Emerging Materials, Wearables, and Diagnostic Advancements in Therapeutic Treatment of Brain Diseases
Abstract
:1. Introduction
2. Common Brain Diseases
2.1. Autoimmune Brain Diseases (ABD)
2.2. Epilepsy
2.3. Brain Infections
2.4. Brain Illness
2.5. Neurodegenerative Brain Diseases
2.6. Neurodevelopmental Disorders
2.7. Strokes and Brain Tumor
3. Advancements in Diagnostics Approaches
3.1. Electroencephalogram (EEG)
3.2. Computed Tomography Scan (CT Scan)
3.3. Angiogram
3.4. Positron Emission Tomography (PET)
3.5. Magnetic Resonance Imaging (MRI)
3.6. Mass Spectrometer and Chromatography
3.7. Functional Near-Infrared Spectroscopy
4. Advancements in Treatment Approaches
4.1. Use of Nanotechnology
4.1.1. Drug Delivery
4.1.2. Biomarker Detection
4.1.3. Disease Specific Treatments
4.2. Wearable Sensors
4.3. Bioprinting
Biomaterials Used in Bioprinting
5. Role of Receptors
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 11-HT | 11-Hydrooxytestosterone |
| ABD | Autoimmune brain diseases |
| AD | Alzheimer’s disease |
| ADHD | Attention-deficit/hyperactivity disorder |
| AI | Artificial Intelligence |
| ANN | Artificial neural networks |
| Anti-DNER | Anti-Delta/Noch like endothelial growth factor |
| Anti-NMDA | Anti-N-methyl D-Aspartate |
| APDs | Annihilation photodiode detectors |
| BBB | Blood brain barrier |
| BCSFB | Blood-cerebrospinal fluid barrier |
| BD | Brain decode |
| CNS | Central Nervous System |
| CSF | blood-cerebrospinal fluid |
| CT | Computed tomography |
| DAMPS | Damage associated molecular patterns |
| DSA | Digital subtraction angiography |
| EBT | Electron beam tomography |
| ECM | Extra cellular matrix |
| EEG | Electro encephalography |
| EGF | Endothelial growth factor |
| FDA | Food and drug administration |
| fNIRS | Functional near infrared spectroscopy |
| GAD67 | Glutamic acid decarboxylase—67 |
| GBM | Glioblastoma |
| GBM | Glioblastoma multiforme |
| GlyR | Glycine receptor |
| HDA | Histamine deacetylase |
| IC | Intracranial circulatory |
| IoT | Internet of things |
| LC | Liquid chromatography |
| LC-MS/MS | Liquid chromatography tandem mass spectroscopy |
| MEMS | Microelectrical mechanical systems |
| ML | Machine Learning |
| MRI | Magnetic resonance imaging |
| MS | Multiple sclerosis |
| NCLS | Neuronal ceroid lipofuscinoses |
| NDD | Neurodevelopmental disorder |
| NPs | Nanoparticles |
| O/W | Oil in water |
| PAMAM | Polyamidoamine |
| PD | Parkinson’s disease |
| PDMS/CNT | Poly-di-methyl siloxane/carbon nanotubes |
| PEG | Polyethylene glycol |
| PET | Positron emission tomography |
| PLGA | Polylactide Glycolic Acid |
| PNIPAAm | Poly(N-isopropylacrylamide) |
| PSMA | Prostate specific membrane antigen |
| PTSD | Post-traumatic stress disorder |
| PU | Polyurethane |
| PVN | Perivascular niche |
| RMT | Receptor-mediated transcytosis |
| ROS | Reactive oxygen species |
| SAP | Self-assembled peptides |
| SiPM | Silicon photomultiplier |
| TBI | Traumatic brain injury |
| TOF | Time of flight |
| TUH | Temple University Hospital |
| UCH-L1 | Ubiquitin C-terminal hydrolase-L1 |
| VEGF | Vascular endothelial growth factor |
| W/O | Water in oil |
References
- Khellaf, A.; Khan, D.Z.; Helmy, A. Recent Advances in Traumatic Brain Injury. J. Neurol. 2019, 266, 2878–2889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonkar, R.; Jha, A.; Viswanadh, M.K.; Burande, A.S.; Pawde, D.M.; Patel, K.K.; Singh, M.; Koch, B.; Muthu, M.S. Gold Liposomes for Brain-Targeted Drug Delivery: Formulation and Brain Distribution Kinetics. Mater. Sci. Eng. C 2021, 120, 111652. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Zhang, X.; Mu, H.; Meng, Q.; Jiang, Y.; Wang, Y.; Lu, X.; Wang, A.; Liu, S.; Zhang, Y.; et al. RVG29-Modified Docetaxel-Loaded Nanoparticles for Brain-Targeted Glioma Therapy. Int. J. Pharm. 2018, 543, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Liu, J.; Li, W.; Wang, D.; Huang, Y.; Jia, C.; Li, Z.; Murtaza, M.; Wang, H.; Song, J.; et al. A Flexible, Robust, and Gel-Free Electroencephalogram Electrode for Noninvasive Brain-Computer Interfaces. Nano Lett. 2019, 19, 6853–6861. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, Y.; Chen, Y.; Yang, Z.; Zhang, L.; Xiao, W.; Yang, J.; Guo, L.; Wu, Y. Dual-Targeting for Brain-Specific Liposomes Drug Delivery System: Synthesis and Preliminary Evaluation. Bioorganic Med. Chem. 2018, 26, 4677–4686. [Google Scholar] [CrossRef]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Front. Cell Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef] [Green Version]
- Bolte, A.C.; Dutta, A.B.; Hurt, M.E.; Smirnov, I.; Kovacs, M.A.; McKee, C.A.; Ennerfelt, H.E.; Shapiro, D.; Nguyen, B.H.; Frost, E.L.; et al. Meningeal Lymphatic Dysfunction Exacerbates Traumatic Brain Injury Pathogenesis. Nat. Commun. 2020, 11, 4524. [Google Scholar] [CrossRef]
- Bhowmick, S.; D’Mello, V.; Caruso, D.; Wallerstein, A.; Abdul-Muneer, P.M. Impairment of Pericyte-Endothelium Crosstalk Leads to Blood-Brain Barrier Dysfunction Following Traumatic Brain Injury. Exp. Neurol. 2019, 317, 260–270. [Google Scholar] [CrossRef]
- Sollmann, N.; Beer, A.J.; Kirchhoff, F. SARS-CoV-2 Infection and the Brain: Direct Evidence for Brain Changes in Milder Cases. Signal Transduct. Target. Ther. 2022, 7, 230. [Google Scholar] [CrossRef]
- Natoli, S.; Oliveira, V.; Calabresi, P.; Maia, L.F.; Pisani, A. Does SARS-Cov-2 Invade the Brain? Translational Lessons from Animal Models. Eur. J. Neurol. 2020, 27, 1764–1773. [Google Scholar] [CrossRef]
- Barber, C.N.; Raben, D.M. Lipid Metabolism Crosstalk in the Brain: Glia and Neurons. Front. Cell Neurosci. 2019, 13, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Jiang, J.; Xie, D.; Wang, S.; Bi, K.; Duan, H.; Yang, J.; He, J. Transient Security Transistors Self-Supported on Biodegradable Natural-Polymer Membranes for Brain-Inspired Neuromorphic Applications. Nanoscale 2018, 10, 14893–14901. [Google Scholar] [CrossRef] [PubMed]
- Ladak, A.A.; Enam, S.A.; Ibrahim, M.T. A Review of the Molecular Mechanisms of Traumatic Brain Injury. World Neurosurg. 2019, 131, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Sullan, M.J.; Asken, B.M.; Jaffee, M.S.; DeKosky, S.T.; Bauer, R.M. Glymphatic System Disruption as a Mediator of Brain Trauma and Chronic Traumatic Encephalopathy. Neurosci. Biobehav. Rev. 2018, 84, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Munley, K.M.; Wade, K.L.; Pradhan, D.S. Uncovering the Seasonal Brain: Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) as a Biochemical Approach for Studying Seasonal Social Behaviors. Horm. Behav. 2022, 142, 105161. [Google Scholar] [CrossRef]
- Mahjoub, I.; Mahjoub, M.A.; Rekik, I.; Weiner, M.; Aisen, P.; Petersen, R.; Jack, C.; Jagust, W.; Trojanowki, J.; Toga, A.; et al. Brain Multiplexes Reveal Morphological Connectional Biomarkers Fingerprinting Late Brain Dementia States. Sci. Rep. 2018, 8, 4103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Shen, Z.; Anraku, Y.; Kataoka, K.; Chen, X. Nanomaterial-Based Blood-Brain-Barrier (BBB) Crossing Strategies. Biomaterials 2019, 224, 119491. [Google Scholar] [CrossRef]
- Bao, Q.; Hu, P.; Xu, Y.; Cheng, T.; Wei, C.; Pan, L.; Shi, J. Simultaneous Blood-Brain Barrier Crossing and Protection for Stroke Treatment Based on Edaravone-Loaded Ceria Nanoparticles. ACS Nano 2018, 12, 6794–6805. [Google Scholar] [CrossRef]
- Manojkumar, K.; Kandeeban, R.; Brindha, R.; Sangeetha, V.; Saminathan, K. Non-Precious Metal-Based Integrated Electrodes for Overall Alkaline Water Splitting. J. Indian Chem. Soc. 2022, 99, 100775. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Reddy, M.v.; Zaghib, K.; Armand, M.; Ramakrishna, S. Growth Mechanism of Micro/Nano Metal Dendrites and Cumulative Strategies for Countering Its Impacts in Metal Ion Batteries: A Review. Nanomaterials 2021, 11, 2476. [Google Scholar] [CrossRef]
- Brindha, R.; Mohanraj, R.; Manojkumar, P.; Selvam, M.; Sakthipandi, K. Hybrid Electrochemical Behaviour of La1−xCaxMnO3 Nano Perovskites and Recycled Polar Interspersed Graphene for Metal-Air Battery System. J. Electrochem. Soc. 2020, 167, 120539. [Google Scholar] [CrossRef]
- Mohanraj, R.; Brindha, R.; Kandeeban, R.; Mahendhar, M.; Saminathan, K.; Ayyappadasan, G. Electrochemical Detection of 5-Hydroxytryptamine Using Sustainable SnO2-Graphite Nanocomposite Modified Electrode. Mater. Lett. 2021, 305, 130796. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Sundarrajan, S.; Chellappan, V.; Reddy, M.V.; Ramakrishna, S.; Zaghib, K. Recent Development in Carbon-LiFePO4 Cathodes for Lithium-Ion Batteries: A Mini Review. Batteries 2022, 8, 133. [Google Scholar] [CrossRef]
- Kumar, K.K.; Brindha, R.; Nandhini, M.; Selvam, M.; Saminathan, K.; Sakthipandi, K. Water-Suspended Graphene as Electrolyte Additive in Zinc-Air Alkaline Battery System. Ionics 2019, 25, 1699–1706. [Google Scholar] [CrossRef]
- Brindha, R.; Ajith, S.S.R.; Nandhini, M.; Selvam, M.; Subannajui, K.; Khotmungkhun, K.; Sakthipandi, K. Evaluation of Anticorrosive Behaviour of ZnO Nanotetra-Pods on a AZ91-Grade Mg Alloy. Bull. Mater. Sci. 2019, 42, 221. [Google Scholar] [CrossRef] [Green Version]
- Ramasubramanian, B.; Chinglenthoiba, C.; Huiqing, X.; Xiping, N.; Hui, H.K.; Valiyaveettil, S.; Ramakrishna, S.; Chellappan, V. Sustainable Fe-MOF@carbon Nanocomposite Electrode for Supercapacitor. Surf. Interfaces 2022, 34, 102397. [Google Scholar] [CrossRef]
- Kandeeban, R.; Brindha, R.; Manojkumar, K.; Batoo, K.M.; Raslan, E.H.; Hadi, M.; Imran, A.; Saminathan, K. Revealing the Synergetic Electrocatalyst Behaviour of Kish Graphite Recovered from Polyethylene Plastics. Mater. Lett. 2021, 297, 129740. [Google Scholar] [CrossRef]
- Wang, C.; Wu, B.; Wu, Y.; Song, X.; Zhang, S.; Liu, Z. Camouflaging Nanoparticles with Brain Metastatic Tumor Cell Membranes: A New Strategy to Traverse Blood–Brain Barrier for Imaging and Therapy of Brain Tumors. Adv. Funct. Mater. 2020, 30, 1909369. [Google Scholar] [CrossRef]
- del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Meza-Toledo, J.A.; Mendoza-Muñoz, N.; González-Torres, M.; Florán, B.; Cortés, H.; Leyva-Gómez, G. Formulations of Curcumin Nanoparticles for Brain Diseases. Biomolecules 2019, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Impact of Nanoparticles on Brain Health: An Up to Date Overview. J. Clin. Med. 2018, 7, 490. [Google Scholar] [CrossRef]
- Mukhtar, M.; Bilal, M.; Rahdar, A.; Barani, M.; Arshad, R.; Behl, T.; Brisc, C.; Banica, F.; Bungau, S. Nanomaterials for Diagnosis and Treatment of Brain Cancer: Recent Updates. Chemosensors 2020, 8, 117. [Google Scholar] [CrossRef]
- Silva, G.A. A New Frontier: The Convergence of Nanotechnology, Brain Machine Interfaces, and Artificial Intelligence. Front. Neurosci. 2018, 12, 843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnabel, R.B.; Hasenfuß, G.; Buchmann, S.; Kahl, K.G.; Aeschbacher, S.; Osswald, S.; Angermann, C.E. Heart and Brain Interactions: Pathophysiology and Management of Cardio-Psycho-Neurological Disorders. Herz 2021, 46, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Yamada, K. Machine Learning Studies on Major Brain Diseases: 5-Year Trends of 2014–2018. Jpn. J. Radiol. 2018, 37, 34–72. [Google Scholar] [CrossRef]
- Soomro, T.A.; Zheng, L.; Afifi, A.J.; Ali, A.; Soomro, S.; Yin, M.; Gao, J. Image Segmentation for MR Brain Tumor Detection Using Machine Learning: A Review. IEEE Rev. Biomed. Eng. 2022, 1. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Signalling and Disorders of the Central Nervous System. Nat. Rev. Drug Discov. 2008, 7, 575–590. [Google Scholar] [CrossRef]
- Deleidi, M.; Jäggle, M.; Rubino, G. Immune Ageing, Dysmetabolism and Inflammation in Neurological Diseases. Front. Neurosci. 2015, 9, 172. [Google Scholar] [CrossRef] [Green Version]
- Wilson, M.; Andronesi, O.; Barker, P.B.; Bartha, R.; Bizzi, A.; Bolan, P.J.; Brindle, K.M.; Choi, I.Y.; Cudalbu, C.; Dydak, U.; et al. Methodological Consensus on Clinical Proton MRS of the Brain: Review and Recommendations. Magn. Reson. Med. 2019, 82, 527–550. [Google Scholar] [CrossRef] [Green Version]
- Thomi, G.; Surbek, D.; Haesler, V.; Joerger-Messerli, M.; Schoeberlein, A. Exosomes Derived from Umbilical Cord Mesenchymal Stem Cells Reduce Microglia-Mediated Neuroinflammation in Perinatal Brain Injury. Stem. Cell Res. Ther. 2019, 10, 105. [Google Scholar] [CrossRef]
- Ceprian, M.; Fulton, D. Glial Cell AMPA Receptors in Nervous System Health, Injury and Disease. Int. J. Mol. Sci. 2019, 20, 2450. [Google Scholar] [CrossRef]
- Reed, C.B.; Feltri, M.L.; Wilson, E.R. Peripheral Glia Diversity. J. Anat. 2022, 241, 1219–1234. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.R.; Andriessen, A.S.; Chen, G.; Wang, K.; Jiang, C.; Maixner, W.; Ji, R.R. Central Nervous System Targets: Glial Cell Mechanisms in Chronic Pain. Neurotherapeutics 2020, 17, 846–860. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.N.; Olofsson, L.E. The Role of the Gut Microbiota in Development, Function and Disorders of the Central Nervous System and the Enteric Nervous System. J. Neuroendocrinol. 2019, 31, e12684. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lu, J.; Shao, A.; Zhang, J.H.; Zhang, J. Glial Cells: Role of the Immune Response in Ischemic Stroke. Front. Immunol. 2020, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Pöyhönen, S.; Er, S.; Domanskyi, A.; Airavaara, M. Effects of Neurotrophic Factors in Glial Cells in the Central Nervous System: Expression and Properties in Neurodegeneration and Injury. Front. Physiol. 2019, 10, 486. [Google Scholar] [CrossRef]
- Lillicrap, T.P.; Santoro, A.; Marris, L.; Akerman, C.J.; Hinton, G. Backpropagation and the Brain. Nat. Rev. Neurosci. 2020, 21, 335–346. [Google Scholar] [CrossRef]
- Keller, D.; Erö, C.; Markram, H. Cell Densities in the Mouse Brain: A Systematic Review. Front. Neuroanat. 2018, 12, 83. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Zhou, J.W. Neuroinflammation in the Central Nervous System: Symphony of Glial Cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef]
- Sanson, A.; Riva, M.A. Anti-Stress Properties of Atypical Antipsychotics. Pharmaceuticals 2020, 13, 322. [Google Scholar] [CrossRef]
- Jia, Z.; Chen, X.; Tang, W.; Zhao, D.; Yu, S. Atypical Functional Connectivity between the Anterior Cingulate Cortex and Other Brain Regions in a Rat Model of Recurrent Headache. Mol. Pain. 2019, 15, 1744806919842483. [Google Scholar] [CrossRef]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; el Khoury, J. Microglia in Neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Janelidze, S.; Surova, Y.; Widner, H.; Zetterberg, H.; Hansson, O. Cerebrospinal Fluid Concentrations of Inflammatory Markers in Parkinson’s Disease and Atypical Parkinsonian Disorders. Sci. Rep. 2018, 8, 13276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, Z.D.; Danyeli, L.V.; Woelfer, M.; Lamers, F.; Wagner, G.; Sobanski, T.; Walter, M. Linking Atypical Depression and Insulin Resistance-Related Disorders via Low-Grade Chronic Inflammation: Integrating the Phenotypic, Molecular and Neuroanatomical Dimensions. Brain Behav. Immun. 2021, 93, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Cellucci, T.; van Mater, H.; Graus, F.; Muscal, E.; Gallentine, W.; Klein-Gitelman, M.S.; Benseler, S.M.; Frankovich, J.; Gorman, M.P.; van Haren, K.; et al. Clinical Approach to the Diagnosis of Autoimmune Encephalitis in the Pediatric Patient. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, 663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gialluisi, A.; Andlauer, T.F.M.; Mirza-Schreiber, N.; Moll, K.; Becker, J.; Hoffmann, P.; Ludwig, K.U.; Czamara, D.; St Pourcain, B.; Brandler, W.; et al. Genome-Wide Association Scan Identifies New Variants Associated with a Cognitive Predictor of Dyslexia. Transl. Psychiatry 2019, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Middha, M.; Bicak, M.; Sjoberg, D.D.; Vertosick, E.; Dahlin, A.; Häggström, C.; Hallmans, G.; Rönn, A.C.; Stattin, P.; et al. Genome-Wide Scan Identifies Role for AOX1 in Prostate Cancer Survival. Eur. Urol. 2018, 74, 710–719. [Google Scholar] [CrossRef]
- Barake, F.; Bravo-Zehnder, M.; González, A. Progress in the Mechanism of Neuronal Surface P Antigen Modulating Hippocampal Function and Implications for Autoimmune Brain Disease. Curr. Opin. Neurol. 2022, 35, 436–442. [Google Scholar] [CrossRef]
- He, Z.; Xu, B.; Buxbaum, J.; Ionita-Laza, I. A Genome-Wide Scan Statistic Framework for Whole-Genome Sequence Data Analysis. Nat. Commun. 2019, 10, 3018. [Google Scholar] [CrossRef] [Green Version]
- van’t Westeinde, A.; Padilla, N.; Siqueiros Sanchez, M.; Fletcher-Sandersjöö, S.; Kämpe, O.; Bensing, S.; Lajic, S. Brain Structure in Autoimmune Addison’s Disease. Cereb. Cortex 2022, 389. [Google Scholar] [CrossRef]
- Dai, W.L.; Bao, Y.N.; Fan, J.F.; Ma, B.; Li, S.S.; Zhao, W.L.; Yu, B.Y.; Liu, J.H. Blockade of Spinal Dopamine D1/D2 Receptor Suppresses Activation of NMDA Receptor through Gαq and Src Kinase to Attenuate Chronic Bone Cancer Pain. J. Adv. Res. 2021, 28, 139–148. [Google Scholar] [CrossRef]
- Hamada, M.; Zaidan, B.B.; Zaidan, A.A. A Systematic Review for Human EEG Brain Signals Based Emotion Classification, Feature Extraction, Brain Condition, Group Comparison. J. Med. Syst. 2018, 42, 162. [Google Scholar] [CrossRef] [PubMed]
- Pape, K.; Tamouza, R.; Leboyer, M.; Zipp, F. Immunoneuropsychiatry—Novel Perspectives on Brain Disorders. Nat. Rev. Neurol. 2019, 15, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Blood-Brain Delivery Methods Using Nanotechnology. Pharmaceutics 2018, 10, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florendo, M.; Figacz, A.; Srinageshwar, B.; Sharma, A.; Swanson, D.; Dunbar, G.L.; Rossignol, J. Use of Polyamidoamine Dendrimers in Brain Diseases. Molecules 2018, 23, 2238. [Google Scholar] [CrossRef] [Green Version]
- Mifflin, K.; Baker, G.B.; Kerr, B.J. Effect of Voluntary Wheel Running on Neuroactive Steroid Levels in Murine Experimental Autoimmune Encephalomyelitis. Neurosci. Lett. 2018, 685, 150–154. [Google Scholar] [CrossRef]
- Todorov, M.I.; Paetzold, J.C.; Schoppe, O.; Tetteh, G.; Shit, S.; Efremov, V.; Todorov-Völgyi, K.; Düring, M.; Dichgans, M.; Piraud, M.; et al. Machine Learning Analysis of Whole Mouse Brain Vasculature. Nat. Methods 2020, 17, 442–449. [Google Scholar] [CrossRef]
- Akamatsu, Y.; Hanafy, K.A. Cell Death and Recovery in Traumatic Brain Injury. Neurotherapeutics 2020, 17, 446–456. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; Dijkhuizen, R.M.; Magnus, T. Neuroinflammation, Stroke, Blood-Brain Barrier Dysfunction, and Imaging Modalities. Stroke 2022, 53, 1473–1486. [Google Scholar] [CrossRef]
- Rodriguez-Otormin, F.; Duro-Castano, A.; Conejos-Sánchez, I.; Vicent, M.J. Envisioning the Future of Polymer Therapeutics for Brain Disorders. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1532. [Google Scholar] [CrossRef] [Green Version]
- Na, S.; Wang, L.v. Photoacoustic Computed Tomography for Functional Human Brain Imaging [Invited]. Biomed. Opt. Express 2021, 12, 4056–4083. [Google Scholar] [CrossRef]
- Hartmann, K.G.; Schirrmeister, R.T.; Ball, T. EEG-GAN: Generative Adversarial Networks for Electroencephalograhic (EEG) Brain Signals. arXiv 2018, arXiv:1806.01875. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Götz, J. The Blood-Brain Barrier: Physiology and Strategies for Drug Delivery. Adv. Drug Deliv. Rev. 2019, 165–166, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, C.; He, D.; Jiang, N.; Bai, Y.; Xin, Y. Rapamycin and MCC950 Modified Gut Microbiota in Experimental Autoimmune Encephalomyelitis Mouse by Brain Gut Axis. Life Sci. 2020, 253, 117747. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Sang, H.; Wang, J. Naringenin Attenuates Experimental Autoimmune Encephalomyelitis by Protecting the Intact of Blood-Brain Barrier and Controlling Inflammatory Cell Migration. J. Nutr. Biochem. 2021, 89, 108560. [Google Scholar] [CrossRef] [PubMed]
- Shobatake, R.; Kumazawa, A.; Koyama, N.; Takahashi, N. Autoimmune Encephalitis Associated with Anti-N-Methyl-D-Aspartate Receptor and Anti-Hu Antibodies Successfully Treated with Carboplatin and Etoposide for Small-Cell Lung Cancer. Intern. Med. 2022, 9707–9722. [Google Scholar] [CrossRef] [PubMed]
- O’donovan, B.; Mandel-Brehm, C.; Vazquez, S.E.; Liu, J.; Parent, A.V.; Anderson, M.S.; Kassimatis, T.; Zekeridou, A.; Hauser, S.L.; Pittock, S.J.; et al. High-Resolution Epitope Mapping of Anti-Hu and Anti-Yo Autoimmunity by Programmable Phage Display. Brain Commun. 2020, 2, fcaa059. [Google Scholar] [CrossRef] [PubMed]
- Sell, J.; Haselmann, H.; Hallermann, S.; Hust, M.; Geis, C. Autoimmune Encephalitis: Novel Therapeutic Targets at the Preclinical Level. Expert Opin. Ther. Targets 2020, 25, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Bordonne, M.; Chawki, M.B.; Doyen, M.; Kas, A.; Guedj, E.; Tyvaert, L.; Verger, A. Brain 18F-FDG PET for the Diagnosis of Autoimmune Encephalitis: A Systematic Review and a Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3847–3858. [Google Scholar] [CrossRef]
- Shen, C.H.; Fang, G.L.; Yang, F.; Cai, M.T.; Zheng, Y.; Fang, W.; Guo, Y.; Zhang, Y.X.; Ding, M.P. Seizures and Risk of Epilepsy in Anti-NMDAR, Anti-LGI1, and Anti-GABABR Encephalitis. Ann. Clin. Transl. Neurol. 2020, 7, 1392–1399. [Google Scholar] [CrossRef]
- Wei, Y.C.; Tseng, J.R.; Wu, C.L.; Su, F.C.; Weng, W.C.; Hsu, C.C.; Chang, K.H.; Wu, C.F.; Hsiao, I.T.; Lin, C.P. Different FDG-PET Metabolic Patterns of Anti-AMPAR and Anti-NMDAR Encephalitis: Case Report and Literature Review. Brain Behav. 2020, 10, e01540. [Google Scholar] [CrossRef]
- Ariño, H.; Muñoz-Lopetegi, A.; Martinez-Hernandez, E.; Armangue, T.; Rosa-Justicia, M.; Escudero, D.; Matos, N.; Graus, F.; Sugranyes, G.; Castro-Fornieles, J.; et al. Sleep Disorders in Anti-NMDAR Encephalitis. Neurology 2020, 95, e671–e684. [Google Scholar] [CrossRef] [PubMed]
- Seery, N.; Butzkueven, H.; O’Brien, T.J.; Monif, M. Contemporary Advances in Anti-NMDAR Antibody (Ab)-Mediated Encephalitis. Autoimmun. Rev. 2022, 21, 103057. [Google Scholar] [CrossRef] [PubMed]
- Omi, T.; Kinoshita, M.; Nishikawa, A.; Tomioka, T.; Ohmori, K.; Fukada, K.; Matsunaga, H. Clinical Relapse of Anti-AMPA Receptor Encephalitis Associated with Recurrence of Thymoma. Intern. Med. 2018, 57, 9682-17. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Wen, B.; Chi, Z.; Zhao, X. The Well Responsiveness of Drug-Resistant Focal Seizures in Anti-AMPA2 Receptor Encephalitis to Perampanel Treatment. Neurol. Sci. 2021, 43, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Mohamadpour, M.; Whitney, K.; Bergold, P.J. The Importance of Therapeutic Time Window in the Treatment of Traumatic Brain Injury. Front. Neurosci. 2019, 13, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, R.; Burch, D.; Bazant, M.; Ceder, G. Particle Size Dependence of the Ionic Diffusivity. Nano Lett. 2010, 10, 4123–4127. [Google Scholar] [CrossRef]
- Milenkovic, J.; Hrenovic, J.; Goic-Barisic, I.; Tomic, M.; Djonlagic, J.; Rajic, N. Synergistic Anti-Biofouling Effect of Ag-Exchanged Zeolite and D-Tyrosine on PVC Composite against the Clinical Isolate of Acinetobacter Baumannii. Biofouling 2014, 30, 965–973. [Google Scholar] [CrossRef]
- Yeung, M.S.Y.; Djelloul, M.; Steiner, E.; Bernard, S.; Salehpour, M.; Possnert, G.; Brundin, L.; Frisén, J. Dynamics of Oligodendrocyte Generation in Multiple Sclerosis. Nature 2019, 566, 538–542. [Google Scholar] [CrossRef]
- Hauser, S.L.; Cree, B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. J. Assoc. Med. Am. 2021, 325, 765–779. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple Sclerosis—A Review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Milh, M.; Roubertoux, P.; Biba, N.; Chavany, J.; Spiga Ghata, A.; Fulachier, C.; Collins, S.C.; Wagner, C.; Roux, J.C.; Yalcin, B.; et al. A Knock-in Mouse Model for KCNQ2-Related Epileptic Encephalopathy Displays Spontaneous Generalized Seizures and Cognitive Impairment. Epilepsia 2020, 61, 868–878. [Google Scholar] [CrossRef] [Green Version]
- Allers, K.; Essue, B.M.; Hackett, M.L.; Muhunthan, J.; Anderson, C.S.; Pickles, K.; Scheibe, F.; Jan, S. The Economic Impact of Epilepsy: A Systematic Review. BMC Neurol. 2015, 15, 245. [Google Scholar] [CrossRef] [Green Version]
- Karoly, P.J.; Rao, V.R.; Gregg, N.M.; Worrell, G.A.; Bernard, C.; Cook, M.J.; Baud, M.O. Cycles in Epilepsy. Nat. Rev. Neurol. 2021, 17, 267–284. [Google Scholar] [CrossRef]
- Perkins, K.L.; Arranz, A.M.; Yamaguchi, Y.; Hrabetova, S. Brain Extracellular Space, Hyaluronan, and the Prevention of Epileptic Seizures. Rev. Neurosci. 2017, 28, 869–892. [Google Scholar] [CrossRef]
- Helmstaedter, C.; Witt, J.A. Epilepsy and Cognition—A Bidirectional Relationship? Seizure 2017, 49, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, N. Epilepsy and COVID-19: Updated Evidence and Narrative Review. Epilepsy Behav. 2021, 116, 107785. [Google Scholar] [CrossRef]
- Xu, C.; Gong, Y.; Wang, Y.; Chen, Z. New Advances in Pharmacoresistant Epilepsy towards Precise Management-from Prognosis to Treatments. Pharmacol. Ther. 2022, 233, 108026. [Google Scholar] [CrossRef]
- Bayat, A.; Bayat, M.; Rubboli, G.; Møller, R.S. Epilepsy Syndromes in the First Year of Life and Usefulness of Genetic Testing for Precision Therapy. Genes 2021, 12, 1051. [Google Scholar] [CrossRef]
- Tsai, M.L.; Chen, C.L.; Hsieh, K.L.C.; Miser, J.S.; Chang, H.; Liu, Y.L.; Wong, T.T. Seizure Characteristics Are Related to Tumor Pathology in Children with Brain Tumors. Epilepsy Res. 2018, 147, 15–21. [Google Scholar] [CrossRef]
- Romoli, M.; Sen, A.; Parnetti, L.; Calabresi, P.; Costa, C. Amyloid-β: A Potential Link between Epilepsy and Cognitive Decline. Nat. Rev. Neurol. 2021, 17, 469–485. [Google Scholar] [CrossRef]
- Khambhati, A.N.; Shafi, A.; Rao, V.R.; Chang, E.F. Long-Term Brain Network Reorganization Predicts Responsive Neurostimulation Outcomes for Focal Epilepsy. Sci. Transl. Med. 2021, 13, eabf6588. [Google Scholar] [CrossRef]
- Larivière, S.; Royer, J.; Rodríguez-Cruces, R.; Paquola, C.; Caligiuri, M.E.; Gambardella, A.; Concha, L.; Keller, S.S.; Cendes, F.; Yasuda, C.L.; et al. Structural Network Alterations in Focal and Generalized Epilepsy Assessed in a Worldwide ENIGMA Study Follow Axes of Epilepsy Risk Gene Expression. Nat. Commun. 2022, 13, 4320. [Google Scholar] [CrossRef]
- Vezzani, A.; Balosso, S.; Ravizza, T. Neuroinflammatory Pathways as Treatment Targets and Biomarkers in Epilepsy. Nat. Rev. Neurol. 2019, 15, 459–472. [Google Scholar] [CrossRef]
- Pitchaimuthu, K.; Wu, Q.Z.; Carter, O.; Nguyen, B.N.; Ahn, S.; Egan, G.F.; McKendrick, A.M. Occipital GABA Levels in Older Adults and Their Relationship to Visual Perceptual Suppression. Sci. Rep. 2017, 7, 14231. [Google Scholar] [CrossRef] [Green Version]
- Mikkelsen, M.; Singh, K.D.; Sumner, P.; Evans, C.J. Comparison of the Repeatability of GABA-Edited Magnetic Resonance Spectroscopy with and without Macromolecule Suppression. Magn. Reson. Med. 2016, 75, 946–953. [Google Scholar] [CrossRef]
- Cook, E.; Hammett, S.T.; Larsson, J. GABA Predicts Visual Intelligence. Neurosci. Lett. 2016, 632, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Derk, J.; Jones, H.E.; Como, C.; Pawlikowski, B.; Siegenthaler, J.A. Living on the Edge of the CNS: Meninges Cell Diversity in Health and Disease. Front. Cell Neurosci. 2021, 15, 245. [Google Scholar] [CrossRef]
- Oordt-Speets, A.M.; Bolijn, R.; van Hoorn, R.C.; Bhavsar, A.; Kyaw, M.H. Global Etiology of Bacterial Meningitis: A Systematic Review and Meta-Analysis. PLoS ONE 2018, 13, e0198772. [Google Scholar] [CrossRef] [Green Version]
- Thanabalasuriar, A.; Scott, B.N.V.; Peiseler, M.; Willson, M.E.; Zeng, Z.; Warrener, P.; Keller, A.E.; Surewaard, B.G.J.; Dozier, E.A.; Korhonen, J.T.; et al. Neutrophil Extracellular Traps Confine Pseudomonas Aeruginosa Ocular Biofilms and Restrict Brain Invasion. Cell Host Microbe 2019, 25, 526–536.e4. [Google Scholar] [CrossRef]
- Holdaway, I.; Reid, I.; Young, N.; Thomas, M. Meningitis in Adults: Diagnosis and Management. Intern. Med. J. 2018, 48, 1294–1307. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, L.; Albecka, A.; Mallery, D.L.; Kellner, M.J.; Paul, D.; Carter, A.P.; James, L.C.; Lancaster, M.A. SARS-CoV-2 Infects the Brain Choroid Plexus and Disrupts the Blood-CSF Barrier in Human Brain Organoids. Cell Stem Cell 2020, 27, 951–961.e5. [Google Scholar] [CrossRef] [PubMed]
- de Melo, G.D.; Lazarini, F.; Levallois, S.; Hautefort, C.; Michel, V.; Larrous, F.; Verillaud, B.; Aparicio, C.; Wagner, S.; Gheusi, G.; et al. COVID-19-Related Anosmia Is Associated with Viral Persistence and Inflammation in Human Olfactory Epithelium and Brain Infection in Hamsters. Sci. Transl. Med. 2021, 13, 8396. [Google Scholar] [CrossRef] [PubMed]
- Wichgers Schreur, P.J.; van Keulen, L.; Anjema, D.; Kant, J.; Kortekaas, J. Microencephaly in Fetal Piglets Following in Utero Inoculation of Zika Virus Article. Emerg. Microbes Infect. 2018, 7, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbaiyan, R.; Ganesan, A.; Ramasubramanian, B. Self-Potent Anti-Microbial and Anti-Fouling Action of Silver Nanoparticles Derived from Lichen-Associated Bacteria. Appl. Nanosci. 2022, 12, 2397–2408. [Google Scholar] [CrossRef]
- Brindha, R.; Kandeeban, R.; Kamal, K.S.; Manojkumar, K.; Nithya, V.; Saminathan, K. Andrographis Paniculata Absorbed ZnO Nanofibers as a Potential Antimicrobial Agent for Biomedical Applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 045002. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Sundarrajan, S.; Rao, R.P.; Reddy, M.V.; Chellappan, V.; Ramakrishna, S. Novel Low-Carbon Energy Solutions for Powering Emerging Wearables, Smart Textiles, and Medical Devices. Energy Environ. Sci. 2022, 15, 4928–4981. [Google Scholar] [CrossRef]
- Adams Waldorf, K.M.; Nelson, B.R.; Stencel-Baerenwald, J.E.; Studholme, C.; Kapur, R.P.; Armistead, B.; Walker, C.L.; Merillat, S.; Vornhagen, J.; Tisoncik-Go, J.; et al. Congenital Zika Virus Infection as a Silent Pathology with Loss of Neurogenic Output in the Fetal Brain. Nat. Med. 2018, 24, 368–374. [Google Scholar] [CrossRef]
- Figueiredo, C.P.; Barros-Aragão, F.G.Q.; Neris, R.L.S.; Frost, P.S.; Soares, C.; Souza, I.N.O.; Zeidler, J.D.; Zamberlan, D.C.; de Sousa, V.L.; Souza, A.S.; et al. Zika Virus Replicates in Adult Human Brain Tissue and Impairs Synapses and Memory in Mice. Nat. Commun. 2019, 10, 3890. [Google Scholar] [CrossRef] [Green Version]
- Mustafá, Y.M.; Meuren, L.M.; Coelho, S.V.A.; de Arruda, L.B. Pathways Exploited by Flaviviruses to Counteract the Blood-Brain Barrier and Invade the Central Nervous System. Front. Microbiol. 2019, 10, 525. [Google Scholar] [CrossRef]
- Hasel, P.; Rose, I.V.L.; Sadick, J.S.; Kim, R.D.; Liddelow, S.A. Neuroinflammatory Astrocyte Subtypes in the Mouse Brain. Nat. Neurosci. 2021, 24, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; McManus, R.M.; Latz, E. Inflammasome Signalling in Brain Function and Neurodegenerative Disease. Nat. Rev. Neurosci. 2018, 19, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of Gut-Brain Axis, Gut Microbial Composition, and Probiotic Intervention in Alzheimer’s Disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.A.; Banks, W.A. Age-Associated Changes in the Immune System and Blood–Brain Barrier Functions. Int. J. Mol. Sci. 2019, 20, 1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deloid, G.M.; Sohal, I.S.; Lorente, L.R.; Molina, R.M.; Pyrgiotakis, G.; Stevanovic, A.; Zhang, R.; McClements, D.J.; Geitner, N.K.; Bousfield, D.W.; et al. Reducing Intestinal Digestion and Absorption of Fat Using a Nature-Derived Biopolymer: Interference of Triglyceride Hydrolysis by Nanocellulose. ACS Nano 2018, 12, 6469–6479. [Google Scholar] [CrossRef]
- Komori, T. Updating the Grading Criteria for Adult Diffuse Gliomas: Beyond the WHO2016CNS Classification. Brain Tumor Pathol. 2020, 37, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Zhen, Y.; Reddy, V.S.; Ramasubramanian, B.; Ramakrishna, S. Three-Dimensional AgNps@Mxene@PEDOT:PSS Composite Hybrid Foam as a Piezoresistive Pressure Sensor with Ultra-Broad Working Range. J. Mater. Sci. 2022, 1–20. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Choi, W.W.Y.; Muffat, J.; Li, Y. Modeling Developmental Brain Diseases Using Human Pluripotent Stem Cells-Derived Brain Organoids – Progress and Perspective. J. Mol. Biol. 2022, 434, 167386. [Google Scholar] [CrossRef]
- Watanabe, S.; Shirogane, Y.; Sato, Y.; Hashiguchi, T.; Yanagi, Y. New Insights into Measles Virus Brain Infections. Trends Microbiol. 2019, 27, 164–175. [Google Scholar] [CrossRef]
- Al-Obaidi, M.M.J.; Desa, M.N.M. Mechanisms of Blood Brain Barrier Disruption by Different Types of Bacteria, and Bacterial–Host Interactions Facilitate the Bacterial Pathogen Invading the Brain. Cell. Mol. Neurobiol. 2018, 38, 1349–1368. [Google Scholar] [CrossRef]
- Fenster, R.J.; Lebois, L.A.M.; Ressler, K.J.; Suh, J. Brain Circuit Dysfunction in Post-Traumatic Stress Disorder: From Mouse to Man. Nat. Rev. Neurosci. 2018, 19, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Betlazar, C.; Harrison-Brown, M.; Middleton, R.J.; Banati, R.; Liu, G.J. Cellular Sources and Regional Variations in the Expression of the Neuroinflammatory Marker Translocator Protein (TSPO) in the Normal Brain. Int. J. Mol. Sci. 2018, 19, 2707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girgenti, M.J.; Wang, J.; Ji, D.; Cruz, D.A.; Alvarez, V.E.; Benedek, D.; Brady, C.; Davis, D.A.; Holtzheimer, P.E.; Keane, T.M.; et al. Transcriptomic Organization of the Human Brain in Post-Traumatic Stress Disorder. Nat. Neurosci. 2020, 24, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.A. Post-Traumatic Stress Disorder: A State-of-the-Art Review of Evidence and Challenges. World Psychiatry 2019, 18, 259–269. [Google Scholar] [CrossRef] [Green Version]
- de Erausquin, G.A.; Snyder, H.; Carrillo, M.; Hosseini, A.A.; Brugha, T.S.; Seshadri, S. The Chronic Neuropsychiatric Sequelae of COVID-19: The Need for a Prospective Study of Viral Impact on Brain Functioning. Alzheimer’s Dement. 2021, 17, 1056–1065. [Google Scholar] [CrossRef]
- Liu, C.; Goel, P.; Kaeser, P.S. Spatial and Temporal Scales of Dopamine Transmission. Nat. Rev. Neurosci. 2021, 22, 345–358. [Google Scholar] [CrossRef]
- Fukuyama, K.; Kato, R.; Murata, M.; Shiroyama, T.; Okada, M. Clozapine Normalizes a Glutamatergic Transmission Abnormality Induced by an Impaired NMDA Receptor in the Thalamocortical Pathway via the Activation of a Group III Metabotropic Glutamate Receptor. Biomolecules 2019, 9, 234. [Google Scholar] [CrossRef] [Green Version]
- Etchepare, L.; Gréa, H.; Durand, P.; Bouchet, D.; Groc, L. NMDA Receptor Membrane Dynamics Tunes the Firing Pattern of Midbrain Dopaminergic Neurons. J. Physiol. 2021, 599, 2933–2951. [Google Scholar] [CrossRef]
- Nesbit, M.O.; Chai, A.; Axerio-Cilies, P.; Phillips, A.G.; Wang, Y.T.; Held, K. The Selective Dopamine D1 Receptor Agonist SKF81297 Modulates NMDA Receptor Currents Independently of D1 Receptors. Neuropharmacology 2022, 207, 108967. [Google Scholar] [CrossRef]
- Pan, X.; Kaminga, A.C.; Wen, S.W.; Wu, X.; Acheampong, K.; Liu, A. Dopamine and Dopamine Receptors in Alzheimer’s Disease: A Systematic Review and Network Meta-Analysis. Front. Aging Neurosci. 2019, 10, 175. [Google Scholar] [CrossRef]
- Umek, N.; Geršak, B.; Vintar, N.; Šoštarič, M.; Mavri, J. Dopamine Autoxidation Is Controlled by Acidic PH. Front. Mol. Neurosci. 2018, 11, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Hu, K.; Wang, D.; Zubi, Y.; Lee, S.T.; Puthongkham, P.; Mirkin, M.V.; Venton, B.J. Cavity Carbon-Nanopipette Electrodes for Dopamine Detection. Anal. Chem. 2019, 91, 4618–4624. [Google Scholar] [CrossRef] [PubMed]
- Stępnicki, P.; Kondej, M.; Kaczor, A.A. Current Concepts and Treatments of Schizophrenia. Molecules 2018, 23, 2087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braff, D.L. The Importance of Endophenotypes in Schizophrenia Research. Schizophr. Res. 2015, 163, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cloitre, M.; Hyland, P.; Bisson, J.I.; Brewin, C.R.; Roberts, N.P.; Karatzias, T.; Shevlin, M. ICD-11 Posttraumatic Stress Disorder and Complex Posttraumatic Stress Disorder in the United States: A Population-Based Study. J. Trauma. Stress 2019, 32, 833–842. [Google Scholar] [CrossRef]
- Simon, N.; Roberts, N.P.; Lewis, C.E.; van Gelderen, M.J.; Bisson, J.I. Associations between Perceived Social Support, Posttraumatic Stress Disorder (PTSD) and Complex PTSD (CPTSD): Implications for Treatment. Eur. J. Psychotraumatology 2019, 10, 1573129. [Google Scholar] [CrossRef]
- Sun, L.; Sun, Z.; Wu, L.; Zhu, Z.; Zhang, F.; Shang, Z.; Jia, Y.; Gu, J.; Zhou, Y.; Wang, Y.; et al. Prevalence and Risk Factors for Acute Posttraumatic Stress Disorder during the COVID-19 Outbreak. J. Affect. Disord. 2021, 283, 123–129. [Google Scholar] [CrossRef]
- Forte, G.; Favieri, F.; Tambelli, R.; Casagrande, M. COVID-19 Pandemic in the Italian Population: Validation of a Post-Traumatic Stress Disorder Questionnaire and Prevalence of PTSD Symptomatology. Int. J. Environ. Res. Public Health 2020, 17, 4151. [Google Scholar] [CrossRef]
- Lewis, C.; Roberts, N.P.; Gibson, S.; Bisson, J.I. Dropout from Psychological Therapies for Post-Traumatic Stress Disorder (PTSD) in Adults: Systematic Review and Meta-Analysis. Eur. J. Psychotraumatology 2020, 11, 1709709. [Google Scholar] [CrossRef] [Green Version]
- Labbadia, J.; Morimoto, R.I. Huntington’s Disease: Underlying Molecular Mechanisms and Emerging Concepts. Trends Biochem. Sci. 2013, 38, 378–385. [Google Scholar] [CrossRef]
- Rauf, A.; Badoni, H.; Abu-Izneid, T.; Olatunde, A.; Rahman, M.M.; Painuli, S.; Semwal, P.; Wilairatana, P.; Mubarak, M.S. Neuroinflammatory Markers: Key Indicators in the Pathology of Neurodegenerative Diseases. Molecules 2022, 27, 3194. [Google Scholar] [CrossRef] [PubMed]
- Voet, S.; Srinivasan, S.; Lamkanfi, M.; Loo, G. van Inflammasomes in Neuroinflammatory and Neurodegenerative Diseases. EMBO Mol. Med. 2019, 11, e10248. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Slanzi, A.; Iannoto, G.; Rossi, B.; Zenaro, E.; Constantin, G. In Vitro Models of Neurodegenerative Diseases. Front. Cell Dev. Biol. 2020, 8, 328. [Google Scholar] [CrossRef] [PubMed]
- Vicente Miranda, H.; El-Agnaf, O.M.A.; Outeiro, T.F. Glycation in Parkinson’s Disease and Alzheimer’s Disease. Mov. Disord. 2016, 31, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, T.; Goñi, F. Immunotherapeutic Approaches for Alzheimer’s Disease. Neuron 2015, 85, 1162–1176. [Google Scholar] [CrossRef] [Green Version]
- Bondi, M.W.; Edmonds, E.C.; Salmon, D.P. Alzheimer’s Disease: Past, Present, and Future. J. Int. Neuropsychol. Soc. 2017, 23, 818–831. [Google Scholar] [CrossRef] [Green Version]
- Barnat, M.; Capizzi, M.; Aparicio, E.; Boluda, S.; Wennagel, D.; Kacher, R.; Kassem, R.; Lenoir, S.; Agasse, F.; Bra, B.Y.; et al. Huntington’s Disease Alters Human Neurodevelopment. Science 2020, 369, 787–793. [Google Scholar] [CrossRef]
- Shannon, K.M.; Fraint, A. Therapeutic Advances in Huntington’s Disease. Mov. Disord. 2015, 30, 1539–1546. [Google Scholar] [CrossRef]
- McColgan, P.; Tabrizi, S.J. Huntington’s Disease: A Clinical Review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Astle, D.E.; Holmes, J.; Kievit, R.; Gathercole, S.E. Annual Research Review: The Transdiagnostic Revolution in Neurodevelopmental Disorders. J. Child. Psychol. Psychiatry 2022, 63, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.N. Translational Animal Models of Autism and Neurodevelopmental Disorders. Dialogues Clin. Neurosci. 2022, 14, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, C.J. The Cerebellum and Neurodevelopmental Disorders. Cerebellum 2015, 15, 34–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summers, J.; Baribeau, D.; Mockford, M.; Goldhopf, L.; Ambrozewicz, P.; Szatmari, P.; Vorstman, J. Supporting Children with Neurodevelopmental Disorders during the COVID-19 Pandemic. J. Am. Acad. Child Adolesc. Psychiatry 2020, 60, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hoekzema, K.; Vecchio, D.; Wu, H.; Sulovari, A.; Coe, B.P.; Gillentine, M.A.; Wilfert, A.B.; Perez-Jurado, L.A.; Kvarnung, M.; et al. Large-Scale Targeted Sequencing Identifies Risk Genes for Neurodevelopmental Disorders. Nat. Commun. 2020, 11, 4932. [Google Scholar] [CrossRef] [PubMed]
- Han, V.X.; Patel, S.; Jones, H.F.; Nielsen, T.C.; Mohammad, S.S.; Hofer, M.J.; Gold, W.; Brilot, F.; Lain, S.J.; Nassar, N.; et al. Maternal Acute and Chronic Inflammation in Pregnancy Is Associated with Common Neurodevelopmental Disorders: A Systematic Review. Transl. Psychiatry 2021, 11, 71. [Google Scholar] [CrossRef]
- Zengeler, K.E.; Lukens, J.R. Innate Immunity at the Crossroads of Healthy Brain Maturation and Neurodevelopmental Disorders. Nat. Rev. Immunol. 2021, 21, 454–468. [Google Scholar] [CrossRef]
- Chen, G.T.; Geschwind, D.H. Challenges and Opportunities for Precision Medicine in Neurodevelopmental Disorders. Adv. Drug Deliv. Rev. 2022, 191, 114564. [Google Scholar] [CrossRef]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global Prevalence of Autism: A Systematic Review Update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef]
- Marotta, R.; Risoleo, M.C.; Messina, G.; Parisi, L.; Carotenuto, M.; Vetri, L.; Roccella, M. The Neurochemistry of Autism. Brain Sci. 2020, 10, 163. [Google Scholar] [CrossRef]
- Snowling, M.J.; Hulme, C.; Nation, K. Defining and Understanding Dyslexia: Past, Present and Future. Oxf. Rev. Educ. 2020, 46, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Kowalski, K.; Piotrowski, P.; Grąźlewski, T.; Samochowiec, J. Neurodevelopmental Aspects of Adverse Childhood Experiences in Psychosis: Relevance of the Allostatic Load Concept. Psychoneuroendocrinology 2022, 143, 105850. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Anrather, J. Stroke Research at a Crossroad: Asking the Brain for Directions. Nat. Neurosci. 2011, 14, 1363–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakor, J.; Ahadian, S.; Niakan, A.; Banton, E.; Nasrollahi, F.; Hasani-Sadrabadi, M.M.; Khademhosseini, A. Engineered Hydrogels for Brain Tumor Culture and Therapy. Bio-Des. Manuf. 2020, 3, 203–226. [Google Scholar] [CrossRef]
- Yang, C.; Hawkins, K.E.; Doré, S.; Candelario-Jalil, E. Neuroinflammatory Mechanisms of Blood-Brain Barrier Damage in Ischemic Stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef]
- Kreienkamp, H.J.; Wagner, M.; Weigand, H.; McConkie-Rossell, A.; McDonald, M.; Keren, B.; Mignot, C.; Gauthier, J.; Soucy, J.F.; Michaud, J.L.; et al. Variant-Specific Effects Define the Phenotypic Spectrum of HNRNPH2-Associated Neurodevelopmental Disorders in Males. Hum. Genet. 2022, 141, 257–272. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of Glioblastoma: State of the Art and Future Directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- le Rhun, E.; Preusser, M.; Roth, P.; Reardon, D.A.; van den Bent, M.; Wen, P.; Reifenberger, G.; Weller, M. Molecular Targeted Therapy of Glioblastoma. Cancer Treat. Rev. 2019, 80, 101896. [Google Scholar] [CrossRef]
- Birzu, C.; French, P.; Caccese, M.; Cerretti, G.; Idbaih, A.; Zagonel, V.; Lombardi, G. Recurrent Glioblastoma: From Molecular Landscape to New Treatment Perspectives. Cancers 2020, 13, 47. [Google Scholar] [CrossRef]
- Hilf, N.; Kuttruff-Coqui, S.; Frenzel, K.; Bukur, V.; Stevanović, S.; Gouttefangeas, C.; Platten, M.; Tabatabai, G.; Dutoit, V.; van der Burg, S.H.; et al. Actively Personalized Vaccination Trial for Newly Diagnosed Glioblastoma. Nature 2018, 565, 240–245. [Google Scholar] [CrossRef]
- Jackson, C.M.; Choi, J.; Lim, M. Mechanisms of Immunotherapy Resistance: Lessons from Glioblastoma. Nat. Immunol. 2019, 20, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, V.; Yang, Y.; Schubert, M.C.; Reyhan, E.; Tetzlaff, S.K.; Wißmann, N.; Botz, M.; Soyka, S.J.; Beretta, C.A.; Pramatarov, R.L.; et al. Glioblastoma Hijacks Neuronal Mechanisms for Brain Invasion. Cell 2022, 185, 2899–2917.e31. [Google Scholar] [CrossRef] [PubMed]
- Suzuka, J.; Tsuda, M.; Wang, L.; Kohsaka, S.; Kishida, K.; Semba, S.; Sugino, H.; Aburatani, S.; Frauenlob, M.; Kurokawa, T.; et al. Rapid Reprogramming of Tumour Cells into Cancer Stem Cells on Double-Network Hydrogels. Nat. Biomed. Eng. 2021, 5, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Iranmanesh, Y.; Jiang, B.; Favour, O.C.; Dou, Z.; Wu, J.; Li, J.; Sun, C. Mitochondria’s Role in the Maintenance of Cancer Stem Cells in Glioblastoma. Front. Oncol. 2021, 11, 101. [Google Scholar] [CrossRef]
- Su, C.; Zhang, J.; Yarden, Y.; Fu, L. The Key Roles of Cancer Stem Cell-Derived Extracellular Vesicles. Signal Transduct. Target. Ther. 2021, 6, 109. [Google Scholar] [CrossRef]
- Biserova, K.; Jakovlevs, A.; Uljanovs, R.; Strumfa, I. Cancer Stem Cells: Significance in Origin, Pathogenesis and Treatment of Glioblastoma. Cells 2021, 10, 621. [Google Scholar] [CrossRef]
- Jiang, J.S.; Hua, Y.; Zhou, X.J.; Shen, D.D.; Shi, J.L.; Ge, M.; Geng, Q.N.; Jia, Z.Z. Quantitative Assessment of Tumor Cell Proliferation in Brain Gliomas with Dynamic Contrast-Enhanced MRI. Acad. Radiol. 2019, 26, 1215–1221. [Google Scholar] [CrossRef]
- Perez, A.; Huse, J.T. The Evolving Classification of Diffuse Gliomas: World Health Organization Updates for 2021. Curr. Neurol. Neurosci. Rep. 2021, 21, 67. [Google Scholar] [CrossRef]
- Salehinejad, M.A.; Ghanavati, E.; Glinski, B.; Hallajian, A.H.; Azarkolah, A. A Systematic Review of Randomized Controlled Trials on Efficacy and Safety of Transcranial Direct Current Stimulation in Major Neurodevelopmental Disorders: ADHD, Autism, and Dyslexia. Brain Behav. 2022, 12, e2724. [Google Scholar] [CrossRef]
- Hwang, E.I.; Sayour, E.J.; Flores, C.T.; Grant, G.; Wechsler-Reya, R.; Hoang-Minh, L.B.; Kieran, M.W.; Salcido, J.; Prins, R.M.; Figg, J.W.; et al. The Current Landscape of Immunotherapy for Pediatric Brain Tumors. Nat. Cancer 2022, 3, 11–24. [Google Scholar] [CrossRef]
- Iroh Tam, P.Y.; Bendel, C.M. Diagnostics for Neonatal Sepsis: Current Approaches and Future Directions. Pediatric Res. 2017, 82, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Weiss, Z.F.; Leon, A.; Koo, S. The Evolving Landscape of Fungal Diagnostics, Current and Emerging Microbiological Approaches. J. Fungi 2021, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Altaheri, H.; Muhammad, G.; Alsulaiman, M.; Amin, S.U.; Altuwaijri, G.A.; Abdul, W.; Bencherif, M.A.; Faisal, M. Deep Learning Techniques for Classification of Electroencephalogram (EEG) Motor Imagery (MI) Signals: A Review. Neural Comput. Appl. 2021, 1–42. [Google Scholar] [CrossRef]
- Halim, Z.; Rehan, M. On Identification of Driving-Induced Stress Using Electroencephalogram Signals: A Framework Based on Wearable Safety-Critical Scheme and Machine Learning. Inf. Fusion 2020, 53, 66–79. [Google Scholar] [CrossRef]
- Craik, A.; He, Y.; Contreras-Vidal, J.L. Deep Learning for Electroencephalogram (EEG) Classification Tasks: A Review. J. Neural. Eng. 2019, 16, 031001. [Google Scholar] [CrossRef]
- Supriya, S.; Siuly, S.; Wang, H.; Zhang, Y. Automated Epilepsy Detection Techniques from Electroencephalogram Signals: A Review Study. Health Inf. Sci. Syst. 2020, 8, 33. [Google Scholar] [CrossRef]
- Gemein, L.A.W.; Schirrmeister, R.T.; Chrabąszcz, P.; Wilson, D.; Boedecker, J.; Schulze-Bonhage, A.; Hutter, F.; Ball, T. Machine-Learning-Based Diagnostics of EEG Pathology. Neuroimage 2020, 220, 117021. [Google Scholar] [CrossRef]
- Barshutina, M.N.; Volkov, V.S.; Arsenin, A.V.; Yakubovsky, D.I.; Melezhik, A.V.; Blokhin, A.N.; Tkachev, A.G.; Lopachev, A.V.; Kondrashov, V.A. Biocompatible, Electroconductive, and Highly Stretchable Hybrid Silicone Composites Based on Few-Layer Graphene and Cnts. Nanomaterials 2021, 11, 1143. [Google Scholar] [CrossRef]
- Jiang, X.; Bian, G.B.; Tian, Z. Removal of Artifacts from EEG Signals: A Review. Sensors 2019, 19, 987. [Google Scholar] [CrossRef] [Green Version]
- Acharya, U.R.; Hagiwara, Y.; Deshpande, S.N.; Suren, S.; Koh, J.E.W.; Oh, S.L.; Arunkumar, N.; Ciaccio, E.J.; Lim, C.M. Characterization of Focal EEG Signals: A Review. Future Gener. Comput. Syst. 2019, 91, 290–299. [Google Scholar] [CrossRef]
- Nam, J.; Lim, H.K.; Kim, N.H.; Park, J.K.; Kang, E.S.; Kim, Y.T.; Heo, C.; Lee, O.S.; Kim, S.G.; Yun, W.S.; et al. Supramolecular Peptide Hydrogel-Based Soft Neural Interface Augments Brain Signals through a Three-Dimensional Electrical Network. ACS Nano 2020, 14, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Yang, R.; Huang, M.; Zeng, N.; Liu, X. A Review on Transfer Learning in EEG Signal Analysis. Neurocomputing 2021, 421, 1–14. [Google Scholar] [CrossRef]
- Namazi, H.; Menon, A.; Krejcar, O. Analysis of the Correlation between Static Visual Stimuli, Eye Movements, and Brain Signals. Fluct. Noise Lett. 2021, 20, 2150056. [Google Scholar] [CrossRef]
- Khosla, A.; Khandnor, P.; Chand, T. A Comparative Analysis of Signal Processing and Classification Methods for Different Applications Based on EEG Signals. Biocybern. Biomed. Eng. 2020, 40, 649–690. [Google Scholar] [CrossRef]
- Salem Ghahfarrokhi, S.; Khodadadi, H. Human Brain Tumor Diagnosis Using the Combination of the Complexity Measures and Texture Features through Magnetic Resonance Image. Biomed. Signal. Process. Control. 2020, 61, 102025. [Google Scholar] [CrossRef]
- Elf, K.; Ronne-Engström, E.; Semnic, R.; Rostami-Berglund, E.; Sundblom, J.; Zetterling, M. Continuous EEG Monitoring after Brain Tumor Surgery. Acta Neurochir. 2019, 161, 1835–1843. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Ashraf, I.; Alhaisoni, M.; Damaševičius, R.; Scherer, R.; Rehman, A.; Bukhari, S.A.C. Multimodal Brain Tumor Classification Using Deep Learning and Robust Feature Selection: A Machine Learning Application for Radiologists. Diagnostics 2020, 10, 565. [Google Scholar] [CrossRef]
- Amin, J.; Sharif, M.; Haldorai, A.; Yasmin, M.; Nayak, R.S. Brain Tumor Detection and Classification Using Machine Learning: A Comprehensive Survey. Complex Intell. Syst. 2021, 8, 3161–3183. [Google Scholar] [CrossRef]
- Merk, T.; Peterson, V.; Köhler, R.; Haufe, S.; Richardson, R.M.; Neumann, W.J. Machine Learning Based Brain Signal Decoding for Intelligent Adaptive Deep Brain Stimulation. Exp. Neurol. 2022, 351, 113993. [Google Scholar] [CrossRef]
- Chen, Y.H.; op de Beeck, M.; Vanderheyden, L.; Carrette, E.; Mihajlović, V.; Vanstreels, K.; Grundlehner, B.; Gadeyne, S.; Boon, P.; van Hoof, C. Soft, Comfortable Polymer Dry Electrodes for High Quality ECG and EEG Recording. Sensors 2014, 14, 23758–23780. [Google Scholar] [CrossRef]
- Wang, L.; Dou, W.; Chen, J.; Lu, K.; Zhang, F.; Abdulaziz, M.; Su, W.; Li, A.; Xu, C.; Sun, Y. A CNT-PDMS Wearable Device for Simultaneous Measurement of Wrist Pulse Pressure and Cardiac Electrical Activity. Mater. Sci. Eng. C 2020, 117, 111345. [Google Scholar] [CrossRef]
- Kirbas Cilingir, E.; Seven, E.S.; Zhou, Y.; Walters, B.M.; Mintz, K.J.; Pandey, R.R.; Wikramanayake, A.H.; Chusuei, C.C.; Vanni, S.; Graham, R.M.; et al. Metformin Derived Carbon Dots: Highly Biocompatible Fluorescent Nanomaterials as Mitochondrial Targeting and Blood-Brain Barrier Penetrating Biomarkers. J. Colloid Interface Sci. 2021, 592, 485–497. [Google Scholar] [CrossRef]
- Furtado, D.; Björnmalm, M.; Ayton, S.; Bush, A.I.; Kempe, K.; Caruso, F. Overcoming the Blood–Brain Barrier: The Role of Nanomaterials in Treating Neurological Diseases. Adv. Mater. 2018, 30, 1801362. [Google Scholar] [CrossRef] [Green Version]
- Zottel, A.; Paska, A.V.; Jovčevska, I. Nanotechnology Meets Oncology: Nanomaterials in Brain Cancer Research, Diagnosis and Therapy. Materials 2019, 12, 1588. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Liu, Y.; Wang, M.; Chen, G.; Jiang, Y.; Yu, J.; Wan, C.; Qi, D.; Xiao, M.; Leow, W.R.; et al. An Artificial Somatic Reflex Arc. Adv. Mater. 2020, 32, 1905399. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jeong, B.; Kim, M.; Jang, R.; Paik, W.; Kang, J.; Chung, W.J.; Hong, G.S.; Kim, N. Emergency Triage of Brain Computed Tomography via Anomaly Detection with a Deep Generative Model. Nat. Commun. 2022, 13, 4251. [Google Scholar] [CrossRef] [PubMed]
- Fletcher’sandersjöö, A.; Tatter, C.; Yang, L.; Pontén, E.; Boman, M.; Lassarén, P.; Forsberg, S.; Grönlund, I.; Tidehag, V.; Rubenson-Wahlin, R.; et al. Stockholm Score of Lesion Detection on Computed Tomography Following Mild Traumatic Brain Injury (SELECT-TBI): Study Protocol for a Multicentre, Retrospective, Observational Cohort Study. BMJ Open 2022, 12, e060679. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Yamada, Y.; Kosugi, K.; Yamada, M.; Narita, K.; Nakahara, T.; Fujiwara, H.; Toda, M.; Jinzaki, M. Effect of Gravity on Brain Structure as Indicated on Upright Computed Tomography. Sci. Rep. 2021, 11, 392. [Google Scholar] [CrossRef]
- Vidhya, V.; Gudigar, A.; Raghavendra, U.; Hegde, A.; Menon, G.R.; Molinari, F.; Ciaccio, E.J.; Acharya, U.R. Automated Detection and Screening of Traumatic Brain Injury (TBI) Using Computed Tomography Images: A Comprehensive Review and Future Perspectives. Int. J. Environ. Res. Public Health 2021, 18, 6499. [Google Scholar] [CrossRef]
- Aqeel, S.; Gupta, A.; Singh, L. A Review on Unknown Repercussions Associated with Metallic Nanoparticles and Their Rectification Techniques. Curr. Nanomater. 2022, 7, 181–192. [Google Scholar] [CrossRef]
- Hamidian, K.; Sarani, M.; Sheikhi, E.; Khatami, M. Cytotoxicity Evaluation of Green Synthesized ZnO and Ag-Doped ZnO Nanoparticles on Brain Glioblastoma Cells. J. Mol. Struct. 2022, 1251, 131962. [Google Scholar] [CrossRef]
- Gravesteijn, B.Y.; Nieboer, D.; Ercole, A.; Lingsma, H.F.; Nelson, D.; van Calster, B.; Steyerberg, E.W.; Åkerlund, C.; Amrein, K.; Andelic, N.; et al. Machine Learning Algorithms Performed No Better than Regression Models for Prognostication in Traumatic Brain Injury. J. Clin. Epidemiol. 2020, 122, 95–107. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, X.; Jiang, H.; Yu, S.; Robins, C.; Armstrong, M.J.; Li, R.; Mei, Z.; Shi, X.; Gerasimov, E.S.; et al. A Machine Learning Approach to Brain Epigenetic Analysis Reveals Kinases Associated with Alzheimer’s Disease. Nat. Commun. 2021, 12, 4472. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jeong, Y.Y.; Jon, S. A Drug-Loaded Aptamer—Gold Nanoparticle Bioconjugate for Combined Ct Imaging and Therapy of Prostate Cancer. ACS Nano 2010, 4, 3689–3696. [Google Scholar] [CrossRef]
- Falahati, M.; Attar, F.; Sharifi, M.; Saboury, A.A.; Salihi, A.; Aziz, F.M.; Kostova, I.; Burda, C.; Priecel, P.; Lopez-Sanchez, J.A.; et al. Gold Nanomaterials as Key Suppliers in Biological and Chemical Sensing, Catalysis, and Medicine. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129435. [Google Scholar] [CrossRef]
- Li, C.H.; Kuo, T.R.; Su, H.J.; Lai, W.Y.; Yang, P.C.; Chen, J.S.; Wang, D.Y.; Wu, Y.C.; Chen, C.C. Fluorescence-Guided Probes of Aptamer-Targeted Gold Nanoparticles with Computed Tomography Imaging Accesses for in Vivo Tumor Resection. Sci. Rep. 2015, 5, 15675. [Google Scholar] [CrossRef] [Green Version]
- Snelling, B.M.; Sur, S.; Shah, S.S.; Khandelwal, P.; Caplan, J.; Haniff, R.; Starke, R.M.; Yavagal, D.R.; Peterson, E.C. Transradial Cerebral Angiography: Techniques and Outcomes. J. Neurointerv. Surg. 2018, 10, 874–881. [Google Scholar] [CrossRef]
- Kumar, G.; Dumitrascu, O.M.; Chiang, C.C.; O’Carroll, C.B.; Alexandrov, A.v. Prediction of Delayed Cerebral Ischemia with Cerebral Angiography: A Meta-Analysis. Neurocritical Care 2018, 30, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Sur, S.; Sedighim, S.; Kassi, A.; Yavagal, D.; Peterson, E.C.; Starke, R.M. Utility of Diagnostic Cerebral Angiography in the Management of Suspected Central Nervous System Vasculitis. J. Clin. Neurosci. 2019, 64, 98–100. [Google Scholar] [CrossRef]
- Sajja, K.C.; Sweid, A.; al Saiegh, F.; Chalouhi, N.; Avery, M.B.; Schmidt, R.F.; Tjoumakaris, S.I.; Gooch, M.R.; Herial, N.; Abbas, R.; et al. Endovascular Robotic: Feasibility and Proof of Principle for Diagnostic Cerebral Angiography and Carotid Artery Stenting. J. Neurointerv. Surg. 2020, 12, 345–349. [Google Scholar] [CrossRef]
- Darsaut, T.E.; Derksen, C.; Farzin, B.; Keough, M.B.; Fahed, R.; Boisseau, W.; Letourneau-Guillon, L.; Januel, A.C.; Weill, A.; Roy, D.; et al. Reliability of the Diagnosis of Cerebral Vasospasm Using Catheter Cerebral Angiography: A Systematic Review and Inter- and Intraobserver Study. Am. J. Neuroradiol. 2021, 42, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, C.; Wu, X.; Cao, X.; Young, G.S.; Chen, H.; Xu, X. A Neural Network Approach to Segment Brain Blood Vessels in Digital Subtraction Angiography. Comput. Methods Programs Biomed. 2020, 185, 105159. [Google Scholar] [CrossRef]
- Schöll, M.; Lockhart, S.N.; Schonhaut, D.R.; O’Neil, J.P.; Janabi, M.; Ossenkoppele, R.; Baker, S.L.; Vogel, J.W.; Faria, J.; Schwimmer, H.D.; et al. PET Imaging of Tau Deposition in the Aging Human Brain. Neuron 2016, 89, 971–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, J.; Zhang, Y. GAN-Based Synthetic Brain PET Image Generation. Brain Inform. 2020, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Sehlin, D.; Syvänen, S.; Ballanger, B.; Barthel, H.; Bischof, G.N.; Boche, D.; Boecker, H.; Bohn, K.P.; Borghammer, P.; Cross, D.; et al. Engineered Antibodies: New Possibilities for Brain PET? Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2848–2858. [Google Scholar] [CrossRef] [Green Version]
- Ouerghi, H.; Mourali, O.; Zagrouba, E. Non-Subsampled Shearlet Transform Based MRI and PET Brain Image Fusion Using Simplified Pulse Coupled Neural Network and Weight Local Features in YIQ Colour Space. IET Image Process 2018, 12, 1873–1880. [Google Scholar] [CrossRef]
- Houghton, S.; Kyron, M.; Lawrence, D.; Hunter, S.C.; Hattie, J.; Carroll, A.; Zadow, C.; Chen, W. Longitudinal Trajectories of Mental Health and Loneliness for Australian Adolescents With-or-without Neurodevelopmental Disorders: The Impact of COVID-19 School Lockdowns. J. Child Psychol. Psychiatry 2022, 63, 1332–1343. [Google Scholar] [CrossRef]
- Mecheter, I.; Alic, L.; Abbod, M.; Amira, A.; Ji, J. MR Image-Based Attenuation Correction of Brain PET Imaging: Review of Literature on Machine Learning Approaches for Segmentation. J. Digit. Imaging 2020, 33, 1224–1241. [Google Scholar] [CrossRef]
- Ladefoged, C.N.; Marner, L.; Hindsholm, A.; Law, I.; Højgaard, L.; Andersen, F.L. Deep Learning Based Attenuation Correction of PET/MRI in Pediatric Brain Tumor Patients: Evaluation in a Clinical Setting. Front. Neurosci. 2019, 13, 1005. [Google Scholar] [CrossRef]
- Laurencin, C.; Lancelot, S.; Gobert, F.; Redouté, J.; Mérida, I.; Iecker, T.; Liger, F.; Irace, Z.; Greusard, E.; Lamberet, L.; et al. Modeling [11C]Yohimbine PET Human Brain Kinetics with Test-Retest Reliability, Competition Sensitivity Studies and Search for a Suitable Reference Region. Neuroimage 2021, 240, 118328. [Google Scholar] [CrossRef]
- Beck, K.; Arumuham, A.; Brugger, S.; McCutcheon, R.A.; Veronese, M.; Santangelo, B.; McGinnity, C.J.; Dunn, J.; Kaar, S.; Singh, N.; et al. The Association between N-Methyl-d-Aspartate Receptor Availability and Glutamate Levels: A Multi-Modal PET-MR Brain Imaging Study in First-Episode Psychosis and Healthy Controls. J. Psychopharmacol. 2022, 36, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Won, J.Y.; Park, H.; Lee, S.; Son, J.W.; Chung, Y.; Ko, G.B.; Kim, K.Y.; Song, J.; Seo, S.; Ryu, Y.; et al. Development and Initial Results of a Brain PET Insert for Simultaneous 7-Tesla PET/MRI Using an FPGA-Only Signal Digitization Method. IEEE Trans. Med. Imaging 2021, 40, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Slomka, P.J.; Pan, T.; Germano, G. Recent Advances and Future Progress in PET Instrumentation. Semin. Nucl. Med. 2016, 46, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.L.; Zhang, Z.; Qu, W.M. Roles of Adenosine and Its Receptors in Sleep–Wake Regulation. Int. Rev. Neurobiol. 2014, 119, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Bahadure, N.B.; Ray, A.K.; Thethi, H.P. Comparative Approach of MRI-Based Brain Tumor Segmentation and Classification Using Genetic Algorithm. J. Digit. Imaging 2018, 31, 477–489. [Google Scholar] [CrossRef]
- Mzoughi, H.; Njeh, I.; Wali, A.; Slima, M.B.; BenHamida, A.; Mhiri, C.; Mahfoudhe, K.B. Deep Multi-Scale 3D Convolutional Neural Network (CNN) for MRI Gliomas Brain Tumor Classification. J. Digit. Imaging 2020, 33, 903–915. [Google Scholar] [CrossRef]
- Almansory, K.O.; Fraioli, F. Combined PET/MRI in Brain Glioma Imaging. Br. J. Hosp. Med. 2019, 80, 380–386. [Google Scholar] [CrossRef]
- Badža, M.M.; Barjaktarović, M.C. Classification of Brain Tumors from MRI Images Using a Convolutional Neural Network. Appl. Sci. 2020, 10, 1999. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, R.; Tao, C.; Huang, X.; Chen, Z.; Li, X.; Zhou, J.; Zeng, Q.; Zhao, B.; Yuan, M.; et al. CuS–NiS2 Nanomaterials for MRI Guided Phototherapy of Gastric Carcinoma via Triggering Mitochondria-Mediated Apoptosis and MLKL/CAPG-Mediated Necroptosis. Nanotoxicology 2020, 14, 774–787. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Hui, D.; Hong, R. Progress in Magnetic Fe3O4 Nanomaterials in Magnetic Resonance Imaging. Nanotechnol. Rev. 2020, 9, 1265–1283. [Google Scholar] [CrossRef]
- Arunkumar, N.; Mohammed, M.A.; Mostafa, S.A.; Ibrahim, D.A.; Rodrigues, J.J.P.C.; de Albuquerque, V.H.C. Fully Automatic Model-Based Segmentation and Classification Approach for MRI Brain Tumor Using Artificial Neural Networks. Concurr. Comput. 2020, 32, e4962. [Google Scholar] [CrossRef]
- Abd-Ellah, M.K.; Awad, A.I.; Khalaf, A.A.M.; Hamed, H.F.A. A Review on Brain Tumor Diagnosis from MRI Images: Practical Implications, Key Achievements, and Lessons Learned. Magn. Reson. Imaging 2019, 61, 300–318. [Google Scholar] [CrossRef]
- Wadhwa, A.; Bhardwaj, A.; Singh Verma, V. A Review on Brain Tumor Segmentation of MRI Images. Magn. Reson. Imaging 2019, 61, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Wood, E.A.; Stopka, S.A.; Zhang, L.; Mattson, S.; Maasz, G.; Pirger, Z.; Vertes, A. Neuropeptide Localization in Lymnaea Stagnalis: From the Central Nervous System to Subcellular Compartments. Front. Mol. Neurosci. 2021, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Syslová, K.; Rambousek, L.; Kuzma, M.; Najmanová, V.; Bubeníková-Valešová, V.; Šlamberová, R.; Kačer, P. Monitoring of Dopamine and Its Metabolites in Brain Microdialysates: Method Combining Freeze-Drying with Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2011, 1218, 3382–3391. [Google Scholar] [CrossRef] [PubMed]
- Yücel, M.A.; Selb, J.J.; Huppert, T.J.; Franceschini, M.A.; Boas, D.A. Functional Near Infrared Spectroscopy: Enabling Routine Functional Brain Imaging. Curr. Opin. Biomed. Eng. 2017, 4, 78–86. [Google Scholar] [CrossRef]
- Ayaz, H.; Onaral, B.; Izzetoglu, K.; Shewokis, P.A.; Mckendrick, R.; Parasuraman, R. Continuous Monitoring of Brain Dynamics with Functional near Infrared Spectroscopy as a Tool for Neuroergonomic Research: Empirical Examples and a Technological Development. Front. Hum. Neurosci. 2013, 7, 871. [Google Scholar] [CrossRef] [Green Version]
- Lloyd-Fox, S.; Blasi, A.; Elwell, C.E. Illuminating the Developing Brain: The Past, Present and Future of Functional near Infrared Spectroscopy. Neurosci. Biobehav. Rev. 2010, 34, 269–284. [Google Scholar] [CrossRef]
- Sajedi, H.; Pardakhti, N. Age Prediction Based on Brain MRI Image: A Survey. J. Med. Syst. 2019, 43, 279. [Google Scholar] [CrossRef]
- Cutini, S.; Brigadoi, S. Unleashing the Future Potential of Functional Near-Infrared Spectroscopy in Brain Sciences. J. Neurosci. Methods 2014, 232, 152–156. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Verma, M.; Karandikar, P.; Furin, J.; Langer, R.; Traverso, G. Nanotechnology Approaches for Global Infectious Diseases. Nat. Nanotechnol. 2021, 16, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Yoo, K.H.; Sym, S.J.; Khang, D. Mesenchymal Stem Cell Therapy Assisted by Nanotechnology: A Possible Combinational Treatment for Brain Tumor and Central Nerve Regeneration. Int. J. Nanomed. 2019, 14, 5925. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Tao, W.; Zou, Y.; Farokhzad, O.C.; Shi, B. Nanotechnology-Based Strategies for SiRNA Brain Delivery for Disease Therapy. Trends Biotechnol. 2018, 36, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Hamano, N.; Li, S.D.; Chougule, M.; Shoyele, S.A.; Gupta, U.; Ajazuddin; et al. Recent Advancements in the Field of Nanotechnology for the Delivery of Anti-Alzheimer Drug in the Brain Region. Expert Opin. Drug Deliv. 2018, 15, 589–617. [Google Scholar] [CrossRef]
- Tan, M.S.A.; Parekh, H.S.; Pandey, P.; Siskind, D.J.; Falconer, J.R. Nose-to-Brain Delivery of Antipsychotics Using Nanotechnology: A Review. Expert Opin. Drug Deliv. 2020, 17, 839–853. [Google Scholar] [CrossRef]
- Moura, R.P.; Martins, C.; Pinto, S.; Sousa, F.; Sarmento, B. Blood-Brain Barrier Receptors and Transporters: An Insight on Their Function and How to Exploit Them through Nanotechnology. Expert Opin. Drug Deliv. 2019, 16, 271–285. [Google Scholar] [CrossRef]
- Kanazawa, T.; Kurano, T.; Ibaraki, H.; Takashima, Y.; Suzuki, T.; Seta, Y. Therapeutic Effects in a Transient Middle Cerebral Artery Occlusion Rat Model by Nose-To-Brain Delivery of Anti-TNF-Alpha SiRNA with Cell-Penetrating Peptide-Modified Polymer Micelles. Pharmaceutics 2019, 11, 478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pokharkar, V.; Suryawanshi, S.; Dhapte-Pawar, V. Exploring Micellar-Based Polymeric Systems for Effective Nose-to-Brain Drug Delivery as Potential Neurotherapeutics. Drug Deliv. Transl. Res. 2019, 10, 1019–1031. [Google Scholar] [CrossRef]
- Gauro, R.; Nandave, M.; Jain, V.K.; Jain, K. Advances in Dendrimer-Mediated Targeted Drug Delivery to the Brain. J. Nanoparticle Res. 2021, 23, 76. [Google Scholar] [CrossRef]
- Gothwal, A.; Kumar, H.; Nakhate, K.T.; Ajazuddin; Dutta, A.; Borah, A.; Gupta, U. Lactoferrin Coupled Lower Generation PAMAM Dendrimers for Brain Targeted Delivery of Memantine in Aluminum-Chloride-Induced Alzheimer’s Disease in Mice. Bioconjug. Chem. 2019, 30, 2573–2583. [Google Scholar] [CrossRef]
- Bonasia, C.G.; Abdulahad, W.H.; Rutgers, A.; Heeringa, P.; Bos, N.A. B Cell Activation and Escape of Tolerance Checkpoints: Recent Insights from Studying Autoreactive B Cells. Cells 2021, 10, 1190. [Google Scholar] [CrossRef] [PubMed]
- Zucca, F.A.; Vanna, R.; Cupaioli, F.A.; Bellei, C.; de Palma, A.; di Silvestre, D.; Mauri, P.; Grassi, S.; Prinetti, A.; Casella, L.; et al. Neuromelanin Organelles Are Specialized Autolysosomes That Accumulate Undegraded Proteins and Lipids in Aging Human Brain and Are Likely Involved in Parkinson’s Disease. NPJ Parkinson’s Dis. 2018, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- You, Y.; Ikezu, T. Emerging Roles of Extracellular Vesicles in Neurodegenerative Disorders. Neurobiol. Dis. 2019, 130, 104512. [Google Scholar] [CrossRef]
- Wang, Y.; Fathali, H.; Mishra, D.; Olsson, T.; Keighron, J.D.; Skibicka, K.P.; Cans, A.S. Counting the Number of Glutamate Molecules in Single Synaptic Vesicles. J. Am. Chem. Soc. 2019, 141, 17507–17511. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Zaldívar, H.M.; Polakovicova, I.; Salas-Huenuleo, E.; Corvalán, A.H.; Kogan, M.J.; Yefi, C.P.; Andia, M.E. Extracellular Vesicles through the Blood–Brain Barrier: A Review. Fluids Barriers CNS 2022, 19, 60. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Zueva, I.V.; Petrov, K.A.; Lukashenko, S.S.; Nizameev, I.R.; Kulik, N.V.; Voloshina, A.D.; Almasy, L.; Kadirov, M.K.; Masson, P.; et al. Mixed Cationic Liposomes for Brain Delivery of Drugs by the Intranasal Route: The Acetylcholinesterase Reactivator 2-PAM as Encapsulated Drug Model. Colloids Surf. B Biointerfaces 2018, 171, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.S.; Oh, K.T.; Choi, H.G.; Lim, S.J. Liposomal Formulations for Nose-to-Brain Delivery: Recent Advances and Future Perspectives. Pharmaceutics 2019, 11, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saint-Pol, J.; Gosselet, F.; Duban-Deweer, S.; Pottiez, G.; Karamanos, Y. Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells 2020, 9, 851. [Google Scholar] [CrossRef] [Green Version]
- Gharbavi, M.; Amani, J.; Kheiri-Manjili, H.; Danafar, H.; Sharafi, A. Niosome: A Promising Nanocarrier for Natural Drug Delivery through Blood-Brain Barrier. Adv. Pharmacol. Sci. 2018, 2018, 6847971. [Google Scholar] [CrossRef]
- Xie, H.; Li, L.; Sun, Y.; Wang, Y.; Gao, S.; Tian, Y.; Ma, X.; Guo, C.; Bo, F.; Zhang, L. An Available Strategy for Nasal Brain Transport of Nanocomposite Based on PAMAM Dendrimers via In Situ Gel. Nanomaterials 2019, 9, 147. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Kim, M.; Kim, G.Y.; Choi, J.S.; Lee, M. Brain Gene Delivery Using Histidine and Arginine-Modified Dendrimers for Ischemic Stroke Therapy. J. Control. Release 2021, 330, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Porterfield, J.E.; Smith, E.; Sharma, R.; Kannan, S.; Kannan, R.M. Effect of Mannose Targeting of Hydroxyl PAMAM Dendrimers on Cellular and Organ Biodistribution in a Neonatal Brain Injury Model. J. Control. Release 2018, 283, 175–189. [Google Scholar] [CrossRef]
- Santos, S.D.; Xavier, M.; Leite, D.M.; Moreira, D.A.; Custódio, B.; Torrado, M.; Castro, R.; Leiro, V.; Rodrigues, J.; Tomás, H.; et al. PAMAM Dendrimers: Blood-Brain Barrier Transport and Neuronal Uptake after Focal Brain Ischemia. J. Control. Release 2018, 291, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Liu, C.; Pang, Z. Dendrimer-Based Drug Delivery Systems for Brain Targeting. Biomolecules 2019, 9, 790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moscariello, P.; Raabe, M.; Liu, W.; Bernhardt, S.; Qi, H.; Kaiser, U.; Wu, Y.; Weil, T.; Luhmann, H.J.; Hedrich, J.; et al. Unraveling In Vivo Brain Transport of Protein-Coated Fluorescent Nanodiamonds. Small 2019, 15, 1902992. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, Z.; Peroutka-Bigus, N.; Bellaire, B.; Jergens, A.; Wannemuehler, M.; Wang, Q. Gut Organoid as a New Platform to Study Alginate and Chitosan Mediated PLGA Nanoparticles for Drug Delivery. Mar. Drugs 2021, 19, 282. [Google Scholar] [CrossRef]
- Zhi, K.; Raji, B.; Nookala, A.R.; Khan, M.M.; Nguyen, X.H.; Sakshi, S.; Pourmotabbed, T.; Yallapu, M.M.; Kochat, H.; Tadrous, E.; et al. PLGA Nanoparticle-Based Formulations to Cross the Blood–Brain Barrier for Drug Delivery: From R&D to CGMP. Pharmaceutics 2021, 13, 500. [Google Scholar] [CrossRef]
- Li, Y.; Yin, K.; Diao, Y.; Fang, M.; Yang, J.; Zhang, J.; Cao, H.; Liu, X.; Jiang, J. A Biopolymer-Gated Ionotronic Junctionless Oxide Transistor Array for Spatiotemporal Pain-Perception Emulation in Nociceptor Network. Nanoscale 2022, 14, 2316–2326. [Google Scholar] [CrossRef] [PubMed]
- Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of Hybrid PLGA Nanoparticles: Future of Smart Drug Delivery and Theranostics Medicine. Mater. Des. 2020, 193, 108805. [Google Scholar] [CrossRef]
- Qu, Z.S.; Li, L.; Sun, X.J.; Zhao, Y.W.; Zhang, J.; Geng, Z.; Fu, J.L.; Ren, Q.G. Glycogen Synthase Kinase-3 Regulates Production of Amyloid- β Peptides and Tau Phosphorylation in Diabetic Rat Brain. Sci. World J. 2014, 2014, 878123. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Liu, H.; Zhang, Y.; Chen, G.; Li, Z.; Wang, Q. An Activatable NIR-II Nanoprobe for In Vivo Early Real-Time Diagnosis of Traumatic Brain Injury. Angew. Chem. Int. Ed. 2020, 59, 247–252. [Google Scholar] [CrossRef]
- Rabanel, J.M.; Piec, P.A.; Landri, S.; Patten, S.A.; Ramassamy, C. Transport of PEGylated-PLA Nanoparticles across a Blood Brain Barrier Model, Entry into Neuronal Cells and in Vivo Brain Bioavailability. J. Control Release 2020, 328, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Aravind, A.; Pfister, B.J.; Chandra, N.; Haorah, J. Animal Models of Traumatic Brain Injury and Assessment of Injury Severity. Mol. Neurobiol. 2019, 56, 5332–5345. [Google Scholar] [CrossRef]
- Bouthour, W.; Mégevand, P.; Donoghue, J.; Lüscher, C.; Birbaumer, N.; Krack, P. Biomarkers for Closed-Loop Deep Brain Stimulation in Parkinson Disease and Beyond. Nat. Rev. Neurol. 2019, 15, 343–352. [Google Scholar] [CrossRef]
- Ledig, C.; Schuh, A.; Guerrero, R.; Heckemann, R.A.; Rueckert, D. Structural Brain Imaging in Alzheimer’s Disease and Mild Cognitive Impairment: Biomarker Analysis and Shared Morphometry Database. Sci. Rep. 2018, 8, 11258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelmalik, P.A.; Draghic, N.; Ling, G.S.F. Management of Moderate and Severe Traumatic Brain Injury. Transfusion 2019, 59, 1529–1538. [Google Scholar] [CrossRef] [Green Version]
- Shi, K.; Zhang, J.; Dong, J.F.; Shi, F.D. Dissemination of Brain Inflammation in Traumatic Brain Injury. Cell. Mol. Immunol. 2019, 16, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Jarrahi, A.; Braun, M.; Ahluwalia, M.; Gupta, R.v.; Wilson, M.; Munie, S.; Ahluwalia, P.; Vender, J.R.; Vale, F.L.; Dhandapani, K.M.; et al. Revisiting Traumatic Brain Injury: From Molecular Mechanisms to Therapeutic Interventions. Biomedicines 2020, 8, 389. [Google Scholar] [CrossRef]
- Yue, J.K.; Yuh, E.L.; Korley, F.K.; Winkler, E.A.; Sun, X.; Puffer, R.C.; Deng, H.; Choy, W.; Chandra, A.; Taylor, S.R.; et al. Association between Plasma GFAP Concentrations and MRI Abnormalities in Patients with CT-Negative Traumatic Brain Injury in the TRACK-TBI Cohort: A Prospective Multicentre Study. Lancet Neurol. 2019, 18, 953–961. [Google Scholar] [CrossRef]
- Maggio, M.G.; de Luca, R.; Molonia, F.; Porcari, B.; Destro, M.; Casella, C.; Salvati, R.; Bramanti, P.; Calabro, R.S. Cognitive Rehabilitation in Patients with Traumatic Brain Injury: A Narrative Review on the Emerging Use of Virtual Reality. J. Clin. Neurosci. 2019, 61, 1–4. [Google Scholar] [CrossRef]
- O’Brien, W.T.; Pham, L.; Symons, G.F.; Monif, M.; Shultz, S.R.; McDonald, S.J. The NLRP3 Inflammasome in Traumatic Brain Injury: Potential as a Biomarker and Therapeutic Target. J. Neuroinflammation 2020, 17, 104. [Google Scholar] [CrossRef]
- Wang, K.K.; Yang, Z.; Zhu, T.; Shi, Y.; Rubenstein, R.; Tyndall, J.A.; Manley, G.T. An Update on Diagnostic and Prognostic Biomarkers for Traumatic Brain Injury. Expert Rev. Mol. Deagn. 2018, 18, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Needham, E.J.; Helmy, A.; Zanier, E.R.; Jones, J.L.; Coles, A.J.; Menon, D.K. The Immunological Response to Traumatic Brain Injury. J. Neuroimmunol. 2019, 332, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.S.; Stein, S.C.; Swanson, R.; Guan, S.; Garcia, L.; Mehta, D.; Smith, D.H. Blood Biomarkers for Traumatic Brain Injury: A Quantitative Assessment of Diagnostic and Prognostic Accuracy. Front. Neurol. 2019, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Ruozi, B.; Belletti, D.; Sharma, H.S.; Sharma, A.; Muresanu, D.F.; Mössler, H.; Forni, F.; Vandelli, M.A.; Tosi, G. PLGA Nanoparticles Loaded Cerebrolysin: Studies on Their Preparation and Investigation of the Effect of Storage and Serum Stability with Reference to Traumatic Brain Injury. Mol. Neurobiol. 2015, 52, 899–912. [Google Scholar] [CrossRef]
- Bailey, Z.S.; Nilson, E.; Bates, J.A.; Oyalowo, A.; Hockey, K.S.; Sajja, V.S.S.S.; Thorpe, C.; Rogers, H.; Dunn, B.; Frey, A.S.; et al. Cerium Oxide Nanoparticles Improve Outcome after In Vitro and In Vivo Mild Traumatic Brain Injury. J. Neurotrauma 2020, 37, 1452–1462. [Google Scholar] [CrossRef] [Green Version]
- Pavlovic, D.; Pekic, S.; Stojanovic, M.; Popovic, V. Traumatic Brain Injury: Neuropathological, Neurocognitive and Neurobehavioral Sequelae. Pituitary 2019, 22, 270–282. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, R.; Jain, D.K.; Saraf, A. Enhancement of Oral Bioavailability of Poorly Water Soluble Carvedilol by Chitosan Nanoparticles: Optimization and Pharmacokinetic Study. Int. J. Biol. Macromol. 2019, 135, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Razzino, C.A.; Serafín, V.; Gamella, M.; Pedrero, M.; Montero-Calle, A.; Barderas, R.; Calero, M.; Lobo, A.O.; Yáñez-Sedeño, P.; Campuzano, S.; et al. An Electrochemical Immunosensor Using Gold Nanoparticles-PAMAM-Nanostructured Screen-Printed Carbon Electrodes for Tau Protein Determination in Plasma and Brain Tissues from Alzheimer Patients. Biosens. Bioelectron. 2020, 163, 112238. [Google Scholar] [CrossRef]
- van Giau, V.; Bagyinszky, E.; Yang, Y.S.; Youn, Y.C.; An, S.S.A.; Kim, S.Y. Genetic Analyses of Early-Onset Alzheimer’s Disease Using next Generation Sequencing. Sci. Rep. 2019, 9, 8368. [Google Scholar] [CrossRef]
- Wegmann, S.; Bennett, R.E.; Delorme, L.; Robbins, A.B.; Hu, M.; McKenzie, D.; Kirk, M.J.; Schiantarelli, J.; Tunio, N.; Amaral, A.C.; et al. Experimental Evidence for the Age Dependence of Tau Protein Spread in the Brain. Sci. Adv. 2019, 5, 6404–6430. [Google Scholar] [CrossRef] [Green Version]
- Ułamek-Kozioł, M.; Czuczwar, S.J.; Januszewski, S.; Pluta, R. Proteomic and Genomic Changes in Tau Protein, Which Are Associated with Alzheimer’s Disease after Ischemia-Reperfusion Brain Injury. Int. J. Mol. Sci. 2020, 21, 892. [Google Scholar] [CrossRef] [Green Version]
- Sundström, A.; Adolfsson, A.N.; Nordin, M.; Adolfsson, R. Loneliness Increases the Risk of All-Cause Dementia and Alzheimer’s Disease. J. Gerontol. Ser. B 2020, 75, 919–926. [Google Scholar] [CrossRef] [Green Version]
- Monzio Compagnoni, G.; di Fonzo, A.; Corti, S.; Comi, G.P.; Bresolin, N.; Masliah, E. The Role of Mitochondria in Neurodegenerative Diseases: The Lesson from Alzheimer’s Disease and Parkinson’s Disease. Mol. Neurobiol. 2020, 57, 2959–2980. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.H.; Kang, S.; Lee, W.; Choi, H.; Chung, S.; Kim, J.I.; Mook-Jung, I. A Breakdown in Metabolic Reprogramming Causes Microglia Dysfunction in Alzheimer’s Disease. Cell Metab. 2019, 30, 493–507.e6. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R.; Muñoz-Torrero, D.; Dembinski, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Uwishema, O.; Mahmoud, A.; Sun, J.; Correia, I.F.S.; Bejjani, N.; Alwan, M.; Nicholas, A.; Oluyemisi, A.; Dost, B. Is Alzheimer’s Disease an Infectious Neurological Disease? A Review of the Literature. Brain Behav. 2022, 12, e2728. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative Stress, Dysfunctional Glucose Metabolism and Alzheimer Disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Henstridge, C.M.; Hyman, B.T.; Spires-Jones, T.L. Beyond the Neuron–Cellular Interactions Early in Alzheimer Disease Pathogenesis. Nat. Rev. Neurosci. 2019, 20, 94–108. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Tramontin, N.; da Silva, S.; Arruda, R.; Ugioni, K.S.; Canteiro, P.B.; de Bem Silveira, G.; Mendes, C.; Silveira, P.C.L.; Muller, A.P. Gold Nanoparticles Treatment Reverses Brain Damage in Alzheimer’s Disease Model. Mol. Neurobiol. 2019, 57, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Sivanesan, S.; Rajeshkumar, S. Gold Nanoparticles in Diagnosis and Treatment of Alzheimer’s Disease. Nanobiotechnology Neurodegener. Dis. 2019, 289–306. [Google Scholar] [CrossRef]
- Serafín, V.; Razzino, C.A.; Gamella, M.; Pedrero, M.; Povedano, E.; Montero-Calle, A.; Barderas, R.; Calero, M.; Lobo, A.O.; Yáñez-Sedeño, P.; et al. Disposable Immunoplatforms for the Simultaneous Determination of Biomarkers for Neurodegenerative Disorders Using Poly(Amidoamine) Dendrimer/Gold Nanoparticle Nanocomposite. Anal. Bioanal. Chem. 2020, 413, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.H.G.; Foffani, G.; Obeso, J.; Sánchez-Ferro, A. New Sensor and Wearable Technologies to Aid in the Diagnosis and Treatment Monitoring of Parkinson’s Disease. Annu. Rev. Biomed. Eng. 2019, 21, 111–143. [Google Scholar] [CrossRef]
- Byrom, B.; McCarthy, M.; Schueler, P.; Muehlhausen, W. Brain Monitoring Devices in Neuroscience Clinical Research: The Potential of Remote Monitoring Using Sensors, Wearables, and Mobile Devices. Clin. Pharmacol. Ther. 2018, 104, 59–71. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.; Kim, H.; Yeo, W.H. Recent Advances in Wearable Sensors and Portable Electronics for Sleep Monitoring. iScience 2021, 24, 102461. [Google Scholar] [CrossRef]
- Habets, J.G.V.; Heijmans, M.; Kuijf, M.L.; Janssen, M.L.F.; Temel, Y.; Kubben, P.L. An Update on Adaptive Deep Brain Stimulation in Parkinson’s Disease. Mov. Disord. 2018, 33, 1834–1843. [Google Scholar] [CrossRef] [Green Version]
- Romero, L.E.; Chatterjee, P.; Armentano, R.L. An IoT Approach for Integration of Computational Intelligence and Wearable Sensors for Parkinson’s Disease Diagnosis and Monitoring. Health Technol. 2016, 6, 167–172. [Google Scholar] [CrossRef]
- Yin, R.; Wang, D.; Zhao, S.; Lou, Z.; Shen, G. Wearable Sensors-Enabled Human–Machine Interaction Systems: From Design to Application. Adv. Funct. Mater. 2021, 31, 2008936. [Google Scholar] [CrossRef]
- Reddy, V.S.; Tian, Y.; Zhang, C.; Ye, Z.; Roy, K.; Chinnappan, A.; Ramakrishna, S.; Liu, W.; Ghosh, R. A Review on Electrospun Nanofibers Based Advanced Applications: From Health Care to Energy Devices. Polymers 2021, 13, 3746. [Google Scholar] [CrossRef]
- Reddy, V.S.; Agarwal, B.; Ye, Z.; Zhang, C.; Roy, K.; Chinnappan, A.; Narayan, R.J.; Ramakrishna, S.; Ghosh, R. Recent Advancement in Biofluid-Based Glucose Sensors Using Invasive, Minimally Invasive, and Non-Invasive Technologies: A Review. Nanomaterials 2022, 12, 1082. [Google Scholar] [CrossRef]
- Ghosh, R.; Pin, K.Y.; Reddy, V.S.; Jayathilaka, W.A.D.M.; Ji, D.; Serrano-García, W.; Bhargava, S.K.; Ramakrishna, S.; Chinnappan, A. Micro/Nanofiber-Based Noninvasive Devices for Health Monitoring Diagnosis and Rehabilitation. Appl. Phys. Rev. 2020, 7, 041309. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Hasan, A.; Kaarela, O.; Byambaa, B.; Sheikhi, A.; Gaharwar, A.K.; Khademhosseini, A. Advancing Frontiers in Bone Bioprinting. Adv. Healthc. Mater. 2019, 8, 1801048. [Google Scholar] [CrossRef] [PubMed]
- Ravanbakhsh, H.; Karamzadeh, V.; Bao, G.; Mongeau, L.; Juncker, D.; Zhang, Y.S. Emerging Technologies in Multi-Material Bioprinting. Adv. Mater. 2021, 33, 2104730. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.; Li, L.H.; Zhang, J.; Li, G.K.; Zavabeti, A.; Li, Y. Enhanced Piezoelectric Properties Enabled by Engineered Low-Dimensional Nanomaterials. ACS Appl. Nano. Mater. 2022, 5, 12126–12142. [Google Scholar] [CrossRef]
- Lee, E.K.; Yoo, H. Self-Powered Sensors: New Opportunities and Challenges from Two-Dimensional Nanomaterials. Molecules 2021, 26, 5056. [Google Scholar] [CrossRef]
- Jayathilaka, W.A.D.M.; Qi, K.; Qin, Y.; Chinnappan, A.; Serrano-García, W.; Baskar, C.; Wang, H.; He, J.; Cui, S.; Thomas, S.W.; et al. Significance of Nanomaterials in Wearables: A Review on Wearable Actuators and Sensors. Adv. Mater. 2019, 31, 1805921. [Google Scholar] [CrossRef]
- Levato, R.; Visser, J.; Planell, J.A.; Engel, E.; Malda, J.; Mateos-Timoneda, M.A. Biofabrication of Tissue Constructs by 3D Bioprinting of Cell-Laden Microcarriers. Biofabrication 2014, 6, 035020. [Google Scholar] [CrossRef]
- Murphy, S.v.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.; Ozbolat, I.T. 3D Bioprinting of Cells, Tissues and Organs. Sci. Rep. 2020, 10, 14023. [Google Scholar] [CrossRef] [PubMed]
- Decante, G.; Costa, J.B.; Silva-Correia, J.; Collins, M.N.; Reis, R.L.; Oliveira, J.M. Engineering Bioinks for 3D Bioprinting. Biofabrication 2021, 13, 032001. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D Bioprinting for Engineering Complex Tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Qu, X.; Zhu, J.; Ma, X.; Patel, S.; Liu, J.; Wang, P.; Lai, C.S.E.; Gou, M.; Xu, Y.; et al. Direct 3D Bioprinting of Prevascularized Tissue Constructs with Complex Microarchitecture. Biomaterials 2017, 124, 106–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchroithner, B.; Hartmann, D.; Mayr, S.; Oh, Y.J.; Sivun, D.; Karner, A.; Buchegger, B.; Griesser, T.; Hinterdorfer, P.; Klar, T.A.; et al. 3D Multiphoton Lithography Using Biocompatible Polymers with Specific Mechanical Properties. Nanoscale Adv. 2020, 2, 2422–2428. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Bakht, S.M.; Gomez-Florit, M.; Rial, R.; Monteiro, R.F.; Teixeira, S.P.B.; Taboada, P.; Reis, R.L.; Domingues, R.M.A.; Gomes, M.E. Magnetically-Assisted 3D Bioprinting of Anisotropic Tissue-Mimetic Constructs. Adv Funct Mater 2022, 32, 2208940. [Google Scholar] [CrossRef]
- Mao, S.; Pang, Y.; Liu, T.; Shao, Y.; He, J.; Yang, H.; Mao, Y.; Sun, W. Bioprinting of in Vitro Tumor Models for Personalized Cancer Treatment: A Review. Biofabrication 2020, 12, 042001. [Google Scholar] [CrossRef]
- Marín-Morales, J.; Higuera-Trujillo, J.L.; Greco, A.; Guixeres, J.; Llinares, C.; Scilingo, E.P.; Alcañiz, M.; Valenza, G. Affective Computing in Virtual Reality: Emotion Recognition from Brain and Heartbeat Dynamics Using Wearable Sensors. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Hsieh, F.Y.; Hsu, S.-h. 3D Bioprinting: A New Insight into the Therapeutic Strategy of Neural Tissue Regeneration. Organogenesis 2016, 11, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.; Kim, H.S.; Hwang, D.Y. Stem Cells as Promising Therapeutic Options for Neurological Disorders. J. Cell Biochem. 2013, 114, 743–753. [Google Scholar] [CrossRef]
- Tasnim, N.; de la Vega, L.; Anil Kumar, S.; Abelseth, L.; Alonzo, M.; Amereh, M.; Joddar, B.; Willerth, S.M. 3D Bioprinting Stem Cell Derived Tissues. Cell. Mol. Bioeng. 2018, 11, 219–240. [Google Scholar] [CrossRef]
- Lee, S.J.; Esworthy, T.; Stake, S.; Miao, S.; Zuo, Y.Y.; Harris, B.T.; Zhang, L.G. Advances in 3D Bioprinting for Neural Tissue Engineering. Adv. Biosyst. 2018, 2, 1700213. [Google Scholar] [CrossRef]
- Alexander Heinrich, M.; Bansal, R.; Lammers, T.; Shrike Zhang, Y.; Michel Schiffelers, R.; Prakash, J.; Heinrich, M.A.; Bansal, R.; Prakash, J.; Lammers, T.; et al. 3D-Bioprinted Mini-Brain: A Glioblastoma Model to Study Cellular Interactions and Therapeutics. Adv. Mater. 2019, 31, 1806590. [Google Scholar] [CrossRef]
- Ngo, M.T.; Harley, B.A.C. Progress in Mimicking Brain Microenvironments to Understand and Treat Neurological Disorders. APL Bioeng. 2021, 5, 020902. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.; Lee, Y.; Li, Z.; Hu, J.; Park, S.S.; Kim, K. Optimized 3D Bioprinting Technology Based on Machine Learning: A Review of Recent Trends and Advances. Micromachines 2022, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Ngo, M.T.; Harley, B.A.C. Perivascular Signals Alter Global Gene Expression Profile of Glioblastoma and Response to Temozolomide in a Gelatin Hydrogel. Biomaterials 2019, 198, 122–134. [Google Scholar] [CrossRef]
- Tirella, A.; Liberto, T.; Ahluwalia, A. Riboflavin and Collagen: New Crosslinking Methods to Tailor the Stiffness of Hydrogels. Mater. Lett. 2012, 74, 58–61. [Google Scholar] [CrossRef]
- Han, J.; Kim, D.S.; Jang, H.; Kim, H.R.; Kang, H.W. Bioprinting of Three-Dimensional Dentin–Pulp Complex with Local Differentiation of Human Dental Pulp Stem Cells. J. Tissue Eng. 2019, 10, 2041731419845849. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Dai, X.; Zhang, X.; Zhang, J.; Xu, T.; Lan, Q. Coaxial Extrusion Bioprinted Shell-Core Hydrogel Microfibers Mimic Glioma Microenvironment and Enhance the Drug Resistance of Cancer Cells. Colloids Surf. B Biointerfaces 2018, 171, 291–299. [Google Scholar] [CrossRef]
- Cha, J.; Kim, P. Biomimetic Strategies for the Glioblastoma Microenvironment. Front. Mater. 2017, 4, 45. [Google Scholar] [CrossRef] [Green Version]
- Nishiguchi, A.; Zhang, H.; Schweizerhof, S.; Schulte, M.F.; Mourran, A.; Möller, M. 4D Printing of a Light-Driven Soft Actuator with Programmed Printing Density. ACS Appl. Mater. Interfaces 2020, 12, 12176–12185. [Google Scholar] [CrossRef]
- Li, Q.; Lin, H.; Wang, O.; Qiu, X.; Kidambi, S.; Deleyrolle, L.P.; Reynolds, B.A.; Lei, Y. Scalable Production of Glioblastoma Tumor-Initiating Cells in 3 Dimension Thermoreversible Hydrogels. Sci. Rep. 2016, 6, 31915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, M.; Rich, J.N.; Chen, S. Biomaterials and 3D Bioprinting Strategies to Model Glioblastoma and the Blood–Brain Barrier. Adv. Mater. 2021, 33, 2004776. [Google Scholar] [CrossRef] [PubMed]
- Raphael, B.; Khalil, T.; Workman, V.L.; Smith, A.; Brown, C.P.; Streuli, C.; Saiani, A.; Domingos, M. 3D Cell Bioprinting of Self-Assembling Peptide-Based Hydrogels. Mater. Lett. 2017, 190, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Boonstra, E.; de Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as Food Supplements: The Effects of GABA on Brain and Behavior. Front. Psychol. 2015, 6, 1520. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.H.; Gardier, A.M. Fast-Acting Antidepressant Activity of Ketamine: Highlights on Brain Serotonin, Glutamate, and GABA Neurotransmission in Preclinical Studies. Pharmacol. Ther. 2019, 199, 58–90. [Google Scholar] [CrossRef]
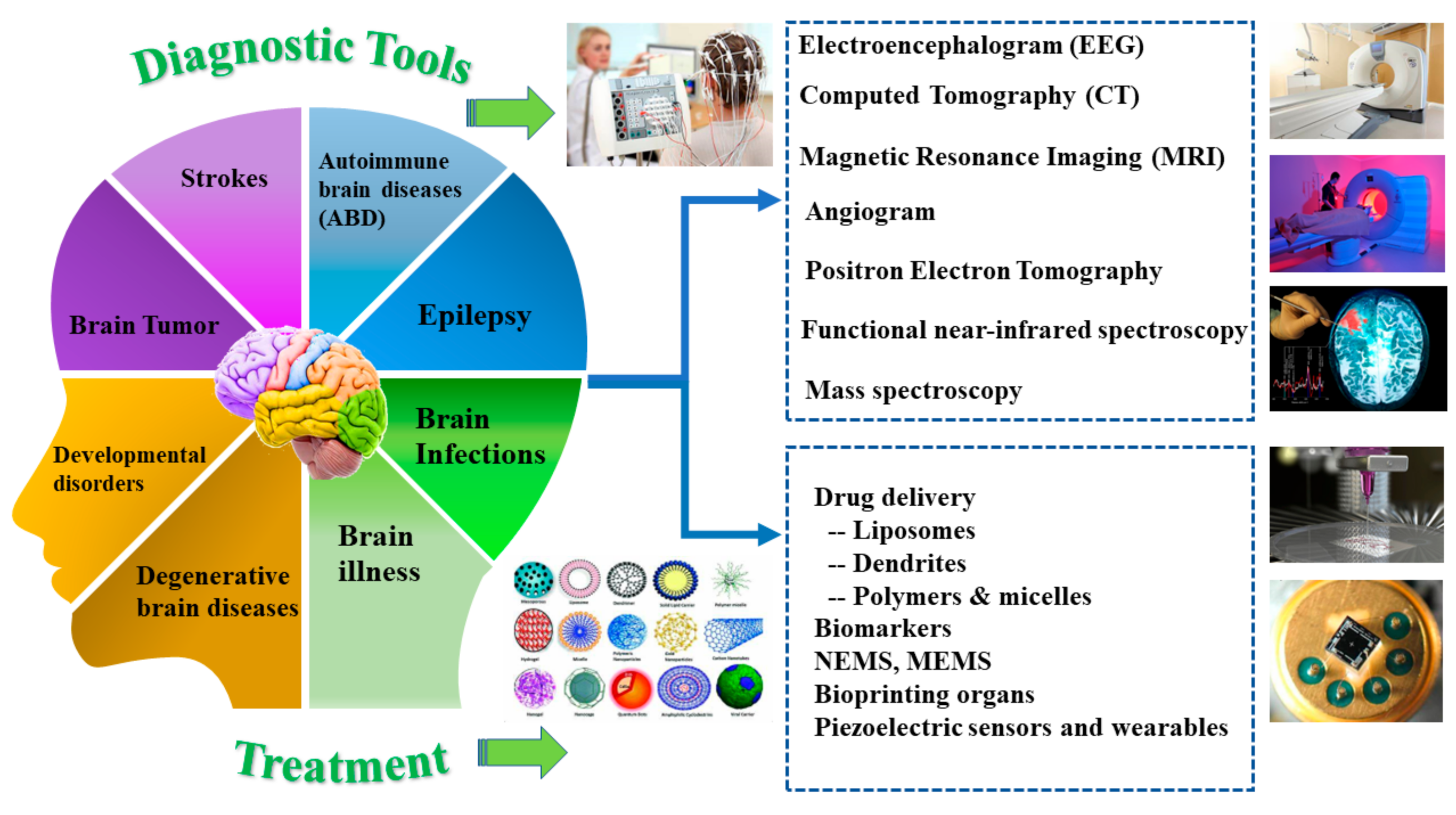
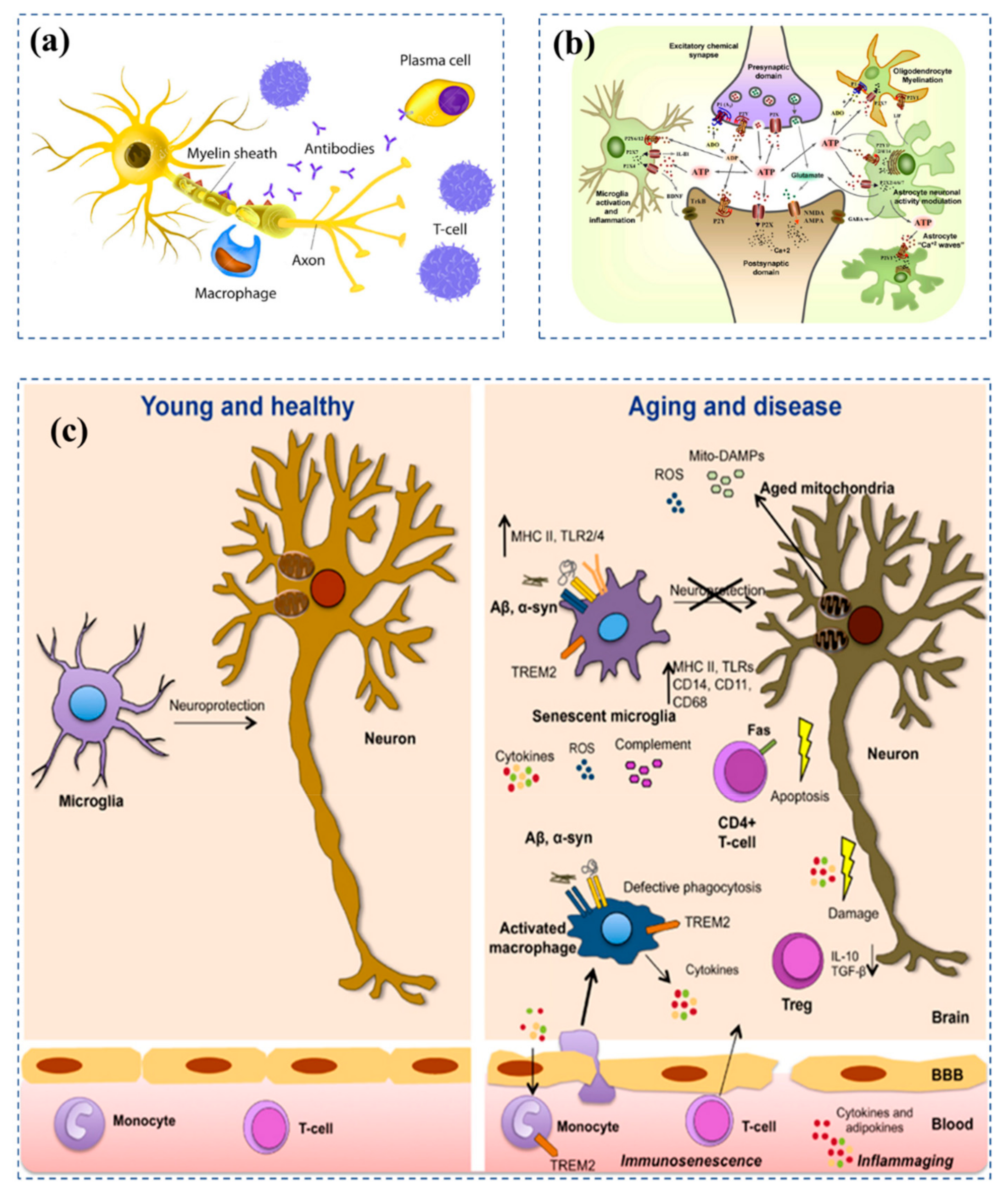

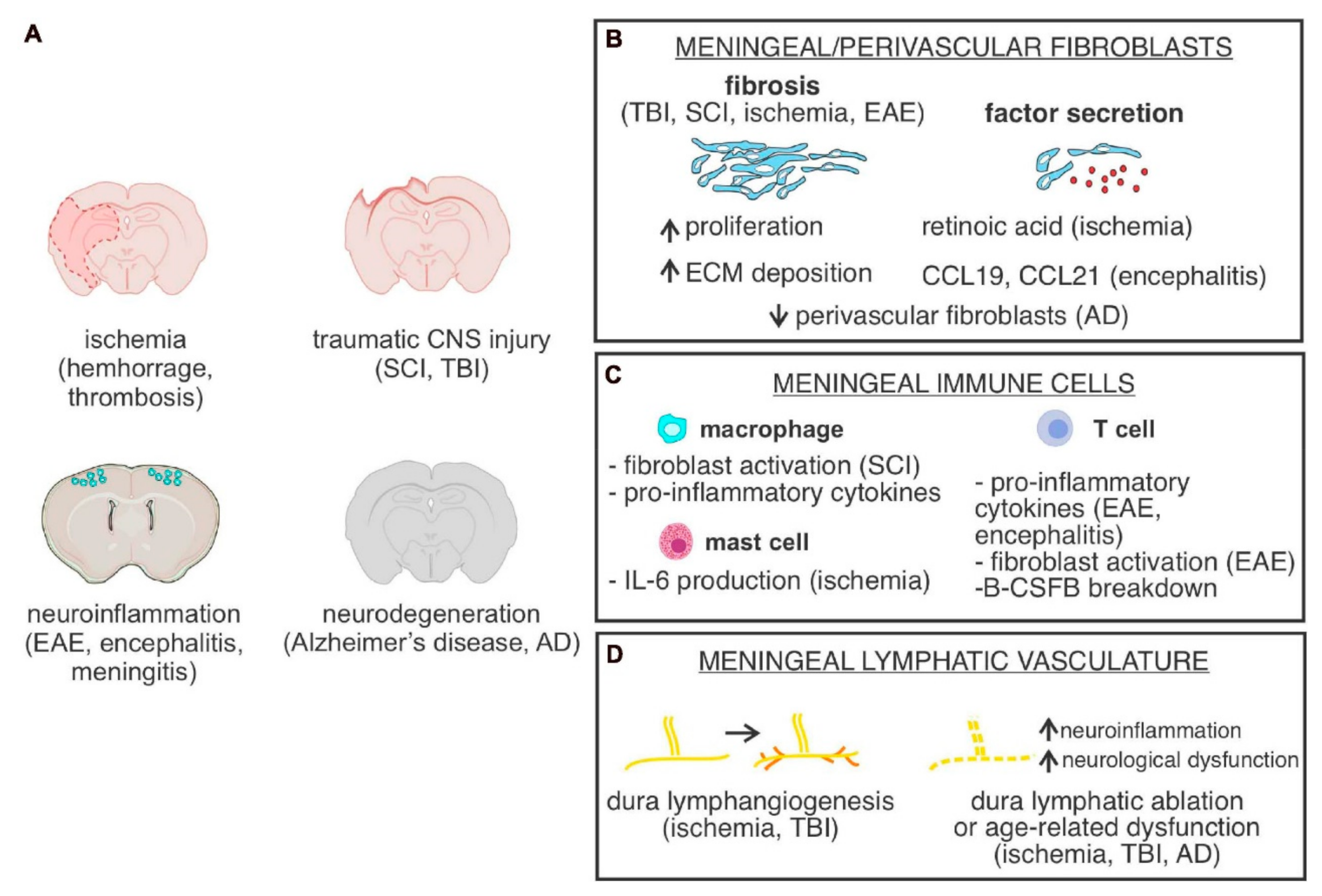
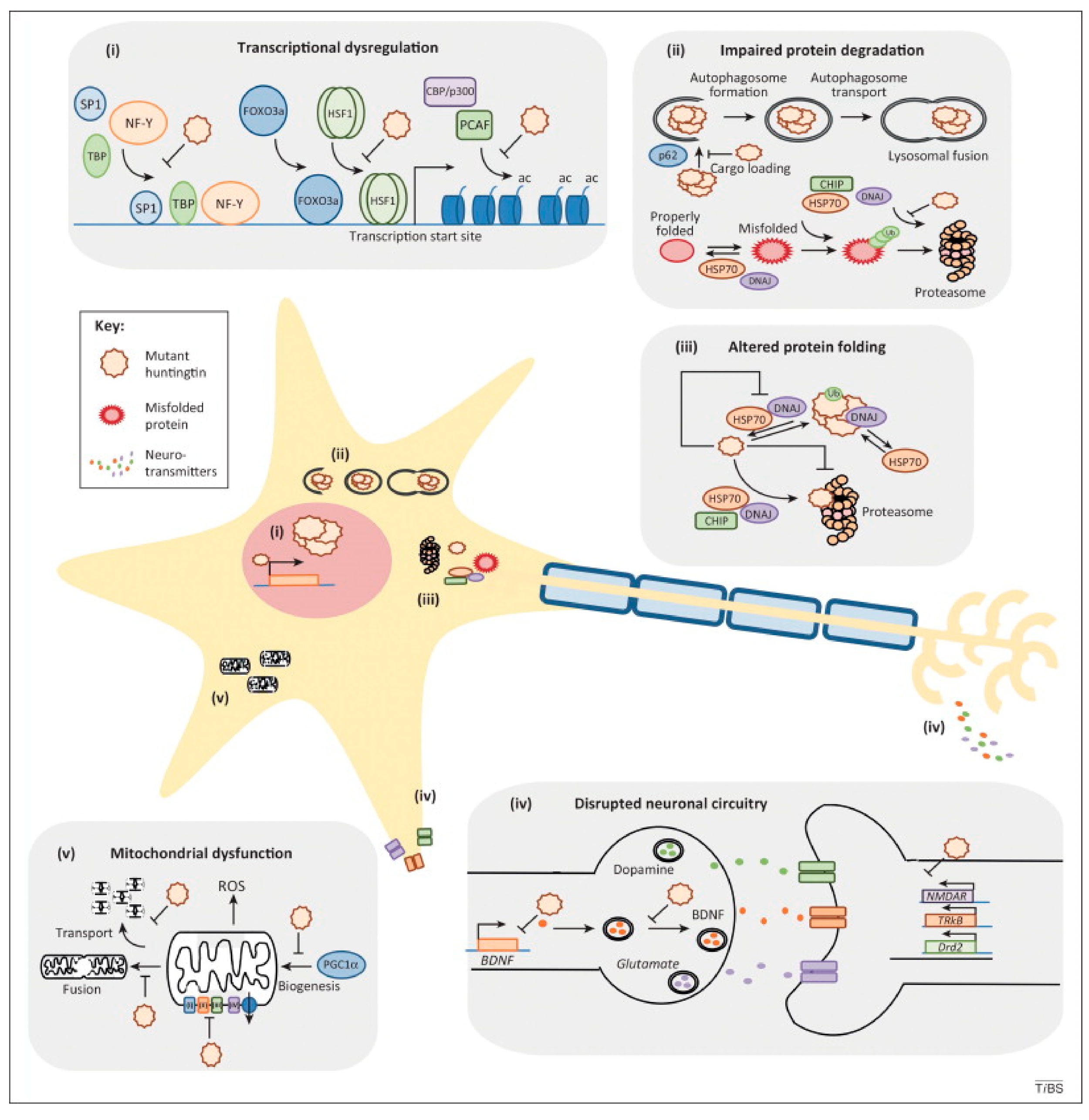
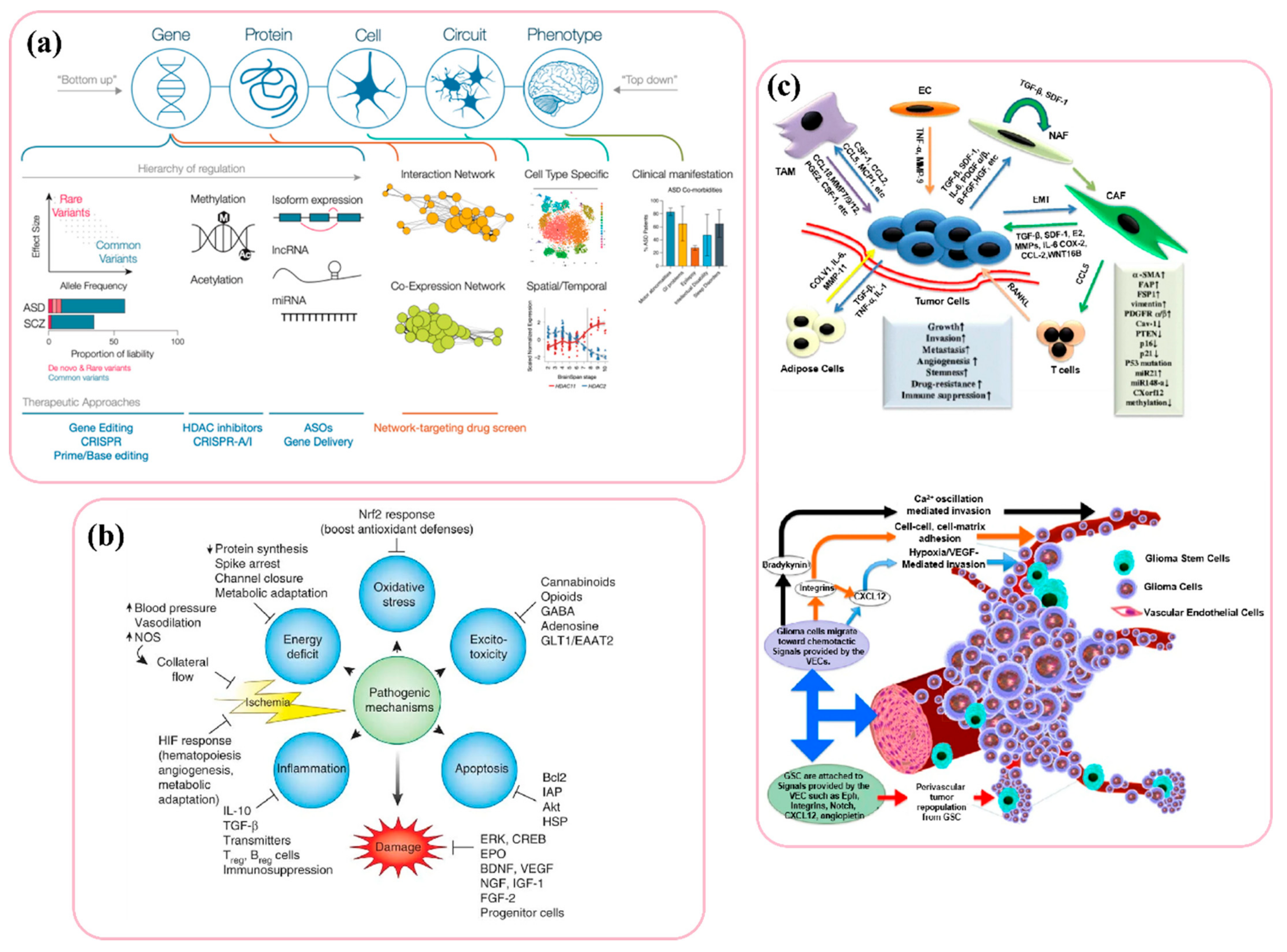
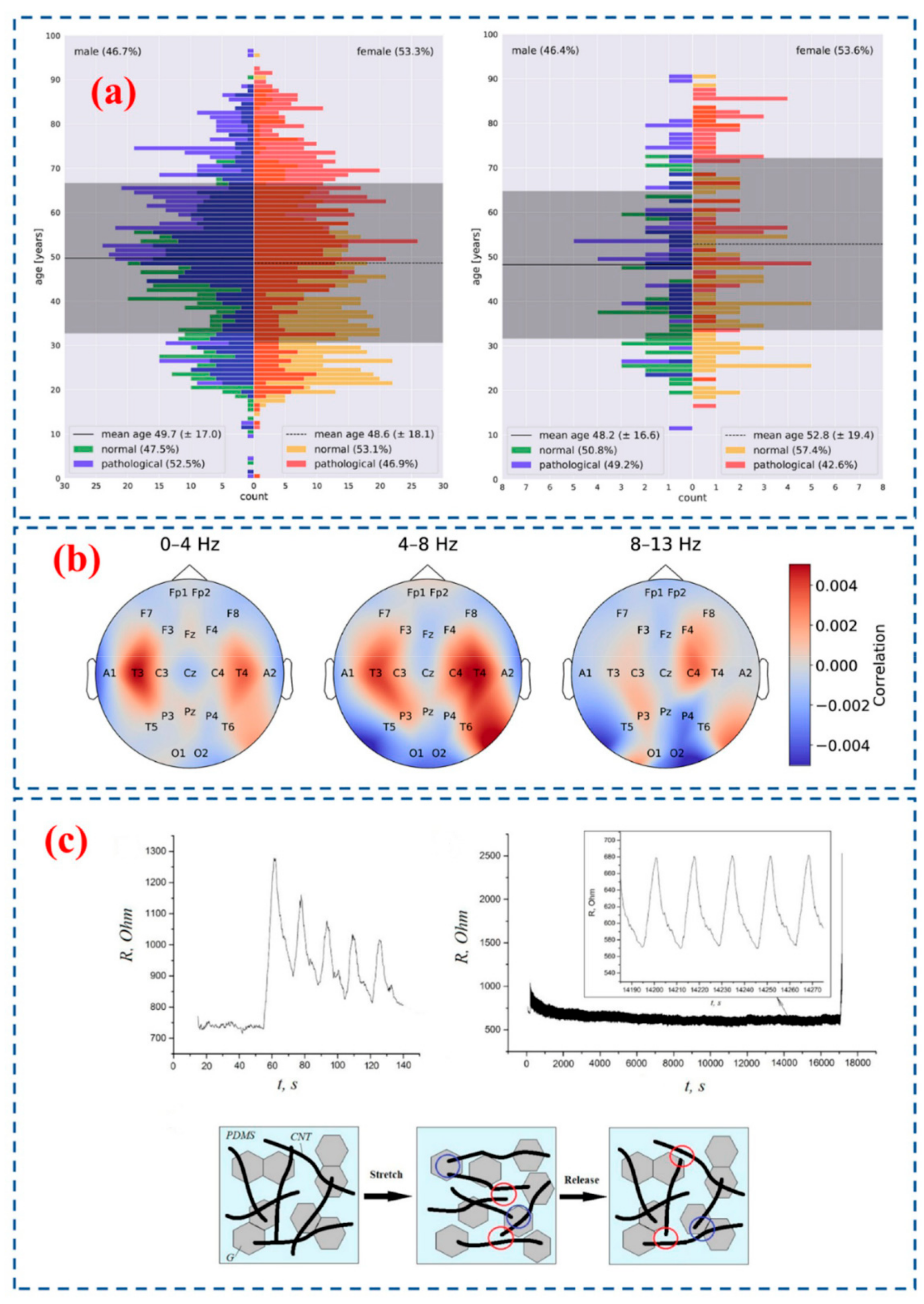
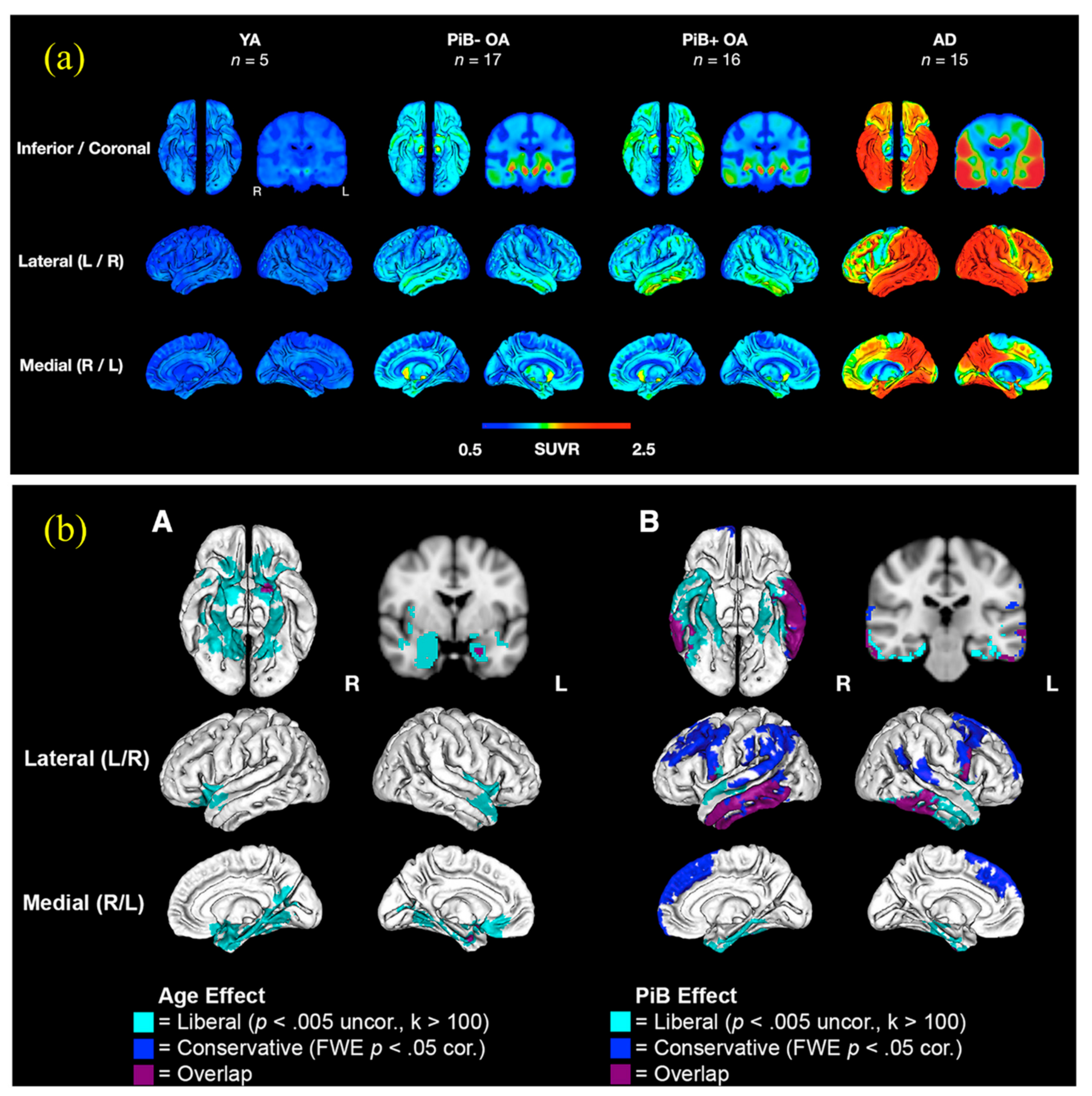
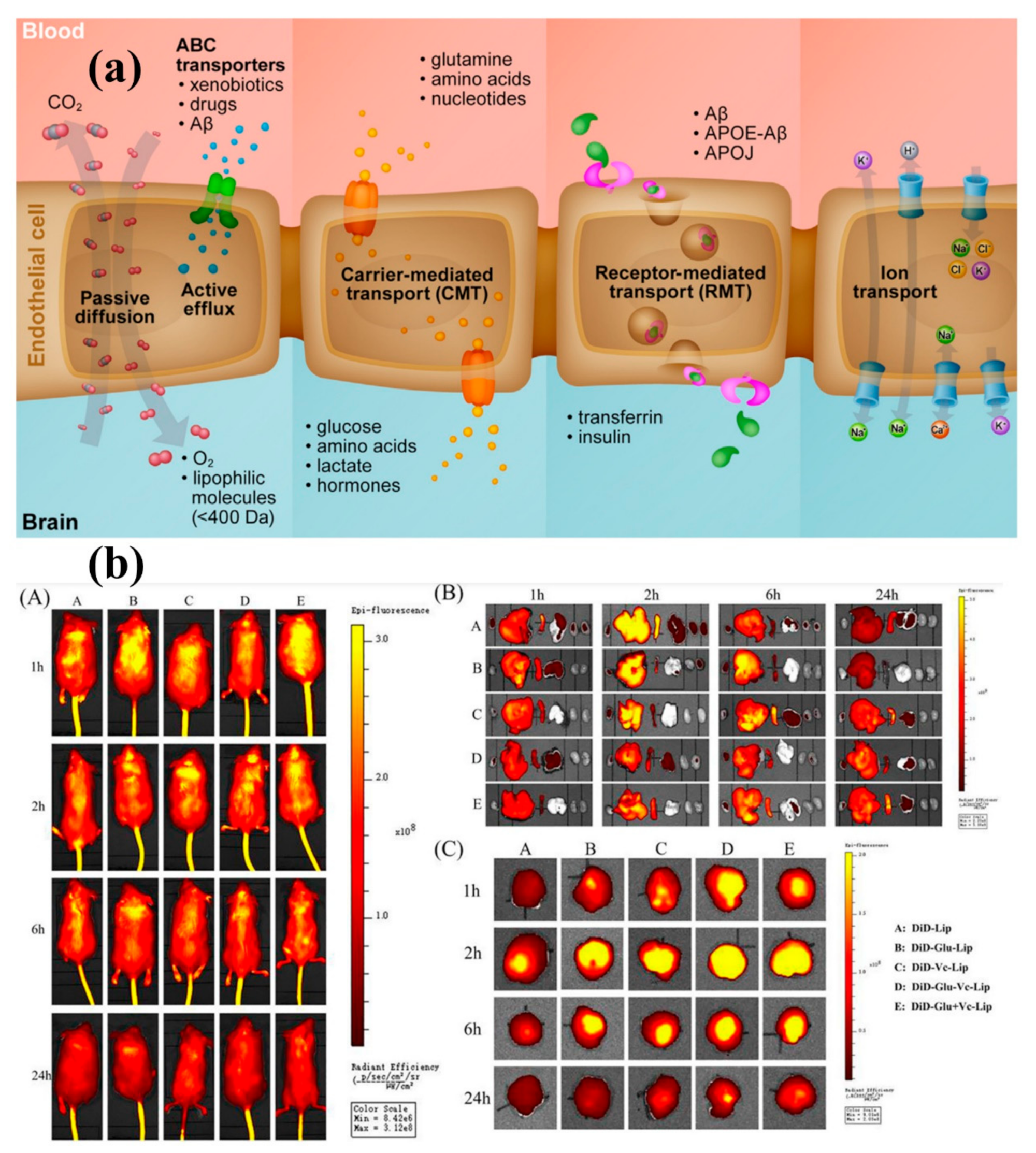
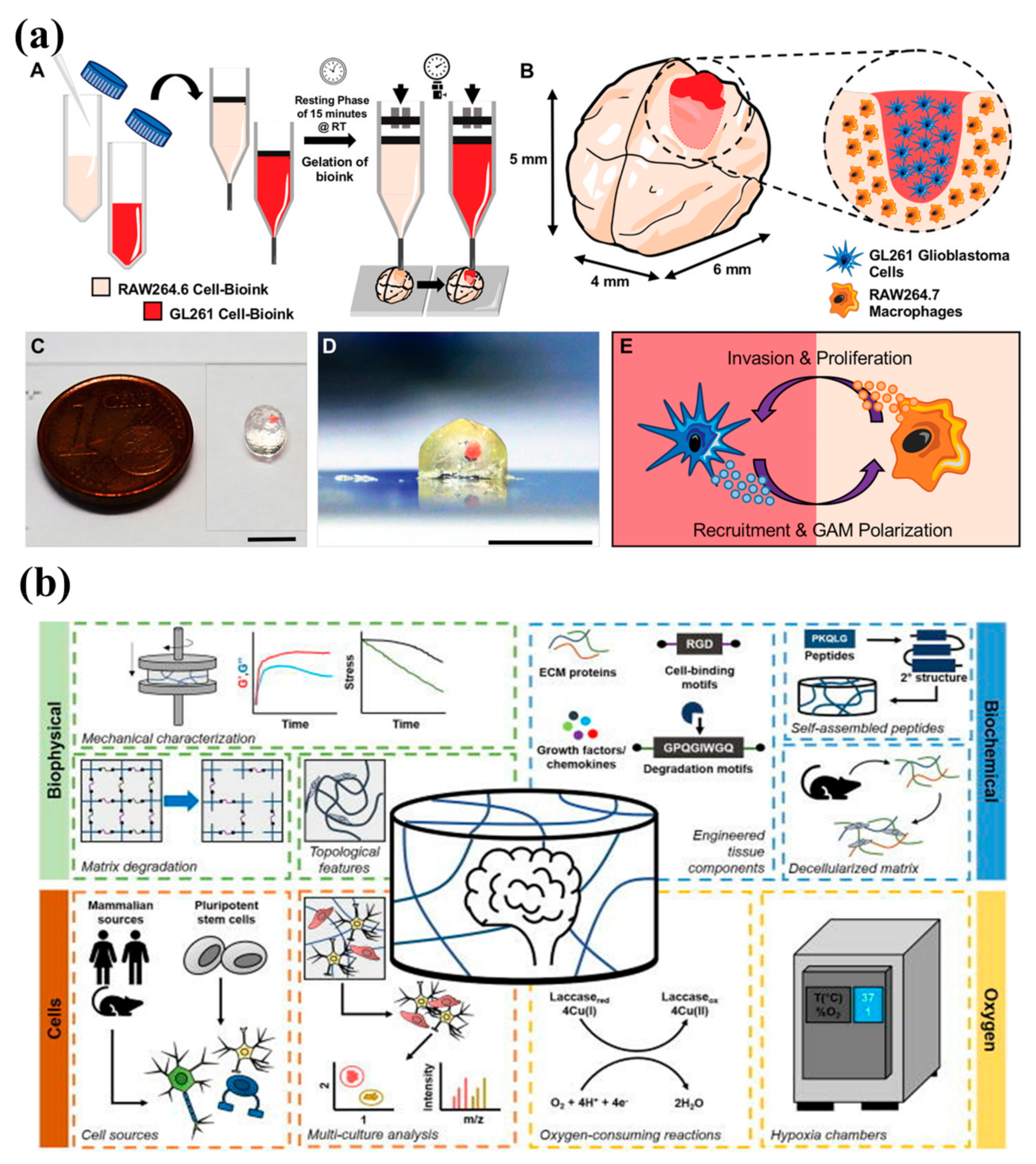
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramasubramanian, B.; Reddy, V.S.; Chellappan, V.; Ramakrishna, S. Emerging Materials, Wearables, and Diagnostic Advancements in Therapeutic Treatment of Brain Diseases. Biosensors 2022, 12, 1176. https://doi.org/10.3390/bios12121176
Ramasubramanian B, Reddy VS, Chellappan V, Ramakrishna S. Emerging Materials, Wearables, and Diagnostic Advancements in Therapeutic Treatment of Brain Diseases. Biosensors. 2022; 12(12):1176. https://doi.org/10.3390/bios12121176
Chicago/Turabian StyleRamasubramanian, Brindha, Vundrala Sumedha Reddy, Vijila Chellappan, and Seeram Ramakrishna. 2022. "Emerging Materials, Wearables, and Diagnostic Advancements in Therapeutic Treatment of Brain Diseases" Biosensors 12, no. 12: 1176. https://doi.org/10.3390/bios12121176
APA StyleRamasubramanian, B., Reddy, V. S., Chellappan, V., & Ramakrishna, S. (2022). Emerging Materials, Wearables, and Diagnostic Advancements in Therapeutic Treatment of Brain Diseases. Biosensors, 12(12), 1176. https://doi.org/10.3390/bios12121176







