Smartphone-Based Chemiluminescent Origami µPAD for the Rapid Assessment of Glucose Blood Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
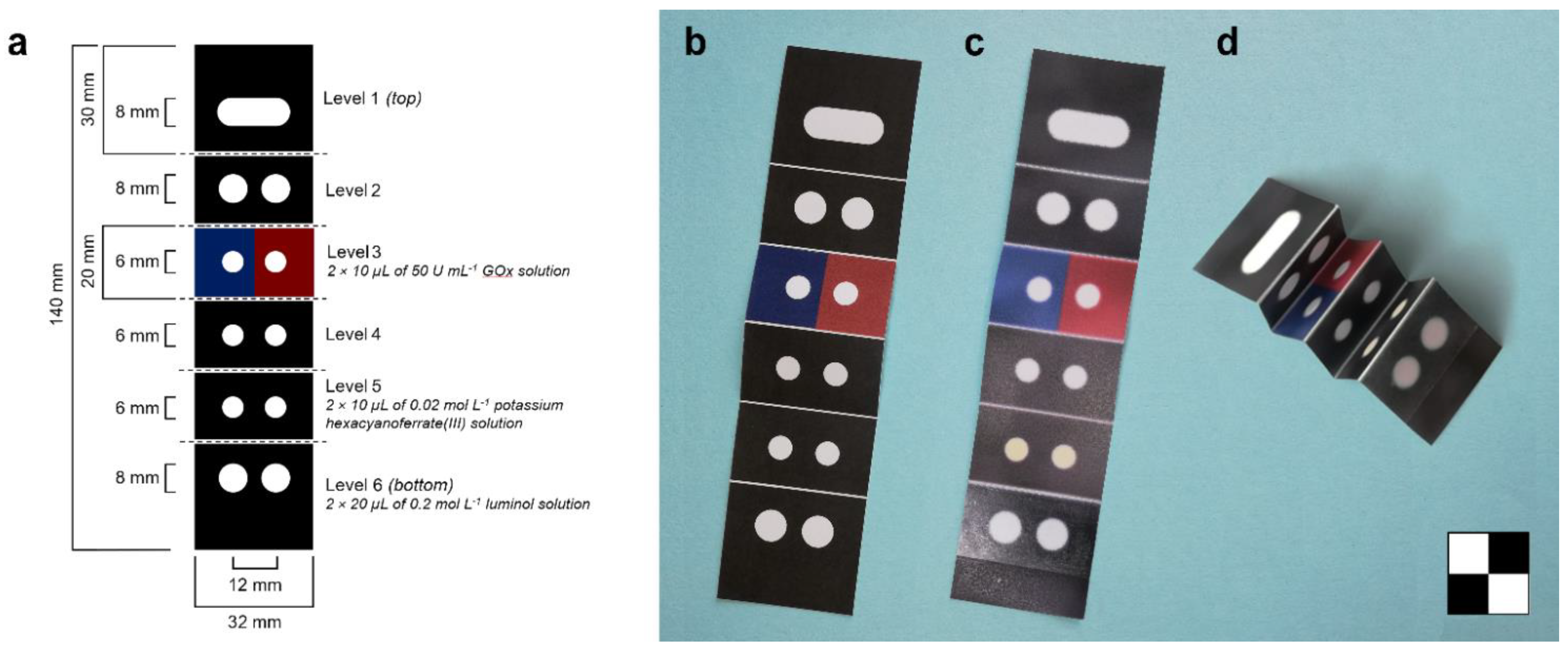
2.3. Origami Paper Biosensor
2.4. Assay Device
- A biosensor holder keeping the origami µPAD neatly folded during the assay to ensure homogeneous migration of the carbonate transport buffer. Four small NdFeB magnets (N45 grade, 5 mm diameter, 1 mm height) were embedded into the two halves of the holder to apply pressure on the folded biosensor, and the upper and lower openings allowed delivery of the transport buffer and imaging of the CL emission, respectively;
- A dark box consisting of a mirror support element with two flat mirrors (a primary 40 × 40 mm2 mirror and a secondary 25 × 25 mm2 one) angled at approximately 45°, which allowed the origami biosensor to be imaged from below, and a cover to support the smartphone adapter and to avoid interference from ambient light during the measurement. Mirrors (first surface mirrors with protected silver coating, purchased from Edmund Optics, Ltd., York, UK) have a reflectivity above 98% in the 450–2000 nm wavelength range. For the measurement, the cover accepted the biosensor holder and a stainless-steel needle connected with a silicone rubber tube (0.5 mm I.D.) to a 1000 µL Hamilton syringe for dispensing the carbonate transport buffer during the assay;
- A smartphone adapter specifically designed to fit a OnePlus 6 smartphone (OnePlus, Shenzen, China).
2.5. Assay Procedure
3. Results and Discussion
3.1. Design of the Origami µPAD and of the Analytical Device
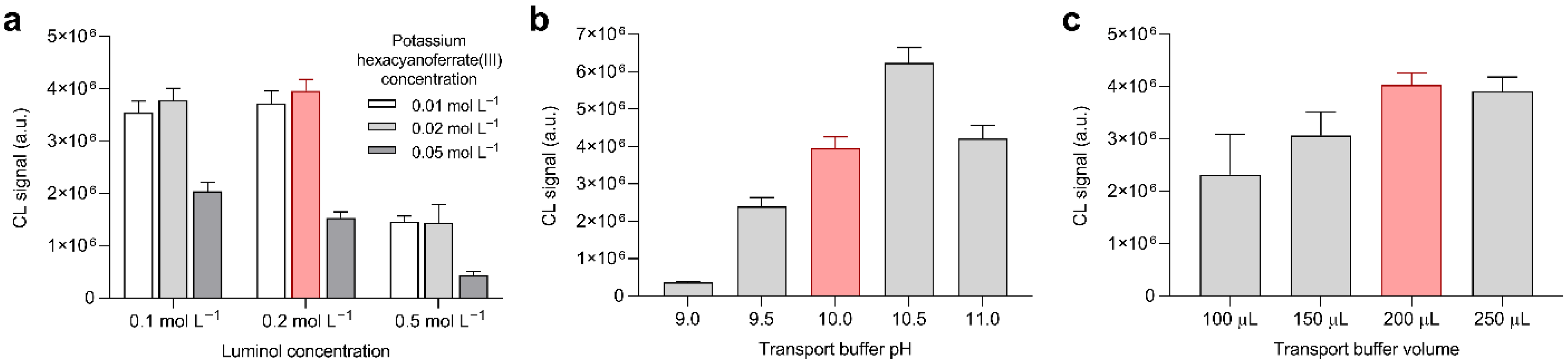
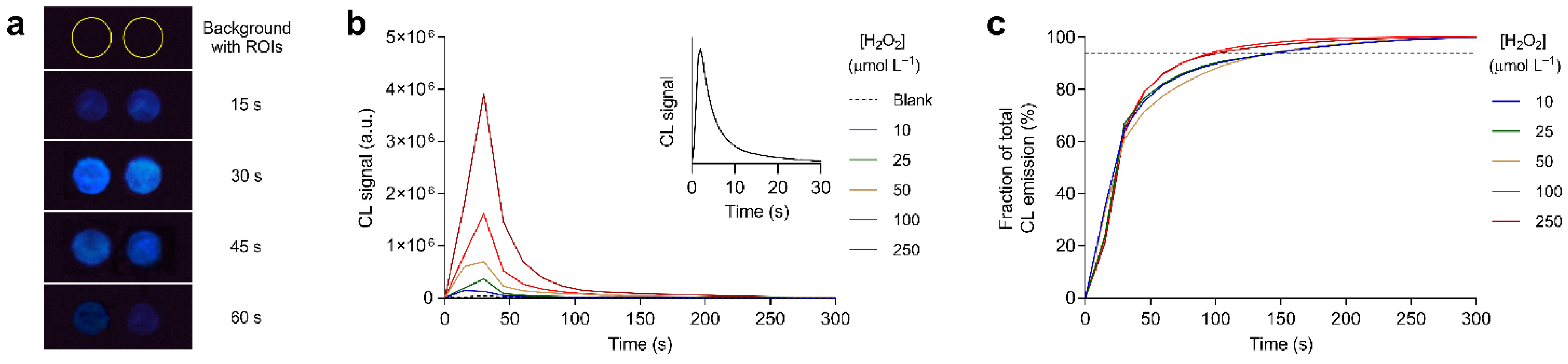
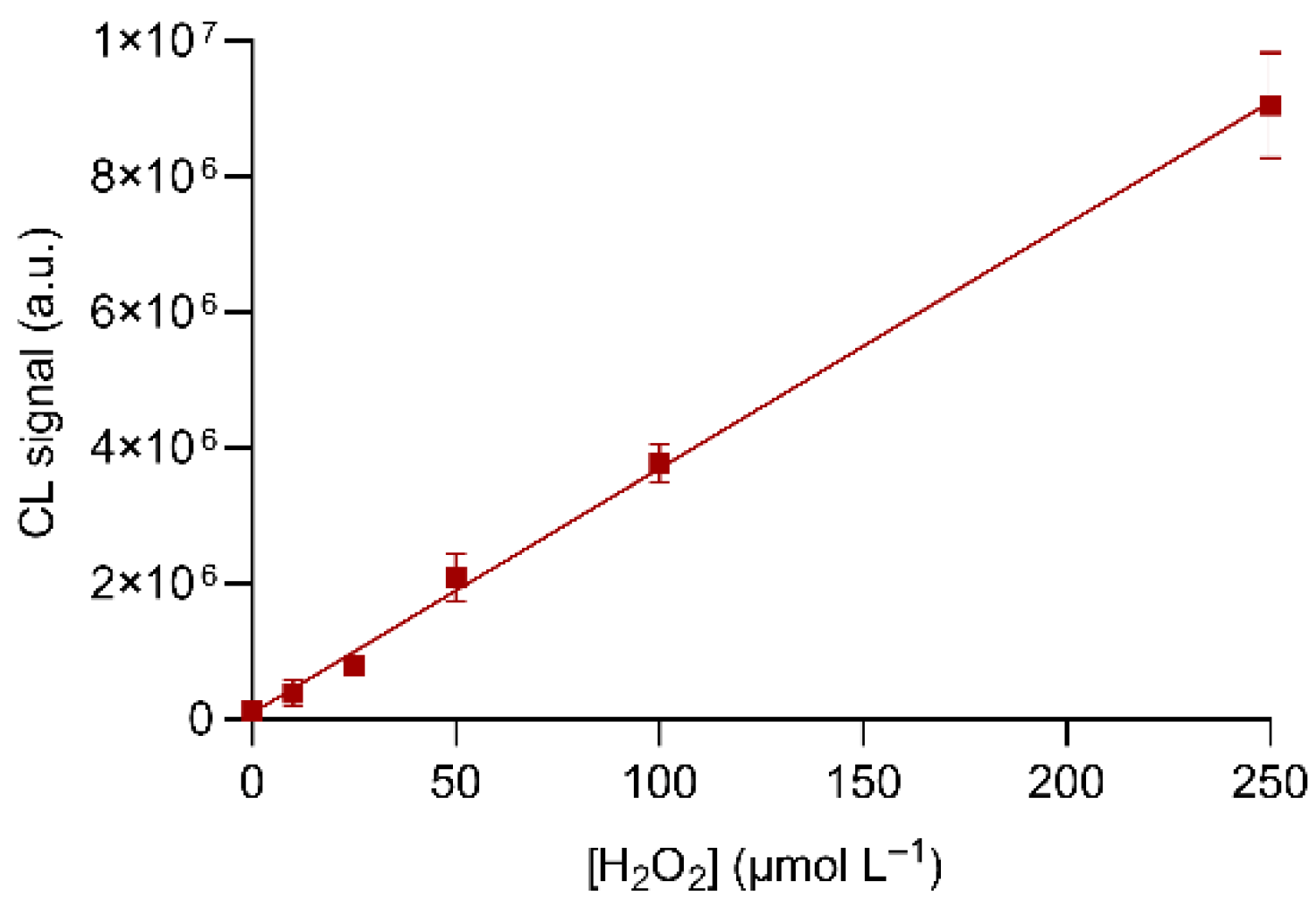
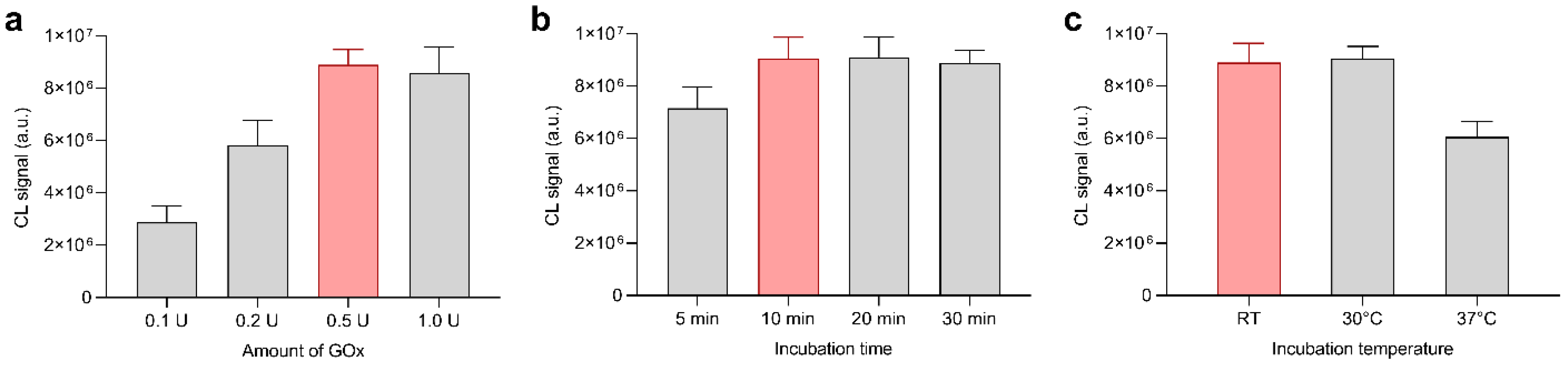
3.2. Optimization of Experimental Parameters: Detection of Hydrogen Peroxide
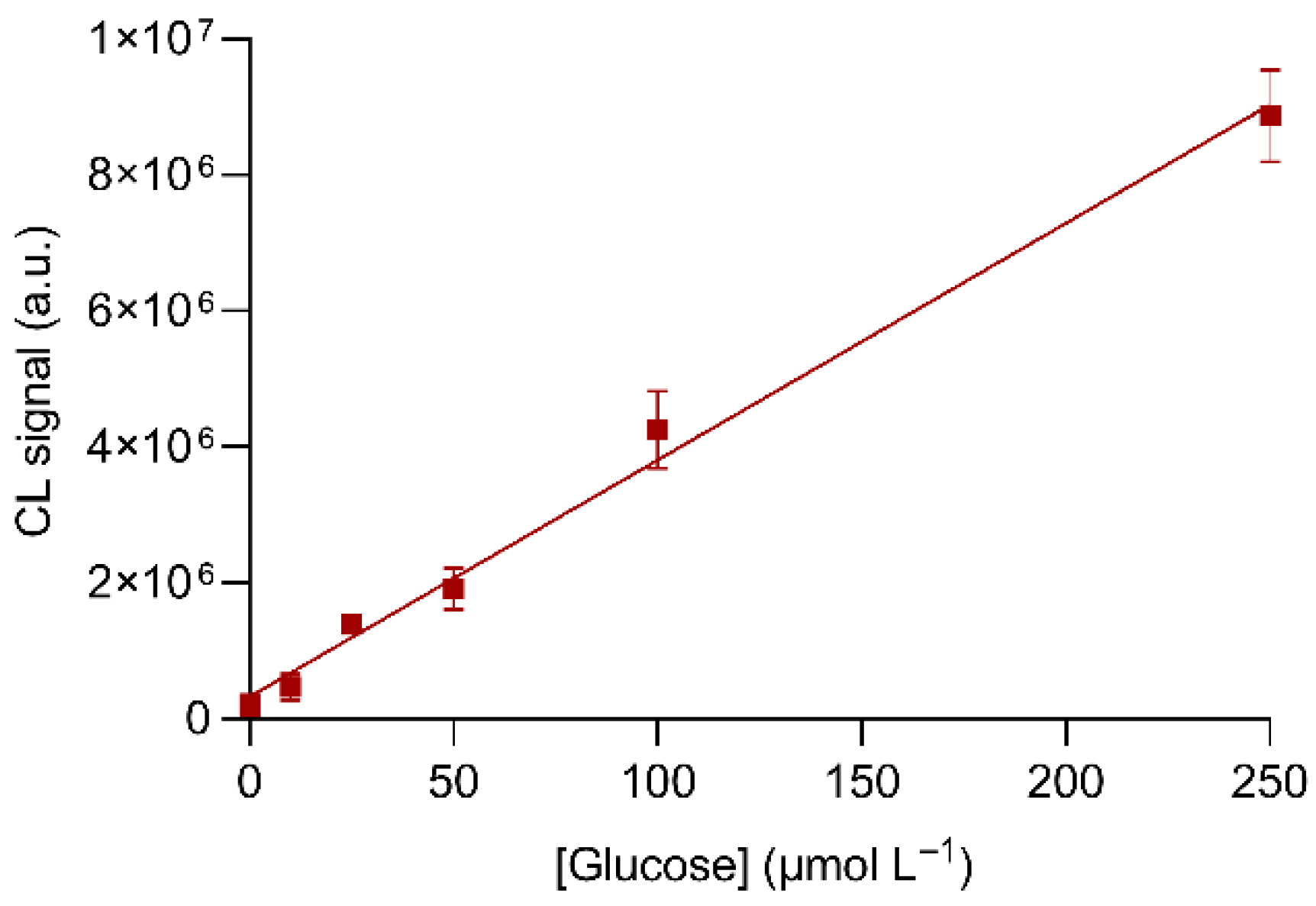
3.3. Optimization of Experimental Parameters: Detection of Glucose
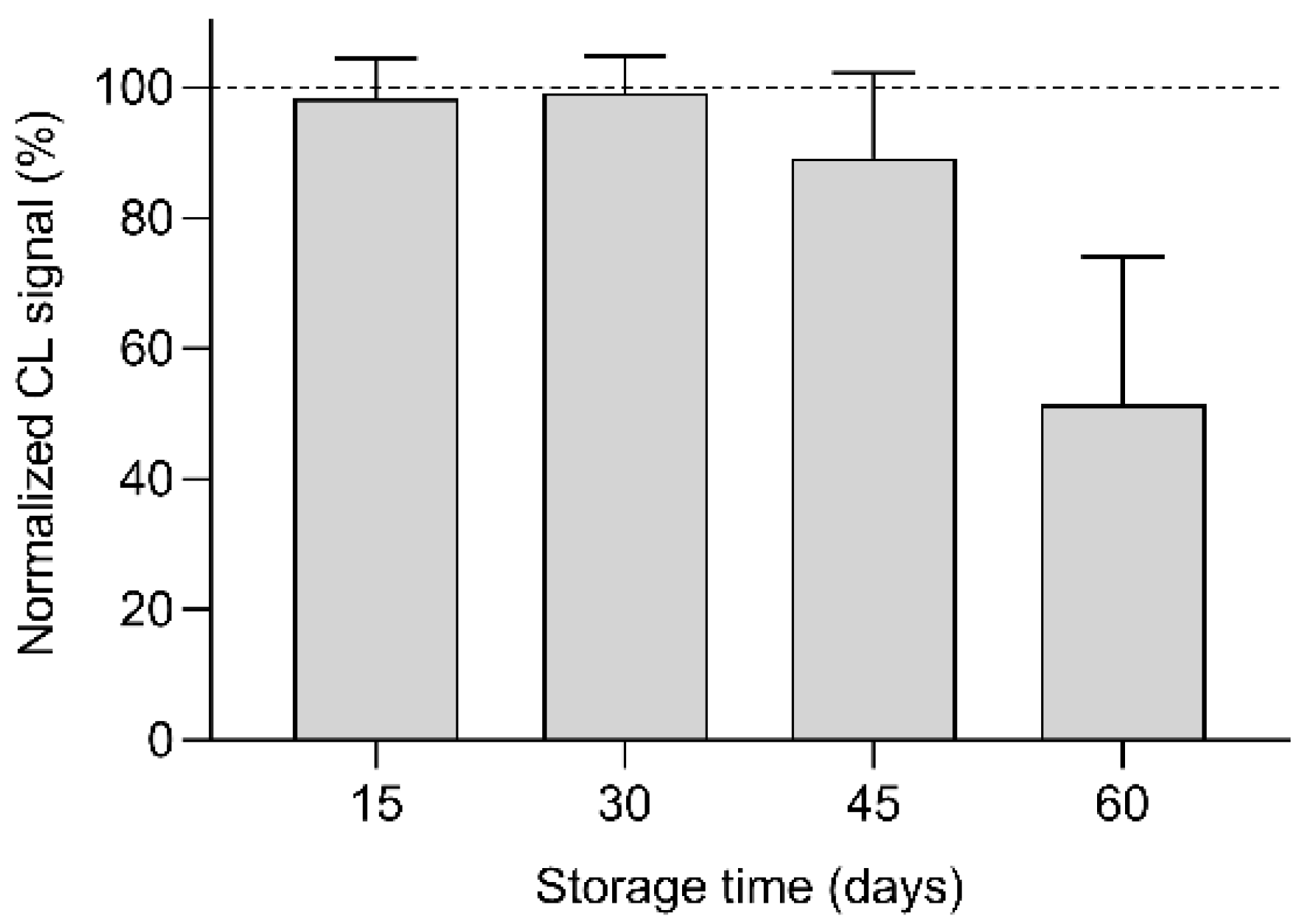
3.4. Biosensor Stability
3.5. Analysis of Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Land, K.J.; Boeras, D.I.; Chen, X.S.; Ramsay, A.R.; Peeling, R.W. REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat. Microbiol. 2019, 4, 46–54. [Google Scholar] [CrossRef]
- Lim, H.; Jafry, A.T.; Lee, J. Fabrication, flow control, and applications of microfluidic paper-based analytical devices. Molecules 2019, 24, 2869. [Google Scholar] [CrossRef] [Green Version]
- Ozer, T.; McMahon, C.; Henry, C.S. Advances in paper-based analytical devices. Annu. Rev. Anal. Chem. 2020, 13, 85–109. [Google Scholar] [CrossRef] [Green Version]
- Suntornsuk, W.; Suntornsuk, L. Recent applications of paper-based point-of-care devices for biomarker detection. Electrophoresis 2020, 41, 287–305. [Google Scholar] [CrossRef]
- Puiu, M.; Mirceski, V.; Bala, C. Paper-based diagnostic platforms and devices. Curr. Opin. Electrochem. 2021, 27, 100726. [Google Scholar] [CrossRef]
- Lee, W.C.; Ng, H.Y.; Hou, C.Y.; Lee, C.T.; Fu, L.M. Recent advances in lab-on-paper diagnostic devices using blood samples. Lab. Chip 2021, 21, 1433–1453. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.T.; Lantigua, D.; Meka, A.; Taing, S.; Pandher, M.; Camci-Unal, G. Paper-based sensors: Emerging themes and applications. Sensors 2018, 18, 2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.W.; Xu, D.D.; Chen, J.; Liu, J.X.; Li, Y.B.; Song, J.; Ma, X.; Guo, J.H. Smartphone-based analytical biosensors. Analyst 2018, 143, 5339–5351. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.X.; Zhang, X.; Fan, Z.Y.; Lv, X.Q.; Su, Y.; Chen, H.D. Recent progress in optical biosensors based on smartphone platforms. Sensors 2017, 17, 2449. [Google Scholar] [CrossRef] [Green Version]
- Ge, L.; Yu, J.H.; Ge, S.G.; Yan, M. Lab-on-paper-based devices using chemiluminescence and electrogenerated chemiluminescence detection. Anal. Bioanal. Chem. 2014, 406, 5613–5630. [Google Scholar] [CrossRef]
- Mirasoli, M.; Guardigli, M.; Michelini, E.; Roda, A. Recent advancements in chemical luminescence-based lab-on-chip and microfluidic platforms for bioanalysis. J. Pharm. Biomed. Anal. 2014, 87, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Arduini, F.; Mirasoli, M.; Zangheri, M.; Fabiani, L.; Colozza, N.; Marchegiani, E.; Simoni, P.; Moscone, D. A challenge in biosensors: Is it better to measure a photon or an electron for ultrasensitive detection? Biosens. Bioelectron. 2020, 155, 112093. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Zangheri, M.; Calabria, D.; Lopreside, A.; Montali, L.; Marchegiani, E.; Trozzi, I.; Guardigli, M.; Mirasoli, M.; Michelini, E. Paper-based immunosensors with bio-chemiluminescence detection. Sensors 2021, 21, 4309. [Google Scholar] [CrossRef]
- Chu, W.R.; Chen, Y.; Liu, W.; Zhao, M.; Li, H.F. Paper-based chemiluminescence immunodevice with temporal controls of reagent transport technique. Sens. Actuator B-Chem. 2017, 250, 324–332. [Google Scholar] [CrossRef]
- Zangheri, M.; Mirasoli, M.; Guardigli, M.; Di Nardo, F.; Anfossi, L.; Baggiani, C.; Simoni, P.; Benassai, M.; Roda, A. Chemiluminescence-based biosensor for monitoring astronauts’ health status during space missions: Results from the International Space Station. Biosens. Bioelectron. 2019, 129, 260–268. [Google Scholar] [CrossRef]
- Ge, L.; Wang, S.M.; Song, X.R.; Ge, S.G.; Yu, J.H. 3D Origami-based multifunction-integrated immunodevice: Low-cost and multiplexed sandwich chemiluminescence immunoassay on microfluidic paper-based analytical device. Lab. Chip 2012, 12, 3150–3158. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cassano, C.L.; Xu, X.; Fan, Z.H. Laminated paper-based analytical devices (LPAD) with origami-enabled chemiluminescence immunoassay for cotinine detection in mouse serum. Anal. Chem. 2013, 85, 10270–10276. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Zangheri, M.; Calabria, D.; Mirasoli, M.; Caliceti, C.; Quintavalla, A.; Lombardo, M.; Trombini, C.; Simoni, P. A simple smartphone-based thermochemiluminescent immunosensor for valproic acid detection using 1,2-dioxetane analogue-doped nanoparticles as a label. Sens. Actuat. B-Chem. 2019, 279, 327–333. [Google Scholar] [CrossRef]
- Yu, J.H.; Wang, S.M.; Ge, L.; Ge, S.G. A novel chemiluminescence paper microfluidic biosensor based on enzymatic reaction for uric acid determination. Biosens. Bioelectron. 2011, 26, 3284–3289. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.C.; Guo, L.; Wang, J.H.; Zhang, K.Q.; He, J.B.; Cui, H. High-resolution temporally resolved chemiluminescence based on double-layered 3D microfluidic paper-based device for multiplexed analysis. Biosens. Bioelectron. 2019, 141, 111472. [Google Scholar] [CrossRef] [PubMed]
- Montali, L.; Calabretta, M.M.; Lopreside, A.; D’Elia, M.; Guardigli, M.; Michelini, E. Multienzyme chemiluminescent foldable biosensor for on-site detection of acetylcholinesterase inhibitors. Biosens. Bioelectron. 2020, 162, 112232. [Google Scholar] [CrossRef] [PubMed]
- Calabria, D.; Mirasoli, M.; Guardigli, M.; Simoni, P.; Zangheri, M.; Severi, P.; Caliceti, C.; Roda, A. Paper-based smartphone chemosensor for reflectometric on-site total polyphenols quantification in olive oil. Sens. Actuat. B-Chem. 2020, 305, 127522. [Google Scholar] [CrossRef]
- Calabria, D.; Guardigli, M.; Severi, P.; Trozzi, I.; Pace, A.; Cinti, S.; Zangheri, M.; Mirasoli, M. A smartphone-based chemosensor to evaluate antioxidants in agri-food matrices by in situ AuNP formation. Sensors 2021, 21, 5432. [Google Scholar] [CrossRef] [PubMed]
- Warm, E.; Laties, G.G. Quantification of hydrogen peroxide in plant extracts by the chemiluminescence reaction with luminol. Phytochemistry 1982, 21, 827–831. [Google Scholar] [CrossRef]
- Zhang, W.C.; Niu, X.H.; Li, X.; He, Y.F.; Song, H.W.; Peng, Y.X.; Pan, J.M.; Qiu, F.X.; Zhao, H.L.; Lan, M.B. A smartphone-integrated ready-to-use paper-based sensor with mesoporous carbon-dispersed Pd nanoparticles as a highly active peroxidase mimic for H2O2 detection. Sens. Actuat. B-Chem. 2018, 265, 412–420. [Google Scholar] [CrossRef]
- Ragavan, K.V.; Ahmed, S.R.; Weng, X.; Neethirajan, S. Chitosan as a peroxidase mimic: Paper based sensor for the detection of hydrogen peroxide. Sens. Actuat. B-Chem. 2018, 272, 8–13. [Google Scholar] [CrossRef]
- Sanchez-Calvo, A.; Costa-Garcia, A.; Blanco-Lopez, M.C. Paper-based electrodes modified with cobalt phthalocyanine colloid for the determination of hydrogen peroxide and glucose. Analyst 2020, 145, 2716–2724. [Google Scholar] [CrossRef] [PubMed]
- Weibel, M.K.; Bright, H.J. The glucose oxidase mechanism: Interpretation of the pH dependence. J. Biol. Chem. 1971, 246, 2734–2744. [Google Scholar] [CrossRef]
- Gouda, M.D.; Singh, S.A.; Rao, A.G.A.; Thakur, M.S.; Karanth, N.G. Thermal inactivation of glucose oxidase. Mechanism and stabilization using additives. J. Biol. Chem. 2003, 278, 24324–24333. [Google Scholar] [CrossRef] [Green Version]
- Jahanshahi-Anbuhi, S.; Kannan, B.; Leung, V.; Pennings, K.; Liu, M.; Carrasquilla, C.; White, D.; Li, Y.F.; Pelton, R.H.; Brennan, J.D.; et al. Simple and ultrastable all-inclusive pullulan tablets for challenging bioassays. Chem. Sci. 2016, 7, 2342–2346. [Google Scholar] [CrossRef] [Green Version]
- Li, H.J.; Wang, D.; Liu, C.L.; Liu, R.; Zhang, C.S. Facile and sensitive chemiluminescence detection of H2O2 and glucose by a gravity/capillary flow and cloth-based low-cost platform. RSC Adv. 2017, 7, 43245–43254. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.H.; Ge, L.; Huang, J.D.; Wang, S.M.; Ge, S.G. Microfluidic paper-based chemiluminescence biosensor for simultaneous determination of glucose and uric acid. Lab. Chip 2011, 11, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, C.S.; Xing, D. Paper-based bipolar electrode-electrochemiluminescence (BPE-ECL) device with battery energy supply and smartphone read-out: A handheld ECL system for biochemical analysis at the point-of-care level. Sens. Actuat. B-Chem. 2016, 237, 308–317. [Google Scholar] [CrossRef]
- Wang, D.; Liang, Y.; Su, Y.; Shang, Q.P.; Zhang, C.S. Sensitivity enhancement of cloth-based closed bipolar electrochemiluminescence glucose sensor via electrode decoration with chitosan/multi-walled carbon nanotubes/graphene quantum dots-gold nanoparticles. Biosens. Bioelectron. 2019, 130, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Finucane, M.M.; Lu, Y.; Singh, G.M.; Cowan, M.J.; Paciorek, C.J.; Lin, J.K.; Farzadfar, F.; Khang, Y.H.; Stevens, G.A.; et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 2011, 378, 31–40. [Google Scholar] [CrossRef]

| Lyphochek® Assayed Chemistry Control | Added Glucose (mmol L−1) | Measured Glucose (mmol L−1) 1 | Bias (%) | |
|---|---|---|---|---|
| Reference Colorimetric Assay | Origami µPAD | |||
| Level 1 | 0.0 | 5.11 ± 0.15 | 5.33 ± 0.28 | +4.3 |
| 2.0 | 7.07 ± 0.14 | 6.95 ± 0.39 | −1.7 | |
| 4.0 | 9.18 ± 0.14 | 9.33 ± 0.35 | +1.6 | |
| 6.0 | 11.12 ± 0.28 | 11.19 ± 0.53 | +0.6 | |
| 8.0 | 13.06 ± 0.23 | 12.38 ± 0.78 | −5.2 | |
| Level 2 | 0.0 | 16.53 ± 0.44 | 2 | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabria, D.; Zangheri, M.; Trozzi, I.; Lazzarini, E.; Pace, A.; Mirasoli, M.; Guardigli, M. Smartphone-Based Chemiluminescent Origami µPAD for the Rapid Assessment of Glucose Blood Levels. Biosensors 2021, 11, 381. https://doi.org/10.3390/bios11100381
Calabria D, Zangheri M, Trozzi I, Lazzarini E, Pace A, Mirasoli M, Guardigli M. Smartphone-Based Chemiluminescent Origami µPAD for the Rapid Assessment of Glucose Blood Levels. Biosensors. 2021; 11(10):381. https://doi.org/10.3390/bios11100381
Chicago/Turabian StyleCalabria, Donato, Martina Zangheri, Ilaria Trozzi, Elisa Lazzarini, Andrea Pace, Mara Mirasoli, and Massimo Guardigli. 2021. "Smartphone-Based Chemiluminescent Origami µPAD for the Rapid Assessment of Glucose Blood Levels" Biosensors 11, no. 10: 381. https://doi.org/10.3390/bios11100381
APA StyleCalabria, D., Zangheri, M., Trozzi, I., Lazzarini, E., Pace, A., Mirasoli, M., & Guardigli, M. (2021). Smartphone-Based Chemiluminescent Origami µPAD for the Rapid Assessment of Glucose Blood Levels. Biosensors, 11(10), 381. https://doi.org/10.3390/bios11100381







