Rapid Capturing and Chemiluminescent Sensing of Programmed Death Ligand-1 Expressing Extracellular Vesicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aptamers Sequences and Auxiliary Reagents
2.2. Cell Culturing for EVs Isolation
2.3. FeO@TiO Beads Formation
2.4. EVs Morphology, Characterization and Enumeration
2.5. Confocal Microscopy and Zeta Potential
2.6. EVs Accumulation by FeO@TiO Beads
2.7. Clinical Feasibility
2.8. Statistical Testing
3. Results
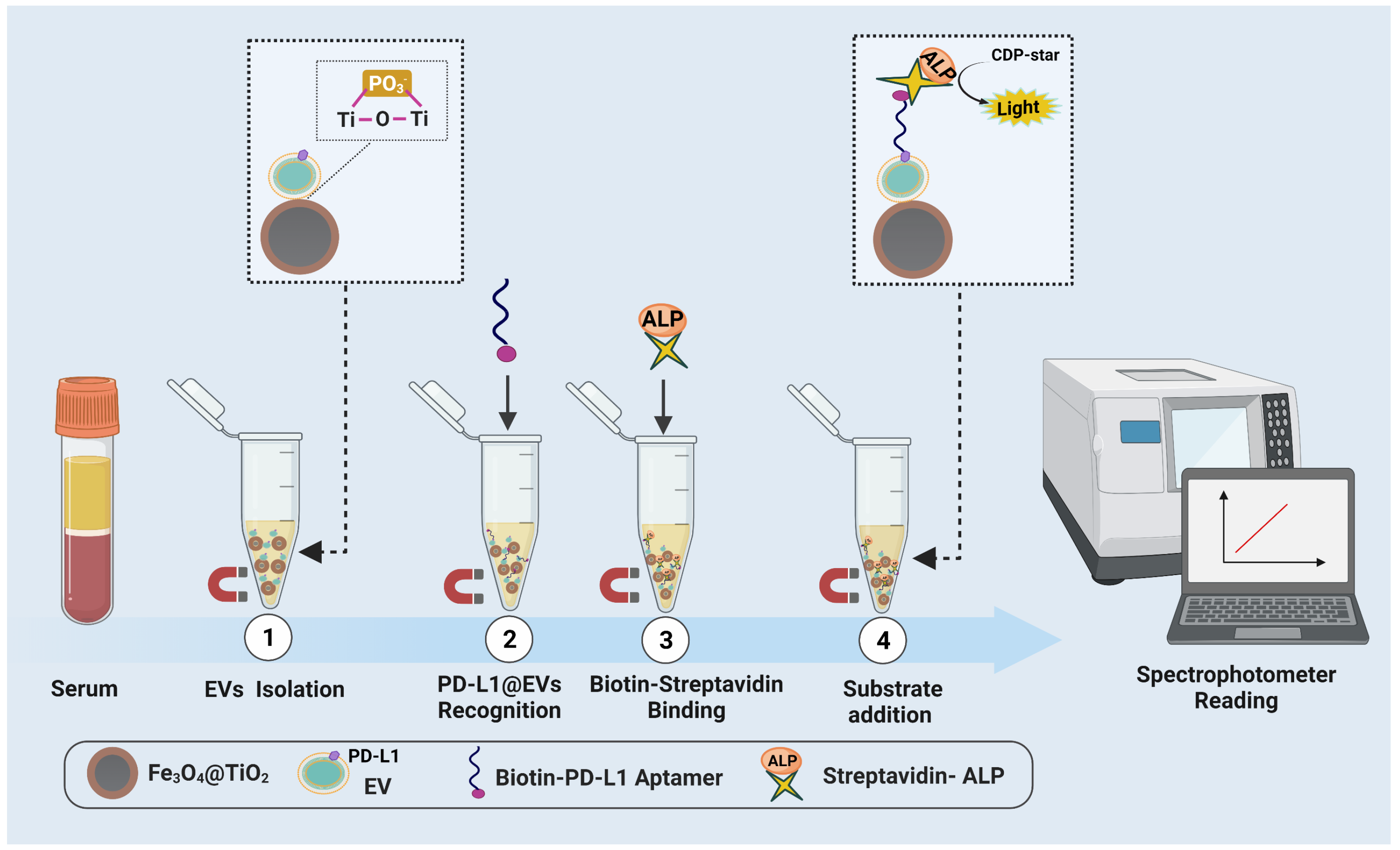
3.1. Principal Design of the Sensing Assay
3.2. FeO@TiO Formation Analysis
3.3. EVs Characterization, Enumeration and PD-L1 Realization
3.4. Assay’s Conditions Optimization
3.5. Confocal Microscopy-Based Visualization of PDL@EVs
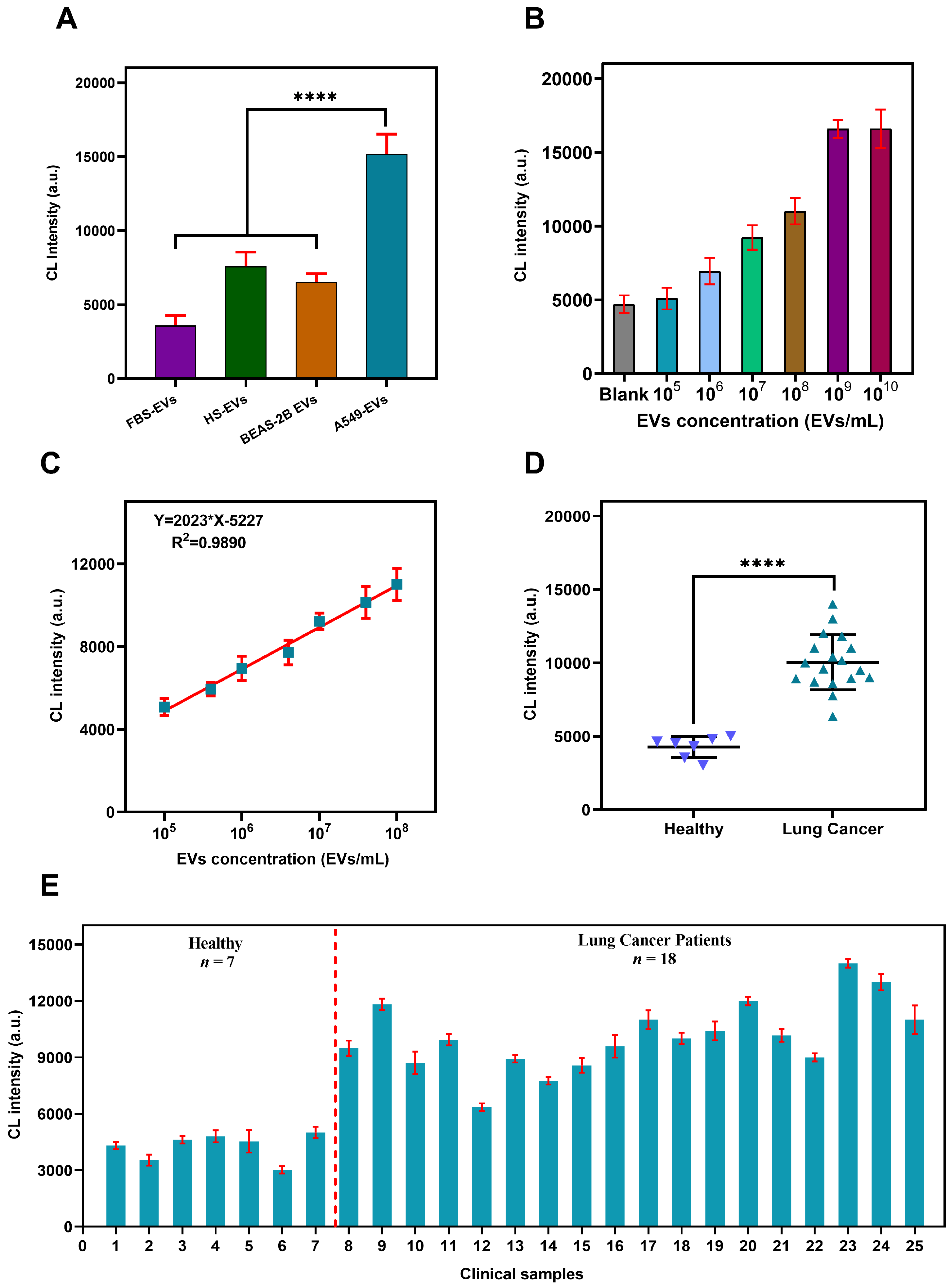
3.6. Verification of Assay’s Specificity, Sensitivity and Accuracy
3.7. Sensing PD-L1@EVs in Clinical Samples
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Yekula, A.; Muralidharan, K.; Kang, K.M.; Wang, L.; Balaj, L.; Carter, B.S. From laboratory to clinic: Translation of extracellular vesicle based cancer biomarkers. Methods 2020, 177, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Daassi, D.; Mahoney, K.M.; Freeman, G.J. The importance of exosomal PDL1 in tumour immune evasion. Nat. Rev. Immunol. 2020, 20, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Yang, J.; Wang, T.; Song, J.; Xia, J.; Wu, L.; Wang, W.; Wu, Q.; Zhu, Z.; Song, Y.; et al. Homogeneous, low-volume, efficient, and sensitive quantitation of circulating exosomal PD-L1 for cancer diagnosis and immunotherapy response prediction. Angew. Chem. 2020, 132, 4830–4835. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Liu, C.; Xiang, X.; Han, S.; Lim, H.Y.; Li, L.; Zhang, X.; Ma, Z.; Yang, L.; Guo, S.; Soo, R.; et al. Blood-based liquid biopsy: Insights into early detection and clinical management of lung cancer. Cancer Lett. 2022, 524, 91–102. [Google Scholar] [CrossRef]
- Boriachek, K.; Masud, M.K.; Palma, C.; Phan, H.P.; Yamauchi, Y.; Hossain, M.S.A.; Nguyen, N.T.; Salomon, C.; Shiddiky, M.J. Avoiding pre-isolation step in exosome analysis: Direct isolation and sensitive detection of exosomes using gold-loaded nanoporous ferric oxide nanozymes. Anal. Chem. 2019, 91, 3827–3834. [Google Scholar] [CrossRef] [Green Version]
- Holcar, M.; Kandušer, M.; Lenassi, M. Blood nanoparticles–influence on extracellular vesicle isolation and characterization. Front. Pharmacol. 2021, 3178. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, C.; Mao, Z.; Bachman, H.; Becker, R.; Rufo, J.; Wang, Z.; Zhang, P.; Mai, J.; Yang, S.; et al. Acoustofluidic centrifuge for nanoparticle enrichment and separation. Sci. Adv. 2021, 7, eabc0467. [Google Scholar] [CrossRef]
- Martín-Gracia, B.; Martín-Barreiro, A.; Cuestas-Ayllón, C.; Grazú, V.; Line, A.; Llorente, A.; Jesús, M.; Moros, M. Nanoparticle-based biosensors for detection of extracellular vesicles in liquid biopsies. J. Mater. Chem. B 2020, 8, 6710–6738. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Jiao, F.; Xia, C.; Zhao, Y.; Ying, W.; Xie, Y.; Guan, X.; Tao, M.; Zhang, Y.; Qin, W.; et al. A novel strategy for facile serum exosome isolation based on specific interactions between phospholipid bilayers and TiO2. Chem. Sci. 2019, 10, 1579–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Sun, N.; Deng, C. Rapid isolation and proteome analysis of urinary exosome based on double interactions of Fe3O4@TiO2-DNA aptamer. Talanta 2021, 221, 121571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Liu, J.W.; Adkins, G.B.; Shen, W.; Trinh, M.P.; Duan, L.Y.; Jiang, J.H.; Zhong, W. Enhancement of the intrinsic peroxidase-like activity of graphitic carbon nitride nanosheets by ssDNAs and its application for detection of exosomes. Anal. Chem. 2017, 89, 12327–12333. [Google Scholar] [CrossRef]
- Yu, X.; He, L.; Pentok, M.; Yang, H.; Yang, Y.; Li, Z.; He, N.; Deng, Y.; Li, S.; Liu, T.; et al. An aptamer-based new method for competitive fluorescence detection of exosomes. Nanoscale 2019, 11, 15589–15595. [Google Scholar] [CrossRef]
- Xie, H.; Di, K.; Huang, R.; Khan, A.; Xia, Y.; Xu, H.; Liu, C.; Tan, T.; Tian, X.; Shen, H.; et al. Extracellular vesicles based electrochemical biosensors for detection of cancer cells: A review. Chin. Chem. Lett. 2020, 31, 1737–1745. [Google Scholar] [CrossRef]
- Lin, B.; Tian, T.; Lu, Y.; Liu, D.; Huang, M.; Zhu, L.; Zhu, Z.; Song, Y.; Yang, C. Tracing tumor-derived exosomal PD-L1 by dual-aptamer activated proximity-induced droplet digital PCR. Angew. Chem. Int. Ed. 2021, 60, 7582–7586. [Google Scholar] [CrossRef]
- Gao, X.; Teng, X.; Dai, Y.; Li, J. Rolling circle Amplification-assisted flow cytometry approach for simultaneous profiling of exosomal surface proteins. ACS Sens. 2021, 6, 3611–3620. [Google Scholar] [CrossRef]
- He, Y.; Wu, Y.; Wang, Y.; Wang, X.; Xing, S.; Li, H.; Guo, S.; Yu, X.; Dai, S.; Zhang, G.; et al. Applying CRISPR/Cas13 to construct exosomal PD-L1 ultrasensitive biosensors for dynamic monitoring of tumor progression in immunotherapy. Adv. Ther. 2020, 3, 2000093. [Google Scholar] [CrossRef]
- Xu, L.; Chopdat, R.; Li, D.; Al-Jamal, K.T. Development of a simple, sensitive and selective colorimetric aptasensor for the detection of cancer-derived exosomes. Biosens. Bioelectron. 2020, 169, 112576. [Google Scholar] [CrossRef]
- Wang, S.; Khan, A.; Huang, R.; Ye, S.; Di, K.; Xiong, T.; Li, Z. Recent advances in single extracellular vesicle detection methods. Biosens. Bioelectron. 2020, 154, 112056. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Liu, Y.; Wang, L.; Adkins, G.B.; Zhong, W. Rapid enrichment and detection of extracellular vesicles enabled by CuS-enclosed microgels. Anal. Chem. 2019, 91, 15951–15958. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Ding, Y.; Gao, Z.; Li, H. S1 nuclease digestion-based rational truncation of PD-L1 aptamer and establishment of a signal dual amplification aptasensor. Sens. Actuators Chem. 2021, 331, 129442. [Google Scholar] [CrossRef]
- McPherson, R.A.; Pincus, M.R. Henry’s Clinical Diagnosis and Management by Laboratory Methods E-Book; Elsevier Health Sciences: Philadelphia, PA, USA, 2021. [Google Scholar]
- Worsfold, P.; Townshend, A.; Poole, C.F.; Miró, M. Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Wang, Y.; Liu, Z.; Wang, X.; Dai, Y.; Li, X.; Gao, S.; Yu, P.; Lin, Q.; Fan, Z.; Ping, Y.; et al. Rapid and quantitative analysis of exosomes by a chemiluminescence immunoassay using superparamagnetic iron oxide particles. J. Biomed. Nanotechnol. 2019, 15, 1792–1800. [Google Scholar] [CrossRef]
- Thierry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants. Curr. Protoc. Cell Biol. 2006, 3, 1–29. [Google Scholar]
- Pang, Y.; Shi, J.; Yang, X.; Wang, C.; Sun, Z.; Xiao, R. Personalized detection of circling exosomal PD-L1 based on Fe3O4@TiO2 isolation and SERS immunoassay. Biosens. Bioelectron. 2020, 148, 111800. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.; Wu, Z.; Wang, J.; Li, B.; Feng, S.; Deng, Y.; Zhang, F.; Zhao, D. A versatile kinetics-controlled coating method to construct uniform porous TiO2 shells for multifunctional core–shell structures. J. Am. Chem. Soc. 2012, 134, 11864–11867. [Google Scholar] [CrossRef]
- Dong, H.Y.; Xie, Q.H.; Pang, D.W.; Chen, G.; Zhang, Z.L. Precise selection of aptamers targeting PD-L1 positive small extracellular vesicles on magnetic chips. Chem. Commun. 2021, 57, 3555–3558. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Y.; Luo, D.; Liu, J.; Yang, E.; Yang, G.; Feng, G.; Chen, Q.; Wu, L. Immunoassay-aptasensor for the determination of tumor-derived exosomes based on the combination of magnetic nanoparticles and hybridization chain reaction. RSC Adv. 2021, 11, 4983–4990. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Yu, X.; Jiang, X.; Li, G.; Zhao, J. Identification of programmed death ligand-1 positive exosomes in breast cancer based on DNA amplification-responsive metal-organic frameworks. Biosens. Bioelectron. 2020, 166, 112452. [Google Scholar] [CrossRef]
- Lee, H.H.; Wang, Y.N.; Xia, W.; Chen, C.H.; Rau, K.M.; Ye, L.; Wei, Y.; Chou, C.K.; Wang, S.C.; Yan, M.; et al. Removal of N-linked glycosylation enhances PD-L1 detection and predicts anti-PD-1/PD-L1 therapeutic efficacy. Cancer Cell 2019, 36, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, L.; Deng, Y.; Wang, M.; Peng, Y.; Yang, J.; Li, G. A simple and sensitive method for exosome detection based on steric hindrance-controlled signal amplification. Chem. Commun. 2020, 56, 13768–13771. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, Y.; Liu, Y.; Ning, L.; Xiang, Y.; Li, G. Bridging exosome and liposome through zirconium–phosphate coordination chemistry: A new method for exosome detection. Chem. Commun. 2019, 55, 2708–2711. [Google Scholar] [CrossRef] [PubMed]
- Kwong Hong Tsang, D.; Lieberthal, T.J.; Watts, C.; Dunlop, I.E.; Ramadan, S.; del Rio Hernandez, A.E.; Klein, N. Chemically functionalised graphene FET biosensor for the label-free sensing of exosomes. Sci. Rep. 2019, 9, 13946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura, S.L.; Martín, C.G.; Martí, M.; Pividori, M.I. Multiplex detection and characterization of breast cancer exosomes by magneto-actuated immunoassay. Talanta 2020, 211, 120657. [Google Scholar] [CrossRef]
- Oliveira-Rodríguez, M.; López-Cobo, S.; Reyburn, H.T.; Costa-García, A.; López-Martín, S.; Yáñez-Mó, M.; Cernuda-Morollón, E.; Paschen, A.; Valés-Gómez, M.; Blanco-López, M.C. Development of a rapid lateral flow immunoassay test for detection of exosomes previously enriched from cell culture medium and body fluids. J. Extracell. Vesicles 2016, 5, 31803. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, M.; Wang, L.; Yan, A.; He, W.; Chen, M.; Lan, J.; Xu, J.; Guan, L.; Chen, J. A visible and colorimetric aptasensor based on DNA-capped single-walled carbon nanotubes for detection of exosomes. Biosens. Bioelectron. 2017, 92, 8–15. [Google Scholar] [CrossRef] [Green Version]



| Method | LOD (EVs/mL) | Sample Type | Prior Isolation (UC/kit/UF) | Linear Range (EVs/mL) | Reference |
|---|---|---|---|---|---|
| Fluorescence | EVs Spiked Serum | Yes | – | [34] | |
| Fluorescence | Culture Medium | Yes | – | [35] | |
| FET | Diluted Serum | Yes | – | [36] | |
| Optical | EVs Spiked Serum | Yes | – | [37] | |
| Lateral flow immunoassay | Culture Medium | Yes | – | [38] | |
| Colorimetric | Diluted Serum | Yes | – | [39] | |
| Colorimetric | Diluted Serum | Yes | – | [14] | |
| Chemiluminescence | Undiluted Serum | No | – | [26] | |
| Chemiluminescence | Undiluted Serum | No | This method |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Di, K.; Khan, H.; He, N.; Li, Z. Rapid Capturing and Chemiluminescent Sensing of Programmed Death Ligand-1 Expressing Extracellular Vesicles. Biosensors 2022, 12, 281. https://doi.org/10.3390/bios12050281
Khan A, Di K, Khan H, He N, Li Z. Rapid Capturing and Chemiluminescent Sensing of Programmed Death Ligand-1 Expressing Extracellular Vesicles. Biosensors. 2022; 12(5):281. https://doi.org/10.3390/bios12050281
Chicago/Turabian StyleKhan, Adeel, Kaili Di, Haroon Khan, Nongyue He, and Zhiyang Li. 2022. "Rapid Capturing and Chemiluminescent Sensing of Programmed Death Ligand-1 Expressing Extracellular Vesicles" Biosensors 12, no. 5: 281. https://doi.org/10.3390/bios12050281
APA StyleKhan, A., Di, K., Khan, H., He, N., & Li, Z. (2022). Rapid Capturing and Chemiluminescent Sensing of Programmed Death Ligand-1 Expressing Extracellular Vesicles. Biosensors, 12(5), 281. https://doi.org/10.3390/bios12050281








