Graphene Oxide-Magnetic Nanoparticles Loaded Polystyrene-Polydopamine Electrospun Nanofibers Based Nanocomposites for Immunosensing Application of C-Reactive Protein
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Instruments
2.3. Synthesis of GO and rGO-MNP
2.4. Preparation of rGO-MNP-PDA, rGO-PDA, and MNP-PDA
2.5. Preparation of PS/rGO-MNP-PDA, PS/rGO-PDA and PS/MNP-PDA Nanofibers
2.6. Preparation of PS/rGO-MNP-PDA/Anti-CRP, PS/rGO-PDA/Anti-CRP, and PS/MNP-PDA/Anti-CRP
2.7. Electrochemical Measurements
3. Results and Discussion
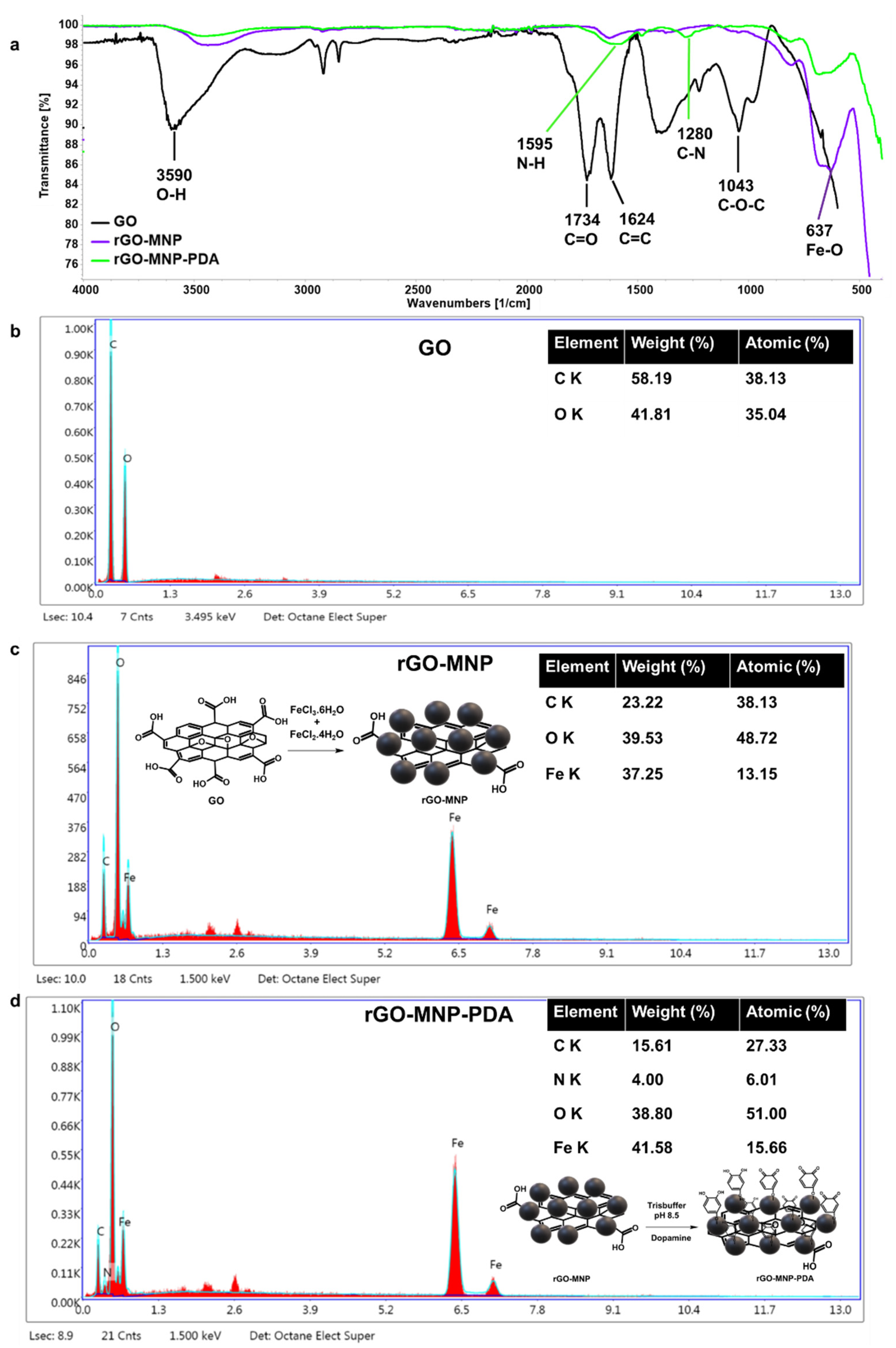
3.1. Characterization of GO and rGO-MNP
3.2. Characterization of PS/rGO-MNP-PDA Electrospun Nanofibers
3.3. Electrochemical Characterization of PS/rGO-MNP-PDA/Anti-CRP
3.4. Application of PS/rGO-MNP-PDA/Anti-CRP for CRP Detection
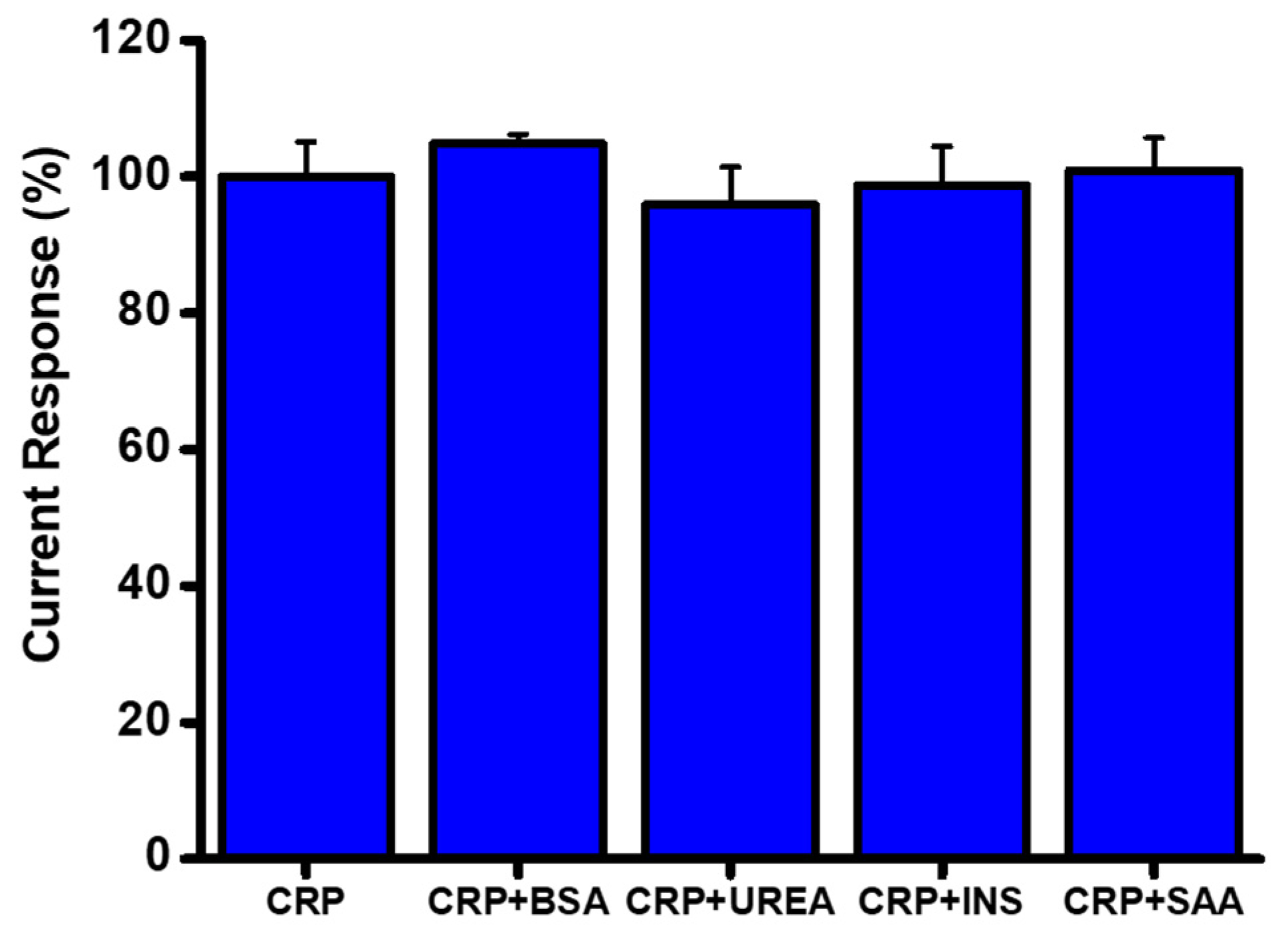
3.5. Sample Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, P.A.; Ambrosone, C. Chapter 7: Molecular epidemiology of genetic polymorphisms in estrogen metabolizing enzymes in human breast cancer. JNCI Monogr. 2000, 2000, 125–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couto, R.A.; Quinaz, M.B. Development of a Nafion/MWCNT-SPCE-based portable sensor for the voltammetric analysis of the anti-tuberculosis drug ethambutol. Sensors 2016, 16, 1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Guo, P.; Li, B.; Fu, L.; Lin, C.-T.; Yu, A.; Lai, G. Enzyme-catalyzed deposition of polydopamine for amplifying the signal inhibition to a novel Prussian blue-nanocomposite and ultrasensitive electrochemical immunosensing. J. Mater. Sci. Technol. 2022, 102, 166–173. [Google Scholar] [CrossRef]
- Mahato, K.; Srivastava, A.; Chandra, P. Paper based diagnostics for personalized health care: Emerging technologies and commercial aspects. Biosens. Bioelectron. 2017, 96, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Jadon, N.; Jain, R. Next-generation polymer nanocomposite-based electrochemical sensors and biosensors: A review. Trends Anal. Chem. 2016, 82, 55–67. [Google Scholar] [CrossRef]
- Smith, S.; Goodge, K.; Delaney, M.; Struzyk, A.; Tansey, N.; Frey, M. A comprehensive review of the covalent immobilization of biomolecules onto electrospun nanofibers. Nanomaterials 2020, 10, 2142. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Barai, D.P.; Bhanvase, B.A.; Saharan, V.K. Reduced graphene oxide-Fe3O4 nanocomposite based nanofluids: Study on ultrasonic assisted synthesis, thermal conductivity, rheology, and convective heat transfer. Ind. Eng. Chem. Res. 2019, 58, 8349–8369. [Google Scholar] [CrossRef]
- Li, D.; Wang, Q.; Huang, F.; Wei, Q. Electrospun nanofibers for enzyme immobilization. In Electrospinning: Nanofabrication and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 765–781. [Google Scholar]
- Lee, S.J.; Tatavarty, R.; Gu, M.B. Electrospun polystyrene–poly (styrene-co-maleic anhydride) nanofiber as a new aptasensor platform. Biosens. Bioelectron. 2012, 38, 302–307. [Google Scholar] [CrossRef]
- Jayaprakash, G.K. Pre-post redox electron transfer regioselectivity at the alanine modified nano graphene electrode interface. Chem. Phys. Lett. 2022, 789, 139295. [Google Scholar] [CrossRef]
- Al-Dhahebi, A.M.; Gopinath, S.C.B.; Saheed, M.S.M. Graphene impregnated electrospun nanofiber sensing materials: A comprehensive overview on bridging laboratory set-up to industry. Nano Converg. 2020, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Orasugh, J.T.; Ray, S.S. Graphene-Based Electrospun Fibrous Materials with Enhanced EMI Shielding: Recent Developments and Future Perspectives. ACS Omega 2022, 7, 33699–33718. [Google Scholar] [CrossRef] [PubMed]
- Che Othman, F.E.; Yusof, N.; González-Benito, J.; Fan, X.; Ismail, A.F. Electrospun composites made of reduced graphene oxide and polyacrylonitrile-based activated carbon nanofibers (rGO/ACNF) for enhanced CO2 adsorption. Polymers 2020, 12, 2117. [Google Scholar] [CrossRef] [PubMed]
- Zang, Z.; Zeng, X.; Wang, M.; Hu, W.; Liu, C.; Tang, X. Tunable photoluminescence of water-soluble AgInZnS–graphene oxide (GO) nanocomposites and their application in-vivo bioimaging. Sens. Actuators B Chem. 2017, 252, 1179–1186. [Google Scholar] [CrossRef]
- Hou, S.; Su, S.; Kasner, M.L.; Shah, P.; Patel, K.; Madarang, C.J. Formation of highly stable dispersions of silane-functionalized reduced graphene oxide. Chem. Phys. Lett. 2010, 501, 68–74. [Google Scholar] [CrossRef]
- Mehdinia, A.; Heydari, S.; Jabbari, A. Synthesis and characterization of reduced graphene oxide-Fe3O4@Polydopamine and application for adsorption of lead ions: Isotherm and kinetic studies. Mater. Chem. Phys. 2020, 239, 121964. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Xu, M.; Wang, Y.; Ran, D.; Yang, S.; Zhang, M. Polydopamine-based molecular imprinting on silica-modified magnetic nanoparticles for recognition and separation of bovine hemoglobin. Analyst 2013, 138, 651–658. [Google Scholar] [CrossRef]
- Lin, L.-S.; Cong, Z.-X.; Cao, J.-B.; Ke, K.-M.; Peng, Q.-L.; Gao, J.; Yang, H.-H.; Liu, G.; Chen, X. Multifunctional Fe3O4@Polydopamine core–shell nanocomposites for intracellular mRNA detection and imaging-guided photothermal therapy. ACS Nano 2014, 8, 3876–3883. [Google Scholar] [CrossRef] [Green Version]
- Martín, M.; Salazar, P.; Villalonga, R.; Campuzano, S.; Pingarrón, J.M.; González-Mora, J.L. Preparation of core–shell Fe3O4@poly(dopamine) magnetic nanoparticles for biosensor construction. J. Mater. Chem. B 2014, 2, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhang, D.; Wang, Y.; Qi, P.; Hou, B. Direct immobilisation of antibodies on a bioinspired architecture as a sensing platform. Biosens. Bioelectron. 2011, 26, 2595–2600. [Google Scholar] [CrossRef] [PubMed]
- Ball, V. Polydopamine nanomaterials: Recent advances in synthesis methods and applications. Front. Bioeng. Biotechnol. 2018, 6, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar-Ferrer, D.; Szewczyk, J.; Coy, E. Recent developments in polydopamine-based photocatalytic nanocomposites for energy production: Physico-chemical properties and perspectives. Catal. Today 2021, 397–399, 316–349. [Google Scholar] [CrossRef]
- He, Z.; Gudavarthy, R.V.; Koza, J.A.; Switzer, J.A. Room-temperature electrochemical reduction of epitaxial magnetite films to epitaxial iron films. J. Am. Chem. Soc. 2011, 133, 12358–12361. [Google Scholar] [CrossRef]
- Chimezie, A.B.; Hajian, R.; Yusof, N.A.; Woi, P.M.; Shams, N. Fabrication of reduced graphene oxide-magnetic nanocomposite (rGO-Fe3O4) as an electrochemical sensor for trace determination of As (III) in water resources. J. Electroanal. Chem. 2017, 796, 33–42. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Zor, E.; Ozcelikay, G.; Ozkan, S.A. Magnetic nanoparticles in developing electrochemical sensors for pharmaceutical and biomedical applications. Talanta 2021, 226, 122108. [Google Scholar] [CrossRef]
- Nejad, F.G.; Tajik, S.; Beitollahi, H.; Sheikhshoaie, I. Magnetic nanomaterials based electrochemical (bio) sensors for food analysis. Talanta 2021, 228, 122075. [Google Scholar] [CrossRef]
- Santosh, T.S.; Parmar, R.; Anand, H.; Srikanth, K.; Saritha, M. A review of salivary diagnostics and its potential implication in detection of COVİD-19. Cureus 2020, 12, e7708. [Google Scholar] [CrossRef] [Green Version]
- Cebrian, A.V.; Carvalho, R.S.; Barreto, A.R.; Maturi, F.E.; Barud, H.S.; Silva, R.R.; Legnani, C.; Cremona, M.; Ribeiro, S.J. Development of conformable substrates for oleds using highly transparent bacterial cellulose modified with recycled polystyrene. Adv. Sustain. Syst. 2022, 6, 2000258. [Google Scholar] [CrossRef]
- Gal, J.-Y.; Fovet, Y.; Adib-Yadzi, M. About a synthetic saliva for in vitro studies. Talanta 2001, 53, 1103–1115. [Google Scholar] [CrossRef]
- Matulevicius, J.; Kliucininkas, L.; Prasauskas, T.; Buivydiene, D.; Martuzevicius, D. The comparative study of aerosol filtration by electrospun polyamide, polyvinyl acetate, polyacrylonitrile and cellulose acetate nanofiber media. J. Aerosol Sci. 2016, 92, 27–37. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Kirbay, F.O.; Yazgan, İ.; Demirkol, D.O. Comparison of Direct and Sandwich Type Immunoassays on Electrospun Nanofibers Using of Metal Organic Frameworks as a Fluorescence Probe. Sens. Actuators B Chem. 2022, 372, 132621. [Google Scholar] [CrossRef]
- Li, P.F.; Xu, Y.; Cheng, X.-H. Chemisorption of thermal reduced graphene oxide nano-layer film on TNTZ surface and its tribological behavior. Surf. Coat. Technol. 2013, 232, 331–339. [Google Scholar] [CrossRef]
- Zubir, N.A.; Yacou, C.; Motuzas, J.; Zhang, X.; Diniz da Costa, J.C. Structural and functional investigation of graphene oxide–Fe3O4 nanocomposites for the heterogeneous Fenton-like reaction. Sci. Rep. 2014, 4, 4594. [Google Scholar] [CrossRef] [Green Version]
- Chin, S.F.; Iyer, K.S.; Raston, C.L. Fabrication of carbon nano-tubes decorated with ultra fine superparamagnetic nano-particles under continuous flow conditions. Lab Chip 2008, 8, 439–442. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Luo, H.; Gu, C.; Zheng, W.; Dai, F.; Wang, X.; Zheng, Z. Facile synthesis of novel size-controlled antibacterial hybrid spheres using silver nanoparticles loaded with poly-dopamine spheres. RSC Adv. 2015, 5, 13470–13477. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Jarusuwannapoom, T.; Hongrojjanawiwat, W.; Jitjaicham, S.; Wannatong, L.; Nithitanakul, M.; Pattamaprom, C.; Koombhongse, P.; Rangkupan, R.; Supaphol, P. Effect of solvents on electro-spinnability of polystyrene solutions and morphological appearance of resulting electrospun polystyrene fibers. Eur. Polym. J. 2005, 41, 409–421. [Google Scholar] [CrossRef]
- Wannatong, L.; Sirivat, A.; Supaphol, P. Effects of solvents on electrospun polymeric fibers: Preliminary study on polystyrene. Polym. Int. 2004, 53, 1851–1859. [Google Scholar] [CrossRef]
- Nitanan, T.; Opanasopit, P.; Akkaramongkolporn, P.; Rojanarata, T.; Ngawhirunpat, T.; Supaphol, P. Effects of processing parameters on morphology of electrospun polystyrene nanofibers. Korean J. Chem. Eng. 2012, 29, 173–181. [Google Scholar] [CrossRef]
- Er, S.; Demirkol, D.O. Graphene oxide incorporated polystyrene electrospun nanofibers for immunosensing of CD36 as a marker of diabetic plasma. Bioelectrochemistry 2022, 145, 108083. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Long, Y.; Zhang, H.; Li, M.; Duvail, J.; Jiang, X.; Yin, H. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Diaz, A.M.; Zhang, Z.; Lee, B.; Luna, F.M.H.; Li Sip, Y.Y.; Lu, X.; Heidings, J.; Tetard, L.; Zhai, L.; Kang, H. Evaluation of single hydrogel nanofiber mechanics using persistence length analysis. ACS Omega 2018, 3, 18304–18310. [Google Scholar] [CrossRef]
- Zulfi, A.; Fauzi, A.; Edikresnha, D.; Munir, M. Synthesis of high-impact polystyrene fibers using electrospinning. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 4th International Conference on Advanced Materials Science and Technology, Malang, Indonesia, 27–28 September 2016; IOP Publishing: Bristol, UK, 2017. [Google Scholar]
- Neděla, O.; Slepička, P.; Švorčík, V. Surface modification of polymer substrates for biomedical applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef]
- Arfin, T. Emerging trends in lab-on-a-chip for biosensing applications. In Functionalized Nanomaterials Based Devices for Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 199–218. [Google Scholar]
- Streeter, I.; Wildgoose, G.G.; Shao, L.; Compton, R.G. Cyclic voltammetry on electrode surfaces covered with porous layers: An analysis of electron transfer kinetics at single-walled carbon nanotube modified electrodes. Sens. Actuators B Chem. 2008, 133, 462–466. [Google Scholar] [CrossRef]
- Zhou, J.; Tsao, H.-K.; Sheng, Y.-J.; Jiang, S. Monte Carlo simulations of antibody adsorption and orientation on charged surfaces. Chem. Phys. 2004, 121, 1050–1057. [Google Scholar] [CrossRef]
- Chen, S.; Liu, L.; Zhou, J.; Jiang, S. Controlling antibody orientation on charged self-assembled monolayers. Langmuir 2003, 19, 2859–2864. [Google Scholar] [CrossRef]
- Talasaz, A.H.; Nemat-Gorgani, M.; Liu, Y.; Ståhl, P.; Dutton, R.W.; Ronaghi, M.; Davis, R.W. Prediction of protein orientation upon immobilization on biological and nonbiological surfaces. Proc. Natl. Acad. Sci. USA 2006, 103, 14773–14778. [Google Scholar] [CrossRef]
- Polk, B.J.; Stelzenmuller, A.; Mijares, G.; MacCrehan, W.; Gaitan, M. Ag/AgCl microelectrodes with improved stability for microfluidics. Sens. Actuators B Chem. 2006, 114, 239–247. [Google Scholar] [CrossRef]
- Ozoemena, O.C.; Mathebula, N.S.; Ehirim, T.J.; Maphumulo, T.; Valikpe, G.M.; Shai, J.L.; Ozoemena, K.I. Onion-like carbon re-inforced electrospun polyacrylonitrile fibres for ultrasensitive electrochemical immunosensing of Vibrio cholerae toxin. Electrochim. Acta 2020, 356, 136816. [Google Scholar] [CrossRef]
- Wang, H.; Pan, M.; Oliver Su, Y.; Tsai, S.; Kao, C.; Sun, S.; Lin, W. Comparison of Differential Pulse Voltammetry (DPV)—A new method of carbamazepine analysis—With Fluorescence Polarization Immunoassay (FPIA). J. Anal. Chem. 2011, 66, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principles of İnstrumental Analysis; Cengage Learning: Boston, MA, USA, 2017. [Google Scholar]
- Zia, T.u.H.; Shah, A.u.H.A. Understanding the adsorption of 1 NLB antibody on polyaniline nanotubes as a function of zeta potential and surface charge density for detection of hepatitis C core antigen: A label-free impedimetric immunosensor. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127076. [Google Scholar] [CrossRef]
- Nossol, E.; Muñoz, R.A.A.; Richter, E.M.; de Souza Borges, P.H.; Silva, S.C.; Rocha, D.P. Sensing Materials: Graphene; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 367–388. [Google Scholar] [CrossRef]
- Kopanczyk, R.; Opris, D.C.; Lickliter, J.; Bridges, E.G.; Nazar, A.M.; Bridges, K.G. C-reactive protein levels in blood and saliva show no correlation in young, healthy adults. FASEB J. 2010, 24, lb409. [Google Scholar] [CrossRef]
- Vashist, S.K.; Luong, J.H. Bioanalytical requirements and regulatory guidelines for immunoassays. In Handbook of İmmunoassay Technologies; Elsevier: Amsterdam, The Netherlands, 2018; pp. 81–95. [Google Scholar]
- Mena, M.; Yanez-Sedeno, P.; Pingarron, J. A comparison of different strategies for the construction of amperometric enzyme biosensors using gold nanoparticle-modified electrodes. Anal. Biochem. 2005, 336, 20–27. [Google Scholar] [CrossRef]
- Maiga, M.; Yalcinkaya, E.E.; Sonmez, B.; Puglia, D.; Yavuz, M.; Demirkol, D.O.; Kenny, J.M.; Timur, S. CTAB modified dellite: A novel support for enzyme immobilization in bio-based electrochemical detection and its in vitro antimicrobial activity. Sens. Actuators B Chem. 2016, 235, 46–55. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Tillman, M.D.; Hubbs, L.M. Limit of detection (LQD)/limit of quantitation (LOQ): Comparison of the empirical and the statistical methods exemplified with GC-MS assays of abused drugs. Clin. Chem. 1994, 40, 1233–1238. [Google Scholar] [CrossRef]
- Carralero, V.; Mena, M.; Gonzalez-Cortes, A.; Yanez-Sedeno, P.; Pingarron, J. Development of a high analytical performance-tyrosinase biosensor based on a composite graphite–Teflon electrode modified with gold nanoparticles. Biosens. Bioelectron. 2006, 22, 730–736. [Google Scholar] [CrossRef]
- Teymourian, H.; Salimi, A.; Khezrian, S. Fe3O4 magnetic nanoparticles/reduced graphene oxide nanosheets as a novel electrochemical and bioeletrochemical sensing platform. Biosens. Bioelectron. 2013, 49, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lister, A.S. Validation of HPLC methods in pharmaceutical analysis. In Separation Science and Technology; Elsevier: Amsterdam, The Netherlands, 2005; pp. 191–217. [Google Scholar]
- Oner, A.; Tufek, E.; Yezer, I.; Birol, A.; Demir, M.; Er, S.; Demirkol, D.O. High generation dendrimer decorated poly-ε-caprolactone/polyacrylic acid electrospun nanofibers for the design of a bioelectrochemical sensing surface. React. Funct. Polym. 2021, 161, 104853. [Google Scholar] [CrossRef]
- Lin, C.-W.; Tsai, Y.-H.; Lu, Y.-P.; Yang, J.-T.; Chen, M.-Y.; Huang, T.-J.; Weng, R.-C.; Tung, C.-W. Application of a Novel Biosensor for Salivary Conductivity in Detecting Chronic Kidney Disease. Biosensors 2022, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.S.; Kora, M.A.A.-A.; El-Shalakany, A.H.; Mashal, B.S.A.A.-B. Clinical significance of saliva urea and creatinine levels in patients with chronic kidney disease. Menoufia Med. J. 2015, 28, 406. [Google Scholar] [CrossRef]
- Zinellu, A.; Paliogiannis, P.; Carru, C.; Mangoni, A.A. Serum amyloid A concentrations, COVID-19 severity and mortality: An updated systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 105, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Carmina, E.; Stanczyk, F.Z.; Lobo, R.A. Evaluation of hormonal status. In Yen and Jaffe’s Reproductive Endocrinology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 887–915.e4. [Google Scholar]
- Desai, G.S.; Mathews, S.T. Saliva as a non-invasive diagnostic tool for inflammation and insulin-resistance. World J. Diabetes 2014, 5, 730. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, M. Validation of analytical methods based on accuracy profiles. J. Chromatogr. A 2007, 1158, 174–183. [Google Scholar] [CrossRef]
- Peng, K.-F.; Zhao, H.-W.; Wu, X.-F. Signal-enhanced electrochemical immunosensor for CD36 based on cascade catalysis of a GOx labeled Prussian blue functionalized Ceria nanohybrid. RSC Adv. 2015, 5, 1812–1817. [Google Scholar] [CrossRef]
- Taverniers, I.; De Loose, M.; Van Bockstaele, E. Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. TrAC Trends Anal. Chem. 2004, 23, 535–552. [Google Scholar] [CrossRef]
- Petruzzi, L.; Maier, T.; Ertl, P.; Hainberger, R. Quantitative detection of C-reactive protein in human saliva using an electrochemical lateral flow device. Biosens. Bioelectron. X 2022, 10, 100136. [Google Scholar] [CrossRef]
- Liu, X.; Ren, X.; Chen, L.; Zou, J.; Li, T.; Tan, L.; Fu, C.; Wu, Q.; Li, C.; Wang, J. Fluorescent hollow ZrO2@CdTe nanoparticles-based lateral flow assay for simultaneous detection of C-reactive protein and troponin T. Mikrochim. Acta 2021, 188, 209. [Google Scholar] [CrossRef]
- Hu, W.; Dang, T.; Li, Z.; Lei, L.; Wang, G.; Li, Y.; Xu, H.; Zhou, Z.; Liu, G.L. C-reaction protein detection in human saliva by nanoplasmonic color imaging. J. Biomed. Nanotechnol. 2019, 15, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, S.; Kwon, K.; Bae, N.-H.; Kwak, B.S.; Cho, W.C.; Lee, S.J.; Jung, H.-I. A photothermal biosensor for detection of C-reactive protein in human saliva. Sens. Actuators B Chem. 2017, 246, 471–476. [Google Scholar] [CrossRef]
- Mishra, S.K.; Sharma, V.; Kumar, D. Biofunctionalized gold nanoparticle-conducting polymer nanocomposite based bioelectrode for CRP detection. Appl. Biochem. Biotechnol. 2014, 174, 984–997. [Google Scholar] [CrossRef] [PubMed]





| Artificial Sample | Added CRP (ng/mL) | Found CRP (ng/mL) | cv % | Recovery % |
|---|---|---|---|---|
| Saliva | 20.00 | 20.07 ± 0.62 | 3.09 | 100.35 |
| Analyte | Technique | Linear Range (ng/mL−1) | LOD (ng/mL−1) | References |
|---|---|---|---|---|
| CRP | LFA | 1–104 | 55 | [77] |
| CRP | LFIA | 10–100 | 1 | [78] |
| CRP | PIS | 5–100 | 5 | [79] |
| CRP | PTB | 0.1–100 | 0.1 | [80] |
| CRP | EIS | 10–104 | 19.38 | [81] |
| CRP | DPV | 0.5–60 | 0.33 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ketmen, S.; Er Zeybekler, S.; Gelen, S.S.; Odaci, D. Graphene Oxide-Magnetic Nanoparticles Loaded Polystyrene-Polydopamine Electrospun Nanofibers Based Nanocomposites for Immunosensing Application of C-Reactive Protein. Biosensors 2022, 12, 1175. https://doi.org/10.3390/bios12121175
Ketmen S, Er Zeybekler S, Gelen SS, Odaci D. Graphene Oxide-Magnetic Nanoparticles Loaded Polystyrene-Polydopamine Electrospun Nanofibers Based Nanocomposites for Immunosensing Application of C-Reactive Protein. Biosensors. 2022; 12(12):1175. https://doi.org/10.3390/bios12121175
Chicago/Turabian StyleKetmen, Simge, Simge Er Zeybekler, Sultan Sacide Gelen, and Dilek Odaci. 2022. "Graphene Oxide-Magnetic Nanoparticles Loaded Polystyrene-Polydopamine Electrospun Nanofibers Based Nanocomposites for Immunosensing Application of C-Reactive Protein" Biosensors 12, no. 12: 1175. https://doi.org/10.3390/bios12121175






