D.T. Simultaneous concentration and detection of biomarkers on paper. Lab Chip 2014, 14, 3021–3028. [Google Scholar] [CrossRef]
- Katis, I.N.; He, P.J.W.; Eason, R.W.; Sones, C.L. Improved sensitivity and limit-of-detection of lateral flow devices using spatial constrictions of the flow-path. Biosens. Bioelectron. 2018, 113, 95–100. [Google Scholar] [CrossRef]
- Xiong, Y.; Pei, K.; Wu, Y.; Duan, H.; Lai, W.; Xiong, Y. Plasmonic ELISA based on enzyme-assisted etching of Au nanorods for the highly sensitive detection of aflatoxin B1 in corn samples. Sens. Actuators B Chem. 2018, 267, 320–327. [Google Scholar] [CrossRef]
- Fang, B.; Hu, S.; Wang, C.; Yuan, M.; Huang, Z.; Xing, K.; Liu, D.; Peng, J.; Lai, W. Lateral flow immunoassays combining enrichment and colorimetry-fluorescence quantitative detection of sulfamethazine in milk based on trifunctional magnetic nanobeads. Food Control 2019, 98, 268–273. [Google Scholar] [CrossRef]
- Tang, D.; Sauceda, J.C.; Lin, Z.; Ott, S.; Basova, E.; Goryacheva, I.; Biselli, S.; Lin, J.; Niessner, R.; Knopp, D. Magnetic nanogold microspheres-based lateral-flow immunodipstick for rapid detection of aflatoxin B2 in food. Biosens. Bioelectron. 2009, 25, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wei, H.P. Determination of reduced glutathione by Fluorescence quenching and recovery method based on ZnS quantum dots. Anal. Chem. 2013, 41, 1102–1106. [Google Scholar]
- Zhang, Y.; Wang, L.; Wang, W.-L.; Yang, C.; Feng, Y.; Shi, X. Visual-afterglow dual-mode immunochromatographic strip for 17β-estradiol detection in milk. Talanta 2021, 232, 122427. [Google Scholar] [CrossRef] [PubMed]
- You, P.Y.; Li, F.C.; Liu, M.H.; Chan, Y.H. Colorimetric and fluorescent dual-mode immunoassay based on plasmon enhanced fluorescence of polymer dots for detection of PSA in whole-blood. ACS Appl. Mater. Interfaces 2019, 11, 9841–9849. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, X.; Zhang, M.; Sun, X.; Bai, J.; Peng, Y.; Li, S.; Han, D.; Ren, S.; Wang, J.; et al. A fluorescent amplification strategy for high- sensitive detection of 17β-estradiol based on EXPAR and HCR. Anal. Chim. Acta 2020, 1116, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.M.; Luo, K.J.; Han, J.J.; Zhang, G.G.; Liu, M.; Lai, W.H. Advantages of time-resolved fluorescent nanobeads compared with fluorescent submicrospheres, quantum dots, and colloidal gold as label in lateral flow assays for detection of ractopamine. Biosens. Bioelectron. 2017, 91, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Majdinasab, M.; Zareian, M.; Zhang, Q.; Li, P. Development of a new format of competitive immunochromatographic assay using secondary antibody–europium nanoparticle conjugates for ultrasensitive and quantitative determination of ochratoxin A. Food Chem. 2019, 275, 721–729. [Google Scholar] [CrossRef]
- Deng, H.; Liu, Q.; Wang, X.; Huang, R.; Liu, H.; Lin, Q.; Zhou, X.; Xing, D. Quantum dots-labeled strip biosensor for rapid and sensitive detection of microRNA based on target-recycled nonenzymatic amplification strategy. Biosens. Bioelectron. 2017, 87, 931–940. [Google Scholar] [CrossRef]
- Deng, X.; Wang, C.; Gao, Y.; Li, J.; Wen, W.; Zhang, X.; Wang, S. Applying strand displacement amplification to quantum dots-based fluorescent lateral flow assay strips for HIV-DNA detection. Biosens. Bioelectron. 2018, 105, 211–217. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Cui, M.; Tian, Y.; Zhang, S.; Peng, K.; Xu, H.; Liao, Z.; Wang, H.; Chang, J. A smartphone-based quantitative detection platform of mycotoxins based on multiple-color upconversion nanoparticles. Nanoscale 2018, 10, 15865–15874. [Google Scholar] [CrossRef]
- Jin, B.; Yang, Y.; He, R.; Lee, A.; Bai, D.; Li, F.; Lu, T.J.; Xu, F.; Lin, M. Lateral flow aptamer assay integrated smartphone-based portable device for simultaneous detection of multiple targets using upconversion nanoparticles. Sens. Actuators B Chem. 2018, 276, 48–56. [Google Scholar] [CrossRef]
- Xie, H.Y.; Pang, D.W. Preparation and application of type Ⅱ-Ⅵ quantum Dots in biological detection. Anal. Chem. 2004, 8, 1099–1103. [Google Scholar]
- Yang, D.Z.; Xu, S.K.; Chen, Q.F. Application of fluorescence properties of quantum dots in biological probes. Spectrosc. Spectr. Anal. 2007, 27, 1807–1810. [Google Scholar]
- Yan, L.L.; Kelong, A.; Le, H.L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar]
- Chen, X.; Huang, Y.; Yang, G.; Li, J.; Wang, T.; Schulz, O.H.; Jennings, L.K. Polydopamine integrated nanomaterials and their biomedical applications. Curr. Pharm. Des. 2015, 21, 4262–4275. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Hua, P.P.; Ru, P.L.; Li, Z.; Jian, D.Q. Facile preparation of novel core-shell enzyme-Au-polydopamine-Fe₃O₄ magnetic bionanoparticles for glucosesensor. Biosens. Bioelectron. 2013, 42, 293–299. [Google Scholar]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine surface chemistry: A decade of discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Ma, H.; Li, S.; Zhang, H.; Wei, Y.; Jiang, L. Fabrication of polydopamine-based layer-by-layer nanocomposites for combined pH-sensitive chemotherapy and photothermal therapy. Colloids Surf. A Physicochem. Eng. Asp. 2019, 561, 332–340. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, W.Q.; Chen, Q.; Wang, S.; Mao, Y.; Gong, X.L.; Leung, K.C.; Tian, J.; Wang, H.J.; Xuan, S.H. A facile one-step method to synthesize SiO2@polydopamine core−shell nanospheres for shear thickening fluid. RSC Adv. 2016, 6, 29279–29287. [Google Scholar] [CrossRef]
- Shen, H.; Xu, F.; Xiao, M. A new lateral-flow immunochromatographic strip combined with quantum dot nanobeads and gold nanoflowers for rapid detection of tetrodotoxin. Analyst 2017, 142, 4393–4398. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, L.; Di Nardo, F.; Cavalera, S.; Giovannoli, C.; Spano, G.; Speranskaya, E.S.; Goryacheva, I.Y.; Baggiani, C. A lateral flow immunoassay for straightforward determination of fumonisin mycotoxins based on the quenching of the fluorescence of CdSe/ZnS quantum dots by gold and silver nanoparticles. Microchim. Acta 2018, 185, 94. [Google Scholar] [CrossRef] [PubMed]
- Pease, L.F.; Elliott, J.T.; Tsai, D.H. Determination of protein aggregation with differential mobility analysis: Application to IgG antibody. Biotechnol. Bioeng. 2008, 101, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.D.; Nogueira, B.R.; Rostelato, M.E.C.M. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compd. 2019, 798, 714–740. [Google Scholar] [CrossRef]
- Zhang, L.; Su, H.L.; Cai, J.L.; Cheng, D.F.; Ma, Y.J.; Zhang, J.P.; Zhou, C.Q.; Liu, S.Y.; Shi, H.C.; Zhang, Y.J.; et al. A multifunctional platform for tumor angiogenesis-targeted chemo-thermal therapy using polydopamine-coated gold nanorods. ACS Nano 2016, 10, 10404–10417. [Google Scholar] [CrossRef]
- Sureshkumar, M.; Lee, C.K. Polydopamine coated magnetic-chitin (MCT) particles as a new matrix for enzyme immobilization. Carbohydr. Polym. 2011, 84, 775–780. [Google Scholar] [CrossRef]
- Brandon, D.L.; Binder, R.G.; Bates, A.H. Monoclonal antibody for multiresidue ELISA of benzimidazole anthelmintics in liver. J. Agric. Food Chem. 1994, 42, 1588–1594. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Peng, D.P.; Pan, Y.H.; Wang, Y.L.; Chen, D.M.; Zhou, Q.; Liu, Z.L.; Yuan, Z.H. A novel hapten and monoclonal-based enzymelinked immunosorbent assay for 3-methyl-quinoxaline-2-carboxylic acid in edible animal tissues. Anal. Methods 2015, 7, 6588–6594. [Google Scholar] [CrossRef]
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing albumin as a carrier for cancer therapies. Adv. Drug Deliv. Rev. 2018, 130, 73–89. [Google Scholar] [CrossRef]
- Zhou, Z.; Yu, M.; Yang, H. FRET-based sensor for imaging chromium (III) in living cells. Chem. Commun. 2008, 29, 3387–3389. [Google Scholar] [CrossRef] [Green Version]
- Li, S.L.; Zhong, X.C.; Jian, B.C.; Kai, M.K.; Qiao, L.P.; Jin, H.G.; Huang, H.Y.; Gang, L.; Xiao, Y.C. Multifunctional Fe₃O₄@polydopamine core-shell nanocomposites for intracellular mRNA detection and imaging-guided photothermal therapy. ACS Nano 2014, 8, 3876–3883. [Google Scholar]
- Jiang, W.H.; Wu, X.S.; Cu, N.; Peng, Z.X.; Cui, H.F.; Wan, Y.P. Establishment of a rapid detection method for carbendazim enzyme-linked immunosorbent. Food Mach. 2021, 37, 94–98. [Google Scholar]
- Wan, Y.P.; Wu, X.S.; Gu, F.F.; Cui, N.; He, F.Y. Study on Gold Immunochromatography Assay for Rapid Detection of Carbendazim. Food Ind. 2021, 42, 279–282. [Google Scholar]
- Wang, J.; Li, Z.J. Synthesis of aspartic acid-functionalized graphene quantum dot-gold composite and its application in electrochemical detection of chlorpyrifos, carbendazim and acetamiprid in apple. Anal. Lab. 2021, 1–10. [Google Scholar] [CrossRef]
- Fan, N.Y.; Cai, X.; Li, C.X. Determination of carbendazim residues in vegetables by spectrophotometry. Gansu Sci. Technol. 2009, 25, 52–53. [Google Scholar]

| Sample | Concentration of CBD (ng/mL) | 5% Methanol-PBS | Dilution | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Colorimetry Signal | Fluorescence Signal | Colorimetry Signal | Fluorescence Signal | ||||||||
| 2× | 5× | 10× | 20× | 2× | 5× | 10× | 20× | ||||
| Strawberry | 0 | – | – | – | – | – | – | – | – | – | – |
| 250 | ± | + | – | ± | ± | ± | – | ± | + | + | |
| 500 | + | + | – | ± | + | + | – | ± | + | + | |
| Cucumber | 0 | – | – | – | – | – | – | – | – | – | – |
| 250 | ± | + | – | ± | ± | ± | – | ± | + | + | |
| 500 | + | + | – | ± | + | + | – | ± | + | + | |
| Sample | Concentration of CBD (mg/kg) | Au@PDAs-QDs-LFIA | LC—MS/MS | |||
|---|---|---|---|---|---|---|
| Colorimetry Signal | Fluorescence Signal | Found (mg/kg) | Recovery (%) | CV (%) | ||
| Strawberry | 0.25 | – | + | 0.247 | 98.6 | 2.9 |
| 0.5 | ± | + | 0.450 | 90.1 | 6.2 | |
| 1 | ± | + | 0.816 | 81.6 | 2.8 | |
| Cucumber | 1 | ± | + | 0.872 | 87.2 | 3.1 |
| 2 | ± | + | 1.58 | 79.0 | 5.2 | |
| 4 | + | + | 3.708 | 92.7 | 4.8 | |
| Sample | Sample Number | Au@PDAs-QDs-LFIA | LC—MS/MS | |
|---|---|---|---|---|
| Colorimetry Signal | Fluorescence Signal | Found (ng/mL) | ||
| Strawberry | 1 | - | - | ND |
| 2 | - | - | ND | |
| 3 | - | - | ND | |
| 4 | - | - | ND | |
| 5 | - | - | ND | |
| 6 | - | - | ND | |
| 7 | - | + | 23 ± 0.015 | |
| 8 | - | - | ND | |
| 9 | - | + | 48 ± 0.076 | |
| 10 | - | - | ND | |
| Cucumber | 1 | - | - | ND |
| 2 | - | + | 16 ± 0.036 | |
| 3 | - | - | ND | |
| 4 | - | - | ND | |
| 5 | - | - | ND | |
| 6 | - | - | ND | |
| 7 | - | - | ND | |
| 8 | - | - | ND | |
| 9 | - | - | ND | |
| 10 | - | - | ND | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, X.; Wang, Y.; Jiang, L.; Zhang, H.; Zhao, Y.; Liu, P.; Liu, J.; Hammock, B.D.; Zhang, C. A Polydopamine-Coated Gold Nanoparticles Quenching Quantum Dots-Based Dual-Readout Lateral Flow Immunoassay for Sensitive Detection of Carbendazim in Agriproducts. Biosensors 2022, 12, 83. https://doi.org/10.3390/bios12020083
Mao X, Wang Y, Jiang L, Zhang H, Zhao Y, Liu P, Liu J, Hammock BD, Zhang C. A Polydopamine-Coated Gold Nanoparticles Quenching Quantum Dots-Based Dual-Readout Lateral Flow Immunoassay for Sensitive Detection of Carbendazim in Agriproducts. Biosensors. 2022; 12(2):83. https://doi.org/10.3390/bios12020083
Chicago/Turabian StyleMao, Xinxin, Yulong Wang, Lan Jiang, Hanxiaoya Zhang, Yun Zhao, Pengyan Liu, Juanjuan Liu, Bruce D. Hammock, and Cunzheng Zhang. 2022. "A Polydopamine-Coated Gold Nanoparticles Quenching Quantum Dots-Based Dual-Readout Lateral Flow Immunoassay for Sensitive Detection of Carbendazim in Agriproducts" Biosensors 12, no. 2: 83. https://doi.org/10.3390/bios12020083
APA StyleMao, X., Wang, Y., Jiang, L., Zhang, H., Zhao, Y., Liu, P., Liu, J., Hammock, B. D., & Zhang, C. (2022). A Polydopamine-Coated Gold Nanoparticles Quenching Quantum Dots-Based Dual-Readout Lateral Flow Immunoassay for Sensitive Detection of Carbendazim in Agriproducts. Biosensors, 12(2), 83. https://doi.org/10.3390/bios12020083





