A Lateral Flow Device for Point-of-Care Detection of Doxorubicin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Lateral Flow Device Assembly and Electrostatic Immobilization of the Capture Probe
2.3. Assay Procedure and Data Processing
2.4. Application in Urine Samples
3. Results
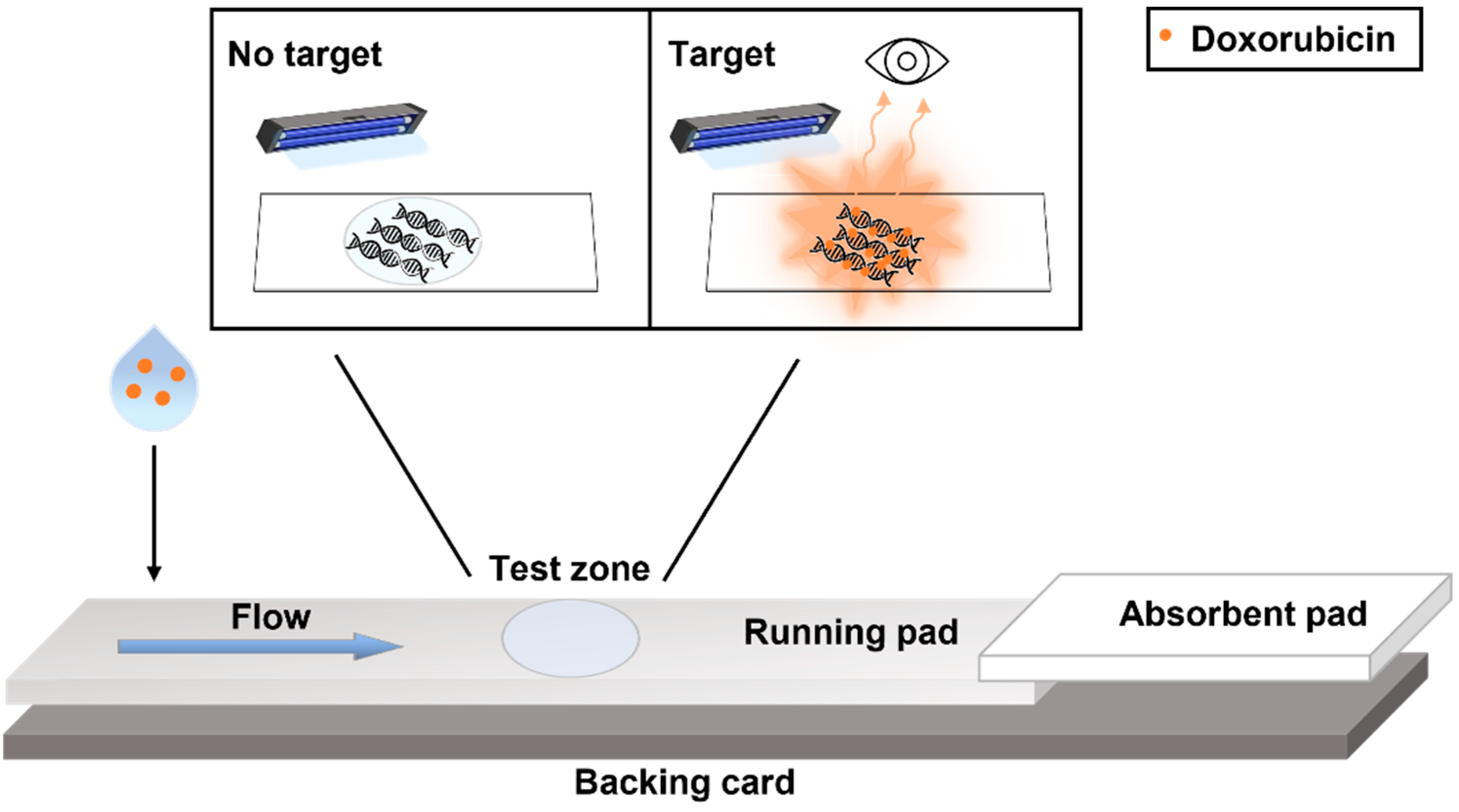
3.1. Device Design and Detection Mechanism
3.2. Optimization of the Capture Probe
3.3. Optimization of the Flow
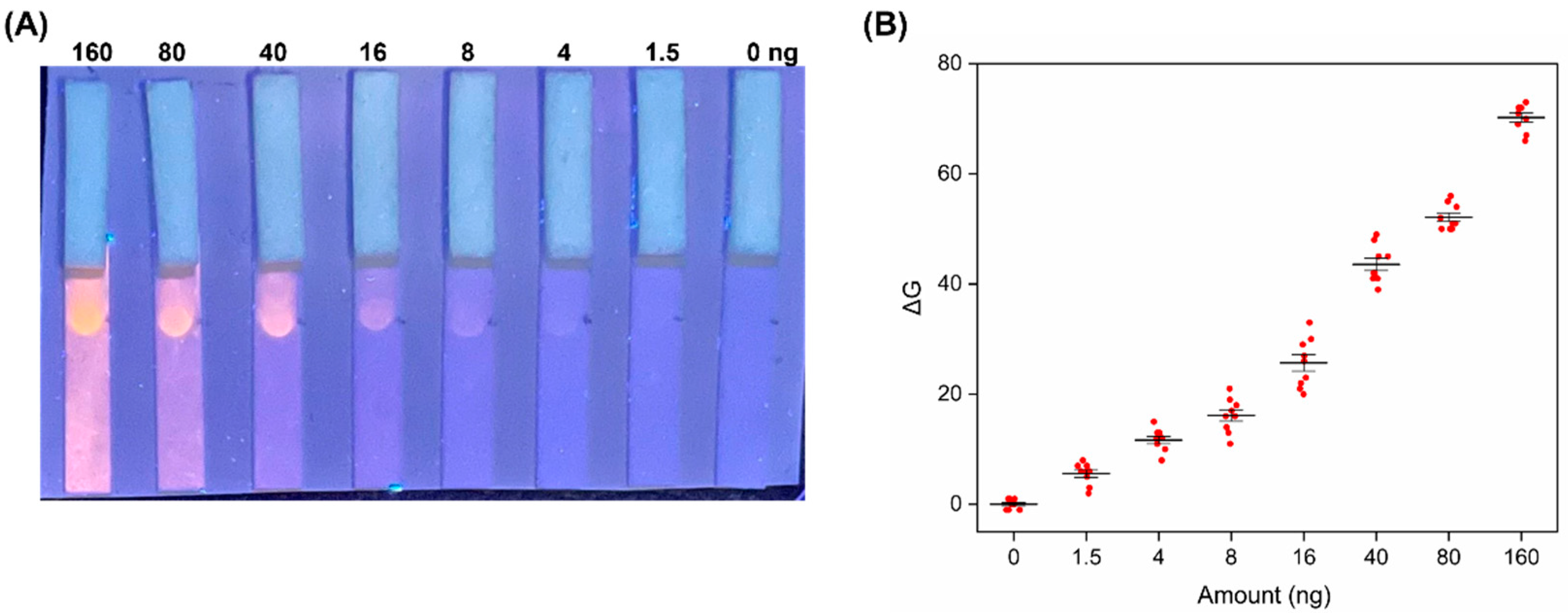
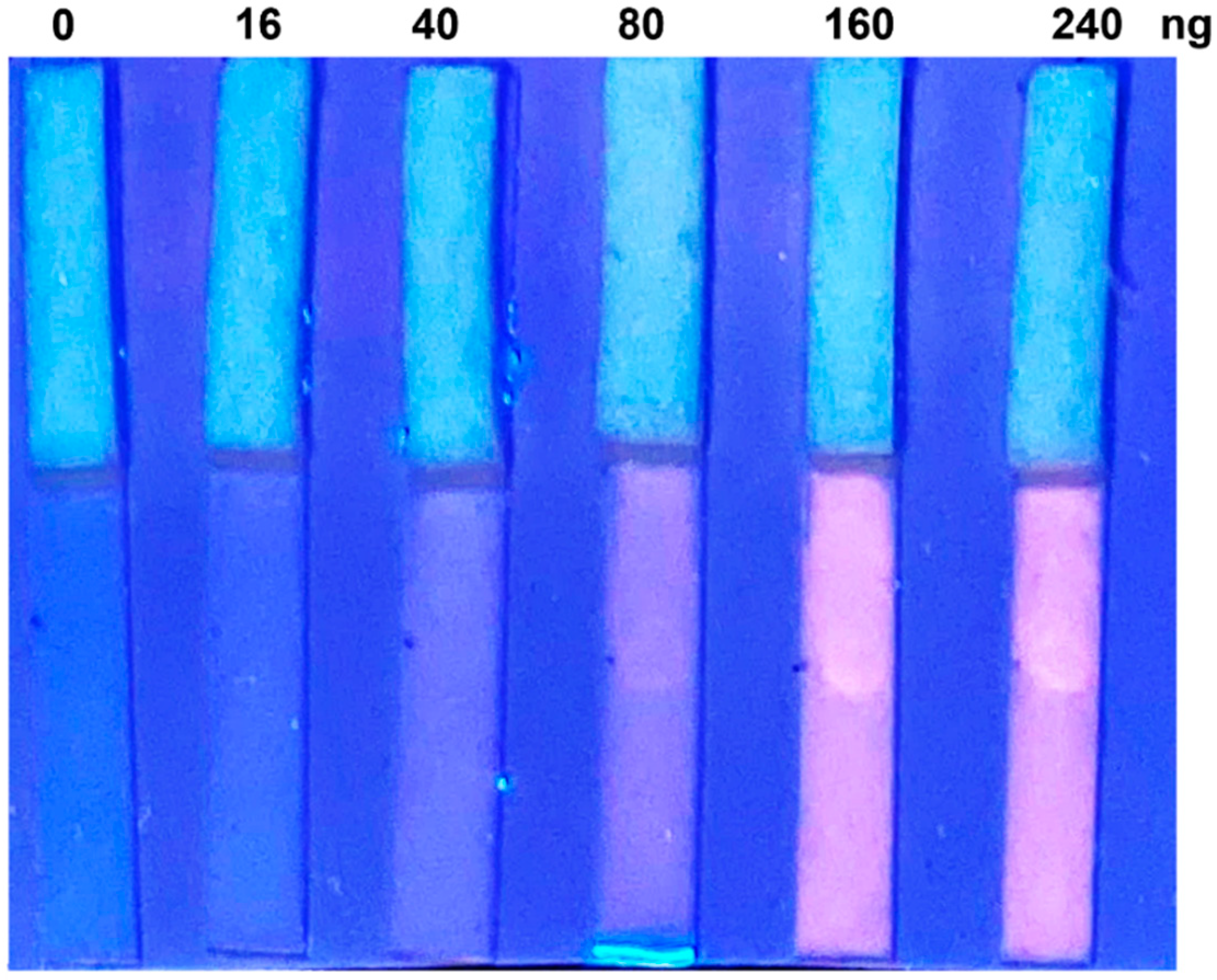
3.4. Analytical Performance
3.5. Detection in Urine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvalho, C.; Santos, R.; Cardoso, S.; Correia, S.; Oliveira, P.; Santos, M.; Moreira, P. Doxorubicin: The Good, the Bad and the Ugly Effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Chen, T.; Shen, H.M.; Deng, Z.Y.; Yang, Z.Z.; Zhao, R.L.; Wang, L.; Feng, Z.P.; Liu, C.; Li, W.H.; Liu, Z.J. A herbal formula, SYKT, reverses doxorubicin-induced myelosuppression and cardiotoxicity by inhibiting ROS-mediated apoptosis. Mol. Med. Rep. 2017, 15, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Marler-Hausen, T.; Holt, C.; Headley, C.; Sessink, P. Use of a closed-system drug transfer device reduces contamination with doxorubicin during bolus injection. Br. J. Nurs. 2020, 29, S15–S21. [Google Scholar] [CrossRef] [PubMed]
- Solimando, D.A.; Wilson, J.P. Demonstration of skin fluorescence following exposure to doxorubicin. Cancer Nurs. 1983, 6, 313–315. [Google Scholar]
- Álvarez-Cedrón, L.; Sayalero, M.L.; Lanao, J.M. High-performance liquid chromatographic validated assay of doxorubicin in rat plasma and tissues. J. Chromatogr. B Biomed. Sci. Appl. 1999, 721, 271–278. [Google Scholar] [CrossRef]
- Yang, M.; Yan, Y.; Liu, E.; Hu, X.; Hao, H.; Fan, J. Polyethyleneimine-functionalized carbon dots as a fluorescent probe for doxorubicin hydrochloride by an inner filter effect. Opt. Mater. 2021, 112, 110743. [Google Scholar] [CrossRef]
- El-Maghrabey, M.; Kishikawa, N.; Kamimura, S.; Ohyama, K.; Kuroda, N. Design of a dual functionalized chemiluminescence ultrasensitive probe for quinones based on their redox cycle. Application to the determination of doxorubicin in lyophilized powder and human serum. Sens. Actuators B Chem. 2021, 329, 129226. [Google Scholar] [CrossRef]
- Baurain, R.; Deprez-De Campeneere, D.; Trouet, A. Determination of daunorubicin, doxorubicin and their fluorescent metabolites by high-pressure liquid chromatography: Plasma levels in DBA2 mice. Cancer Chemother. Pharmacol. 1979, 2, 11–14. [Google Scholar] [CrossRef]
- Semreen, M.H.; Alniss, H.Y.; Mousa, M.K.; El-Awady, R.; Khan, F.; Al-Rub, K.A. Quantitative determination of doxorubicin in the exosomes of A549/MCF-7 cancer cells and human plasma using ultra performance liquid chromatography-tandem mass spectrometry. Saudi Pharm. J. 2018, 26, 1027–1034. [Google Scholar] [CrossRef]
- Ricciarello, R.; Pichini, S.; Pacifici, R.; Altieri, I.; Pellegrini, M.; Fattorossi, A.; Zuccaro, P. Simultaneous determination of epirubicin, doxorubicin and their principal metabolites in human plasma by high-performance liquid chromatography and electrochemical detection. J. Chromatogr. B Biomed. Appl. 1998, 707, 219–225. [Google Scholar] [CrossRef]
- Alarfaj, N.A.; El-Tohamy, M.F. New functionalized polymeric sensor based nio/mgo nanocomposite for potentiometric determination of doxorubicin hydrochloride in commercial injections and human plasma. Polymers 2020, 12, 3066. [Google Scholar] [CrossRef] [PubMed]
- Karukstis, K.K.; Thompson, E.H.Z.; Whiles, J.A.; Rosenfeld, R.J. Deciphering the fluorescence signature of daunomycin and doxorubicin. Biophys. Chem. 1998, 73, 249–263. [Google Scholar] [CrossRef]
- Motlagh, N.S.H.; Parvin, P.; Ghasemi, F.; Atyabi, F. Fluorescence properties of several chemotherapy drugs: Doxorubicin, paclitaxel and bleomycin. Biomed. Opt. Express 2016, 7, 2400. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Nguyen, T.T.; Nghiem, T.H.L.; Nguyen, D.T.; Tran, T.T.H.; Vu, D.; Nguyen, T.B.N.; Nguyen, T.M.H.; Nguyen, V.T.; Nguyen, M.H. Optical properties of doxorubicin hydrochloride load and release on silica nanoparticle platform. Molecules 2021, 26, 3968. [Google Scholar] [CrossRef] [PubMed]
- Van Raalte, J.; Rice, C.; Moss, C.E. Visible-light system for detecting doxorubicin contamination on skin and surfaces. Am. J. Hosp. Pharm. 1990, 47, 1067–1074. [Google Scholar] [CrossRef]
- Teymourian, H.; Parrilla, M.; Sempionatto, J.R.; Montiel, N.F.; Barfidokht, A.; Van Echelpoel, R.; De Wael, K.; Wang, J. Wearable Electrochemical Sensors for the Monitoring and Screening of Drugs. ACS Sens. 2020, 5, 2679–2700. [Google Scholar] [CrossRef] [PubMed]
- Hassani Moghadam, F.; Taher, M.A.; Karimi-Maleh, H. Doxorubicin anticancer drug monitoring by ds-dna-based electrochemical biosensor in clinical samples. Micromachines 2021, 12, 808. [Google Scholar] [CrossRef]
- Sikora, T.; Morawska, K.; Lisowski, W.; Rytel, P.; Dylong, A. Application of Optical Methods for Determination of Concentration of Doxorubicin in Blood and Plasma. Pharmaceuticals 2022, 15, 112. [Google Scholar] [CrossRef]
- Charbaji, A.; Heidari-Bafroui, H.; Anagnostopoulos, C.; Faghri, M. A new paper-based microfluidic device for improved detection of nitrate in water. Sensors 2021, 21, 102. [Google Scholar] [CrossRef]
- Uhrovčík, J. Strategy for determination of LOD and LOQ values—Some basic aspects. Talanta 2014, 119, 178–180. [Google Scholar] [CrossRef]
- Sarigul, N.; Korkmaz, F.; Kurultak, İ. A New Artificial Urine Protocol to Better Imitate Human Urine. Sci. Rep. 2019, 9, 20159. [Google Scholar] [CrossRef] [PubMed]
- Javani, A.; Javadi-Zarnaghi, F.; Rasaee, M.J. Development of a colorimetric nucleic acid-based lateral flow assay with non-biotinylated capture DNA. Appl. Biol. Chem. 2017, 60, 637–645. [Google Scholar] [CrossRef]
- Liu, J.; Mazumdar, D.; Lu, Y. A simple and sensitive “dipstick” test in serum based on lateral flow separation of aptamer-linked nanostructures. Angew. Chemie Int. Ed. 2006, 45, 7955–7959. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, H.; Onodera, K.; Fujita, H.; Sakamoto, T.; Akitomi, J.; Kaneko, N.; Shiratori, I.; Kuwahara, M.; Horii, K.; Waga, I. Selection, Characterization and Application of Artificial DNA Aptamer Containing Appended Bases with Sub-nanomolar Affinity for a Salivary Biomarker. Sci. Rep. 2017, 7, 42716. [Google Scholar] [CrossRef]
- Fang, Z.; Huang, J.; Lie, P.; Xiao, Z.; Ouyang, C.; Wu, Q.; Wu, Y.; Liu, G.; Zeng, L. Lateral flow nucleic acid biosensor for Cu2+ detection in aqueous solution with high sensitivity and selectivity. Chem. Commun. 2010, 46, 9043–9045. [Google Scholar] [CrossRef]
- Jauset-Rubio, M.; Svobodová, M.; Mairal, T.; McNeil, C.; Keegan, N.; Saeed, A.; Abbas, M.N.; El-Shahawi, M.S.; Bashammakh, A.S.; Alyoubi, A.O.; et al. Ultrasensitive, rapid and inexpensive detection of DNA using paper based lateral flow assay. Sci. Rep. 2016, 6, 37732. [Google Scholar] [CrossRef]
- Ying, N.; Ju, C.; Li, Z.; Liu, W.; Wan, J. Visual detection of nucleic acids based on lateral flow biosensor and hybridization chain reaction amplification. Talanta 2017, 164, 432–438. [Google Scholar] [CrossRef]
- Wang, L.; Ma, W.; Chen, W.; Liu, L.; Ma, W.; Zhu, Y.; Xu, L.; Kuang, H.; Xu, C. An aptamer-based chromatographic strip assay for sensitive toxin semi-quantitative detection. Biosens. Bioelectron. 2011, 26, 3059–3062. [Google Scholar] [CrossRef]
- Nimse, S.B.; Song, K.; Sonawane, M.D.; Sayyed, D.R.; Kim, T. Immobilization techniques for microarray: Challenges and applications. Sensors 2014, 14, 22208–22229. [Google Scholar] [CrossRef]
- Singh, V.; Zharnikov, M.; Gulino, A.; Gupta, T. DNA immobilization, delivery and cleavage on solid supports. J. Mater. Chem. 2011, 21, 10602–10618. [Google Scholar] [CrossRef]
- James Cleaves, H.; Crapster-Pregont, E.; Jonsson, C.M.; Jonsson, C.L.; Sverjensky, D.A.; Hazen, R.A. The adsorption of short single-stranded DNA oligomers to mineral surfaces. Chemosphere 2011, 83, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Parolo, C.; Sena-Torralba, A.; Bergua, J.F.; Calucho, E.; Fuentes-Chust, C.; Hu, L.; Rivas, L.; Álvarez-Diduk, R.; Nguyen, E.P.; Cinti, S.; et al. Tutorial: Design and fabrication of nanoparticle-based lateral-flow immunoassays. Nat. Protoc. 2020, 15, 3788–3816. [Google Scholar] [CrossRef] [PubMed]
- Sastry, C.S.P.; Lingeswara Rao, J.S.V.M. Determination of doxorubicin hydrochloride by visible spectrophotometry. Talanta 1996, 43, 1827–1835. [Google Scholar] [CrossRef]
- Pradhan, N.; Rajkhowa, H.; Giri, H.; Shrestha, B. Simultaneous spectrophotometric estimation of moxifloxacin hydrochloride and doxorubicin hydrochloride. Int. J. Pharm. Pharm. Sci. 2015, 7, 21–26. [Google Scholar]
- Jouyban, A.; Samadi, A.; Jouyban-Gharamaleki, V.; Khoubnasabjafari, M. A microscale spectrophotometric method for quantification of doxorubicin in exhaled breath condensate. Anal. Methods 2019, 11, 648–653. [Google Scholar] [CrossRef]
- Kauffman, M.; Kauffman, M.; Zhu, H.; Jia, Z.; Li, Y. Fluorescence-Based Assays for Measuring Doxorubicin in Biological Systems. React. Oxyg. Species 2016, 2, 432–439. [Google Scholar] [CrossRef]
- Tavallali, H.; Jahanbekam, A. Flow injection spectrophotometric determination of doxorubicin hydrochloride in urine samples. Int. J. PharmTech Res. 2010, 2, 1943–1947. [Google Scholar]
- Maliszewska, O.; Plenis, A.; Olędzka, I.; Kowalski, P.; Miękus, N.; Bień, E.; Krawczyk, M.A.; Adamkiewicz-Drożynska, E.; Bączek, T. Optimization of LC method for the quantification of doxorubicin in plasma and urine samples in view of pharmacokinetic, biomedical and drug monitoring therapy studies. J. Pharm. Biomed. Anal. 2018, 158, 376–385. [Google Scholar] [CrossRef]
| Sample Type | LOD (µM) | Preparative Procedure | Visual Detection (Instrument-Free) | Ref. |
|---|---|---|---|---|
| Pharmaceutical formulations | 0.034–0.22 | - | X | [33] |
| Pharmaceutical formulations | 0.46 | - | X | [34] |
| Exhaled breath condensate | 0.004 | Water bath 70 °C for 10 min | X | [35] |
| Skin and surfaces | 1.8 | - | ✓ | [15] |
| Blood and plasma | 0.92 | Extraction with ethyl acetate and drying | X | [18] |
| Cultured cells | 0.18 | Washing and lysis | X | [36] |
| Urine samples | 0.055 | Flow injection | X | [37] |
| Surfaces and urine samples | 0.10 | - | ✓ | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pomili, T.; Gatto, F.; Pompa, P.P. A Lateral Flow Device for Point-of-Care Detection of Doxorubicin. Biosensors 2022, 12, 896. https://doi.org/10.3390/bios12100896
Pomili T, Gatto F, Pompa PP. A Lateral Flow Device for Point-of-Care Detection of Doxorubicin. Biosensors. 2022; 12(10):896. https://doi.org/10.3390/bios12100896
Chicago/Turabian StylePomili, Tania, Francesca Gatto, and Pier Paolo Pompa. 2022. "A Lateral Flow Device for Point-of-Care Detection of Doxorubicin" Biosensors 12, no. 10: 896. https://doi.org/10.3390/bios12100896
APA StylePomili, T., Gatto, F., & Pompa, P. P. (2022). A Lateral Flow Device for Point-of-Care Detection of Doxorubicin. Biosensors, 12(10), 896. https://doi.org/10.3390/bios12100896






