A Novel Enzyme-Free Ratiometric Fluorescence Immunoassay Based on Silver Nanoparticles for the Detection of Dibutyl Phthalate from Environmental Waters
Abstract
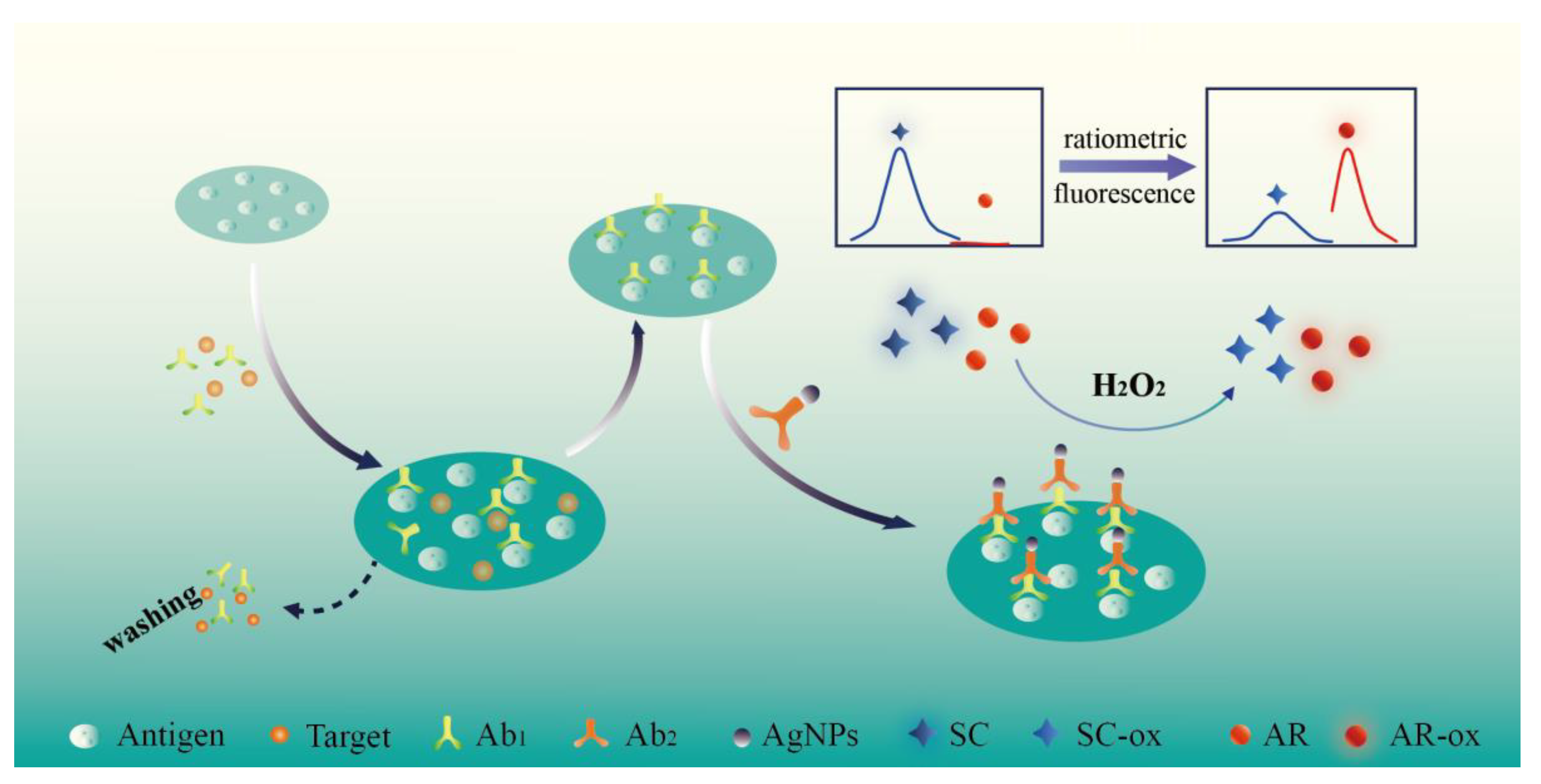
:1. Introduction
2. Experiments and Materials
2.1. Synthesis of Ag Nanoparticles (AgNPs)
2.2. Synthesis of AgNPs@Ab2
2.3. Catalytic Oxidation Assay
2.4. The Development of Ratiometric Fluorescence ELISA for DBP
2.5. LC–MS/MS Analysis
3. Result and Discussion
3.1. The Characterization of AgNPs and AgNPs@Ab2
3.2. The Peroxidase-like Activity of the Nanoparticles
3.3. Optimization of Experimental Conditions
3.4. Assay Validation and Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, Y.; An, T.; Ji, Y.; Li, G.; Zhao, C. Eco-toxicity and human estrogenic exposure risks from OH-initiated photochemical transformation of four phthalates in water: A computational study. Environ. Pollut. 2015, 206, 510–517. [Google Scholar] [CrossRef]
- Montevecchi, G.; Masino, F.; Zanasi, L.; Antonelli, A. Determination of phthalate esters in distillates by ultrasound-vortex-assisted dispersive liquid-liquid micro-extraction (USVADLLME) coupled with gas chromatography/mass spectrometry. Food Chem. 2017, 221, 1354–1360. [Google Scholar] [CrossRef]
- Wang, M.; Yang, F.; Liu, L.; Cheng, C.; Yang, Y. Ionic Liquid-Based Surfactant Extraction Coupled with Magnetic Dispersive μ-Solid Phase Extraction for the Determination of Phthalate Esters in Packaging Milk Samples by HPLC. Food Anal. Methods 2016, 10, 1745–1754. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, S.; Zhao, J.; Li, H. Effect of dibutyl phthalate on expression of connexin 43 and testosterone production of leydig cells in adult rats. Environ. Toxicol. Pharmacol. 2016, 47, 131–135. [Google Scholar] [CrossRef]
- Janjua, N.R.; Mortensen, G.K.; Andersson, A.M.; Kongshoj, B.; Wulf, H.C. Systemic Uptake of Diethyl Phthalate, Dibutyl Phthalate, and Butyl Paraben Following Whole-Body Topical Application and Reproductive and Thyroid Hormone Levels in Humans. Environ. Sci. Technol. 2007, 41, 5564–5570. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, K.; Liu, J. Immunochemical detection of emerging organic contaminants in environmental waters. TrAC Trends Anal. Chem. 2017, 87, 49–57. [Google Scholar] [CrossRef]
- Han, X.; Liu, D. Detection of the toxic substance dibutyl phthalate in Antarctic krill. Antarct. Sci. 2017, 29, 511–516. [Google Scholar] [CrossRef]
- Ibrahim, N.; Osman, R.; Abdullah, A.; Saim, N. Determination of Phthalate Plasticisers in Palm Oil Using Online Solid Phase Extraction-Liquid Chromatography (SPE-LC). J. Chem. 2014, 2014, 682975. [Google Scholar] [CrossRef] [Green Version]
- Iha, K.; Inada, M.; Kawada, N.; Nakaishi, K.; Watabe, S.; Tan, Y.H.; Shen, C.; Ke, L.Y.; Yoshimura, T.; Ito, E. Ultrasensitive ELISA Developed for Diagnosis. Diagnostics 2019, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.-P.; Yang, H.-W.; Li, N.-S.; Chen, Y.T.; Pang, H.-H.; Pang, S.T. Instrument-Free Detection of FXYD3 Using Vial-Based Immunosensor for Earlier and Faster Urothelial Carcinoma Diagnosis. ACS Sens. 2020, 5, 928–935. [Google Scholar] [CrossRef]
- Xiong, Y.; Leng, Y.; Li, X.; Huang, X.; Xiong, Y. Emerging strategies to enhance the sensitivity of competitive ELISA for detection of chemical contaminants in food samples. TrAC Trends Anal. Chem. 2020, 126, 115861. [Google Scholar] [CrossRef]
- Bao, K.; Liu, X.; Xu, Q.; Su, B.; Liu, Z.; Cao, H.; Chen, Q. Nanobody multimerization strategy to enhance the sensitivity of competitive ELISA for detection of ochratoxin A in coffee samples. Food Control 2021, 127, 108167. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, B.; Sun, J.; Yi, Y.; Li, M.; Du, D.; Zhu, F.; Luan, J. Highly efficient detection of salbutamol in environmental water samples by an enzyme immunoassay. Sci. Total Environ. 2018, 613, 861–865. [Google Scholar] [CrossRef]
- Pan, Y.; Wei, X.; Guo, X.; Wang, H.; Song, H.; Pan, C.; Xu, N. Immunoassay based on Au-Ag bimetallic nanoclusters for colorimetric/fluorescent double biosensing of dicofol. Biosens. Bioelectron. 2021, 194, 113611. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, M.; Wang, W.; Jiang, Q.; Wang, F.; Pang, D.-W.; Liu, X. Plasmonic and Photothermal Immunoassay via Enzyme-Triggered Crystal Growth on Gold Nanostars. Anal. Chem. 2019, 91, 2086–2092. [Google Scholar] [CrossRef]
- Vdovenko, M.M.; Stepanova, A.S.; Eremin, S.A.; Van Cuong, N.; Uskova, N.A.; Yu Sakharov, I. Quantification of 2,4-dichlorophenoxyacetic acid in oranges and mandarins by chemiluminescent ELISA. Food Chem. 2013, 141, 865–868. [Google Scholar] [CrossRef]
- Xuan, Z.; Li, M.; Rong, P.; Wang, W.; Li, Y.; Liu, D. Plasmonic ELISA based on the controlled growth of silver nanoparticles. Nanoscale 2016, 8, 17271–17277. [Google Scholar] [CrossRef]
- Pang, Y.-H.; Guo, L.-L.; Shen, X.-F.; Yang, N.-C.; Yang, C. Rolling circle amplified DNAzyme followed with covalent organic frameworks: Cascade signal amplification of electrochemical ELISA for alfatoxin M1 sensing. Electrochim. Acta 2020, 341, 136055. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, W.; Peng, W.; Zhao, Q.; Piao, J.; Zhang, B.; Wu, X.; Wang, H.; Gong, X.; Chang, J. Enhanced Fluorescence ELISA Based on HAT Triggering Fluorescence “Turn-on” with Enzyme–Antibody Dual Labeled AuNP Probes for Ultrasensitive Detection of AFP and HBsAg. ACS Appl. Mater. Interfaces 2017, 9, 9369–9377. [Google Scholar] [CrossRef]
- Mao, G.; Cai, Q.; Wang, F.; Luo, C.; Ji, X.; He, Z. One-Step Synthesis of Rox-DNA Functionalized CdZnTeS Quantum Dots for the Visual Detection of Hydrogen Peroxide and Blood Glucose. Anal. Chem. 2017, 89, 11628–11635. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, C.; Jin, J.; Huang, L.; Yu, W.; Su, B.; Hu, J. Ratiometric Fluorescent Lateral Flow Immunoassay for Point-of-Care Testing of Acute Myocardial Infarction. Angew. Chem. Int. Ed. Engl. 2021, 60, 13042–13049. [Google Scholar] [CrossRef]
- Zhu, N.; Li, X.; Liu, Y.; Liu, J.; Wang, Y.; Wu, X.; Zhang, Z. Dual amplified ratiometric fluorescence ELISA based on G-quadruplex/hemin DNAzyme using tetrahedral DNA nanostructure as scaffold for ultrasensitive detection of dibutyl phthalate in aquatic system. Sci. Total Environ. 2021, 784, 147212. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, L.; Du, W.; Li, H.; Yang, D.; Zhu, C. Hierarchical manganese dioxide nanoflowers enable accurate ratiometric fluorescence enzyme-linked immunosorbent assay. Nanoscale 2018, 10, 21893–21897. [Google Scholar] [CrossRef]
- Luo, L.; Song, Y.; Zhu, C.; Fu, S.; Shi, Q.; Sun, Y.-M.; Jia, B.; Du, D.; Xu, Z.-L.; Lin, Y. Fluorescent silicon nanoparticles-based ratiometric fluorescence immunoassay for sensitive detection of ethyl carbamate in red wine. Sens. Actuators B Chem. 2018, 255, 2742–2749. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, S.; Lu, S.; Liu, G.; Sun, J.; Yang, X. Fluorometric and Colorimetric Dual-Readout Immunoassay Based on an Alkaline Phosphatase-Triggered Reaction. Anal. Chem. 2019, 91, 7828–7834. [Google Scholar] [CrossRef]
- Fan, Y.; Lv, M.; Xue, Y.; Li, J.; Wang, E. In Situ Fluorogenic Reaction Generated via Ascorbic Acid for the Construction of Universal Sensing Platform. Anal. Chem. 2021, 93, 6873–6880. [Google Scholar] [CrossRef]
- Zhao, D.; Li, J.; Peng, C.; Zhu, S.; Sun, J.; Yang, X. Fluorescence Immunoassay Based on the Alkaline Phosphatase Triggered in Situ Fluorogenic Reaction of o-Phenylenediamine and Ascorbic Acid. Anal. Chem. 2019, 91, 2978–2984. [Google Scholar] [CrossRef]
- Zhao, L.J.; Yu, R.J.; Ma, W.; Han, H.X.; Tian, H.; Qian, R.C.; Long, Y.T. Sensitive detection of protein biomarkers using silver nanoparticles enhanced immunofluorescence assay. Theranostics 2017, 7, 876–883. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, N.; Zou, Y.; Wu, X.; Qu, G.; Shi, J. A novel, enzyme-linked immunosorbent assay based on the catalysis of AuNCs@BSA-induced signal amplification for the detection of dibutyl phthalate. Talanta 2018, 179, 64–69. [Google Scholar] [CrossRef]
- Schiesaro, I.; Battocchio, C.; Venditti, I.; Prosposito, P.; Burratti, L.; Centomo, P.; Meneghini, C. Structural characterization of 3d metal adsorbed AgNPs. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 123, 114162. [Google Scholar] [CrossRef]
- Tran, L.; Park, S. Highly sensitive detection of dengue biomarker using streptavidin-conjugated quantum dots. Sci. Rep. 2021, 11, 15196. [Google Scholar] [CrossRef]
- Alvandi, H.; Dorosti, N.; Afshar, F. Synthesis of AgNPs and Ag@MoS2 nanocomposites by dracocephalum kotschyi aqueous extract and their antiacetylcholinesterase activities. Mater. Technol. 2021, 1–12. [Google Scholar] [CrossRef]
- Du, P.; Niu, Q.; Chen, J.; Chen, Y.; Zhao, J.; Lu, X. “Switch-On” Fluorescence Detection of Glucose with High Specificity and Sensitivity Based on Silver Nanoparticles Supported on Porphyrin Metal–Organic Frameworks. Anal. Chem. 2020, 92, 7980–7986. [Google Scholar] [CrossRef]
- Jiang, Z.J.; Liu, C.Y.; Sun, L.W. Catalytic Properties of Silver Nanoparticles Supported on Silica Spheres. J. Phys. Chem. B 2005, 109, 1730–1735. [Google Scholar] [CrossRef]
- Jiang, C.; Bai, Z.; Yuan, F.; Ruan, Z.; Wang, W. A colorimetric sensor based on Glutathione-AgNPs as peroxidase mimetics for the sensitive detection of Thiamine (Vitamin B1). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 265, 120348. [Google Scholar] [CrossRef]
- Fan, D.; Shang, C.; Gu, W.; Wang, E.; Dong, S. Introducing Ratiometric Fluorescence to MnO2 Nanosheet-Based Biosensing: A Simple, Label-Free Ratiometric Fluorescent Sensor Programmed by Cascade Logic Circuit for Ultrasensitive GSH Detection. ACS Appl. Mater. Interfaces 2017, 9, 25870–25877. [Google Scholar] [CrossRef]




| Samples | Background (ng/mL) | Added (ng/mL) | Found (ng/mL) | Recovery (%) | CV a (%) |
|---|---|---|---|---|---|
| Pure water | ND b | 5 | 4.36 | 87.20 | 3.73 |
| 15 | 15.31 | 102.07 | 2.57 | ||
| 60 | 58.95 | 98.25 | 4.85 | ||
| River water | 10.46 | 5 | 16.14 | 104.40 | 4.97 |
| 15 | 23.78 | 93.40 | 2.71 | ||
| 60 | 71.42 | 101.36 | 3.68 | ||
| Tap water | ND b | 5 | 4.83 | 96.60 | 6.54 |
| 15 | 13.67 | 91.13 | 5.23 | ||
| 60 | 59.25 | 98.75 | 3.93 | ||
| Pond water | 21.71 | 5 | 25.97 | 97.23 | 2.91 |
| 15 | 39.14 | 106.62 | 4.75 | ||
| 60 | 75.37 | 92.24 | 3.46 |
| Samples | Background (ng/mL) | ELISA (ng/mL) | Our Method (ng/mL) | CV a (%) |
|---|---|---|---|---|
| S1 | ND b | ND | 1.12 | 3.57 |
| S2 | ND | ND | ND | ND |
| S3 | ND | ND | 3.97 | 2.35 |
| S4 | ND | ND | ND | ND |
| S5 | ND | 23.81 | 24.15 | 7.31 |
| S6 | ND | 16.57 | 19.62 | 4.68 |
| S7 | ND | ND | ND | ND |
| S8 | ND | ND | ND | ND |
| S9 | ND | 11.21 | 10.53 | 2.14 |
| S10 | ND | ND | ND | ND |
| S11 | ND | 9.62 | 8.36 | 3.95 |
| S12 | ND | 19.87 | 20.65 | 2.93 |
| S13 | ND | ND | ND | ND |
| S14 | ND | ND | ND | ND |
| S15 | ND | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, H.; Yao, N.; Zeng, K.; Zhu, N.; Wang, Y.; Zhao, B.; Zhang, Z. A Novel Enzyme-Free Ratiometric Fluorescence Immunoassay Based on Silver Nanoparticles for the Detection of Dibutyl Phthalate from Environmental Waters. Biosensors 2022, 12, 125. https://doi.org/10.3390/bios12020125
Meng H, Yao N, Zeng K, Zhu N, Wang Y, Zhao B, Zhang Z. A Novel Enzyme-Free Ratiometric Fluorescence Immunoassay Based on Silver Nanoparticles for the Detection of Dibutyl Phthalate from Environmental Waters. Biosensors. 2022; 12(2):125. https://doi.org/10.3390/bios12020125
Chicago/Turabian StyleMeng, Hui, Nannan Yao, Kun Zeng, Nuanfei Zhu, Yue Wang, Biying Zhao, and Zhen Zhang. 2022. "A Novel Enzyme-Free Ratiometric Fluorescence Immunoassay Based on Silver Nanoparticles for the Detection of Dibutyl Phthalate from Environmental Waters" Biosensors 12, no. 2: 125. https://doi.org/10.3390/bios12020125
APA StyleMeng, H., Yao, N., Zeng, K., Zhu, N., Wang, Y., Zhao, B., & Zhang, Z. (2022). A Novel Enzyme-Free Ratiometric Fluorescence Immunoassay Based on Silver Nanoparticles for the Detection of Dibutyl Phthalate from Environmental Waters. Biosensors, 12(2), 125. https://doi.org/10.3390/bios12020125






