A Systematic Review of Virtual Reality and Robot Therapy as Recent Rehabilitation Technologies Using EEG-Brain–Computer Interface Based on Movement-Related Cortical Potentials
Abstract
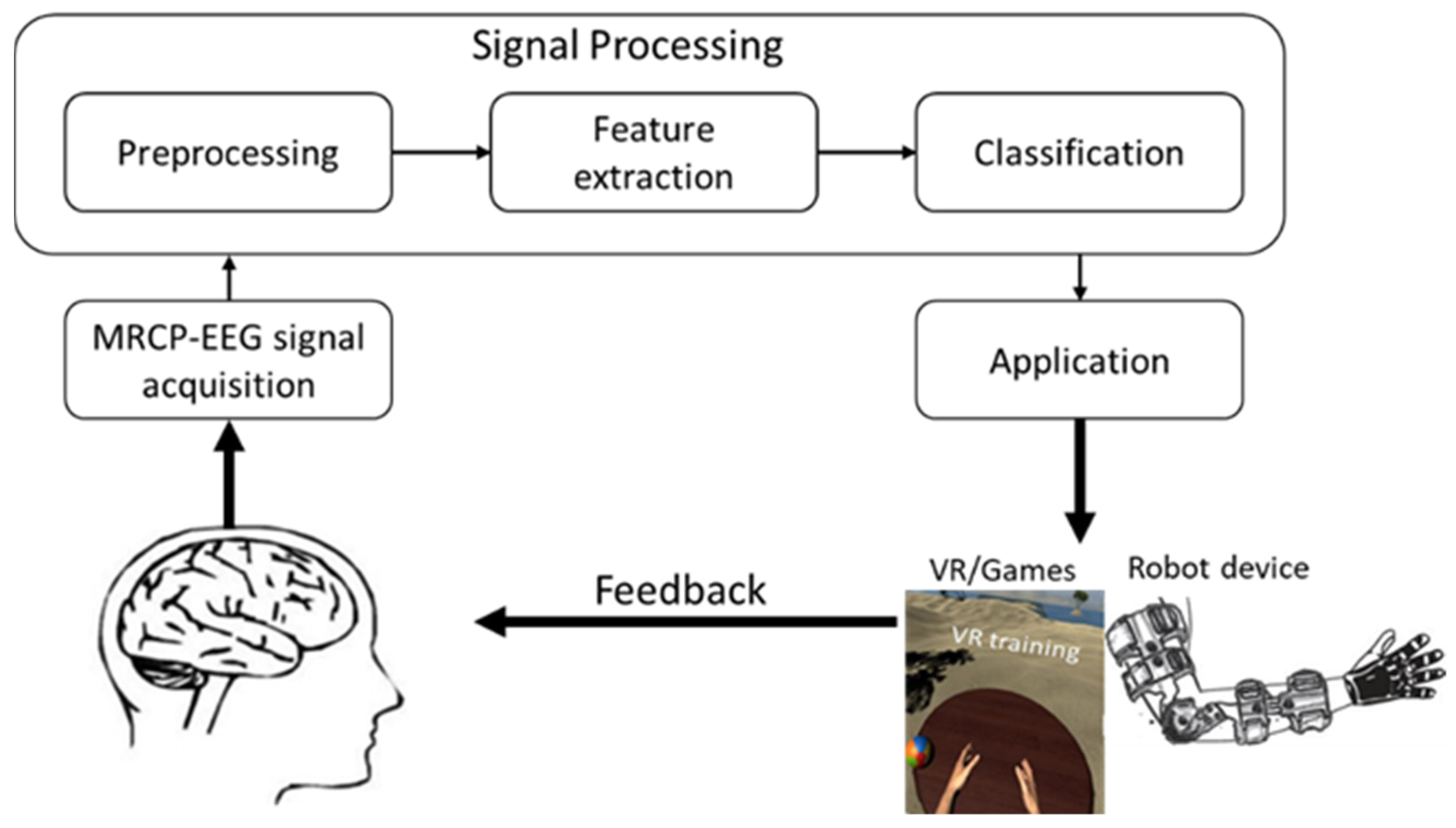
1. Introduction

- A comprehensive survey of RT- and VR-MRCP-based BCI neurorehabilitation approaches using a systematic literature review and bibliographic overlay visualization;
- Identification of the MRCP signal processing approaches, including classifiers and performance measures;
- A review of the MRCP signal preprocessing methods, including epochs, selected electrodes, and applied bandpass filters in RT and VR-MRCP-based BCI neurorehabilitation approaches;
- Provision of the measure for the methodological quality of studies based on the Physiotherapy Evidence Database (PEDro) scale;
- Determining the potential challenges and suggesting solutions for RT- and VR-MRCP-based BCI rehabilitation techniques.
2. Methods
2.1. Search Methods
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment Method
3. Synthesis and Analysis
3.1. Key Items Coincidence Analysis
3.2. Identified Article Results
3.3. General Information of the Subjects
3.4. Applied Rehabilitation Methods
3.5. Signal Acquisition and Processing
3.6. Performance of the Applied Method
4. Discussion
Limitation
5. Future Direction
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Raggi, A.; Monasta, L.; Beghi, E.; Caso, V.; Castelpietra, G.; Mondello, S.; Giussani, G.; Logroscino, G.; Magnani, F.G.; Piccininni, M.; et al. Incidence, Prevalence and Disability Associated with Neurological Disorders in Italy between 1990 and 2019: An Analysis Based on the Global Burden of Disease Study 2019. J. Neurol. 2022, 269, 2080–2098. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Neurology Collaborators. Global, Regional, and National Burden of Neurological Disorders, 1990-2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet. Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Hatem, S.M.; Saussez, G.; della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Chen, X.; Yuan, H.; Liu, W. A Stacking Model Using URL and HTML Features for Phishing Webpage Detection. Futur. Gener. Comput. Syst. 2019, 94, 27–39. [Google Scholar] [CrossRef]
- Nicolelis, M.A.L. Actions from Thoughts. Nature 2001, 409, 403–407. [Google Scholar] [CrossRef]
- Tripathi, P.; Ansari, M.A.; Akhtar, F.; Bin Heyat, M.B.; Mehrotra, R.; Yatoo, A.H.; Teelhawod, B.N.; Asfaw, A.B.; Baig, A.A. Automatic Epileptic Seizure Detection Based on the Discrete Wavelet Transform Approach Using an Artificial Neural Network Classifier on the Scalp Electroencephalogram Signal. Comput. Intell. Healthc. Appl. 2022, 157–173. [Google Scholar] [CrossRef]
- AlShorman, O.; Masadeh, M.; Bin Heyat, B.; Akhtar, F.; Almahasneh, H.; Ashraf, G.M.; Alexiou, A. Frontal Lobe Real-Time EEG Analysis Using Machine Learning Techniques for Mental Stress Detection. J. Integr. Neurosci. 2022, 21, 020. [Google Scholar] [CrossRef]
- Bin Heyat, B.; Lai, D.; Khan, F.I.; Zhang, Y. Sleep Bruxism Detection Using Decision Tree Method by the Combination of C4-P4 and C4-A1 Channels of Scalp EEG. IEEE Access 2019, 7, 102542–102553. [Google Scholar] [CrossRef]
- Bin Heyat, B.; Akhtar, F.; Khan, A.; Noor, A.; Benjdira, B.; Qamar, Y.; Abbas, S.J.; Lai, D. A Novel Hybrid Machine Learning Classification for the Detection of Bruxism Patients Using Physiological Signals. Appl. Sci. 2020, 10, 7410. [Google Scholar] [CrossRef]
- Abiri, R.; Borhani, S.; Sellers, E.W.; Jiang, Y.; Zhao, X. A Comprehensive Review of EEG-Based Brain-Computer Interface Paradigms. Artic. J. Neural Eng. 2018, 16, 011001. [Google Scholar] [CrossRef] [PubMed]
- Bin Heyat, M.B.; Akhtar, F.; Abbas, S.J.; Al-Sarem, M.; Alqarafi, A.; Stalin, A.; Abbasi, R.; Muaad, A.Y.; Lai, D.; Wu, K. Wearable Flexible Electronics Based Cardiac Electrode for Researcher Mental Stress Detection System Using Machine Learning Models on Single Lead Electrocardiogram Signal. Biosensors 2022, 12, 427. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Alonso, L.F.; Gomez-Gil, J. Brain Computer Interfaces, a Review. Sensors 2012, 12, 1211–1279. [Google Scholar] [CrossRef]
- Chen, W.; Chen, S.-K.; Liu, Y.-H.; Chen, Y.-J.; Chen, C.-S. An Electric Wheelchair Manipulating System Using SSVEP-Based BCI System. Biosensors 2022, 12, 772. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.; Naghdy, G.; Naghdy, F.; Murray, G.; Du, H. Assessment of Neuroplasticity Using EEG Signal in Rehabilitation of Brain Stem Stroke Patients. In Proceedings of the 2020 IEEE Canadian Conference on Electrical and Computer Engineering, London, ON, Canada, 30 August–2 September 2020; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 30 August 2020; Volume 2020, pp. 1–4. [Google Scholar]
- Shibasaki, H.; Hallett, M. What Is the Bereitschaftspotential? Clin. Neurophysiol. 2006, 117, 2341–2356. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, M.; Hallett, M. The Bereitschaftspotential: What Does It Measure and Where Does It Come From? In The Bereitschaftspotential; Springer: Boston, MA, USA, 2003; pp. 1–17. [Google Scholar] [CrossRef]
- Wu, C.-T.; Dillon, D.G.; Hsu, H.C.; Huang, S.; Barrick, E.; Liu, Y.H. Depression Detection Using Relative EEG Power Induced by Emotionally Positive Images and a Conformal Kernel Support Vector Machine. Appl. Sci. 2018, 8, 1244. [Google Scholar] [CrossRef]
- Jochumsen, M.; Rovsing, C.; Rovsing, H.; Cremoux, S.; Signal, N.; Allen, K.; Taylor, D.; Niazi, I.K. Quantification of Movement-Related EEG Correlates Associated with Motor Training: A Study on Movement-Related Cortical Potentials and Sensorimotor Rhythms. Front. Hum. Neurosci. 2017, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Carducci, F.; Cincotti, F.; Rossini, P.M.; Neuper, C.; Pfurtscheller, G.; Babiloni, F. Human Movement-Related Potentials vs Desynchronization of EEG Alpha Rhythm: A High-Resolution EEG Study. Neuroimage 1999, 10, 658–665. [Google Scholar] [CrossRef]
- Lazcano-Herrera, A.G.; Fuentes-Aguilar, R.Q.; Chairez, I.; Alonso-Valerdi, L.M.; Gonzalez-Mendoza, M.; Alfaro-Ponce, M. Review on BCI Virtual Rehabilitation and Remote Technology Based on EEG for Assistive Devices. Appl. Sci. 2022, 12, 12253. [Google Scholar] [CrossRef]
- Deng, J.; Yao, J.; Dewald, J.P.A. Classification of the Intention to Generate a Shoulder versus Elbow Torque by Means of a Time-Frequency Synthesized Spatial Patterns BCI Algorithm. J. Neural Eng. 2005, 2, 131–138. [Google Scholar] [CrossRef]
- Liu, D.; Chen, W.; Pei, Z.; Wang, J. A Brain-Controlled Lower-Limb Exoskeleton for Human Gait Training. Rev. Sci. Instrum. 2017, 88, 104302. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.J.; Holmes, P.S.; Smith, D. Using the Movement-Related Cortical Portential to Study Motor Skill Learning. J. Mot. Behav. 2011, 43, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, G.; Xie, J.; Chen, C. A Review: Motor Rehabilitation after Stroke with Control Based on Human Intent. Proc. Inst. Mech. Eng. H. 2018, 232, 344–360. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Zhang, B.; Liu, Q.; Hsu, W.C.; Hsiao, Y.T.; Su, J.Y.; Kobayashi, Y.; Fujie, M.G. A Robotic Gait Training System Integrating Split-Belt Treadmill, Footprint Sensing and Synchronous EEG Recording for Neuro-Motor Recovery. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS 2015, 2015, 3573–3577. [Google Scholar] [CrossRef]
- Takahashi, K.; Domen, K.; Sakamoto, T.; Toshima, M.; Otaka, Y.; Seto, M.; Irie, K.; Haga, B.; Takebayashi, T.; Hachisuka, K. Efficacy of Upper Extremity Robotic Therapy in Subacute Poststroke Hemiplegia: An Exploratory Randomized Trial. Stroke 2016, 47, 1385–1388. [Google Scholar] [CrossRef]
- Ali, L.; Bukhari, S.A.C. An Approach Based on Mutually Informed Neural Networks to Optimize the Generalization Capabilities of Decision Support Systems Developed for Heart Failure Prediction. IRBM 2021, 42, 345–352. [Google Scholar] [CrossRef]
- Said, R.R.; Yong, W.Q.; Bin Heyat, M.B.; Ali, L.; Qiang, S.; Ali, A.; Rauf, H.T.; Wu, Z. Design of a Smart Elbow Brace as a Home-Based Rehabilitation Device. Comput. Intell. Neurosci. 2022, 2022, 3754931. [Google Scholar] [CrossRef]
- Fregni, F.; Pascual-Leone, A. Hand Motor Recovery after Stroke: Tuning the Orchestra to Improve Hand Motor Function. Cogn. Behav. Neurol. 2006, 19, 21–33. [Google Scholar] [CrossRef]
- Hallett, M. Plasticity of the Human Motor Cortex and Recovery from Stroke. Brain Res. Brain Res. Rev. 2001, 36, 169–174. [Google Scholar] [CrossRef]
- Zeiler, S.R.; Krakauer, J.W. The Interaction between Training and Plasticity in the Poststroke Brain. Curr. Opin. Neurol. 2013, 26, 609–616. [Google Scholar] [CrossRef]
- Mawase, F.; Uehara, S.; Bastian, A.J.; Celnik, P. Motor Learning Enhances Use-Dependent Plasticity. J. Neurosci. 2017, 37, 2673–2685. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Kim, S.; Lee, B. Effects of Action Observational Training Plus Brain-Computer Interface-Based Functional Electrical Stimulation on Paretic Arm Motor Recovery in Patient with Stroke: A Randomized Controlled Trial. Occup. Ther. Int. 2016, 23, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Neuper, C.; Scherer, R.; Wriessnegger, S.; Pfurtscheller, G. Motor Imagery and Action Observation: Modulation of Sensorimotor Brain Rhythms during Mental Control of a Brain-Computer Interface. Clin. Neurophysiol. 2009, 120, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Modroño, C.; Bermúdez, S.; Cameirão, M.; Pereira, F.; Paulino, T.; Marcano, F.; Hernández-Martín, E.; Plata-Bello, J.; Palenzuela, N.; Núñez-Pádron, D.; et al. Is It Necessary to Show Virtual Limbs in Action Observation Neurorehabilitation Systems? J. Rehabil. Assist. Technol. Eng. 2019, 6, 205566831985914. [Google Scholar] [CrossRef]
- Leeb, R.; Friedman, D.; Müller-Putz, G.R.; Scherer, R.; Slater, M.; Pfurtscheller, G. Self-Paced (Asynchronous) BCI Control of a Wheelchair in Virtual Environments: A Case Study with a Tetraplegic. Comput. Intell. Neurosci. 2007, 2007. [Google Scholar] [CrossRef]
- Alkadhi, H.; Brugger, P.; Boendermaker, S.H.; Crelier, G.; Curt, A.; Hepp-Reymond, M.C.; Kollias, S.S. What Disconnection Tells about Motor Imagery: Evidence from Paraplegic Patients. Cereb. Cortex 2005, 15, 131–140. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Leeb, R.; Friedman, D.; Slater, M. Centrally Controlled Heart Rate Changes during Mental Practice in Immersive Virtual Environment: A Case Study with a Tetraplegic. Int. J. Psychophysiol. 2008, 68, 1–5. [Google Scholar] [CrossRef]
- Rehman, A.; Khan, A.; Ali, M.A.; Khan, M.U.; Khan, S.U.; Ali, L. Performance Analysis of PCA, Sparse PCA, Kernel PCA and Incremental PCA Algorithms for Heart Failure Prediction. In Proceedings of the 2020 International Conference on Electrical, Communication, and Computer Engineering (ICECCE), Istanbul, Turkey, 12–13 June 2020. [Google Scholar] [CrossRef]
- Khan, M.A.; Das, R.; Iversen, H.K.; Puthusserypady, S. Review on Motor Imagery Based BCI Systems for Upper Limb Post-Stroke Neurorehabilitation: From Designing to Application. Comput. Biol. Med. 2020, 123, 103843. [Google Scholar] [CrossRef]
- Kumar, A.; Gao, L.; Pirogova, E.; Fang, Q. A Review of Error-Related Potential-Based Brain-Computer Interfaces for Motor Impaired People. IEEE Access 2019, 7, 142451–142466. [Google Scholar] [CrossRef]
- Al-Qaysi, Z.T.; Ahmed, M.A.; Hammash, N.M.; Hussein, A.F.; Albahri, A.S.; Suzani, M.S.; Al-Bander, B.; Shuwandy, M.L.; Salih, M.M. Systematic Review of Training Environments with Motor Imagery Brain–Computer Interface: Coherent Taxonomy, Open Issues and Recommendation Pathway Solution. Health Technol. 2021, 11, 783–801. [Google Scholar] [CrossRef]
- López-Larraz, E.; Sarasola-Sanz, A.; Irastorza-Landa, N.; Birbaumer, N.; Ramos-Murguialday, A. Brain-Machine Interfaces for Rehabilitation in Stroke: A Review. NeuroRehabilitation 2018, 43, 77–97. [Google Scholar] [CrossRef]
- Rodrigues, J.d.C.; Rebouças Filho, P.P.; Damaševičius, R.; de Albuquerque, V.H.C. EEG-Based Biometric Systems. Neurotechnology 2020, 97–153. [Google Scholar] [CrossRef]
- Basteris, A.; Nijenhuis, S.M.; Stienen, A.H.A.; Buurke, J.H.; Prange, G.B.; Amirabdollahian, F. Training Modalities in Robot-Mediated Upper Limb Rehabilitation in Stroke: A Framework for Classification Based on a Systematic Review. J. Neuroeng. Rehabil. 2014, 11. [Google Scholar] [CrossRef]
- Li, S.; Zhu, Y.; Huang, G.; Zhang, L.; Zhang, Z.; Huang, K. Detection of Movement-Related Cortical Potentials Associated with Emergency and Non-Emergency Tasks. In Proceedings of the IEEE 23rd International Conference on Digital Signal Processing (DSP), Shanghai, China, 19–21 November 2018; pp. 1–5. [Google Scholar]
- PEDro Scale—PEDro. Available online: https://pedro.org.au/english/resources/pedro-scale/ (accessed on 17 May 2021).
- Zhang, Y.; Prasad, S.; Kilicarslan, A.; Contreras-Vidal, J.L. Multiple Kernel Based Region Importance Learning for Neural Classification of Gait States from EEG Signals. Front. Neurosci. 2017, 11. [Google Scholar] [CrossRef]
- Luu, T.P.; He, Y.; Brown, S.; Nakagome, S.; Contreras-Vidal, J.L. A Closed-Loop Brain Computer Interface to a Virtual Reality Avatar: Gait Adaptation to Visual Kinematic Perturbations. In Proceedings of the 2015 International Conference on Virtual Rehabilitation (ICVR), Valencia, Spain, 9–12 June 2015; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 16 December 2015; pp. 30–37. [Google Scholar]
- Donati, A.R.C.; Shokur, S.; Morya, E.; Campos, D.S.F.; Moioli, R.C.; Gitti, C.M.; Augusto, P.B.; Tripodi, S.; Pires, C.G.; Pereira, G.A.; et al. Long-Term Training with a Brain-Machine Interface-Based Gait Protocol Induces Partial Neurological Recovery in Paraplegic Patients. Sci. Rep. 2016, 6, 30383. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.; Naghdy, G.; Naghdy, F.; Murray, G.; Du, H. Investigating the Detection of Intention Signal during Different Exercise Protocols in Robot-Assisted Hand Movement of Stroke Patients and Healthy Subjects Using EEG-BCI System. Adv. Sci. Technol. Eng. Syst. 2019, 4, 300–307. [Google Scholar] [CrossRef]
- Mrachacz-Kersting, N.; Jiang, N.; Stevenson, A.J.; Imran, N.K.; Kostic, V.; Pavlovic, A.; Radovanovic, S.; Djuric-Jovicic, M.; Agosta, F.; Dremstrup, K.; et al. Efficient Neuroplasticity Induction in Chronic Stroke Patients by an Associative Brain-Computer Interface. J Neuro-Physiol 2016, 115, 1410–1421. [Google Scholar] [CrossRef]
- Kilicarslan, A.; Prasad, S.; Grossman, R.G.; Contreras-Vidal, J.L. High Accuracy Decoding of User Intentions Using EEG to Control a Lower-Body Exoskeleton. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Osaka, Japan, 3–7 July 2013; pp. 5606–5609. [Google Scholar]
- Xu, R.; Jiang, N.; Dosen, S.; Lin, C.; Mrachacz-Kersting, N.; Dremstrup, K.; Farina, D. Endogenous Sensory Discrimination and Selection by a Fast Brain Switch for a High Transfer Rate Brain-Computer Interface. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 901–910. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kwak, N.S.; Lee, M.H.; Lee, S.-W. Decoding of Walking Intention under Lower Limb Exoskeleton Environment Using MRCP Feature. In Proceedings of the 7th Graz Brain-Computer Interface Conference 2017GBCIC, Graz, Austria, 18–22 September 2017. [Google Scholar]
- Jeong, J.H.; Kwak, N.S.; Guan, C.; Lee, S.W. Decoding Movement-Related Cortical Potentials Based on Subject-Dependent and Section-Wise Spectral Filtering. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 687–698. [Google Scholar] [CrossRef]
- Jing, Z.; Weihai, C.; Zhang, J.; Fangang, M. Detection of Self-Paced Reaching Movementintention from EEG Signals. In Proceedings of the 13th IEEE Conference on Industrial Electronics and Applications (ICIEA), Wuhan, China, 31 May 2018; Volume 5, pp. 1820–1824. [Google Scholar]
- Xu, R.; Jiang, N.; Mrachacz-Kersting, N.; Lin, C.; Asin Prieto, G.; Moreno, J.C.; Pons, J.L.; Dremstrup, K.; Farina, D. A Closed-Loop Brain-Computer Interface Triggering an Active Ankle-Foot Orthosis for Inducing Cortical Neural Plasticity. IEEE Trans. Biomed. Eng. 2014, 61, 2092–2101. [Google Scholar] [CrossRef]
- Yao, L.; Chen, M.L.; Sheng, X.; Mrachacz-Kersting, N.; Zhu, X.; Farina, D.; Jiang, N. Influence of Spontaneous Rhythm on Movement-Related Cortical Potential -A Preliminary Neurofeedback Study. In Proceedings of the Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer: Berlin/Heidelberg, Germany, 2017; Volume 10284, pp. 90–98. [Google Scholar]
- He, Y.; Nathan, K.; Venkatakrishnan Anusha Rovekamp, R.; Beck, C.; Ozdemir, R.A.; Francisco, G.E.; Contreras-Vidal, J.L. An Integrated Neuro-Robotic Interface for Stroke Rehabilitation Using the NASA X1 Powered Lower Limb Exoskeleton. In Proceedings of the 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 3985–3988. [Google Scholar]
- Maryam, B.; Golshah, N.; Fazel, N.; Geoffrey, M.; Du, H. Patient-Specific Robot-Assisted Stroke Rehabilitation Guided by EEG—A Feasibility Study. In Proceedings of the 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 2841–2844. [Google Scholar]
- Xu, R.; Dosen, S.; Jiang, N.; Yao, L.; Farooq, A.; Jochumsen, M.; Mrachacz-Kersting, N.; Dremstrup, K.; Farina, D. Continuous 2D Control via State-Machine Triggered by Endogenous Sensory Discrimination and a Fast Brain Switch. J. Neural Eng. 2019, 16, 056001. [Google Scholar] [CrossRef] [PubMed]
- López-Larraz, E.; Trincado-Alonso, F.; Rajasekaran, V.; Pérez-Nombela, S.; del-Ama, A.J.; Aranda, J.; Minguez, J.; Gil-Agudo, A.; Montesano, L. Control of an Ambulatory Exoskeleton with a Brain-Machine Interface for Spinal Cord Injury Gait Rehabilitation. Front. Neurosci. 2016, 10. [Google Scholar] [CrossRef]
- Perianes-Rodriguez, A.; Waltman, L.; van Eck, N.J. Constructing Bibliometric Networks: A Comparison between Full and Fractional Counting. J. Informetr. 2016, 10, 1178–1195. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L.; van Eck, N.J.; Waltman, L. Visualizing Bibliometric Networks; Springer: Cham, Switzerland, 2014; pp. 285–320. [Google Scholar]
- Bin Heyat, M.B.; Akhtar, F.; Ansari, M.A.; Khan, A.; Alkahtani, F.; Khan, H.; Lai, D. Progress in Detection of Insomnia Sleep Disorder: A Comprehensive Review. Curr. Drug Targets 2020, 22, 672–684. [Google Scholar] [CrossRef]
- Geng, H.; Li, M.; Tang, J.; Lv, Q.; Li, R.; Wang, L. Early Rehabilitation Exercise after Stroke Improves Neurological Recovery through Enhancing Angiogenesis in Patients and Cerebral Ischemia Rat Model. Int. J. Mol. Sci. 2022, 23, 10508. [Google Scholar] [CrossRef]
- Cortés-Pérez, I.; Nieto-Escamez, F.A.; Obrero-Gaitán, E. Immersive Virtual Reality in Stroke Patients as a New Approach for Reducing Postural Disabilities and Falls Risk: A Case Series. Brain Sci. 2020, 10, 296. [Google Scholar] [CrossRef]
- Arcuri, F.; Porcaro, C.; Ciancarelli, I.; Tonin, P.; Cerasa, A. Electrophysiological Correlates of Virtual-Reality Applications in the Rehabilitation Setting: New Perspectives for Stroke Patients. Electron 2021, 10, 836. [Google Scholar] [CrossRef]
- López-Larraz, E.; Montesano, L.; Gil-Agudo, Á.; Minguez, J. Continuous Decoding of Movement Intention of Upper Limb Self-Initiated Analytic Movements from Pre-Movement EEG Correlates. J. Neuroeng. Rehabil. 2014, 11, 153. [Google Scholar] [CrossRef]
- Rodríguez-Ugarte, M.; Iáñez, E.; Ortíz, M.; Azorín, J.M. Personalized Offline and Pseudo-Online BCI Models to Detect Pedaling Intent. Front. Neuroinform. 2017, 11, 45. [Google Scholar] [CrossRef]
- Ullah, H.; Bin Heyat, M.B.; Alsalman, H.; Khan, H.M.; Akhtar, F.; Gumaei, A.; Mehdi, A.; Muaad, A.Y.; Islam, M.S.; Ali, A.; et al. An Effective and Lightweight Deep Electrocardiography Arrhythmia Recognition Model Using Novel Special and Native Structural Regularization Techniques on Cardiac Signal. J. Healthc. Eng. 2022, 2022, 3408501. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Abbasi, R.; Bin Heyat, M.B.; Akhtar, F.; Abdelgeliel, A.S.; Albogami, S.; Fayad, E.; Iqbal, M.A. Recognition of MRNA N4 Acetylcytidine (Ac4C) by Using Non-Deep vs. Deep Learning. Appl. Sci. 2022, 12, 1344. [Google Scholar] [CrossRef]
- Han, Y.; Liu, C.; Zhang, B.; Zhang, N.; Wang, S.; Han, M.; Ferreira, J.P.; Liu, T.; Zhang, X. Measurement, Evaluation, and Control of Active Intelligent Gait Training Systems—Analysis of the Current State of the Art. Electronics 2022, 11, 1633. [Google Scholar] [CrossRef]
- Xu, R.; Jiang, N.; Lin, C.; Mrachacz-Kersting, N.; Dremstrup, K.; Farina, D. Enhanced Low-Latency Detection of Motor Intention from EEG for Closed-Loop Brain-Computer Interface Applications. IEEE Trans. Biomed. Eng. 2014, 61, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Gizzi, L.; Mrachacz-Kersting, N.; Dremstrup, K.; Farina, D. A Brain-Computer Interface for Single-Trial Detection of Gait Initiation from Movement Related Cortical Potentials. Clin. Neurophysiol. 2015, 126, 154–159. [Google Scholar] [CrossRef]
- Karimi, F.; Kofman, J.; Mrachacz-Kersting, N.; Farina, D.; Jiang, N. Detection of Movement Related Cortical Potentials from EEG Using Constrained ICA for Brain-Computer Interface Applications. Front. Neurosci. 2017, 11, 356. [Google Scholar] [CrossRef]
- Ahmad, F.S.; Ali, L.; Raza-Ul-Mustafa; Khattak, H.A.; Hameed, T.; Wajahat, I.; Kadry, S.; Bukhari, S.A.C. A Hybrid Machine Learning Framework to Predict Mortality in Paralytic Ileus Patients Using Electronic Health Records (EHRs). J. Ambient Intell. Humaniz. Comput. 2021, 12, 3283–3293. [Google Scholar] [CrossRef]
- Ali, L.; Wajahat, I.; Amiri Golilarz, N.; Keshtkar, F.; Bukhari, S.A.C. LDA–GA–SVM: Improved Hepatocellular Carcinoma Prediction through Dimensionality Reduction and Genetically Optimized Support Vector Machine. Neural Comput. Appl. 2021, 33, 2783–2792. [Google Scholar] [CrossRef]
- Akbar, W.; Wu, W.P.; Saleem, S.; Farhan, M.; Saleem, M.A.; Javeed, A.; Ali, L. Development of Hepatitis Disease Detection System by Exploiting Sparsity in Linear Support Vector Machine to Improve Strength of AdaBoost Ensemble Model. Mob. Inf. Syst. 2020, 2020, 8870240. [Google Scholar] [CrossRef]
- Lotte, F.; Bougrain, L.; Cichocki, A.; Clerc, M.; Congedo, M.; Rakotomamonjy, A.; Yger, F. A Review of Classification Algorithms for EEG-Based Brain–Computer Interfaces: A 10 Year Update. J. Neural Eng. 2018, 15, 031005. [Google Scholar] [CrossRef]
- Raudys, S.J.; Jain, A.K. Small Sample Size Effects in Statistical Pattern Recognition: Recommendations for Practitioners. IEEE Trans. Pattern Anal. Mach. Intell. 1991, 13, 252–264. [Google Scholar] [CrossRef]
- Jain, A.K.; Chandrasekaran, B. 39 Dimensionality and Sample Size Considerations in Pattern Recognition Practice. In Handbook of Statistics; Elsevier: Amsterdam, The Netherlands, 1982; Volume 2, pp. 835–855. [Google Scholar] [CrossRef]
- Jochumsen, M.; Samran Navid, M.; Wiberg Nedergaard, R.; Signal, N.; Rashid, U.; Hassan, A.; Haavik, H.; Taylor, D.; Khan Niazi, I. Self-Paced Online vs. Cue-Based Offline Brain-Computer Interfaces for Inducing Neural Plasticity. Brain Sci 2019, 9, 127. [Google Scholar] [CrossRef]
- Mrachacz-Kersting, N.; Kristensen, S.R.; Niazi, I.K.; Farina, D. Precise Temporal Association between Cortical Potentials Evoked by Motor Imagination and Afference Induces Cortical Plasticity. J. Physiol. 2012, 590, 1669–1682. [Google Scholar] [CrossRef]
- Jochumsen, M.; Farina, D.; Khan Niazi, I.; Mrachacz-Kersting, N.; Dremstrup, K. Detection and Classification of Movement-Related Cortical Potentials Associated with Task Force and Speed. Artic. J. Neural Eng. 2013, 10. [Google Scholar] [CrossRef]
- Mrachacz-Kersting, N.; Voigt, M.; Stevenson, A.; Aliakbaryhosseinabadi, S.; Jiang, N.; Dremstrup, K.; Farina, D. The Effect of Type of Afferent Feedback Timed with Motor Imagery on the Induction of Cortical Plasticity. Brain Res. 2017, 1674, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Cunnington, R.; Windischberger, C.; Deecke, L.; Moser, E. The Preparation and Readiness for Voluntary Movement: A High-Field Event-Related FMRI Study of the Bereitschafts-BOLD Response. Neuroimage 2003, 20, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Jochumsen, M.; Knoche, H.; Kjaer, T.W.; Dinesen, B.; Kidmose, P. EEG Headset Evaluation for Detection of Single-Trial Movement Intention for Brain-Computer Interfaces. Sensors 2020, 20, 2804. [Google Scholar] [CrossRef] [PubMed]
- Rodpongpun, S.; Janyalikit, T.; Ratanamahatana, C.A. Influential Factors of an Asynchronous BCI for Movement Intention Detection. Comput. Math. Methods Med. 2020, 2020, 8573754. [Google Scholar] [CrossRef]


| Ref. | [49] | [15] | [50] | [51] | [52] | [53] | [54] | [55] | [56] | [47] | [57] | [58] | [59] | [60] | [61] | [62] | [63] | [64] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| R | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 |
| C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| B1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| I | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| B4 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| P | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 |
| Total | 6 | 7 | 6 | 5 | 5 | 6 | 3 | 5 | 4 | 6 | 5 | 5 | 7 | 4 | 6 | 3 | 5 | 6 |
| Studies | Rehabilitation Methods | Rehabilitation Tool Name |
|---|---|---|
| [15,52,62] | RT-MRCP-based BCI therapy | AMADEO |
| [51] | Lokomat | |
| [54,56,57] | Rex, | |
| [58] | X1 exoskeleton robot | |
| [59] | BCI-MAFO | |
| [47,50,58,63] | VR-MRCP-based BCI therapy | Controlling a virtual walking avatar and 2D cursor |
| Ref. | Electrode | Epochs (s) | Bandpass Filter (Hz) | Classifier | Performance (%) |
|---|---|---|---|---|---|
| [47] | Fz, FC1, FC2, C3, Cz, C4, CP1, CP2, Pz | −2 to 0 | 0.04 to 3 | MF-SVM | Sensitivity was 60.57 ± 14.79 for non-emergency tasks and 44.29 ± 5.73 for emergency tasks. |
| [49] | - | −2 to 1 | 0.1 to 2 | MKL | Accuracy was above 90 for classifying gait states from EEG signals. |
| [52] | C3, FC3, CP3, Cz, T7 | −2 to 0 | 0.1 to 1 | SVM | Accuracy was 79.7 in healthy subjects and 66.64 in patients for movement execution trials. |
| [55] | Cz, Fz, FC1, FC2, C3, C4, CP1, CP2, Pz | −1 to 1 | 0.05 to 3 | LPP-LDA | Sensitivity was 80 for the movement execution task and 70 for the intended task. |
| [56] | Cz, C1, C2, CPz | −2 to 1 | 0.1 to 4 | RLDA | Accuracy was 87.6 for the walking intention task. |
| [57] | C1, C2, CPz, Cz | −2 to 1 | 0.05 to 2 | RLDA | Accuracy was 86 in the generated dataset and 73 in the public dataset for the movement execution task. |
| [58] | Cz, CPz, FCz, C2, C1, CP1, CP2, C3 | −1 to 2 | 0.1 to 1 | LDA | Sensitivity was 83 for movement intention. |
| [59] | Cz, Fz, FC1, FC2, C3, C4, CP1, CP2, Pz | −1.5 to 0.5 | 0.05 to 3 | LPP-LDA | Sensitivity was 73.0 ± 10.3 for movement intention. |
| [63] | Cz, Fz, FC1, FC2, C3, C4 | −2 to 0 | 0.1 to 5 | LPP-LDA | Accuracy was 97 for motor execution and 92 for motor imagery. |
| [64] | FCz, FC2, C1, Cz, C2, CP1, CPz, CP2 | −1 to 1 | 0.1 to 1 | SDA | Sensitivity was 84.44 in healthy subjects and 77.61 in patients for activating exoskeleton movement. |
| Ref. | Subj. | App. | Frequency | Analy. | Paced | Description |
|---|---|---|---|---|---|---|
| [15] | 1 Patient | RT | 3 blocks of 10 min every 3 days a week | Offline | Self | The use of EEG signals to improve the engagement of stroke patients using robot-assisted multisession rehabilitation training. |
| [47] | 7 Healthy | VR | 5 rounds, 5 min per round; 2–3 min of resting after two rounds | Offline | Self | MRCP detection concerning emergency and non-emergency tasks. |
| [49] | 2 Healthy 1 Patient | RT | Multiple sessions in 30 days | Offline | Self | To compare the brain areas used for identifying movement intentions in healthy subjects and individuals with spinal cord injury. |
| [50] | 2 Healthy | VR | Pre-exposure for 8 min, exposure for 15 min, and post-exposure for 8 min for 8 days | Online | Self | The use of the closed-loop BCI-VR technology to control the walking movements of a virtual avatar. |
| [51] | 8 Patient | RT | Cumulated number of hours and sessions recorded after 4, 7, 10, and 12 months | Offline | Self | Long-term training with a BMI gait protocol induces partial neurological recovery in paraplegic patients. |
| [52] | 4 Healthy 2 Patient | RT | 6 blocks consisting of 23 trials | Offline | Self | Using an EEG-BCI system with robot-assistive technologies to improve the effectiveness of the hand motor skills in post-stroke patients. |
| [53] | 21 Patient | RT | 30–50 training trials of dorsiflexion of the foot | Offline | Self | The application of BCI to chronic Stroke led to an increased output of the motor cortex to the target muscle. |
| [54] | 1 Patient | RT | 8 trials followed by a 45-min break | Offline | Self | To decode the motion intentions of a paraplegic person and give him the ability to walk using a lower-body exoskeleton. |
| [55] | 10 Healthy | RT | 30 training trials | Online | Self | The capability of the user to discriminate between a set of external sensory stimuli combined with a fast and reliable BCI brain switch. |
| [56] | 5 Healthy | RT. | 50 trials, 9 s of one-step walking, and 10 s of resting | Offline | Self | Under the powered exoskeleton environment, decoding user intention. |
| [57] | 10 Healthy | RT | 50 trials of resting, walking intention, and exoskeleton walking | Offline | Self | To improve the performance of MRCP decoding. |
| [58] | 6 healthy | VR | 50 trials | Offline | Self | EEG activities were utilized to characterize the intention to move in rehabilitation procedures. |
| [59] | 10 Healthy | RT | 30 trials | Online | Self | MAFO is powered by a BCI for stroke rehabilitation, with evidence of its efficacy in promoting cortical neuroplasticity. |
| [60] | 4 Healthy | RT | 20 trials per run, with 5–8 s between each task | Online | Self | Neurofeedback study for variations in MRCP in real-time. |
| [61] | 2 Patient | RT | 5-min walk with robot-on, robot-off, and no-robot conditions | Offline | Cue | Implementation of multimodal physiological interface with the X1 device during walking. |
| [62] | 3 Patient | RT | 24 training sessions, each lasting for 30 min 3 days a week | Offline | Self | Application of two-stage robot-assisted training for hand motor recovery. |
| [63] | 11 Healthy | VR | 30 trials of ballistic dorsiflexion | Online | Self | Application of the new BCI to dynamic real-life scenarios: feasibility and benefit. |
| [64] | 3 Healthy, 4 Patient | RT | For healthy subjects, 5–10 min of walking with the exoskeleton. For the patients, 20 and 30 min | Online | Self | Analyzing the indicators of the viability of the system for clinical purposes. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Said, R.R.; Heyat, M.B.B.; Song, K.; Tian, C.; Wu, Z. A Systematic Review of Virtual Reality and Robot Therapy as Recent Rehabilitation Technologies Using EEG-Brain–Computer Interface Based on Movement-Related Cortical Potentials. Biosensors 2022, 12, 1134. https://doi.org/10.3390/bios12121134
Said RR, Heyat MBB, Song K, Tian C, Wu Z. A Systematic Review of Virtual Reality and Robot Therapy as Recent Rehabilitation Technologies Using EEG-Brain–Computer Interface Based on Movement-Related Cortical Potentials. Biosensors. 2022; 12(12):1134. https://doi.org/10.3390/bios12121134
Chicago/Turabian StyleSaid, Ramadhan Rashid, Md Belal Bin Heyat, Keer Song, Chao Tian, and Zhe Wu. 2022. "A Systematic Review of Virtual Reality and Robot Therapy as Recent Rehabilitation Technologies Using EEG-Brain–Computer Interface Based on Movement-Related Cortical Potentials" Biosensors 12, no. 12: 1134. https://doi.org/10.3390/bios12121134
APA StyleSaid, R. R., Heyat, M. B. B., Song, K., Tian, C., & Wu, Z. (2022). A Systematic Review of Virtual Reality and Robot Therapy as Recent Rehabilitation Technologies Using EEG-Brain–Computer Interface Based on Movement-Related Cortical Potentials. Biosensors, 12(12), 1134. https://doi.org/10.3390/bios12121134






