Recent Advances in Biosensor Technologies for Point-of-Care Urinalysis
Abstract
1. Introduction
2. Detecting Analytes in Urine for Urinalysis
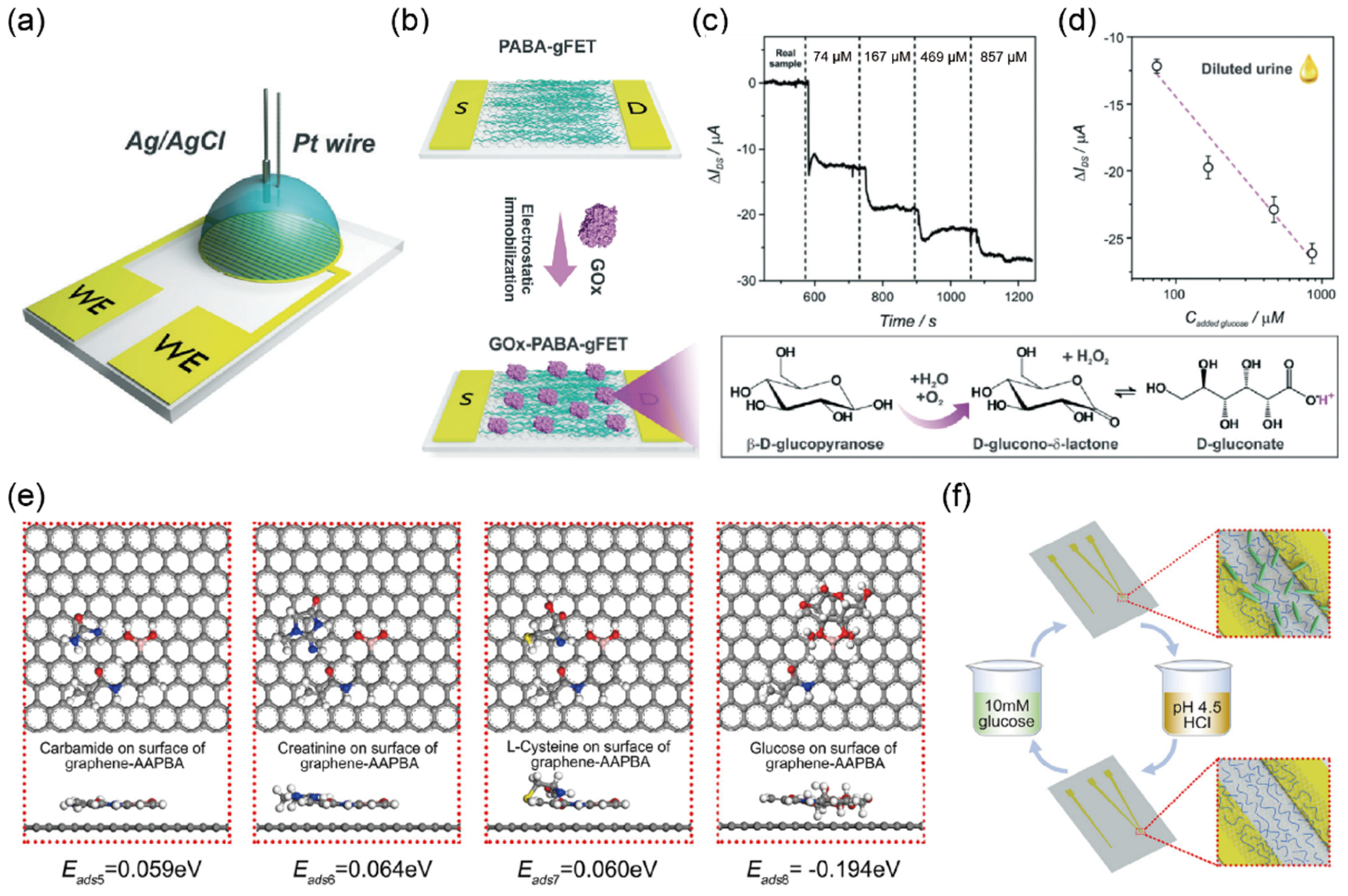
2.1. Detection of Glucose in Urine Sample
2.2. Detection of Hydrogen Ions in Urine Sample
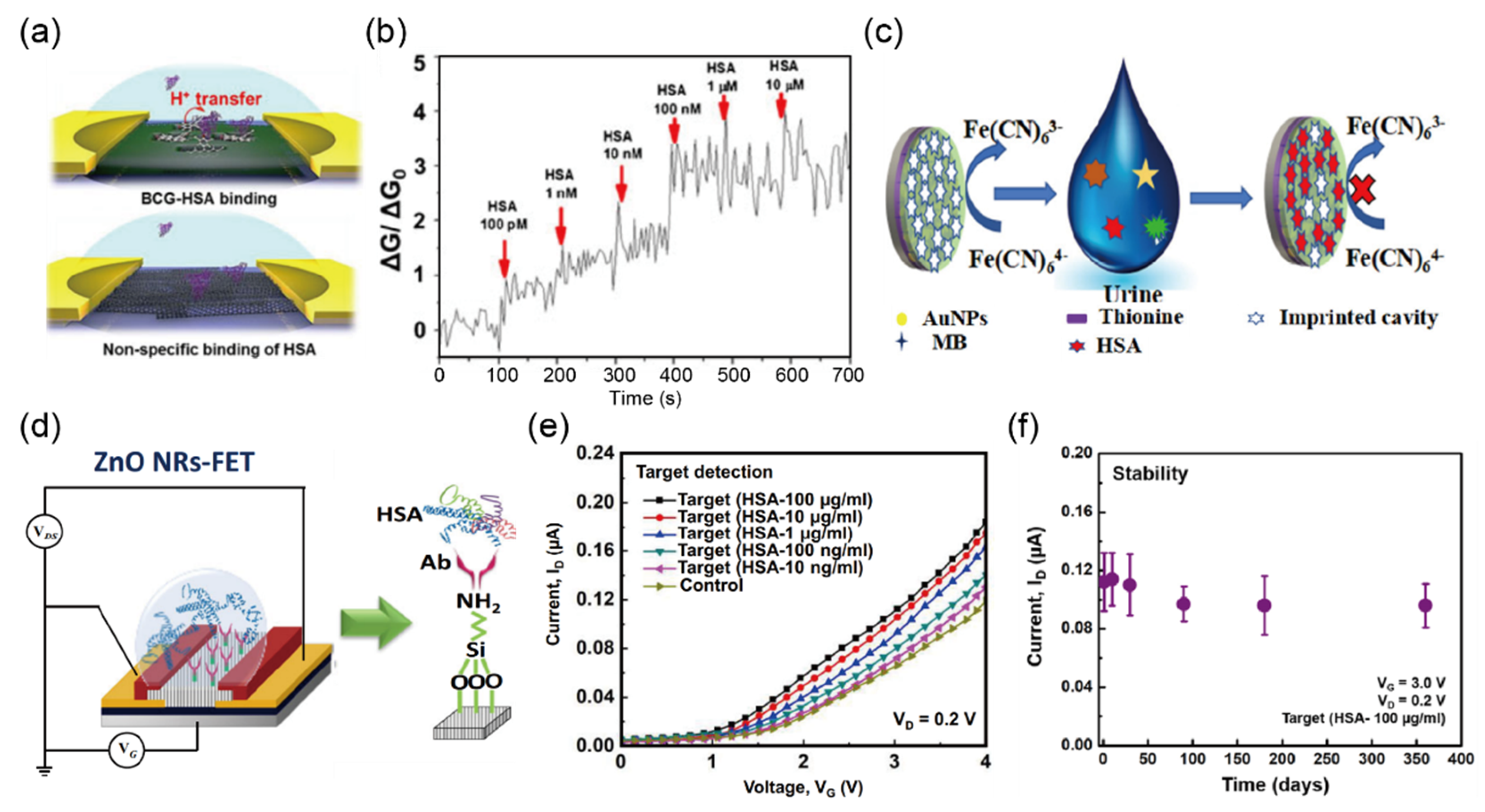
2.3. Detection of Protein (Albumin) in Urine Sample
2.4. Detection of Ketone Bodies in Urine Sample
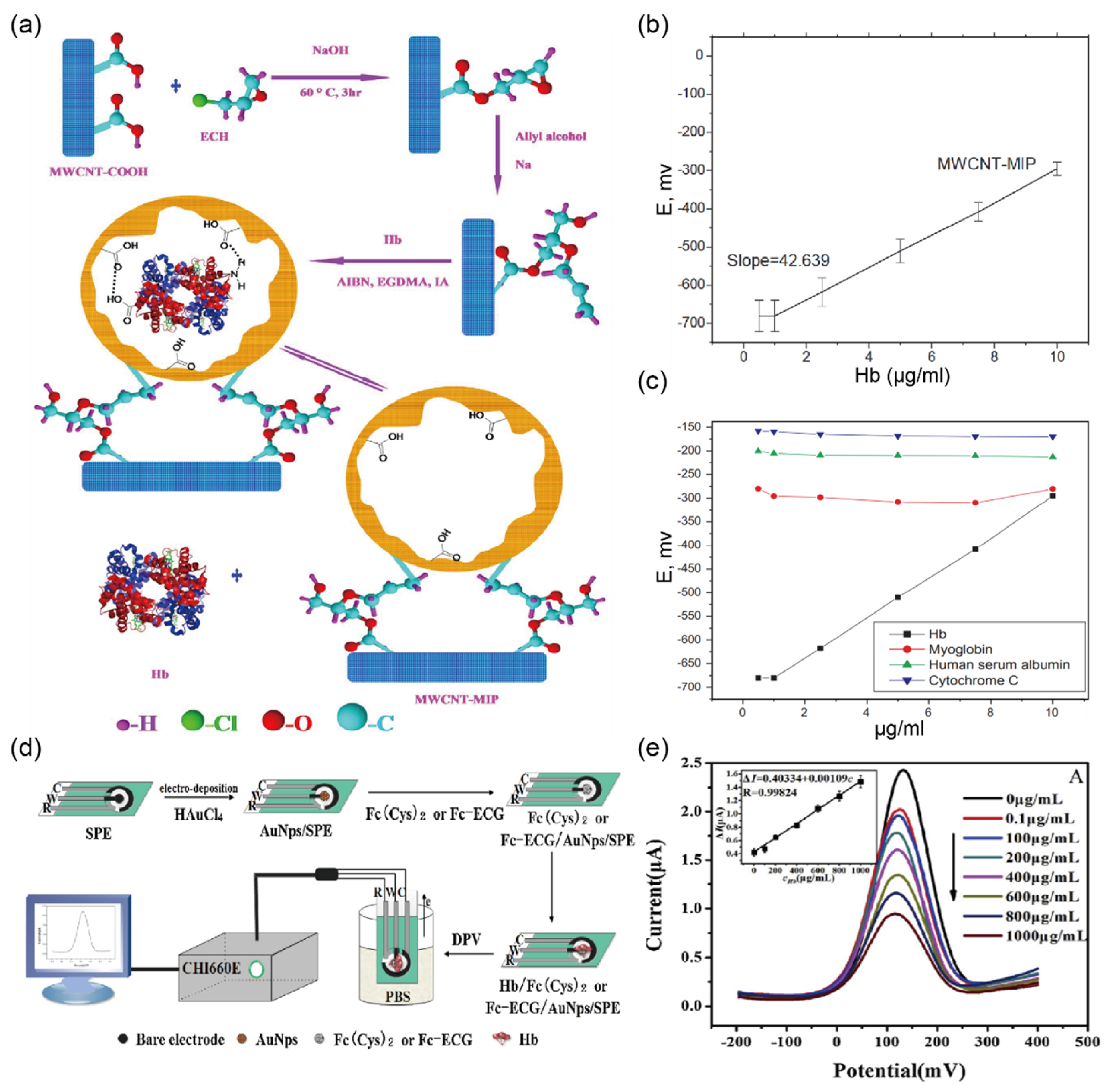
2.5. Detection of Hemoglobin in Urine Sample
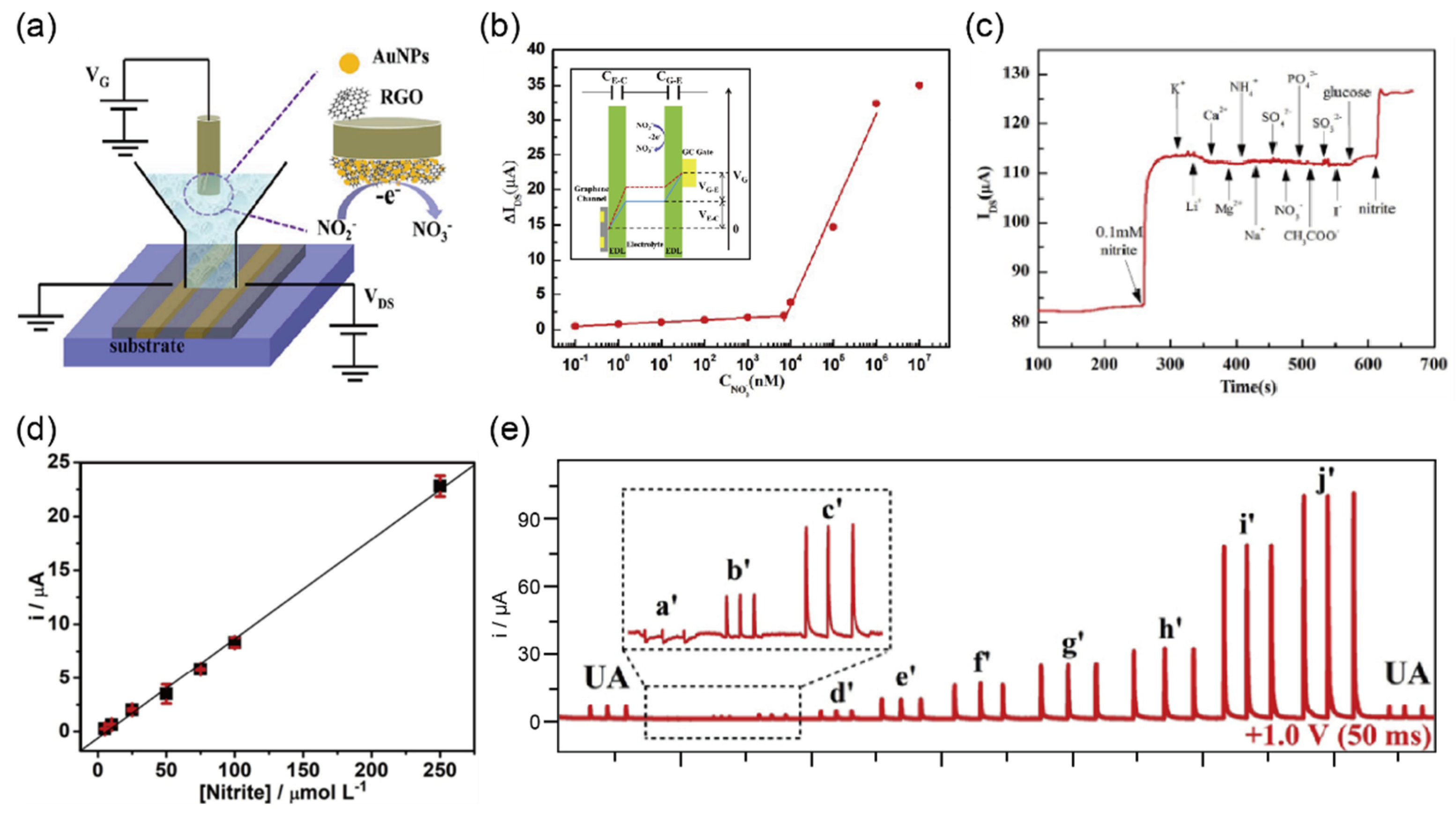
2.6. Detection of Nitrite in Urine Sample
2.7. Detection of Bilirubin in Urine Sample
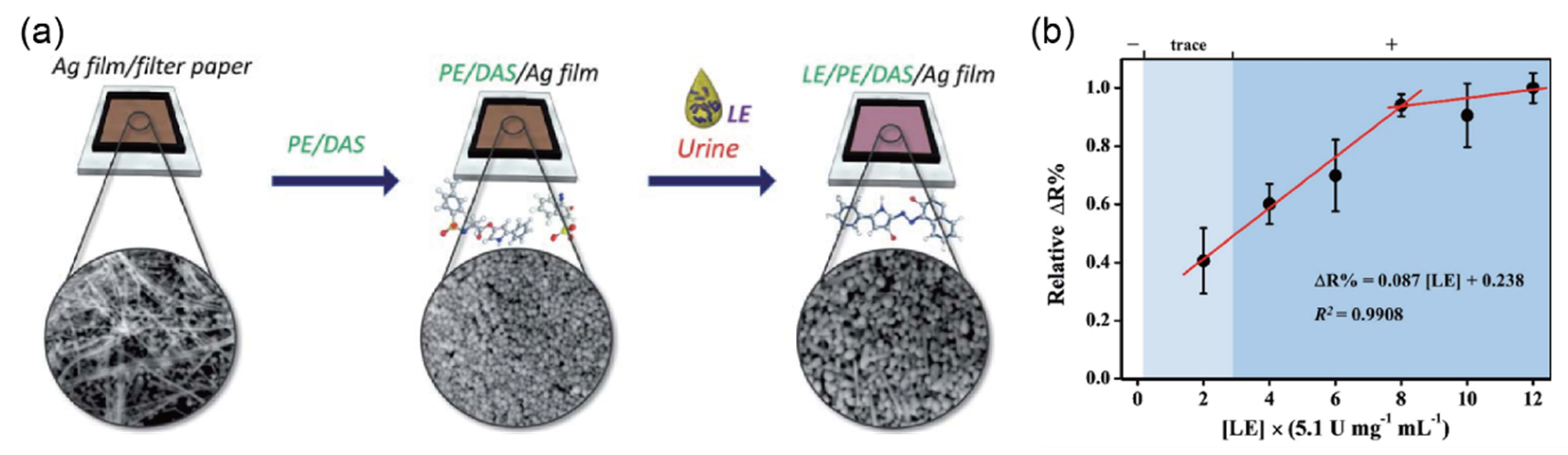
2.8. Detection of Leukocyte Esterase (LE) in Urine Sample
3. Detecting Other Biomolecules in Urine Sample
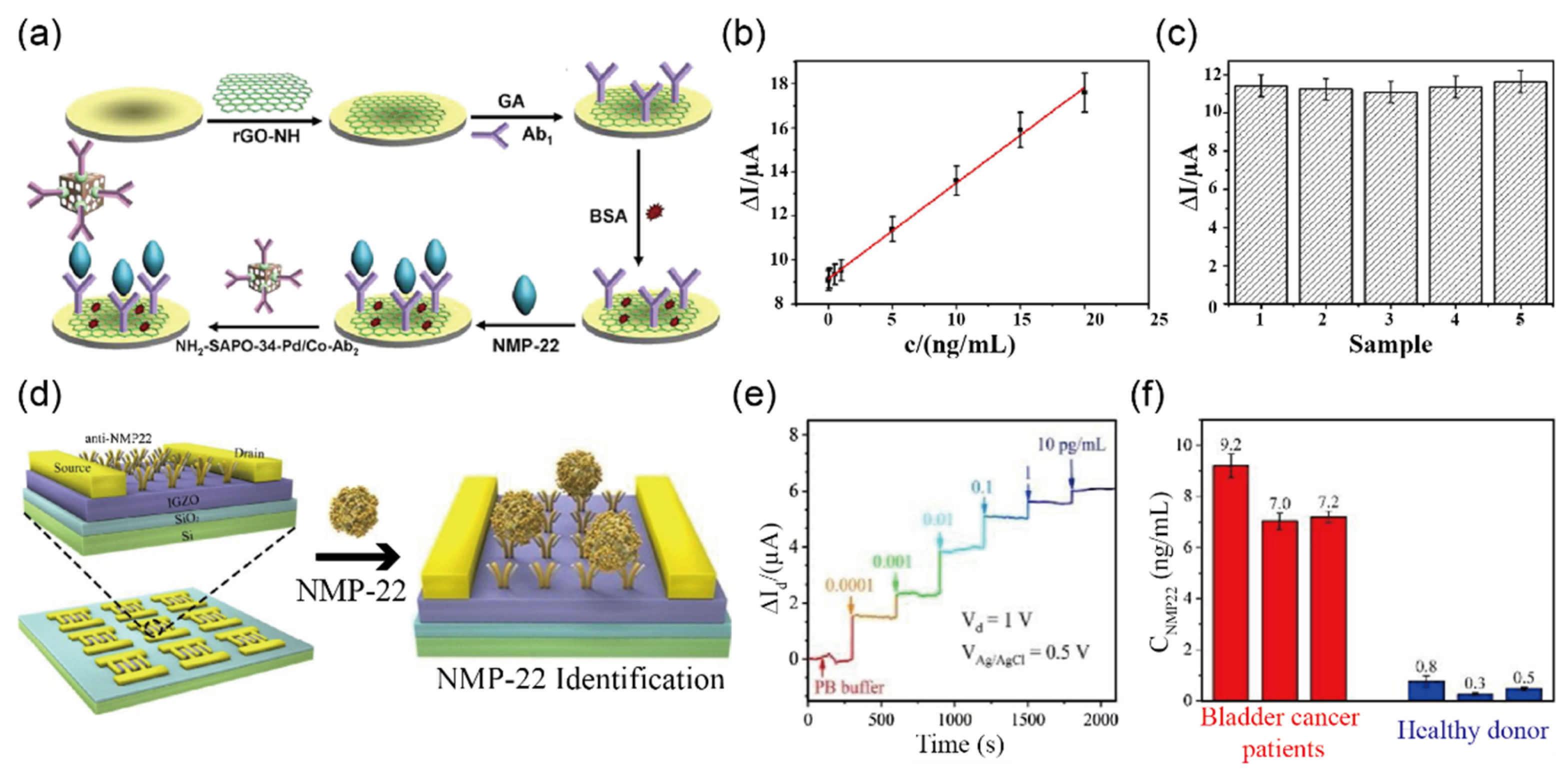
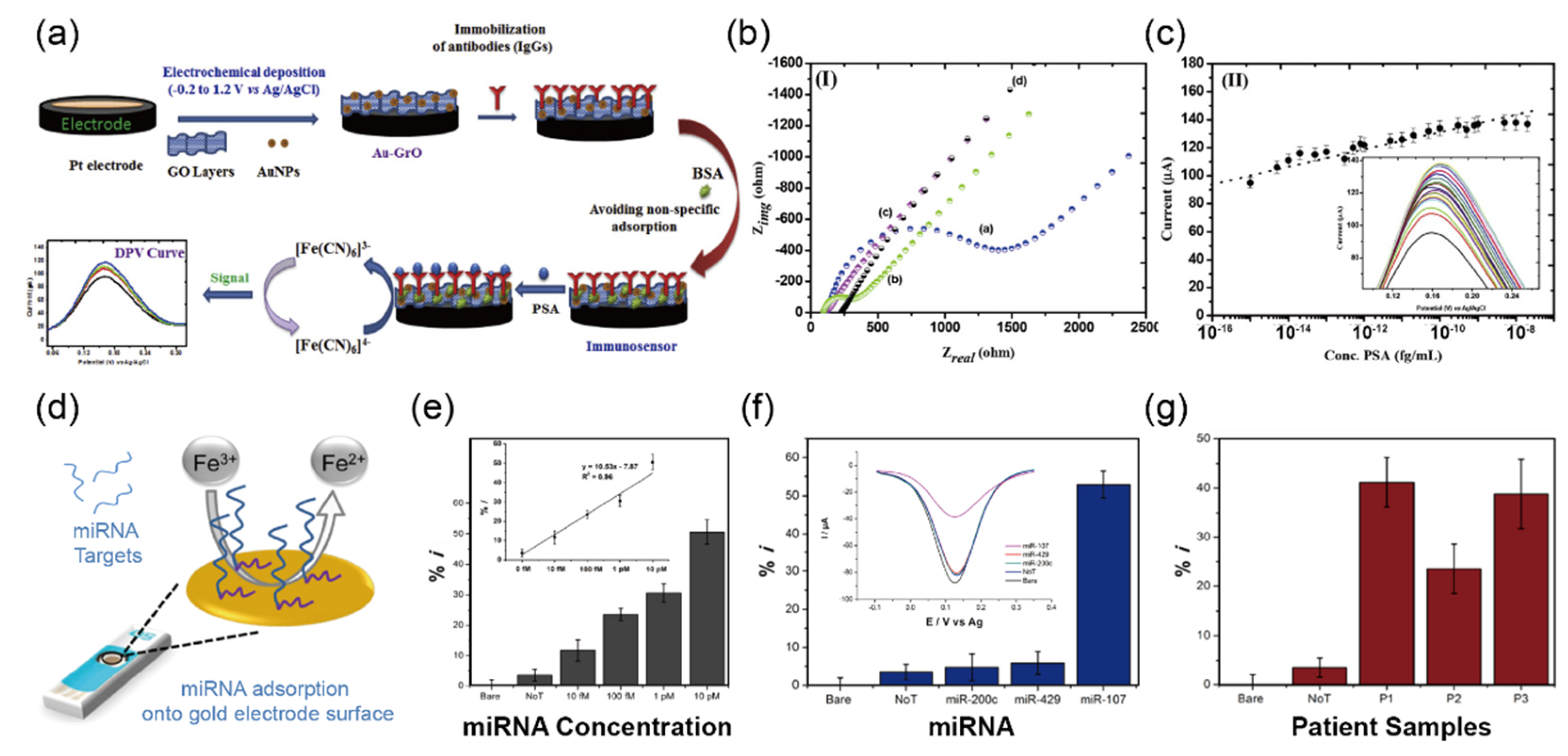
3.1. Detection of Cancer Biomarkers in Urine Sample
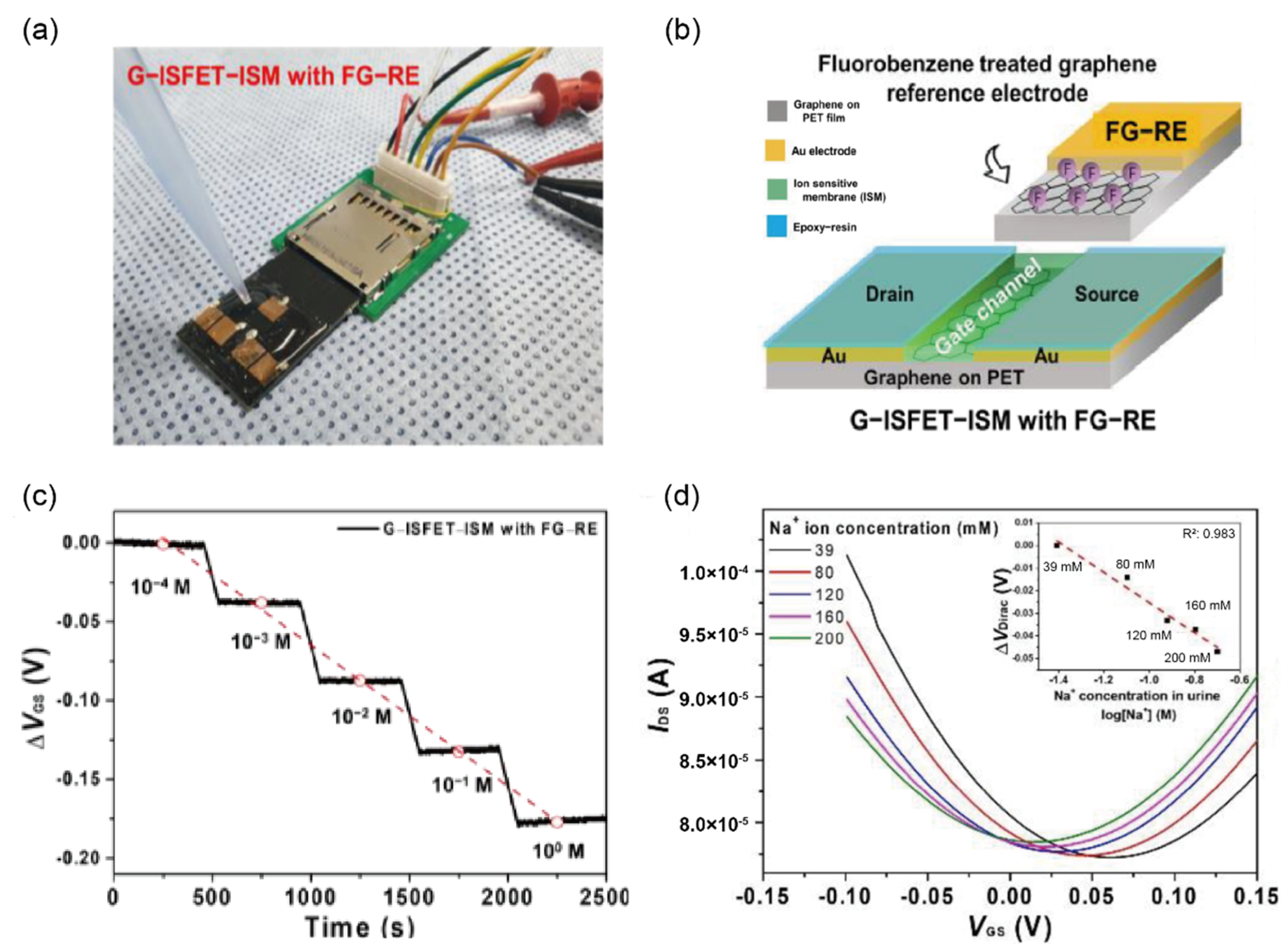
3.2. Detection of Inorganic Ions in Urine Sample
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fogazzi, G.B. The Urinary Sediment. An Integrated View; Penerbit Buku Kompas: Trento, Italy, 2010; ISBN 978-88-214-3016-9. [Google Scholar]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, C.M.; Baldwin, D.; Portmann, B.; Lolin, Y.; Mowat, A.P.; Mieli-Vergani, G. Value of Urinary Copper Excretion after Penicillamine Challenge in the Diagnosis of Wilson’s Disease. Hepatology 1992, 15, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Marsden, J.; Pickering, D. Urine Testing for Diabetic Analysis. Community Eye Health 2015, 28, 77. [Google Scholar]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Hess, B. Acid–Base Metabolism: Implications for Kidney Stosne Formation. Urol. Res. 2006, 34, 134–138. [Google Scholar] [CrossRef]
- Hemmelgarn, B.R.; Manns, B.J.; Lloyd, A.; James, M.T.; Klarenbach, S.; Quinn, R.R.; Wiebe, N.; Tonelli, M. Alberta Kidney Disease Network Relation between Kidney Function, Proteinuria, and Adverse Outcomes. JAMA 2010, 303, 423–429. [Google Scholar] [CrossRef]
- Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; van der Velde, M.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Chronic Kidney Disease Prognosis Consortium. Lower Estimated Glomerular Filtration Rate and Higher Albuminuria Are Associated with Mortality and End-Stage Renal Disease. A Collaborative Meta-Analysis of Kidney Disease Population Cohorts. Kidney Int. 2011, 79, 1331–1340. [Google Scholar] [CrossRef]
- Gilbertson, D.T.; Ebben, J.P.; Foley, R.N.; Weinhandl, E.D.; Bradbury, B.D.; Collins, A.J. Hemoglobin Level Variability: Associations with Mortality. Clin. J. Am. Soc. Nephrol. 2008, 3, 133–138. [Google Scholar] [CrossRef]
- Cashore, W.J. Bilirubin and jaundice in the micropremie. Clin. Perinatol. 2000, 27, 171–179. [Google Scholar] [CrossRef]
- Fan, L.; Tighiouart, H.; Levey, A.S.; Beck, G.J.; Sarnak, M.J. Urinary Sodium Excretion and Kidney Failure in Non-Diabetic Chronic Kidney Disease. Kidney Int. 2014, 86, 582–588. [Google Scholar] [CrossRef]
- Fleming, C.R.; George, L.; Stoner, G.L.; Tarrosa, V.B.; Moyer, T.P. The Importance of Urinary Magnesium Values in Patients With Gut Failure. Mayo Clin. Proc. 1996, 71, 21–24. [Google Scholar] [CrossRef]
- Assadi, F. Diagnosis of Hypokalemia: A Problem-Solving Approach to Clinical Cases. Iran. J. Kidney Dis. 2008, 2, 115–122. [Google Scholar]
- Schrier, R.W. Diagnostic Value of Urinary Sodium, Chloride, Urea, and Flow. J. Am. Soc. Nephrol. 2011, 22, 1610–1613. [Google Scholar] [CrossRef][Green Version]
- Pearce, E.N.; Lazarus, J.H.; Moreno-Reyes, R.; Zimmermann, M.B. Consequences of Iodine Deficiency and Excess in Pregnant Women: An Overview of Current Knowns and Unknowns12. Am. J. Clin. Nutr. 2016, 104, 918S–923S. [Google Scholar] [CrossRef]
- Zimmermann, M.B. The Effects of Iodine Deficiency in Pregnancy and Infancy. Paediatr. Perinat. Epidemiol. 2012, 26 (Suppl. 1), 108–117. [Google Scholar] [CrossRef]
- Givler, D.N.; Givler, A. Asymptomatic Bacteriuria; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Linares, L.A.; Thornton, D.J.; Strymish, J.; Baker, E.; Gupta, K. Electronic Memorandum Decreases Unnecessary Antimicrobial Use for Asymptomatic Bacteriuria and Culture-Negative Pyuria. Infect. Control. Hosp. Epidemiol. 2011, 32, 644–648. [Google Scholar] [CrossRef]
- Kouri, T.; Fogazzi, G.; Gant, V.; Hallander, H.; Hofmann, W.; Guder, W.G. European Urinalysis Guidelines. Scand. J. Clin. Lab. Investig. 2000, 60, 1–96. [Google Scholar] [CrossRef]
- Simerville, J.A.; Maxted, W.C.; Pahira, J.J. Urinalysis: A Comprehensive Review. Am. Fam. Physician 2005, 71, 1153–1162. [Google Scholar]
- Echeverry, G.; Hortin, G.L.; Rai, A.J. Introduction to Urinalysis: Historical Perspectives and Clinical Application. Methods Mol. Biol. 2010, 641, 1–12. [Google Scholar] [CrossRef]
- Pesola, G.R.; Argos, M.; Chen, Y.; Parvez, F.; Ahmed, A.; Hasan, R.; Rakibuz-Zaman, M.; Islam, T.; Eunus, M.; Sarwar, G.; et al. Dipstick Proteinuria as a Predictor of All-Cause and Cardiovascular Disease Mortality in Bangladesh: A Prospective Cohort Study. Prev. Med. 2015, 78, 72–77. [Google Scholar] [CrossRef]
- Iseki, K.; Konta, T.; Asahi, K.; Yamagata, K.; Fujimoto, S.; Tsuruya, K.; Narita, I.; Kasahara, M.; Shibagaki, Y.; Moriyama, T.; et al. Association of Dipstick Hematuria with All-Cause Mortality in the General Population: Results from the Specific Health Check and Guidance Program in Japan. Nephrol. Dial. Transplant. 2018, 33, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Kosmadakis, G.; Filiopoulos, V.; Georgoulias, C.; Smirloglou, D.; Draganis, T.; Michail, S. Quantitative Evaluation of Proteinuria by Estimation of the Protein/Creatinine Ratio in a Random Urine Sample. Ren. Fail. 2010, 32, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Huo, R.; Mohan, C. Current and Emerging Trends in Point-of-Care Urinalysis Tests. Expert Rev. Mol. Diagn. 2020, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Vuljanić, D.; Dojder, A.; Špoljarić, V.; Saračević, A.; Dukić, L.; Leniček-Krleža, J.; Vlašić-Tanasković, J.; Maradin, I.; Grzunov, A.; Vogrinc, Ž.; et al. Analytical Verification of 12 Most Commonly Used Urine Dipsticks in Croatia: Comparability, Repeatability and Accuracy. Biochem. Med. 2019, 29, 010708. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.A.; Pincus, M.R. Henry’s Clinical Diagnosis and Management by Laboratory Methods E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021; ISBN 978-0-323-75508-5. [Google Scholar]
- Pirkle, J.L.; Palavecino, E.L.; Freedman, B.I. Lactobacillus Species Can Cause a False-Positive Test for Hematuria on Dipstick Urinalysis. Am. J. Med. 2013, 126, e3–e4. [Google Scholar] [CrossRef]
- Davis, R.; Jones, J.S.; Barocas, D.A.; Castle, E.P.; Lang, E.K.; Leveillee, R.J.; Messing, E.M.; Miller, S.D.; Peterson, A.C.; Turk, T.M.T.; et al. Diagnosis, Evaluation and Follow-Up of Asymptomatic Microhematuria (AMH) in Adults: AUA Guideline. J. Urol. 2012, 188, 2473–2481. [Google Scholar] [CrossRef]
- Fenoy, G.E.; Marmisollé, W.A.; Knoll, W.; Azzaroni, O. Highly Sensitive Urine Glucose Detection with Graphene Field-Effect Transistors Functionalized with Electropolymerized Nanofilms. Sens. Diagn. 2022, 1, 139–148. [Google Scholar] [CrossRef]
- Huang, C.; Hao, Z.; Wang, Z.; Zhao, X.; Wang, H.; Li, F.; Liu, S.; Pan, Y. A Fully Integrated Graphene-Polymer Field-Effect Transistor Biosensing Device for on-Site Detection of Glucose in Human Urine. Mater. Today Chem. 2022, 23, 100635. [Google Scholar] [CrossRef]
- Rabboh, F.M.; O’Neil, G.D. Voltammetric PH Measurements in Unadulterated Foodstuffs, Urine, and Serum with 3D-Printed Graphene/Poly(Lactic Acid) Electrodes. Anal. Chem. 2020, 92, 14999–15006. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.; Kim, C.-H.; Yoon, M.-H. Approaching the Nernst Detection Limit in an Electrolyte-Gated Metal Oxide Transistor. IEEE Electron Device Lett. 2021, 42, 50–53. [Google Scholar] [CrossRef]
- Chae, M.-S.; Park, J.H.; Son, H.W.; Hwang, K.S.; Kim, T.G. IGZO-Based Electrolyte-Gated Field-Effect Transistor for In Situ Biological Sensing Platform. Sens. Actuators B Chem. 2018, 262, 876–883. [Google Scholar] [CrossRef]
- Kim, B.; Kim, T.H. Determination of Human Serum Albumin Using a Single-Walled Carbon Nanotube-FET Modified with Bromocresol Green. Microchim. Acta 2016, 183, 1513–1518. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, Y.; Guo, M.; Lin, B.; Zhang, L. A Sensitive Determination of Albumin in Urine by Molecularly Imprinted Electrochemical Biosensor Based on Dual-Signal Strategy. Sens. Actuators B Chem. 2019, 288, 564–570. [Google Scholar] [CrossRef]
- Karim, S.S.A.; Nadzirah, S.; Kazmi, J.; Rahim, R.A.; Dee, C.F.; Hamzah, A.A.; Mohamed, M.A. Zinc Oxide Nanorods-Based Immuno-Field-Effect Transistor for Human Serum Albumin Detection. J. Mater. Sci. 2021, 56, 15344–15353. [Google Scholar] [CrossRef]
- Go, A.; Park, S.R.; Ku, Y.; Sun, M.; Yeon, S.; Lee, J.-K.; Lee, S.W.; Lee, M.-H. Highly Sensitive Electrochemical Sensor for Diagnosis of Diabetic Ketoacidosis (DKA) by Measuring Ketone Bodies in Urine. Sensors 2021, 21, 4902. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Alexander, S. A Potentiometric Sensor for the Trace Level Determination of Hemoglobin in Real Samples Using Multiwalled Carbon Nanotube Based Molecular Imprinted Polymer. Eur. Polym. J. 2017, 97, 84–93. [Google Scholar] [CrossRef]
- Han, G.-C.; Su, X.; Hou, J.; Ferranco, A.; Feng, X.-Z.; Zeng, R.; Chen, Z.; Kraatz, H.-B. Disposable Electrochemical Sensors for Hemoglobin Detection Based on Ferrocenoyl Cysteine Conjugates Modified Electrode. Sens. Actuators B Chem. 2019, 282, 130–136. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, M.; He, H.; Cai, Z.; Gao, N.; He, C.; Chang, G.; Wang, X.; He, Y. Highly Sensitive Nitrite Sensor Based on AuNPs/RGO Nanocomposites Modified Graphene Electrochemical Transistors. Biosens. Bioelectron. 2019, 146, 111751. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Silva, P.R.L.; Lima, A.P.; Rocha, D.P.; Oliveira, T.C.; do Prado, T.M.; Fava, E.L.; Fatibello-Filho, O.; Richter, E.M.; Muñoz, R.A.A. 3D-Printed Graphene/Polylactic Acid Electrode for Bioanalysis: Biosensing of Glucose and Simultaneous Determination of Uric Acid and Nitrite in Biological Fluids. Sens. Actuators B Chem. 2020, 307, 127621. [Google Scholar] [CrossRef]
- Thangamuthu, M.; Gabriel, W.; Santschi, C.; Martin, O. Electrochemical Sensor for Bilirubin Detection Using Screen Printed Electrodes Functionalized with Carbon Nanotubes and Graphene. Sensors 2018, 18, 800. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahmed, J.; Asiri, A.M. Selective Bilirubin Sensor Fabrication Based on Doped IAO Nanorods for Environmental Remediation. New J. Chem. 2019, 43, 19298–19307. [Google Scholar] [CrossRef]
- Ho, M.-L.; Liu, W.-F.; Tseng, H.-Y.; Yeh, Y.-T.; Tseng, W.-T.; Chou, Y.-Y.; Huang, X.-R.; Hsu, H.-C.; Ho, L.-I.; Pan, S.-W. Quantitative Determination of Leukocyte Esterase with a Paper-Based Device. RSC Adv. 2020, 10, 27042–27049. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, Y.; Zhang, Y.; Ma, H.; Yan, T.; Du, B.; Wei, Q. Sensitive Electrochemical Immunosensor for Detection of Nuclear Matrix Protein-22 Based on NH2-SAPO-34 Supported Pd/Co Nanoparticles. Sci. Rep. 2016, 6, 24551. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, B.; Yang, Y.; Liang, H.; Yang, Y.; Yuan, Q. Design of High Stability Thin-Film Transistor Biosensor for the Diagnosis of Bladder Cancer. Chin. Chem. Lett. 2020, 31, 1387–1391. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Huang, W.; Wan, G.; Xia, M.; Chen, D.; Zhang, Y.; Wang, Y.; Guo, F.; Tan, J.; et al. Integrated Urinalysis Devices Based on Interface-Engineered Field-Effect Transistor Biosensors Incorporated with Electronic Circuits. Adv. Mater. 2022, 34, 2203224. [Google Scholar] [CrossRef]
- Pal, M.; Khan, R. Graphene Oxide Layer Decorated Gold Nanoparticles Based Immunosensor for the Detection of Prostate Cancer Risk Factor. Anal. Biochem. 2017, 536, 51–58. [Google Scholar] [CrossRef]
- Koo, K.M.; Carrascosa, L.G.; Shiddiky, M.J.A.; Trau, M. Poly(A) Extensions of MiRNAs for Amplification-Free Electrochemical Detection on Screen-Printed Gold Electrodes. Anal. Chem. 2016, 88, 2000–2005. [Google Scholar] [CrossRef]
- Oh, H.G.; Jeon, D.C.; Gianti, M.S.; Cho, H.S.; Jo, D.A.; Indriatmoko, M.N.; Jang, B.K.; Lim, J.M.; Cho, S.; Song, K.S. Two–Dimensional Disposable Graphene Sensor to Detect Na+ Ions. Nanomaterials 2021, 11, 787. [Google Scholar] [CrossRef]
- Iannazzo, D.; Espro, C.; Ferlazzo, A.; Celesti, C.; Branca, C.; Neri, G. Electrochemical and Fluorescent Properties of Crown Ether Functionalized Graphene Quantum Dots for Potassium and Sodium Ions Detection. Nanomaterials 2021, 11, 2897. [Google Scholar] [CrossRef]
- Cunha-Silva, H.; Julia Arcos-Martinez, M. Development of a Selective Chloride Sensing Platform Using a Screen-Printed Platinum Electrode. Talanta 2019, 195, 771–777. [Google Scholar] [CrossRef]
- Khunseeraksa, V.; Kongkaew, S.; Thavarungkul, P.; Kanatharana, P.; Limbut, W. Electrochemical Sensor for the Quantification of Iodide in Urine of Pregnant Women. Microchim. Acta 2020, 187, 591. [Google Scholar] [CrossRef]
- Hwang, C.; Kwak, T.; Kim, C.-H.; Kim, J.H.; Park, S. Quantitative and Rapid Detection of Iodide Ion via Electrolyte-Gated IGZO Thin-Film Transistors. Sens. Actuators B Chem. 2022, 353, 131144. [Google Scholar] [CrossRef]
- Phan, L.M.T.; Vo, T.A.T.; Hoang, T.X.; Selvam, S.P.; Pham, H.L.; Kim, J.Y.; Cho, S. Trending Technology of Glucose Monitoring during COVID-19 Pandemic: Challenges in Personalized Healthcare. Adv. Mater. Technol. 2021, 6, 2100020. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Wei, O.Y.; Teece, S. Urine Dipsticks in Screening for Diabetes Mellitus. Emerg. Med. J. 2006, 23, 138. [Google Scholar] [CrossRef][Green Version]
- Dagogo-Jack, S. Diabetes Mellitus in Developing Countries and Underserved Communities; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; p. 294. [Google Scholar]
- Pickup, J.C.; Hussain, F.; Evans, N.D.; Rolinski, O.J.; Birch, D.J.S. Fluorescence-Based Glucose Sensors. Biosens. Bioelectron. 2005, 20, 2555–2565. [Google Scholar] [CrossRef]
- Rachim, V.P.; Chung, W.-Y. Wearable-Band Type Visible-near Infrared Optical Biosensor for Non-Invasive Blood Glucose Monitoring. Sens. Actuators B Chem. 2019, 286, 173–180. [Google Scholar] [CrossRef]
- Hsieh, H.V.; Pfeiffer, Z.A.; Amiss, T.J.; Sherman, D.B.; Pitner, J.B. Direct Detection of Glucose by Surface Plasmon Resonance with Bacterial Glucose/Galactose-Binding Protein. Biosens. Bioelectron. 2004, 19, 653–660. [Google Scholar] [CrossRef]
- Heller, A.; Feldman, B. Electrochemical Glucose Sensors and Their Applications in Diabetes Management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose Oxidase—An Overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef]
- Robinson, S.; Dhanlaksmi, N. Photonic Crystal Based Biosensor for the Detection of Glucose Concentration in Urine. Photonic Sens. 2017, 7, 11–19. [Google Scholar] [CrossRef]
- Onay, A.; Dogan, Ü.; Ciftci, H.; Cetin, D.; Suludere, Z.; Tamer, U. Amperometric Glucose Sensor Based on the Glucose Oxidase Enzyme Immobilized on Graphite Rod Electrode Modified with Fe3O4-CS-Au Magnetic Nanoparticles. Ionics 2018, 24, 4015–4022. [Google Scholar] [CrossRef]
- Anusha, J.R.; Raj, C.J.; Cho, B.-B.; Fleming, A.T.; Yu, K.-H.; Kim, B.C. Amperometric Glucose Biosensor Based on Glucose Oxidase Immobilized over Chitosan Nanoparticles from Gladius of Uroteuthis Duvauceli. Sens. Actuators B Chem. 2015, 215, 536–543. [Google Scholar] [CrossRef]
- Srinivasan, V.; Pamula, V.K.; Fair, R.B. An Integrated Digital Microfluidic Lab-on-a-Chip for Clinical Diagnostics on Human Physiological Fluids. Lab Chip 2004, 4, 310–315. [Google Scholar] [CrossRef]
- Partanen, J.I.; Covington, A.K. Re-Evaluation of the Second Stoichiometric Dissociation Constants of Phosphoric Acid at Temperatures from (0 to 60) °C in Aqueous Buffer Solutions with or without NaCl or KCl. 2. Tests and Use of the Resulting Hückel Model Equations. J. Chem. Eng. Data 2005, 50, 2065–2073. [Google Scholar] [CrossRef]
- Grases, F.; Costa-Bauzá, A.; Gomila, I.; Ramis, M.; García-Raja, A.; Prieto, R.M. Urinary PH and Renal Lithiasis. Urol. Res. 2012, 40, 41–46. [Google Scholar] [CrossRef]
- Grases, F.; Villacampa, A.I.; Costa-Bauzá, A.; Söhnel, O. Uric Acid Calculi: Types, Etiology and Mechanisms of Formation. Clin. Chim. Acta 2000, 302, 89–104. [Google Scholar] [CrossRef]
- Yao, S.; Wang, M.; Madou, M. A PH Electrode Based on Melt-Oxidized Iridium Oxide. J. Electrochem. Soc. 2001, 148, H29. [Google Scholar] [CrossRef]
- Grases, F.; Rodriguez, A.; Berga, F.; Costa-Bauza, A.; Prieto, R.M.; Burdallo, I.; Cadarso, A.; Jimenez-Jorquera, C.; Baldi, A.; Garganta, R. A New Device for Simple and Accurate Urinary PH Testing by the Stone-Former Patient. SpringerPlus 2014, 3, 209. [Google Scholar] [CrossRef]
- Kwong, T.; Robinson, C.; Spencer, D.; Wiseman, O.J.; Karet Frankl, F.E. Accuracy of Urine PH Testing in a Regional Metabolic Renal Clinic: Is the Dipstick Accurate Enough? Urolithiasis 2013, 41, 129–132. [Google Scholar] [CrossRef]
- Omar, M.; Sarkissian, C.; Jianbo, L.; Calle, J.; Monga, M. Dipstick Spot Urine PH Does Not Accurately Represent 24 Hour Urine PH Measured by an Electrode. Int. Braz. J. Urol. 2016, 42, 546–549. [Google Scholar] [CrossRef][Green Version]
- Bergveld, P. Development of an Ion-Sensitive Solid-State Device for Neurophysiological Measurements. IEEE Trans. Biomed. Eng. 1970, BME-17, 70–71. [Google Scholar] [CrossRef]
- Cai, G.; Qiang, L.; Yang, P.; Chen, Z.; Zhuo, Y.; Li, Y.; Pei, Y.; Wang, G. High-Sensitivity PH Sensor Based on Electrolyte-Gated In2O3 TFT. IEEE Electron Device Lett. 2018, 39, 1409–1412. [Google Scholar] [CrossRef]
- Mani, G.K.; Morohoshi, M.; Yasoda, Y.; Yokoyama, S.; Kimura, H.; Tsuchiya, K. ZnO-Based Microfluidic PH Sensor: A Versatile Approach for Quick Recognition of Circulating Tumor Cells in Blood. ACS Appl. Mater. Interfaces 2017, 9, 5193–5203. [Google Scholar] [CrossRef]
- Fung, C.D.; Cheung, P.W.; Ko, W.H. A Generalized Theory of an Electrolyte-Insulator-Semiconductor Field-Effect Transistor. IEEE Trans. Electron Devices 1986, 33, 8–18. [Google Scholar] [CrossRef]
- Lea, J. The Relationship Between Magnitude of Proteinuria Reduction and Risk of End-Stage Renal Disease: Results of the African American Study of Kidney Disease and Hypertension. Arch. Intern. Med. 2005, 165, 947. [Google Scholar] [CrossRef]
- Seegmiller, J.C.; Miller, W.G.; Bachmann, L.M. Moving Toward Standardization of Urine Albumin Measurements. EJIFCC 2017, 28, 258–267. [Google Scholar]
- Miller, W.G.; Seegmiller, J.C.; Lieske, J.C.; Narva, A.S.; Bachmann, L.M. Standardization of Urine Albumin Measurements: Status and Performance Goals. J. Appl. Lab. Med. 2017, 2, 423–429. [Google Scholar] [CrossRef]
- Johnson, C.A.; Levey, A.S.; Coresh, J.; Levin, A.; LauEknoyan, J.G. Clinical Practice Guidelines for Chronic Kidney Disease in Adults: Part II. Glomerular Filtration Rate, Proteinuria, and Other Markers. Am. Fam. Physician 2004, 70, 1091–1097. [Google Scholar]
- Montañés Bermúdez, R.; Gràcia García, S.; Pérez Surribas, D.; Martínez Castelao, A.; Bover Sanjuán, J.; Bover Sanjuán, J. Consensus Document. Recommendations on Assessing Proteinuria during the Diagnosis and Follow-up of Chronic Kidney Disease. Nefrología 2011, 31, 331–345. [Google Scholar] [CrossRef]
- Japanese Society of Nephrology. Evidence-Based Practice Guideline for the Treatment of CKD. Clin. Exp. Nephrol. 2009, 13, 537–566. [Google Scholar] [CrossRef] [PubMed]
- Klapkova, E.; Fortova, M.; Prusa, R.; Moravcova, L.; Kotaska, K. Determination of Urine Albumin by New Simple High-Performance Liquid Chromatography Method. J. Clin. Lab. Anal. 2016, 30, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.A.; Guerin, M.D. Quantification of Serum Proteins Using Capillary Electrophoresis. Ann. Clin. Biochem. 1995, 32, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Yao, B.; Owyong, T.C.; Prashanth, S.; Wang, C.; Zhang, X.; Wong, W.W.H.; Tang, Y.; Hong, Y. Detection of Urinary Albumin Using a “Turn-on” Fluorescent Probe with Aggregation-Induced Emission Characteristics. Chem. Asian J. 2021, 16, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Krämer, B.K.; Jesse, U.; Ress, K.M.; Schmülling, R.M.; Risler, T. Enzyme-Linked Immunosorbent Assay for Urinary Albumin at Low Concentrations. Clin. Chem. 1987, 33, 609–611. [Google Scholar] [CrossRef]
- Feng, P.; Huang, C.Z.; Li, Y.F. Direct Quantification of Human Serum Albumin in Human Blood Serum without Separation of C-Globulin by the Total Internal Reflected Resonance Light Scattering of Thorium–Sodium Dodecylbenzene Sulfonate at Water/Tetrachloromethane Interface. Anal. Biochem. 2002, 308, 83–89. [Google Scholar] [CrossRef]
- Liang, A.; Lu, Z.; Liu, Q.; Zhang, X.; Wen, G.; Jiang, Z. SERS Quantitative Analysis of Trace HSA with a Coomassie Brilliant Blue G-250 Molecular Probe in Nanogold Sol Substrate. RSC Adv. 2015, 5, 5711–5715. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Song, J.-F. Interface Potential Sensing from Adsorption of Human Serum Albumin (HSA) on Carbon Nanotube (CNT) Monitored by Zero Current Potentiometry for HSA Determination. Biosens. Bioelectron. 2015, 72, 225–229. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Detection of Inflammatory Biomarkers in Saliva and Urine: Potential in Diagnosis, Prevention, and Treatment for Chronic Diseases. Exp. Biol. Med. 2016, 241, 783–799. [Google Scholar] [CrossRef]
- Liu, B.-C.; Zhang, L.; Lv, L.; Wang, Y.; Liu, D.; Zhang, X. Application of Antibody Array Technology in the Analysis of Urinary Cytokine Profiles in Patients with Chronic Kidney Disease. Am. J. Nephrol. 2006, 26, 483–490. [Google Scholar] [CrossRef]
- Walker, H.K.; Hall, W.D.; Hurst, J.W. (Eds.) Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Butterworths: Boston, MA, USA, 1990; ISBN 978-0-409-90077-4. [Google Scholar]
- Orlandi, P.F.; Fujii, N.; Roy, J.; Chen, H.-Y.; Lee Hamm, L.; Sondheimer, J.H.; He, J.; Fischer, M.J.; Rincon-Choles, H.; Krishnan, G.; et al. Hematuria as a Risk Factor for Progression of Chronic Kidney Disease and Death: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. BMC Nephrol. 2018, 19, 150. [Google Scholar] [CrossRef]
- Shimoni, Z.; Glick, J.; Hermush, V.; Froom, P. Sensitivity of the Dipstick in Detecting Bacteremic Urinary Tract Infections in Elderly Hospitalized Patients. PLoS ONE 2017, 12, e0187381. [Google Scholar] [CrossRef]
- Deville, W.L.; Yzermans, J.C.; van Duijn, N.; Bezemer, P.D.; van der Windt, D.; Bouter, L.M. The Urine Dipstick Test Useful to Rule out Infections: A Meta-Analysis of the Accuracy. BMC Urol. 2004, 4, 4. [Google Scholar] [CrossRef]
- Manzanares Palenzuela, C.L.; Novotný, F.; Krupička, P.; Sofer, Z.; Pumera, M. 3D-Printed Graphene/Polylactic Acid Electrodes Promise High Sensitivity in Electroanalysis. Anal. Chem. 2018, 90, 5753–5757. [Google Scholar] [CrossRef]
- Niu, X.; Chen, C.; Teng, Y.; Zhao, H.; Lan, M. Novel Screen-Printed Gold Nano Film Electrode for Trace Mercury(II) Determination Using Anodic Stripping Voltammetry. Anal. Lett. 2012, 45, 764–773. [Google Scholar] [CrossRef]
- Rao, T.N. Validation of Analytical Methods; IntechOpen: London, UK, 2018; ISBN 978-1-78923-085-7. [Google Scholar]
- Kalakonda, A.; Jenkins, B.A.; John, S. Physiology, Bilirubin; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Fossati, P.; Ponti, M.; Prencipe, L.; Tarenghi, G. One-Step Protocol for Assays of Total and Direct Bilirubin with Stable Combined Reagents. Clin. Chem. 1989, 35, 173–176. [Google Scholar] [CrossRef]
- Ellairaja, S.; Shenbagavalli, K.; Ponmariappan, S.; Vasantha, V.S. A Green and Facile Approach for Synthesizing Imine to Develop Optical Biosensor for Wide Range Detection of Bilirubin in Human Biofluids. Biosens. Bioelectron. 2017, 91, 82–88. [Google Scholar] [CrossRef]
- Foley, K.F.; Wasserman, J. Are Unexpected Positive Dipstick Urine Bilirubin Results Clinically Significant? A Retrospective Review. Lab. Med. 2014, 45, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Genshaw, M.A.; Smith, P.J. Minimizing Instrumental Response to Urine Components Causing Atypical Colors with Bilirubin Reagent Strips. Clin. Chem. 1981, 27, 767–768. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, C. Molecularly Imprinted Hydroxyapatite Thin Film for Bilirubin Recognition. Biosens. Bioelectron. 2011, 29, 167–171. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lee, K.-S.; Park, D.-S.; Won, M.-S.; Shim, Y.-B. An Amperometric Bilirubin Biosensor Based on a Conductive Poly-Terthiophene-Mn(II) Complex. Biosens. Bioelectron. 2008, 23, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Syu, M.-J.; Chiu, T.-C.; Lai, C.-Y.; Chang, Y.-S. Amperometric Detection of Bilirubin from a Micro-Sensing Electrode with a Synthetic Bilirubin Imprinted Poly(MAA-Co-EGDMA) Film. Biosens. Bioelectron. 2006, 22, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Grayson, K.; Gregory, E.; Khan, G.; Guinn, B.-A. Urine Biomarkers for the Early Detection of Ovarian Cancer—Are We There Yet? Biomark. Cancer 2019, 11, 1179299X1983097. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Böhle, A.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Hernández, V.; Kaasinen, E.; Palou, J.; Rouprêt, M.; et al. EAU Guidelines on Non–Muscle-Invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur. Urol. 2017, 71, 447–461. [Google Scholar] [CrossRef] [PubMed]
- van der Aa, M.N.M.; Steyerberg, E.W.; Bangma, C.; van Rhijn, B.W.G.; Zwarthoff, E.C.; van der Kwast, T.H. Cystoscopy Revisited as the Gold Standard for Detecting Bladder Cancer Recurrence: Diagnostic Review Bias in the Randomized, Prospective CEFUB Trial. J. Urol. 2010, 183, 76–80. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Ku, J.H. Urinary Markers for Bladder Cancer Diagnosis and Monitoring. Front. Cell Dev. Biol. 2022, 10, 892067. [Google Scholar] [CrossRef]
- Zippe, C.; Pandimngi, L. NMP22 is a sensitive, cost-effective test in patients at risk for bladder cancer. J. Urol. 1999, 161, 62–65. [Google Scholar] [CrossRef]
- Wu, X.; Chai, Y.; Yuan, R.; Zhuo, Y.; Chen, Y. Dual Signal Amplification Strategy for Enzyme-Free Electrochemical Detection of MicroRNAs. Sens. Actuators B Chem. 2014, 203, 296–302. [Google Scholar] [CrossRef]
- Gao, X.; Xu, H.; Baloda, M.; Gurung, A.S.; Xu, L.-P.; Wang, T.; Zhang, X.; Liu, G. Visual Detection of MicroRNA with Lateral Flow Nucleic Acid Biosensor. Biosens. Bioelectron. 2014, 54, 578–584. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lei, K.F.; Tsai, S.-W.; Tsang, N.-M. Development of Graphene-Based Sensors on Paper Substrate for the Measurement of PH Value of Analyte. BioChip J. 2016, 10, 182–188. [Google Scholar] [CrossRef]
- Sohn, I.-Y.; Kim, D.-J.; Jung, J.-H.; Yoon, O.J.; Nguyen Thanh, T.; Tran Quang, T.; Lee, N.-E. PH Sensing Characteristics and Biosensing Application of Solution-Gated Reduced Graphene Oxide Field-Effect Transistors. Biosens. Bioelectron. 2013, 45, 70–76. [Google Scholar] [CrossRef]
- Fu, W.; Nef, C.; Tarasov, A.; Wipf, M.; Stoop, R.; Knopfmacher, O.; Weiss, M.; Calame, M.; Schönenberger, C. High Mobility Graphene Ion-Sensitive Field-Effect Transistors by Noncovalent Functionalization. Nanoscale 2013, 5, 12104–12110. [Google Scholar] [CrossRef]




| Biomarker | Dipstick | Typical Range | Biosensors | |||||
|---|---|---|---|---|---|---|---|---|
| Sensing Material | Detection Method | Linear Range | LOD | Human Sample | Ref. | |||
| Glucose (mM) | Negative, 5, 15, 30, 60, 110 | 0–0.8 mM | GOx–PABA-GFET c | I–V | 10 μM–1 mM | 4.1 μM | urine | [30] |
| P-GFET | I–V | 0.04–10 mM | 1.9 μM | urine | [31] | |||
| pH | 5.0, 6.0, 6.5, 7.0, 7.5, 8.0, 9.0 | 4.5–8 | 3D-printed G/PLA d | CV n | 2.02–11.22 | - | urine | [32] |
| IGZO-EGTFT e | I–V | 3–7 | - | - | [33] | |||
| I–V | 4–10 | - | - | [34] | ||||
| Protein (g/L) | 0.15, 0.3, 1.0, 3.0, 20 | Less than 30 mg/g | BCG-modified f SWCNT- FETs | Conductance | 0.07–70 mg/L | 18.6 μg/L | urine | [35] |
| GE/AuNPs/PTH-MB/MIP g | DPV o | 0.1 ng/L– 0.1 mg/L | 30 pg/L | urine | [36] | |||
| ZnO NRs-FET | I–V | 10 μg/L– 100 mg/L | 9.81 μg/L | serum | [37] | |||
| Ketone (mg/dL) | 5, 15, 40, 100 | Less than 1 mg/dL | Enzyme-depositedAu electrode | CV n | 6.25–100 mg/dL | 6.25 mg/dL | urine | [38] |
| (Cell/μL) | Non-hemolyzed: 10, 80, 100 Hemolyzed: 25, 80, 200 | 0–5 Cell/μL | MWCNT-MIP | Potential measurement | 1.0–10 mg/L | 1.0 mg/L | urine | [39] |
| Fc [CO-Glu-Cys-Gly-OH] | CV n | 0.1–1000 mg/L | 0.03 mg/L | serum | [40] | |||
| Nitrite | Negative, Positive | Less than 7 μM | Au/rGO-GECT h | I–V | 0.1 nM–7 μM 7–1000 μM | 0.1 nM | - | [41] |
| 3D-printed G/PLA | Amperometry | 0.5–250 μM | 0.03 μM | urine | [42] | |||
| Bilirubin (μM) | 17, 35, 70 | - | Er-GO | I–V | 0.1–600 μM | 0.1 ± 0.018 nM | urine | [43] |
| IAO-NRs/Nafion/GCE i | I–V | 0.1 nM–0.01 M | 16.5 ± 0.05 pM | urine | [44] | |||
| Leukocyte esterase (WBC/μL) | 25, 75, 500 | - | PE/DAS/Ag/film j | Resistivity | 20–80 WBC/μL | 20 WBC/μL | urine | [45] |
| NMP a-22 | Less than 5 ng/mL | Less than 5 ng/mL | NH2-SAPO-34-Pd/Co-Ab2 | CV n | 0.33 pg/mL | urine | [46] | |
| IGZO-FET | IGZO-FET | I–V | 2.7 amol/L | urine | [47] | |||
| IGZO-FET | IGZO-FET | I–V | 3.2 × 10−17 g/mL | urine | [48] | |||
| PSA b | 4–10 ng/mL | 4–10 ng/mL | Au-GrO | DPV o | 0.24 fg/mL | serum | [49] | |
| miR-107 | - | - | Screen-Printed Gold Electrodes | DPV o | 10 fM | urine | [50] | |
| Sodium ions | 40–220 mmol/day | 40–220 mmol/day | G-ISFET k with FG-RE | I–V | - | urine | [51] | |
| Potassium ions | 0–10 mM | 0–10 mM | Graphene Quantum Dots | Potentiometric | 1 mM | - | [52] | |
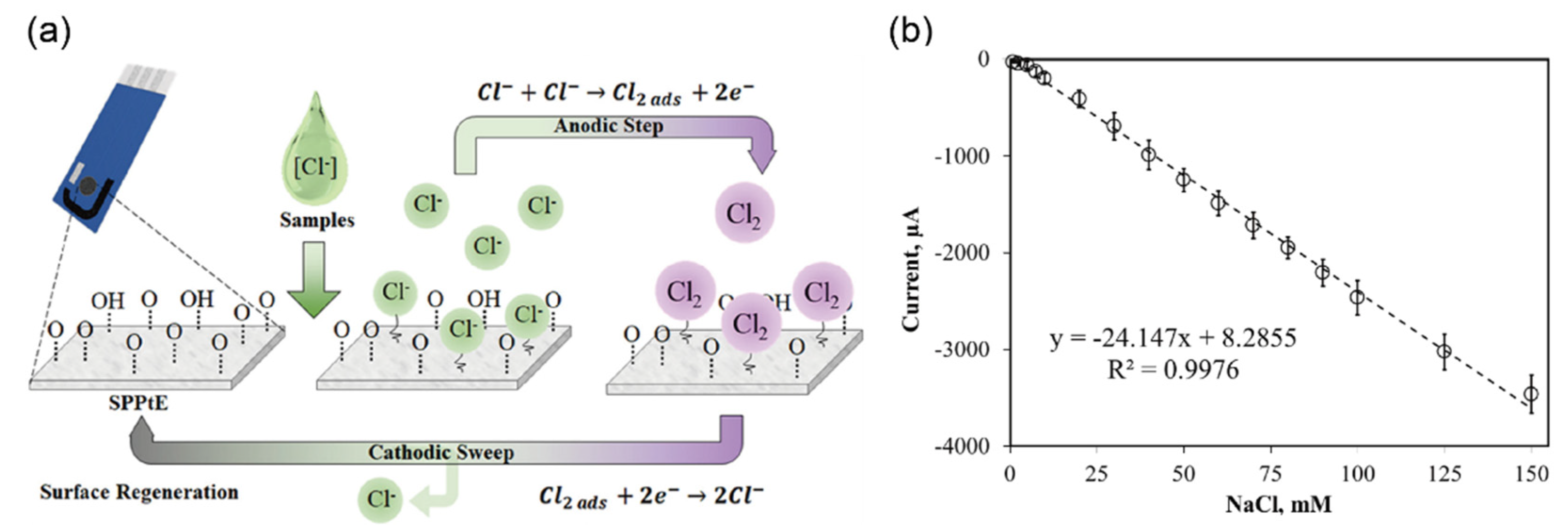
| Chloride ions | 20–40 mM | 20–40 mM | SPPtE l | CV n | 0.76 mM | urine | [53] | |
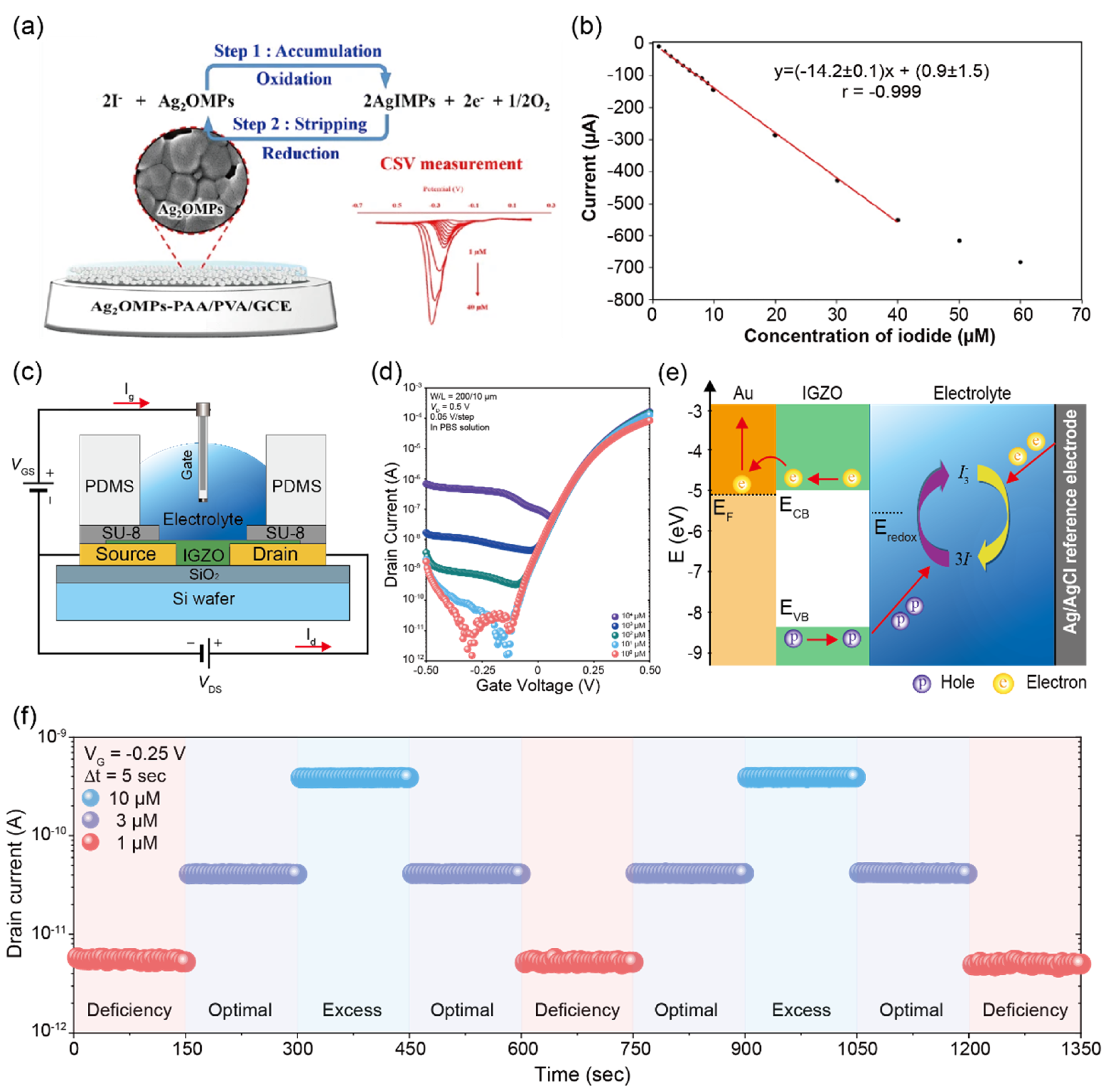
| Iodide ions | 0.3–6.0 μM | 0.3–6.0 μM | Ag2OMPs-PAA/PVA m | CSV p | 0.3 μM | urine | [54] | |
| IGZO | IGZO | I–V | 1 μM | - | [55] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, C.; Lee, W.-J.; Kim, S.D.; Park, S.; Kim, J.H. Recent Advances in Biosensor Technologies for Point-of-Care Urinalysis. Biosensors 2022, 12, 1020. https://doi.org/10.3390/bios12111020
Hwang C, Lee W-J, Kim SD, Park S, Kim JH. Recent Advances in Biosensor Technologies for Point-of-Care Urinalysis. Biosensors. 2022; 12(11):1020. https://doi.org/10.3390/bios12111020
Chicago/Turabian StyleHwang, Chuljin, Won-June Lee, Su Dong Kim, Sungjun Park, and Joo Hee Kim. 2022. "Recent Advances in Biosensor Technologies for Point-of-Care Urinalysis" Biosensors 12, no. 11: 1020. https://doi.org/10.3390/bios12111020
APA StyleHwang, C., Lee, W.-J., Kim, S. D., Park, S., & Kim, J. H. (2022). Recent Advances in Biosensor Technologies for Point-of-Care Urinalysis. Biosensors, 12(11), 1020. https://doi.org/10.3390/bios12111020






