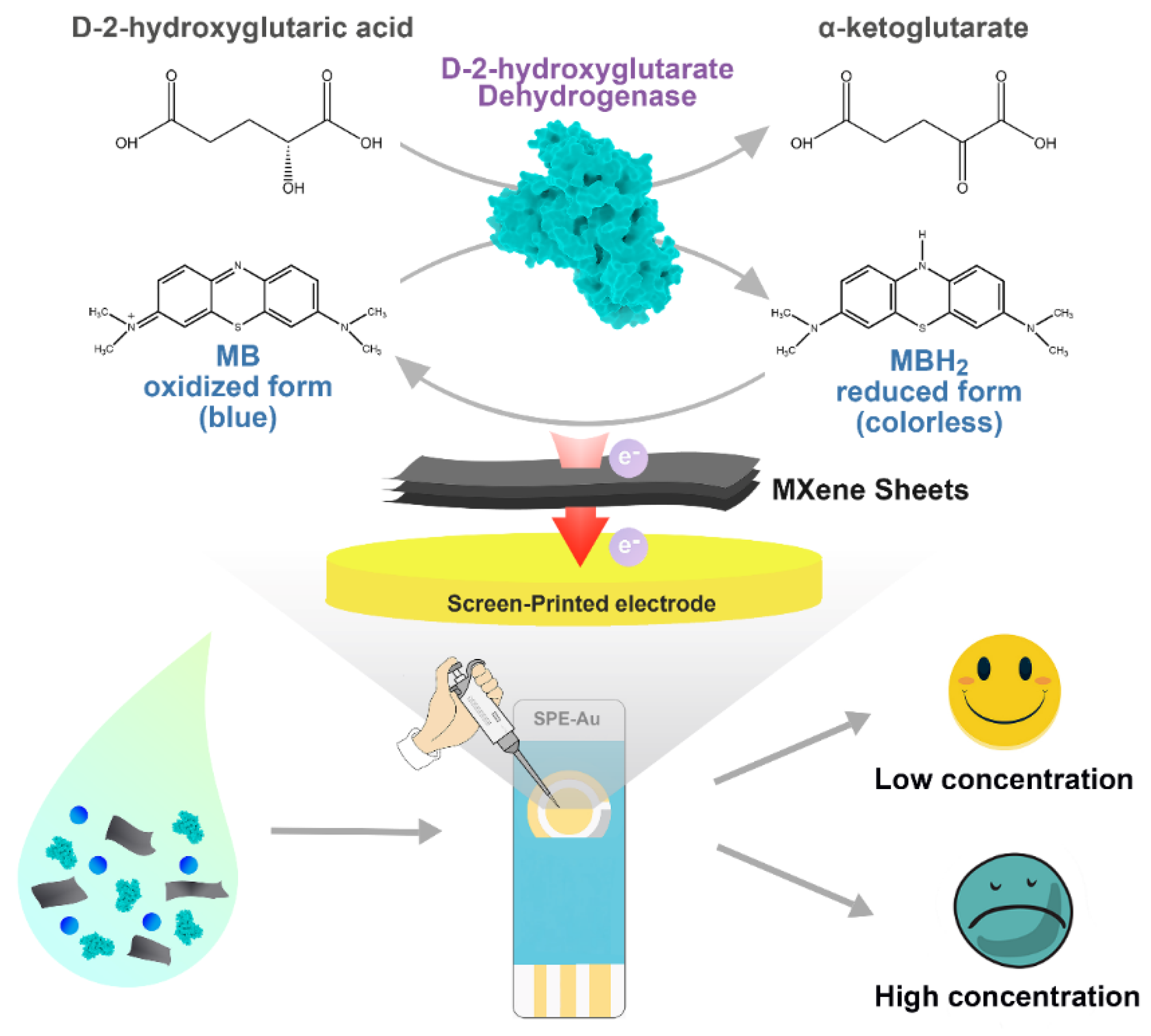
An Enzymatic Biosensor for the Detection of D-2-Hydroxyglutaric Acid in Serum and Urine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus and Reagents
2.2. Synthesis of MXene
2.3. Preparation and Assays of RsD2HGDH
2.4. Construction of MXene-Based Biosensor
2.5. Construction of MXene-Based Biosensor
3. Results
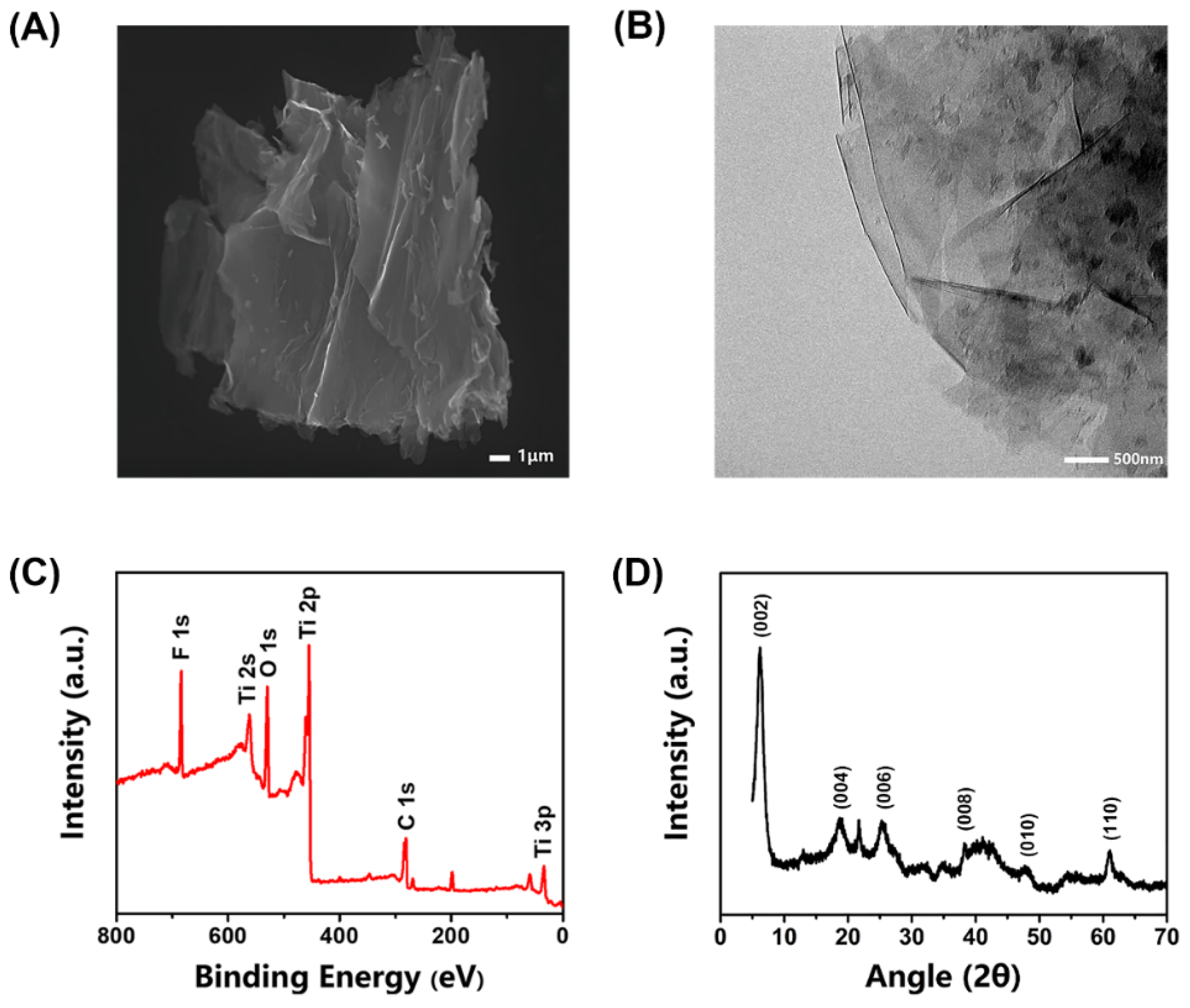
3.1. Characterization of MXene
3.2. Characterization of RsD2HGDH
3.3. Construction of D2HG Biosensor
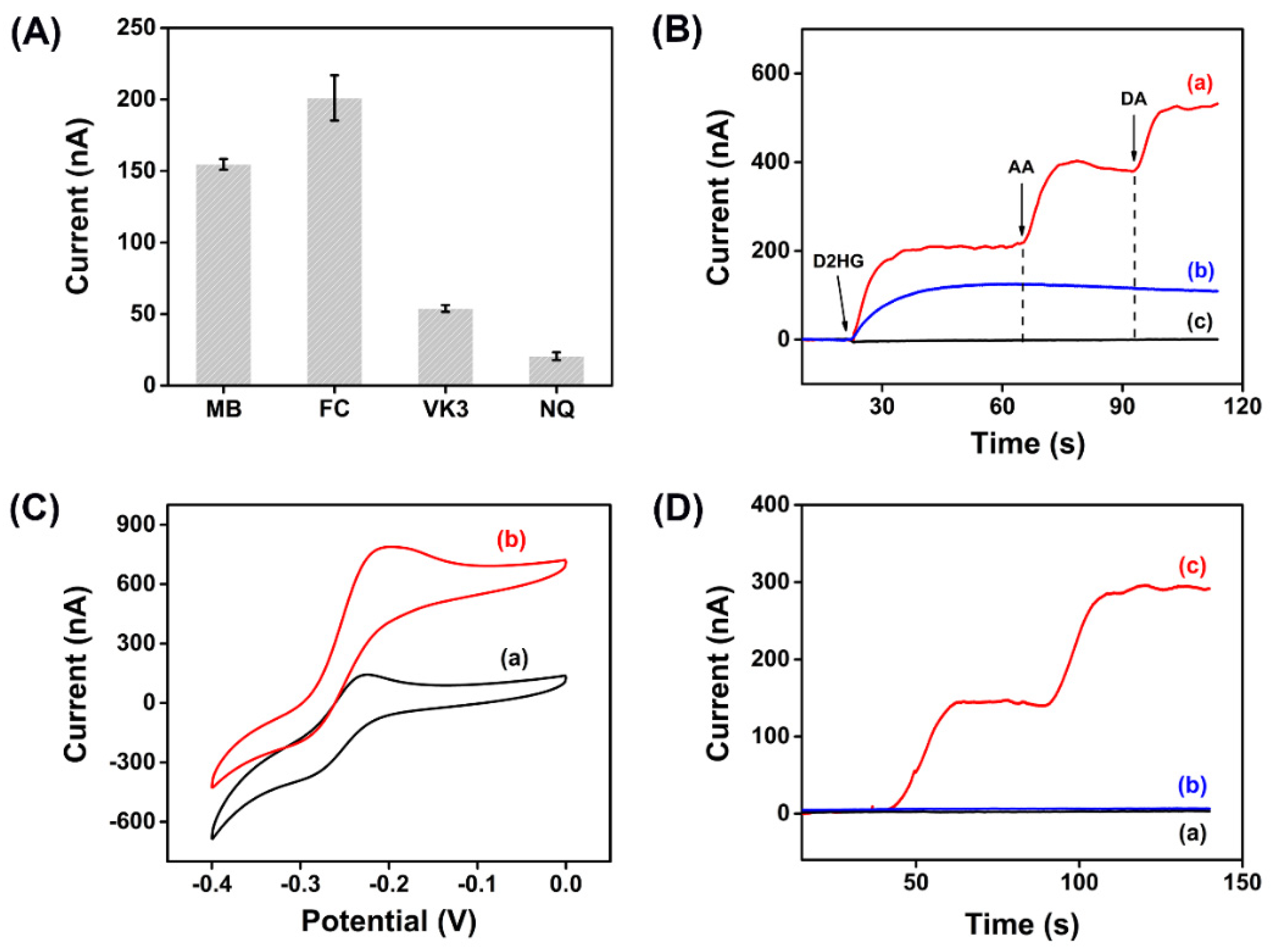
3.4. Optimization of Experiment Parameters
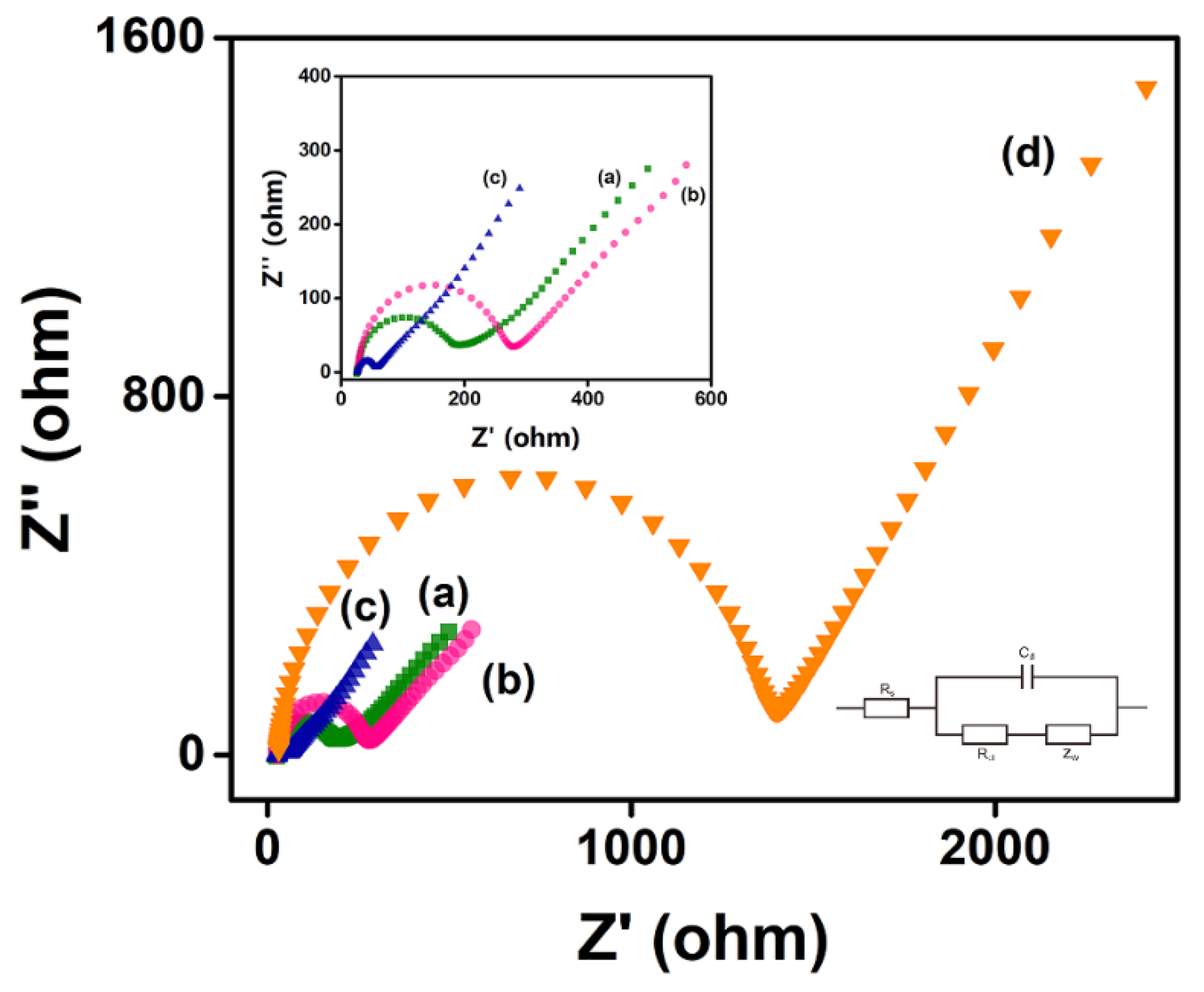
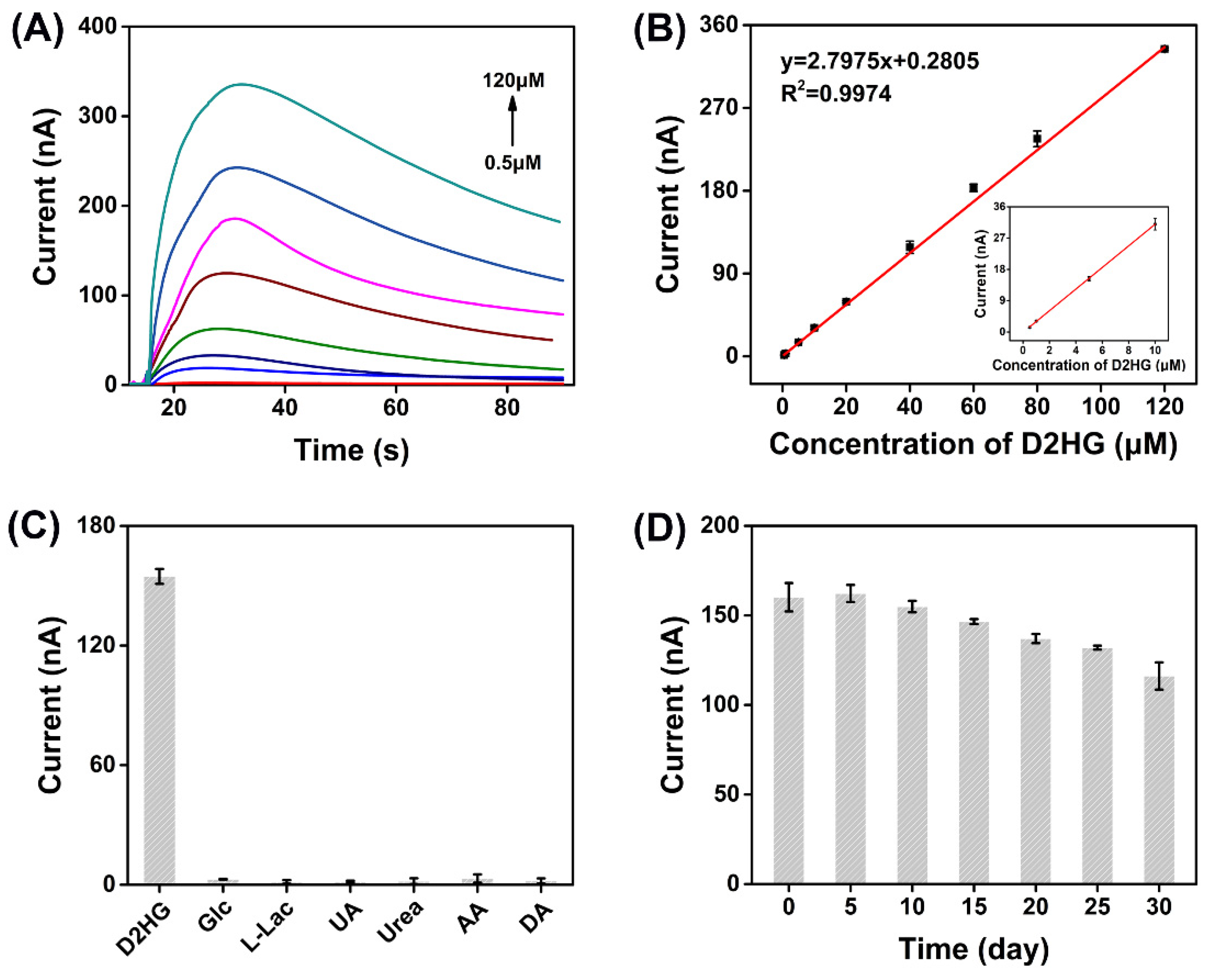
3.5. Electrochemical Measurement
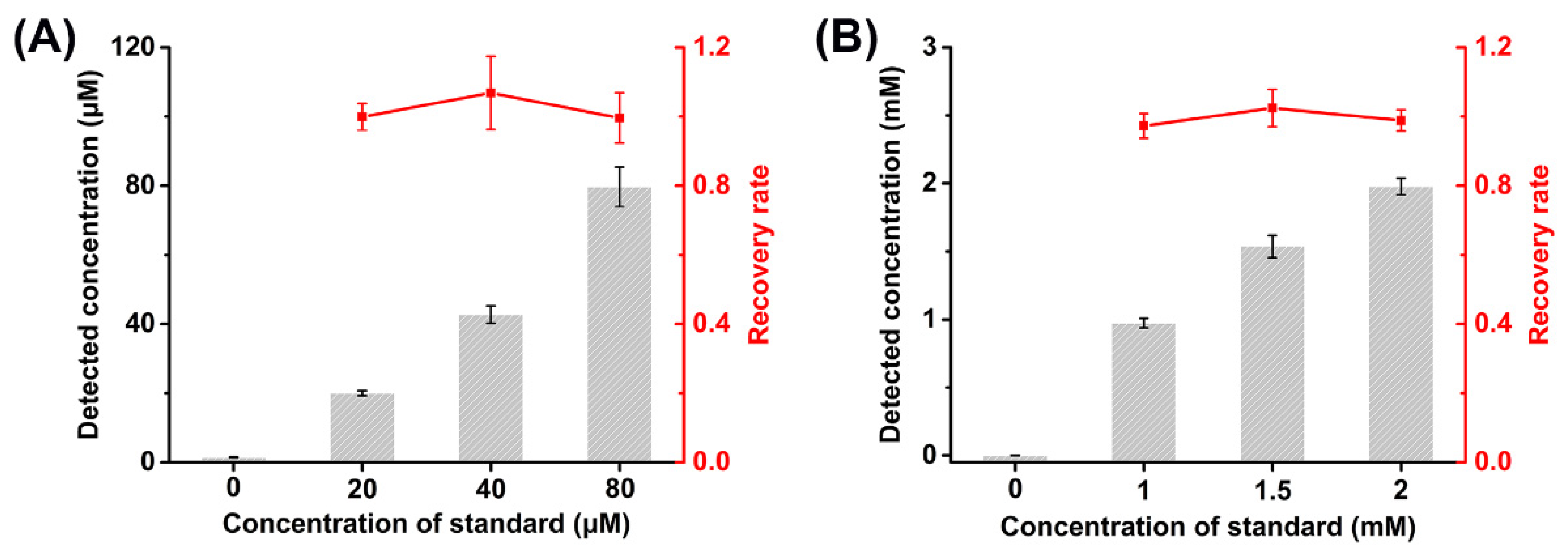
3.6. Real Sample Analysis
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Araujo, W.L.; Ishizaki, K.; Nunes-Nesi, A.; Larson, T.R.; Tohge, T.; Krahnert, I.; Witt, S.; Obata, T.; Schauer, N.; Graham, I.A.; et al. Identification of the 2-Hydroxyglutarate and Isovaleryl-Coa Dehydrogenases as Alternative Electron Donors Linking Lysine Catabolism to the Electron Transport Chain of Arabidopsis Mitochondria. Plant Cell 2010, 22, 1549–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Knaap, M.S.; Jakobs, C.; Hoffmann, G.F.; Duran, M.; Muntau, A.C.; Schweitzer, S.; Kelley, R.I.; Parrot-Roulaud, F.; Amiel, J.; De Lonlay, P.; et al. D-2-Hydroxyglutaric Aciduria: Further Clinical Delineation. J. Inherited Metab. Dis. 1999, 22, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Toplak, M.; Brunner, J.; Schmidt, J.; Macheroux, P. Biochemical Characterization of Human D-2-Hydroxyglutarate Dehydrogenase and Two Disease Related Variants Reveals the Molecular Cause of D-2-Hydroxyglutaric Aciduria. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 140255. [Google Scholar] [CrossRef] [PubMed]
- Struys, E.A. D-2-Hydroxyglutaric Aciduria: Unravelling the Biochemical Pathway and the Genetic Defect. J. Inherit. Metab. Dis. 2006, 29, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Van der Knaap, M.S.; Jakobs, C.; Hoffmann, G.F.; Nyhan, W.L.; Renier, W.O.; Smeitink, J.A.; Catsman-Berrevoets, C.E.; Hjalmarson, O.; Vallance, H.; Sugita, K.; et al. D-2-Hydroxyglutaric Aciduria: Biochemical Marker or Clinical Disease Entity? Ann. Neurol. 1999, 45, 111–119. [Google Scholar] [CrossRef]
- Parsons, D.W.; Jones, S.; Zhang, X.; Lin, J.C.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Siu, I.M.; Gallia, G.L.; et al. An Integrated Genomic Analysis of Human Glioblastoma Multiforme. Science 2008, 321, 1807–1812. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Dong, Q.; Zhang, C.; Kuan, P.F.; Liu, Y.; Jeck, W.R.; Andersen, J.B.; Jiang, W.; Savich, G.L.; Tan, T.X.; et al. Mutations in Isocitrate Dehydrogenase 1 and 2 Occur Frequently in Intrahepatic Cholangiocarcinomas and Share Hypermethylation Targets with Glioblastomas. Oncogene 2013, 32, 3091–3100. [Google Scholar] [CrossRef] [Green Version]
- Amary, M.F.; Bacsi, K.; Maggiani, F.; Damato, S.; Halai, D.; Berisha, F.; Pollock, R.; O’Donnell, P.; Grigoriadis, A.; Diss, T.; et al. IDH1 and IDH2 Mutations Are Frequent Events in Central Chondrosarcoma and Central and Periosteal Chondromas but Not in Other Mesenchymal Tumours. J. Pathol. 2011, 224, 334–343. [Google Scholar] [CrossRef]
- Marcucci, G.; Maharry, K.; Wu, Y.Z.; Radmacher, M.D.; Mrozek, K.; Margeson, D.; Holland, K.B.; Whitman, S.P.; Becker, H.; Schwind, S.; et al. IDH1 and IDH2 Gene Mutations Identify Novel Molecular Subsets within De Novo Cytogenetically Normal Acute Myeloid Leukemia: A Cancer and Leukemia Group B Study. J. Clin. Oncol. 2010, 28, 2348–2355. [Google Scholar] [CrossRef] [Green Version]
- Thol, F.; Damm, F.; Wagner, K.; Gohring, G.; Schlegelberger, B.; Hoelzer, D.; Lubbert, M.; Heit, W.; Kanz, L.; Schlimok, G.; et al. Prognostic Impact of IDH2 Mutations in Cytogenetically Normal Acute Myeloid Leukemia. Blood 2010, 116, 614–616. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.; Ganji, S.K.; DeBerardinis, R.J.; Hatanpaa, K.J.; Rakheja, D.; Kovacs, Z.; Yang, X.L.; Mashimo, T.; Raisanen, J.M.; Marin-Valencia, I.; et al. 2-Hydroxyglutarate Detection by Magnetic Resonance Spectroscopy in IDH-Mutated Patients with Gliomas. Nat. Med. 2012, 18, 624–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahm, F.; Capper, D.; Pusch, S.; Balss, J.; Koch, A.; Langhans, C.D.; Okun, J.G.; von Deimling, A. Detection of 2-Hydroxyglutarate in Formalin-Fixed Paraffin-Embedded Glioma Specimens by Gas Chromatography/Mass Spectrometry. Brain Pathol. 2012, 22, 26–31. [Google Scholar] [CrossRef]
- Pope, W.B.; Prins, R.M.; Albert Thomas, M.; Nagarajan, R.; Yen, K.E.; Bittinger, M.A.; Salamon, N.; Chou, A.P.; Yong, W.H.; Soto, H.; et al. Non-Invasive Detection of 2-Hydroxyglutarate and Other Metabolites in IDH1 Mutant Glioma Patients Using Magnetic Resonance Spectroscopy. J. Neurooncol. 2012, 107, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldham, W.M.; Loscalzo, J. Quantification of 2-Hydroxyglutarate Enantiomers by Liquid Chromatography-Mass Spectrometry. Bio Protoc 2016, 6, e1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.; Li, Z.; Guo, Z.; Shao, S.; Mo, L.; Wei, W.; Xue, M. Single-Cell Profiling of D-2-Hydroxyglutarate Using Surface-Immobilized Resazurin Analogs. Biosens. Bioelectron. 2021, 190, 113368. [Google Scholar] [CrossRef] [PubMed]
- Balss, J.; Pusch, S.; Beck, A.C.; Herold-Mende, C.; Kramer, A.; Thiede, C.; Buckel, W.; Langhans, C.D.; Okun, J.G.; von Deimling, A. Enzymatic Assay for Quantitative Analysis of (D)-2-Hydroxyglutarate. Acta Neuropathol. 2012, 124, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, G.; Yan, X.; Zhu, D.; Lin, P.P.; Wang, Z.; Qu, H.; He, X.; Fu, Y.; Zhu, X.; et al. Fresh Tissue Multi-Omics Profiling Reveals Immune Classification and Suggests Immunotherapy Candidates for Conventional Chondrosarcoma. Clin. Cancer. Res. 2021, 27, 6543–6558. [Google Scholar] [CrossRef]
- Malarz, K.; Mularski, J.; Pacholczyk, M.; Musiol, R. The Landscape of the Anti-Kinase Activity of the IDH1 Inhibitors. Cancers 2020, 12, 536. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.D.; Jang, Y.H.; Yoon, H.C. Cascadic Multienzyme Reaction-Based Electrochemical Biosensors. Adv. Biochem. Eng. Biotechnol. 2014, 140, 221–251. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef] [Green Version]
- Bollella, P.; Gorton, L. Enzyme Based Amperometric Biosensors. Curr. Opin. Electrochem. 2018, 10, 157–173. [Google Scholar] [CrossRef]
- Newman, J.D.; Setford, S.J. Enzymatic Biosensors. Mol. Biotechnol. 2006, 32, 249–268. [Google Scholar] [CrossRef]
- Trojanowicz, M.; vel Krawczyk, T.K. Electrochemical Biosensors Based on Enzymes Immobilized in Electropolymerized Films. Mikrochim. Acta 1995, 121, 167–181. [Google Scholar] [CrossRef]
- Alvarado-Ramirez, L.; Rostro-Alanis, M.; Rodriguez-Rodriguez, J.; Sosa-Hernandez, J.E.; Melchor-Martinez, E.M.; Iqbal, H.M.N.; Parra-Saldivar, R. Enzyme (Single and Multiple) and Nanozyme Biosensors: Recent Developments and Their Novel Applications in the Water-Food-Health Nexus. Biosensors 2021, 11, 410. [Google Scholar] [CrossRef]
- Terry, L.A.; White, S.F.; Tigwell, L.J. The Application of Biosensors to Fresh Produce and the Wider Food Industry. J. Agric. Food Chem. 2005, 53, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.F.; Li, Y.T.; Yang, V.C. Biomedical Application of Immobilized Enzymes. J. Pharm. Sci. 2000, 89, 979–990. [Google Scholar] [CrossRef] [Green Version]
- Mueller, A. Enzyme Electrodes for Medical Sensors. Mini Rev. Med. Chem. 2005, 5, 231–239. [Google Scholar] [CrossRef]
- Zuo, S.; Teng, Y.; Yuan, H.; Lan, M. Development of a Novel Silver Nanoparticles-Enhanced Screen-Printed Amperometric Glucose Biosensor. Anal. Lett. 2008, 41, 1158–1172. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Caruana, D.J. Electrochemical Immobilization of Enzymes. Part V. Microelectrodes for the Detection of Glucose Based on Glucose Oxidase Immobilized in a Poly(Phenol) Film. The Analyst 1992, 117, 1287–1292. [Google Scholar] [CrossRef]
- Ahmad, M.; Pan, C.F.; Luo, Z.X.; Zhu, J. A Single ZnO Nanofiber-Based Highly Sensitive Amperometric Glucose Biosensor. J. Phys. Chem. C 2010, 114, 9308–9313. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Xie, W.; Zhou, Y.; Zhang, Z.; Cass, A. Genetic Modification of Glucose Oxidase for Improving Performance of an Amperometric Glucose Biosensor. Biosens. Bioelectron. 2002, 17, 851–857. [Google Scholar] [CrossRef]
- Zhao, K.; Veksha, A.; Ge, L.; Lisak, G. Near Real-Time Analysis of Para-Cresol in Wastewater with a Laccase-Carbon Nanotube-Based Biosensor. Chemosphere 2021, 269, 128699. [Google Scholar] [CrossRef] [PubMed]
- Özbek, M.A.; Yaşar, A.; Çete, S.; Er, E.; Erk, N. A Novel Biosensor Based on Graphene/Platinum Nanoparticles/Nafion Composites for Determination of Glucose. J. Solid State Electrochem. 2021, 25, 1601–1610. [Google Scholar] [CrossRef]
- Deshmukh, K.; Kovářík, T.; Khadheer Pasha, S.K. State of the Art Recent Progress in Two Dimensional MXenes Based Gas Sensors and Biosensors: A Comprehensive Review. Coord. Chem. Rev. 2020, 424, 213514. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Shin, M.; Lim, J.; Lee, J.Y.; Choi, J.W. Recent Advances in MXene Nanocomposite-Based Biosensors. Biosensors 2020, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lu, X.; Dhanjai; Wu, Z.S.; Dong, Y.; Wang, X.; Zheng, S.; Chen, J. 2D Transition Metal Carbide MXene as a Robust Biosensing Platform for Enzyme Immobilization and Ultrasensitive Detection of Phenol. Biosens. Bioelectron. 2018, 107, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Koyappayil, A.; Chavan, S.G.; Mohammadniaei, M.; Go, A.; Hwang, S.Y.; Lee, M.H. β-Hydroxybutyrate Dehydrogenase Decorated MXene Nanosheets for the Amperometric Determination of β-Hydroxybutyrate. Mikrochim. Acta 2020, 187, 277. [Google Scholar] [CrossRef]
- Hroncekova, S.; Bertok, T.; Hires, M.; Jane, E.; Lorencova, L.; Vikartovska, A.; Tanvir, A.; Kasak, P.; Tkac, J. Ultrasensitive Ti3C2Tx MXene/Chitosan Nanocomposite-Based Amperometric Biosensor for Detection of Potential Prostate Cancer Marker in Urine Samples. Processes 2020, 8, 580. [Google Scholar] [CrossRef]
- Xia, T.; Liu, G.; Wang, J.; Hou, S.; Hou, S. MXene-Based Enzymatic Sensor for Highly Sensitive and Selective Detection of Cholesterol. Biosens. Bioelectron. 2021, 183, 113243. [Google Scholar] [CrossRef]
- Muntau, A.C.; Roschinger, W.; Merkenschlager, A.; van der Knaap, M.S.; Jakobs, C.; Duran, M.; Hoffmann, G.F.; Roscher, A.A. Combined D-2- and L-2-Hydroxyglutaric Aciduria with Neonatal Onset Encephalopathy: A Third Biochemical Variant of 2-Hydroxyglutaric Aciduria? Neuropediatrics 2000, 31, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Urbankowski, P.; Anasori, B.; Hantanasirisakul, K.; Yang, L.; Zhang, L.; Haines, B.; May, S.J.; Billinge, S.J.L.; Gogotsi, Y. 2D Molybdenum and Vanadium Nitrides Synthesized by Ammoniation of 2D Transition Metal Carbides (MXenes). Nanoscale 2017, 9, 17722–17730. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, M.; Cao, M.; Zhang, W.; Kang, Z.; Xu, P.; Ma, C.; Gao, C. D-2-Hydroxyglutarate Dehydrogenase Plays a Dual Role in L-Serine Biosynthesis and D-Malate Utilization in the Bacterium Pseudomonas Stutzeri. J. Biol. Chem. 2018, 293, 15513–15523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozmysłowska-Wojciechowska, A.; Wojciechowski, T.; Ziemkowska, W.; Chlubny, L.; Olszyna, A.; Jastrzębska, A.M. Surface Interactions between 2D Ti3C2/Ti2C MXenes and Lysozyme. Appl. Surf. Sci. 2019, 473, 409–418. [Google Scholar] [CrossRef]
- Halim, J.; Cook, K.M.; Naguib, M.; Eklund, P.; Gogotsi, Y.; Rosen, J.; Barsoum, M.W. X-Ray Photoelectron Spectroscopy of Select Multi-Layered Transition Metal Carbides (MXenes). Appl. Surf. Sci. 2016, 362, 406–417. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Yin, X.; Wu, H.; Hou, Z.; Song, C.; Li, X.; Zhang, L.; Cheng, L. Ti3C2 MXenes with Modified Surface for High-Performance Electromagnetic Absorption and Shielding in the X-Band. ACS Appl. Mater. Interfaces 2016, 8, 21011–21019. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Khazaei, M.; Arai, M.; Sasaki, T.; Chung, C.-Y.; Venkataramanan, N.S.; Estili, M.; Sakka, Y.; Kawazoe, Y. Novel Electronic and Magnetic Properties of Two-Dimensional Transition Metal Carbides and Nitrides. Adv. Funct. Mater. 2013, 23, 2185–2192. [Google Scholar] [CrossRef]
- Das, G.; Prabhu, K.A. Immobilization of Cane Amylase and Acid Phosphatase on Tricalcium Phosphate (TCP) Gel. Enzyme Microb. Technol. 1990, 12, 625–630. [Google Scholar] [CrossRef]
- Lu, X.; Wen, Z.; Li, J. Hydroxyl-Containing Antimony Oxide Bromide Nanorods Combined with Chitosan for Biosensors. Biomaterials 2006, 27, 5740–5747. [Google Scholar] [CrossRef]
- Hichem, H.; Djamila, A.; Hania, A. Optical, Electrical and Photoelectrochemical Characterization of Electropolymerized Poly Methylene Blue on Fluorine Doped Tin Oxide Conducting Glass. Electrochim. Acta 2013, 106, 69–74. [Google Scholar] [CrossRef]
- Liu, J.C.; Mu, S.L. The Electrochemical Polymerization of Methylene Blue and Properties of Polymethylene Blue. Synth. Met. 1999, 107, 159–165. [Google Scholar] [CrossRef]
- Feng, J.J.; Zhao, G.; Xu, J.J.; Chen, H.Y. Direct Electrochemistry and Electrocatalysis of Heme Proteins Immobilized on Gold Nanoparticles Stabilized by Chitosan. Anal. Biochem. 2005, 342, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Gao, G.; Ma, C.; Li, Y.; Shi, J.; Zhou, X.; Zhu, Z. A Hybrid System Integrating Xylose Dehydrogenase and NAD+ Coupled with PtNPs@MWCNTs Composite for the Real-Time Biosensing of Xylose. Analyst 2020, 145, 5563–5570. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.M.; ten Brink, H.J.; Schor, D.S.; Kok, R.M.; Bootsma, A.H.; Hoffmann, G.F.; Jakobs, C. Stable-Isotope Dilution Analysis of D- and L-2-Hydroxyglutaric Acid: Application to the Detection and Prenatal Diagnosis of D- and L-2-Hydroxyglutaric Acidemias. Pediatr. Res. 1993, 34, 277–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, C.; Sweha, S.R.; Pratt, D.; Tamrazi, B.; Panwalkar, P.; Banda, A.; Bayliss, J.; Hawes, D.; Yang, F.; Lee, H.J.; et al. Integrated Metabolic and Epigenomic Reprograming by H3K27M Mutations in Diffuse Intrinsic Pontine Gliomas. Cancer Cell 2020, 38, 334–349.e339. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Zhang, W.; Guo, X.; Liu, Y.; Hu, C.; Guo, S.; Kang, Z.; Xu, X.; Ma, C.; Gao, C.; et al. A D-2-Hydroxyglutarate Biosensor Based on Specific Transcriptional Regulator DhdR. Nat. Commun. 2021, 12, 7108. [Google Scholar] [CrossRef]
| Detection Method | Sensing Element | Coenzyme | LOD (μM) | Linear Range (μM) | Detection Time | Stability | Ref |
|---|---|---|---|---|---|---|---|
| Colorimetry | D2HGDH | NAD+ * | 10 | - | 37 °C, 60 min | −20 °C, <60 days | assay kit |
| Fluorometry | D2HGDH | NAD+ | 2.2 | 1−100 | 37 °C, 60 min | - | [16] |
| Fluorometry | D2HGDH | NAD+ | 0.13 | 0.44–50 | RT, 30 min | - | [17] |
| AlphaScreen ** | DhdR *** | - | 0.1 | 0.3–20 | RT, 60 min | - | [36] |
| Amperometry | D2HGDH | FAD | 0.1 | 0.5–120 | RT, 15 s | 4 °C, >30 days | this work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Li, Z.; Kang, Z.; Ma, C.; Song, H.; Lu, F.; Zhu, Z. An Enzymatic Biosensor for the Detection of D-2-Hydroxyglutaric Acid in Serum and Urine. Biosensors 2022, 12, 66. https://doi.org/10.3390/bios12020066
Wu B, Li Z, Kang Z, Ma C, Song H, Lu F, Zhu Z. An Enzymatic Biosensor for the Detection of D-2-Hydroxyglutaric Acid in Serum and Urine. Biosensors. 2022; 12(2):66. https://doi.org/10.3390/bios12020066
Chicago/Turabian StyleWu, Bo, Zehua Li, Zepeng Kang, Chunling Ma, Haiyan Song, Fuping Lu, and Zhiguang Zhu. 2022. "An Enzymatic Biosensor for the Detection of D-2-Hydroxyglutaric Acid in Serum and Urine" Biosensors 12, no. 2: 66. https://doi.org/10.3390/bios12020066
APA StyleWu, B., Li, Z., Kang, Z., Ma, C., Song, H., Lu, F., & Zhu, Z. (2022). An Enzymatic Biosensor for the Detection of D-2-Hydroxyglutaric Acid in Serum and Urine. Biosensors, 12(2), 66. https://doi.org/10.3390/bios12020066






