Ruthenium and Nickel Molybdate-Decorated 2D Porous Graphitic Carbon Nitrides for Highly Sensitive Cardiac Troponin Biosensor
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Apparatus
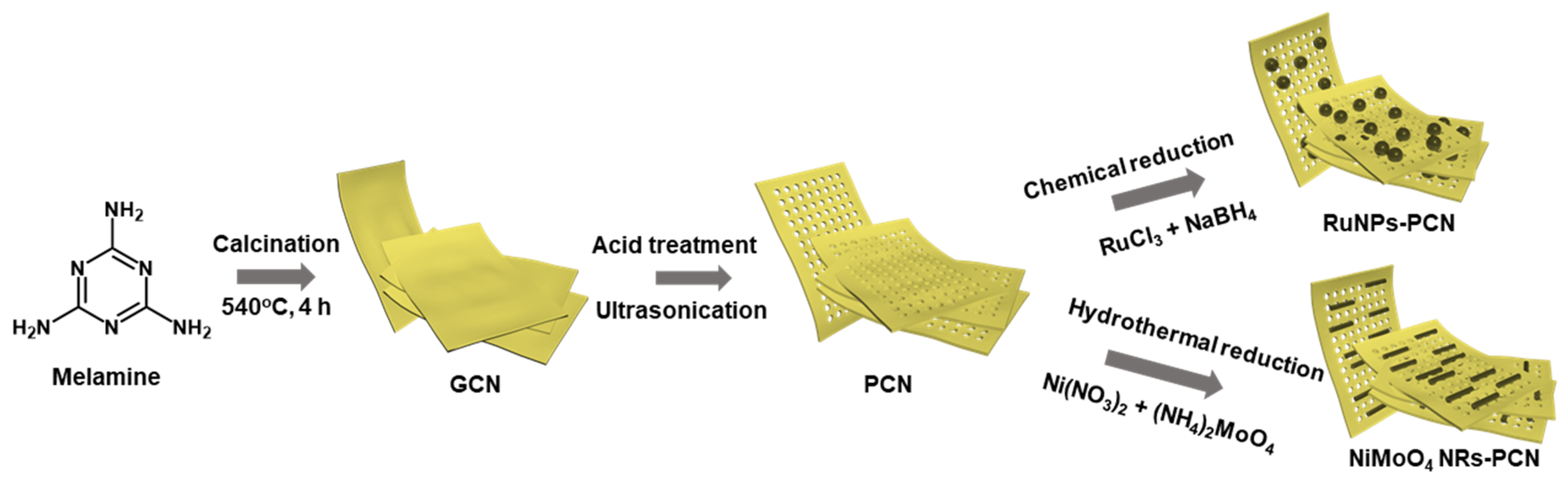
2.2. Nanomaterials Synthesis
2.3. Biosensor Fabrication Steps and Analysis Procedure
2.4. Electrochemical Assay Conditions
3. Results and Discussions
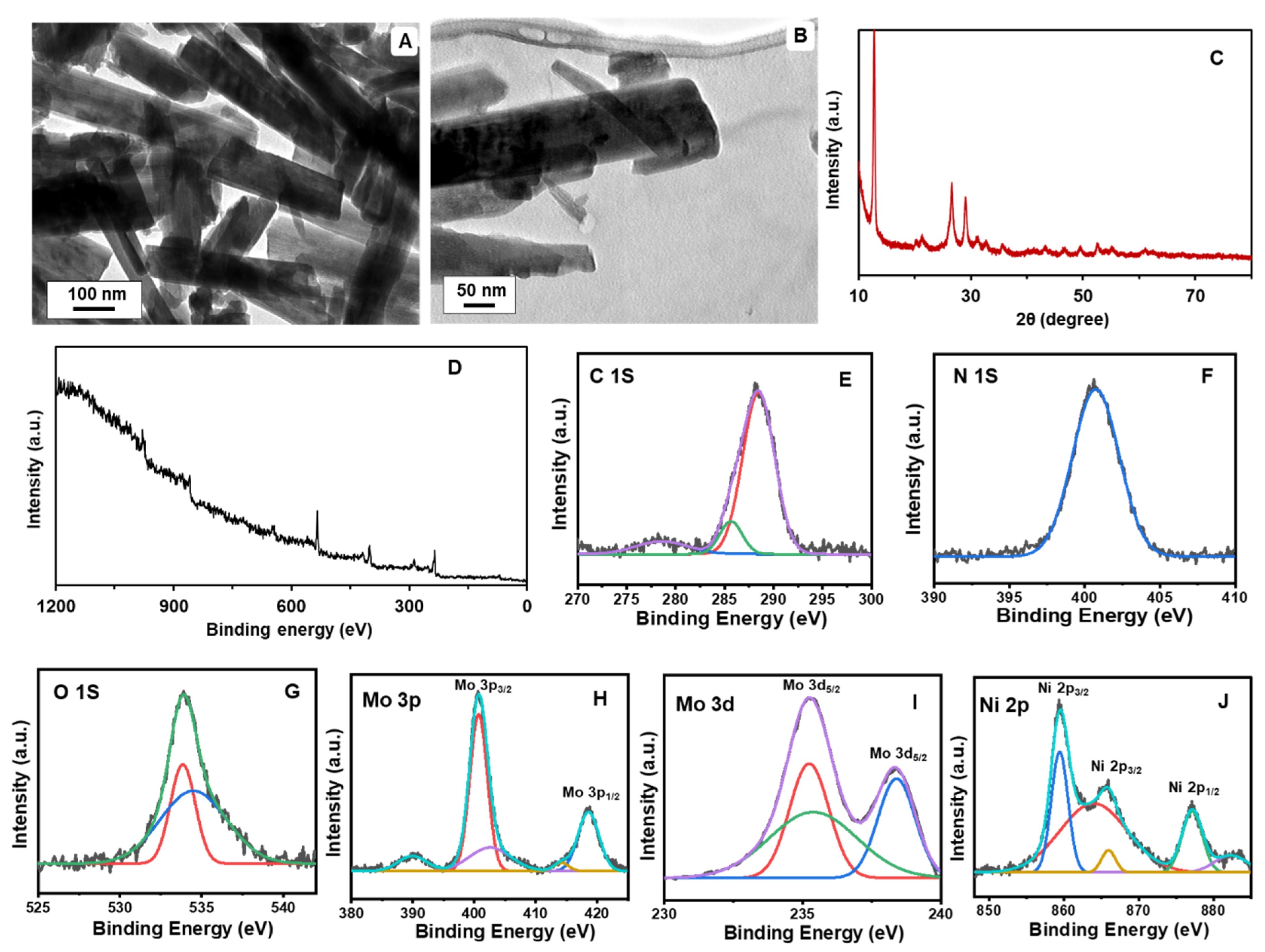
3.1. Characterizations
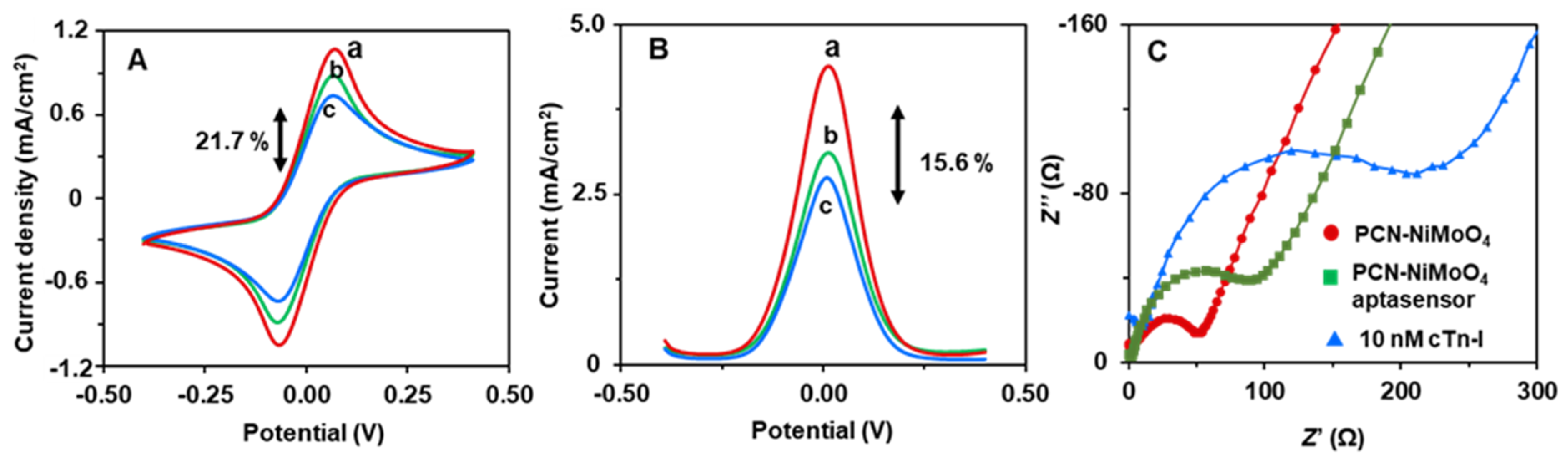
3.2. Electrochemically Active Surface Area
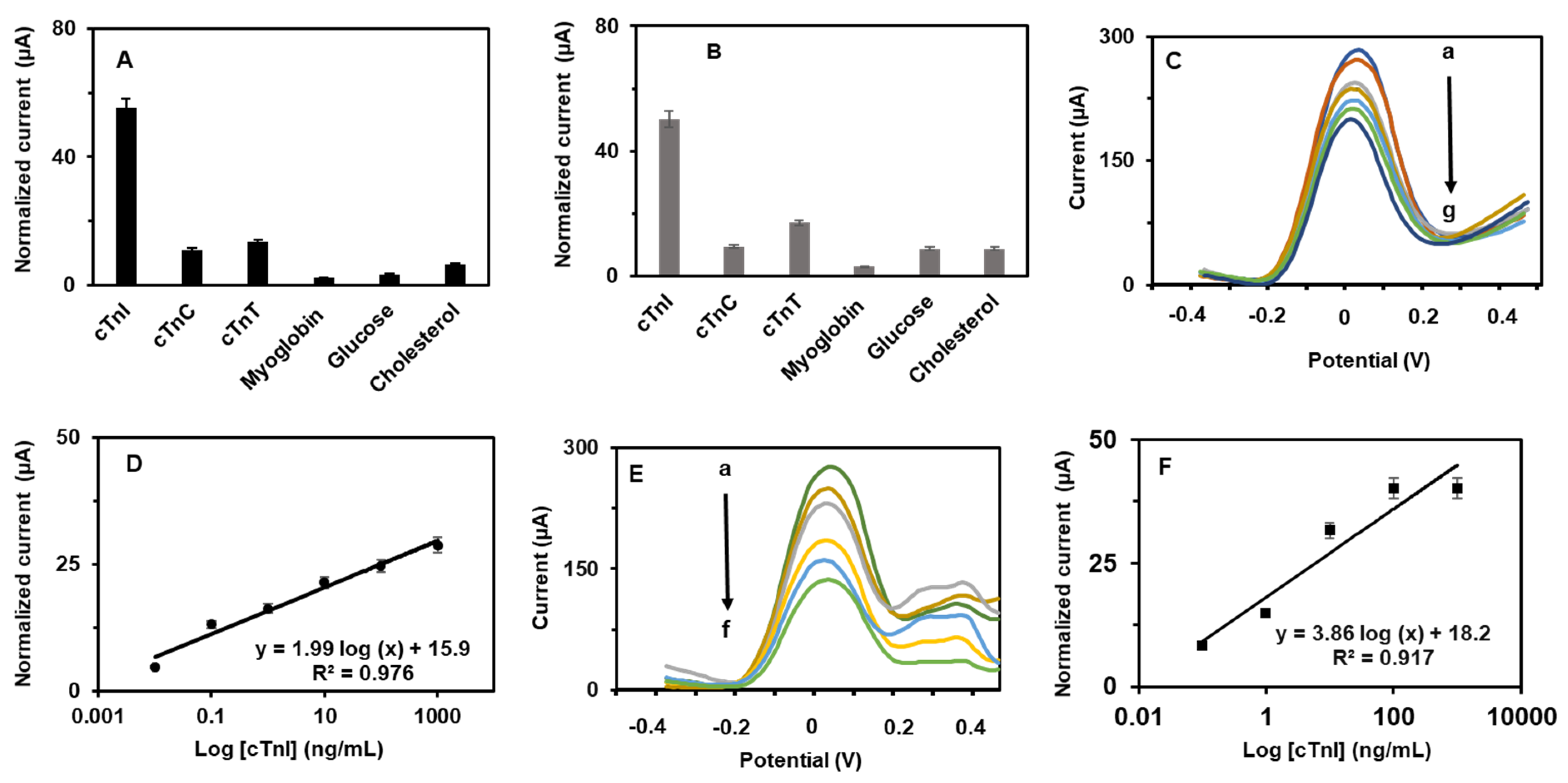
3.3. Aptasensing Performances
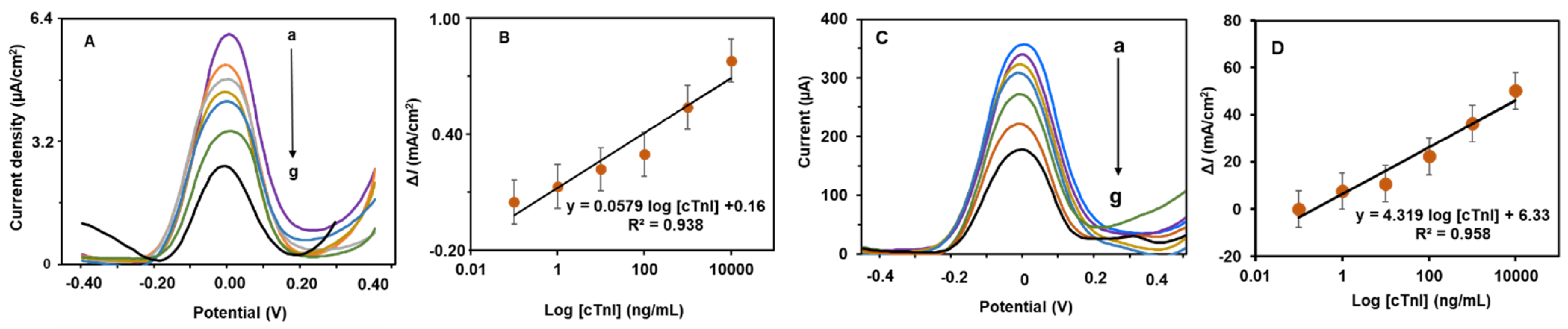
3.4. Determination of cTn-I
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Surya, S.G.; Majhi, S.M.; Agarwal, D.K.; Lahcen, A.A.; Yuvaraja, S.; Chappanda, K.N.; Salama, K.N. A label-free aptasensor FET based on Au nanoparticle decorated Co3O4 nanorods and a SWCNT layer for detection of cardiac troponin T protein. J. Mater. Chem. B 2020, 8, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Tu, D.; Tong, L.; Sarwar, M.; Bhimaraj, A.; Li, C.; Cote, G.L.; di Carlo, D. A review of biosensor technologies for blood biomarkers toward monitoring cardiovascular diseases at the point-of-care. Biosens. Bioelectron. 2021, 171, 112621. [Google Scholar] [CrossRef] [PubMed]
- Savonnet, M.; Rolland, T.; Cubizolles, M.; Roupioz, Y.; Buhot, A. Recent advances in cardiac biomarkers detection: From commercial devices to emerging technologies. J. Pharm. Biomed. Anal. 2021, 194, 113777. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Durmus, C.; Khushaim, W.; Ferreira, D.C.; Timur, S.; Arduini, F.; Salama, K.N. Multiplexed sensing techniques for cardiovascular disease biomarkers—A review. Biosens. Bioelectron. 2022, 216, 114680. [Google Scholar] [CrossRef]
- Pourali, A.; Rashidi, M.R.; Barar, J.; Pavon-Djavid, G.; Omidi, Y. Voltammetric biosensors for analytical detection of cardiac troponin biomarkers in acute myocardial infarction. TrAC Trends Anal. Chem. 2021, 134, 116123. [Google Scholar] [CrossRef]
- Bolotsky, A.; Butler, D.; Dong, C.; Gerace, K.; Glavin, N.R.; Muratore, C.; Robinson, J.A.; Ebrahimi, A. Two-dimensional materials in biosensing and healthcare: From in vitro diagnostics to optogenetics and beyond. ACS Nano 2019, 13, 9781–9810. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Liu, B.; Cheng, H.-M. Preparation of 2D material dispersions and their applications. Chem. Soc. Rev. 2018, 47, 6224–6266. [Google Scholar] [CrossRef]
- Kumar, S.; Lei, Y.; Alshareef, N.H.; Quevedo-Lopez, M.A.; Salama, K.N. Biofunctionalized two-dimensional Ti3C2 MXenes for ultrasensitive detection of cancer biomarker. Biosens. Bioelectron. 2018, 121, 243–249. [Google Scholar] [CrossRef]
- Zhu, L.; Farhat, M.; Salama, K.N.; Chen, P.-Y. Two-dimensional materials-based radio frequency wireless communication and sensing systems for Internet-of-things applications. In Emerging 2D Materials and Devices for the Internet of Things; Elsevier: Amsterdam, The Netherlands, 2020; pp. 29–57. [Google Scholar]
- Liao, G.; He, F.; Li, Q.; Zhong, L.; Zhao, R.; Che, H.; Gao, H.; Fang, B. Emerging graphitic carbon nitride-based materials for biomedical applications. Prog. Mater. Sci. 2020, 112, 100666. [Google Scholar] [CrossRef]
- Rajaji, U.; Chinnapaiyan, S.; Chen, S.-M.; Govindasamy, M.; de Oliveira Filho, J.I.; Khushaim, W.; Mani, V. Design and fabrication of yttrium ferrite garnet-embedded graphitic carbon nitride: A sensitive electrocatalyst for smartphone-enabled point-of-care pesticide (mesotrione) analysis in food samples. ACS Appl. Mater. Interfaces 2021, 13, 24865–24876. [Google Scholar] [CrossRef]
- Rajaji, U.; Selvi, S.V.; Chen, S.-M.; Chinnapaiyan, S.; Chen, T.-W.; Govindasamy, M. A nanocomposite consisting of cuprous oxide supported on graphitic carbon nitride nanosheets for non-enzymatic electrochemical sensing of 8-hydroxy-2′-deoxyguanosine. Microchim. Acta 2020, 187, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhang, H.; Xiang, Q. Porous graphitic carbon nitride for solar photocatalytic applications. Nanoscale Horiz. 2020, 5, 765–786. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fan, H.; Ren, X.; Fang, J.; Ma, J.; Zhao, N. Porous graphitic carbon nitride nanosheets by pre-polymerization for enhanced photocatalysis. Mater. Charact. 2018, 139, 89–99. [Google Scholar] [CrossRef]
- Hou, Y.; Yang, J.; Lei, C.; Yang, B.; Li, Z.; Xie, Y.; Zhang, X.; Lei, L.; Chen, J. Nitrogen vacancy structure driven photoeletrocatalytic degradation of 4-chlorophenol using porous graphitic carbon nitride nanosheets. ACS Sustain. Chem. Eng. 2018, 6, 6497–6506. [Google Scholar] [CrossRef]
- Gupta, N.; Todi, K.; Narayan, T.; Malhotra, B. Graphitic carbon nitride-based nanoplatforms for biosensors: Design strategies and applications. Mater. Today Chem. 2022, 24, 100770. [Google Scholar]
- Idris, A.O.; Oseghe, E.O.; Msagati, T.A.; Kuvarega, A.T.; Feleni, U.; Mamba, B. Graphitic carbon nitride: A highly electroactive nanomaterial for environmental and clinical sensing. Sensors 2020, 20, 5743. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Li, Z.; Yu, X.; Huang, Z.-H.; Kang, F.; Yang, Q.-H. Macroscopic 3D Porous Graphitic Carbon Nitride Monolith for Enhanced Photocatalytic Hydrogen Evolution. Adv. Mater. 2015, 27, 4634–4639. [Google Scholar] [CrossRef]
- Bhowmik, T.; Kundu, M.K.; Barman, S. Highly active and durable Pd nanoparticles-porous graphitic carbon nitride composite for electrocatalytic oxygen reduction reaction. Int. J. Hydrogen Energy 2016, 41, 14768–14777. [Google Scholar]
- Fischer, A.; Antonietti, M.; Thomas, A. Growth confined by the nitrogen source: Synthesis of pure metal nitride nanoparticles in mesoporous graphitic carbon nitride. Adv. Mater. 2007, 19, 264–267. [Google Scholar] [CrossRef]
- Rajkumar, C.; Thirumalraj, B.; Chen, S.-M.; Veerakumar, P.; Liu, S.-B. Ruthenium nanoparticles decorated tungsten oxide as a bifunctional catalyst for electrocatalytic and catalytic applications. ACS Appl. Mater. Interfaces 2017, 9, 31794–31805. [Google Scholar] [CrossRef]
- Gallay, P.; Eguílaz, M.; Rivas, G. Designing electrochemical interfaces based on nanohybrids of avidin functionalized-carbon nanotubes and ruthenium nanoparticles as peroxidase-like nanozyme with supramolecular recognition properties for site-specific anchoring of biotinylated residues. Biosens. Bioelectron. 2020, 148, 111764. [Google Scholar] [CrossRef] [PubMed]
- Veerakumar, P.; Hung, S.-T.; Hung, P.-Q.; Lin, K.-C. Review of the design of ruthenium-based nanomaterials and their sensing applications in electrochemistry. J. Agric. Food Chem. 2022, 70, 8523–8550. [Google Scholar] [CrossRef] [PubMed]
- Lou, B.-S.; Rajaji, U.; Chen, S.-M.; Chen, T.-W. A simple sonochemical assisted synthesis of NiMoO4/chitosan nanocomposite for electrochemical sensing of amlodipine in pharmaceutical and serum samples. Ultrasonic. Sonochem. 2020, 64, 104827. [Google Scholar] [CrossRef]
- Jo, H.; Gu, H.; Jeon, W.; Youn, H.; Her, J.; Kim, S.-K.; Lee, J.; Shin, J.H.; Ban, C. Electrochemical aptasensor of cardiac troponin I for the early diagnosis of acute myocardial infarction. Anal. Chem. 2015, 87, 9869–9875. [Google Scholar] [CrossRef] [PubMed]
- Rauf, S.; Lahcen, A.A.; Aljedaibi, A.; Beduk, T.; Ilton de Oliveira Filho, J.; Salama, K.N. Gold nanostructured laser-scribed graphene: A new electrochemical biosensing platform for potential point-of-care testing of disease biomarkers. Biosens. Bioelectron. 2021, 180, 113116. [Google Scholar] [CrossRef]
- Beduk, T.; de Oliveira Filho, J.I.; Ait Lahcen, A.; Mani, V.; Salama, K.N. Inherent Surface activation of laser-scribed graphene decorated with au and ag nanoparticles: Simultaneous electrochemical behavior toward uric acid and dopamine. Langmuir 2021, 37, 13890–13902. [Google Scholar] [CrossRef] [PubMed]
- Ghanam, A.; Lahcen, A.A.; Beduk, T.; Alshareef, H.N.; Amine, A.; Salama, K.N. Laser scribed graphene: A novel platform for highly sensitive detection of electroactive biomolecules. Biosens. Bioelectron. 2020, 168, 112509. [Google Scholar] [CrossRef]
- Hornberger, E.; Bergmann, A.; Schmies, H.; Kühl, S.; Wang, G.; Drnec, J.; Sandbeck, D.J.; Ramani, V.; Cherevko, S.; Mayrhofer, K.J. In situ stability studies of platinum nanoparticles supported on ruthenium−titanium mixed oxide (RTO) for fuel cell cathodes. ACS Catal. 2018, 8, 9675–9683. [Google Scholar] [CrossRef]
- Cored, J.; García-Ortiz, A.; Iborra, S.; Climent, M.J.; Liu, L.; Chuang, C.-H.; Chan, T.-S.; Escudero, C.; Concepción, P.; Corma, A. Hydrothermal synthesis of ruthenium nanoparticles with a metallic core and a ruthenium carbide shell for low-temperature activation of CO2 to methane. J. Am. Chem. Soc. 2019, 141, 19304–19311. [Google Scholar] [CrossRef]
- Oudghiri-Hassani, H.; Al Wadaani, F. Preparation, characterization and catalytic activity of nickel molybdate (NiMoO4) nanoparticles. Molecules 2018, 23, 273. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, G.; Chen, Y.; Rui, K.; Mao, H.; Dou, S.X.; Sun, W. Iron-doped nickel molybdate with enhanced oxygen evolution kinetics. Chem. Eur. J. 2019, 25, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, Q.; Wu, H.; Chen, Y.; Lu, C.-H.; Chi, Y.; Yang, H.-H. Graphitic carbon nitride materials: Sensing, imaging and therapy. Small 2016, 12, 5376–5393. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.H.; Ghodsi, E.; Abdollahi, S.; Nadri, S. Porous graphene oxide nanostructure as an excellent scaffold for label-free electrochemical biosensor: Detection of cardiac troponin I. Mate. Sci. Eng. C 2016, 69, 447–452. [Google Scholar]
- Singh, N.; Ali, M.A.; Rai, P.; Sharma, A.; Malhotra, B.; John, R. Microporous nanocomposite enabled microfluidic biochip for cardiac biomarker detection. ACS Appl. Mater. Interfaces 2017, 9, 33576–33588. [Google Scholar]
- Rauf, S.; Mani, V.; Lahcen, A.A.; Yuvaraja, S.; Beduk, T.; Salama, K.N. Binary transition metal oxide modified laser-scribed graphene electrochemical aptasensor for the accurate and sensitive screening of acute myocardial infarction. Electrochim. Acta 2021, 386, 138489. [Google Scholar] [CrossRef]
- Sun, D.; Luo, Z.; Lu, J.; Zhang, S.; Che, T.; Chen, Z.; Zhang, L. Electrochemical dual-aptamer-based biosensor for nonenzymatic detection of cardiac troponin I by nanohybrid electrocatalysts labeling combined with DNA nanotetrahedron structure. Biosens. Bioelectron. 2019, 134, 49–56. [Google Scholar]
- Yola, M.L.; Atar, N. Development of cardiac troponin-I biosensor based on boron nitride quantum dots including molecularly imprinted polymer. Biosens. Bioelectron. 2019, 126, 418–424. [Google Scholar] [CrossRef]
- Feng, S.; Yan, M.; Xue, Y.; Huang, J.; Yang, X. Electrochemical immunosensor for cardiac troponin I detection based on covalent organic framework and enzyme-catalyzed signal amplification. Anal. Chem. 2021, 93, 13572–13579. [Google Scholar]
- Beduk, D.; Ilton de Oliveira Filho, J.; Beduk, T.; Harmanci, D.; Zihnioglu, F.; Cicek, C.; Sertoz, R.; Arda, B.; Goksel, T.; Turhan, K.; et al. ‘All In One’ SARS-CoV-2 variant recognition platform: Machine learning-enabled point of care diagnostics. Biosens. Bioelectron. X 2022, 10, 100105. [Google Scholar] [CrossRef]




| Material | Type | Technique | Linear Range (ng/mL) | LOD (pg/mL) | Real Sample |
|---|---|---|---|---|---|
| Porous graphene oxide/GCE [34] | Immunosensor | EIS | 0.1–10 | 70.0 | Clinical samples |
| Zinc ferrite/LSGE [36] | Aptasensor | SWV | 0.001–200 | 1.0 | Serum |
| magnetic metal organic frameworks Fe3O4@UiO-66/Cu@Au/SPGE [37] | Dual-aptasensor | DPV | 0.05–100 | 16.0 | Serum |
| Manganese oxide-reduced graphene oxide/ITO [35] | Immunosensor | EIS | 0.008–20 | 8.0 | Serum |
| cTnI imprinted polypyrrole/boron nitride quantum dots/GCE [38] | MIP sensor | DPV | 0.01–5.00 | 0.5 | Blood serum |
| Au electrode/Ab1 (detection probe); HRP-Ab2-Au-COF (signal probe) [39] | Sandwich Immunosensor | DPV | 0.005-10 | 1.7 | Human serum |
| PCN-RuNPs/LSGE [this work] | Aptamer | SWV | 0.1-10,000 | 50 | Serum |
| PCN-NiMoO4 NRs/LSGE [This work] | Aptamer | SWV | 0.1-10,000 | 70 | Serum |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khushaim, W.; Mani, V.; Peramaiya, K.; Huang, K.-W.; Salama, K.N. Ruthenium and Nickel Molybdate-Decorated 2D Porous Graphitic Carbon Nitrides for Highly Sensitive Cardiac Troponin Biosensor. Biosensors 2022, 12, 783. https://doi.org/10.3390/bios12100783
Khushaim W, Mani V, Peramaiya K, Huang K-W, Salama KN. Ruthenium and Nickel Molybdate-Decorated 2D Porous Graphitic Carbon Nitrides for Highly Sensitive Cardiac Troponin Biosensor. Biosensors. 2022; 12(10):783. https://doi.org/10.3390/bios12100783
Chicago/Turabian StyleKhushaim, Walaa, Veerappan Mani, Karthik Peramaiya, Kuo-Wei Huang, and Khaled Nabil Salama. 2022. "Ruthenium and Nickel Molybdate-Decorated 2D Porous Graphitic Carbon Nitrides for Highly Sensitive Cardiac Troponin Biosensor" Biosensors 12, no. 10: 783. https://doi.org/10.3390/bios12100783
APA StyleKhushaim, W., Mani, V., Peramaiya, K., Huang, K.-W., & Salama, K. N. (2022). Ruthenium and Nickel Molybdate-Decorated 2D Porous Graphitic Carbon Nitrides for Highly Sensitive Cardiac Troponin Biosensor. Biosensors, 12(10), 783. https://doi.org/10.3390/bios12100783







