Au-Ag Alloy Nanoshuttle Mediated Surface Plasmon Coupling for Enhanced Fluorescence Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Au Substrate Preparation and Modification
2.3. Preparation of RhB-PMMA Solution
2.4. Cell Culture
2.5. Chip Preparation for Imaging
2.6. Variable-Angle Nanoplasmonic Fluorescence Microscope
3. Results and Discussion
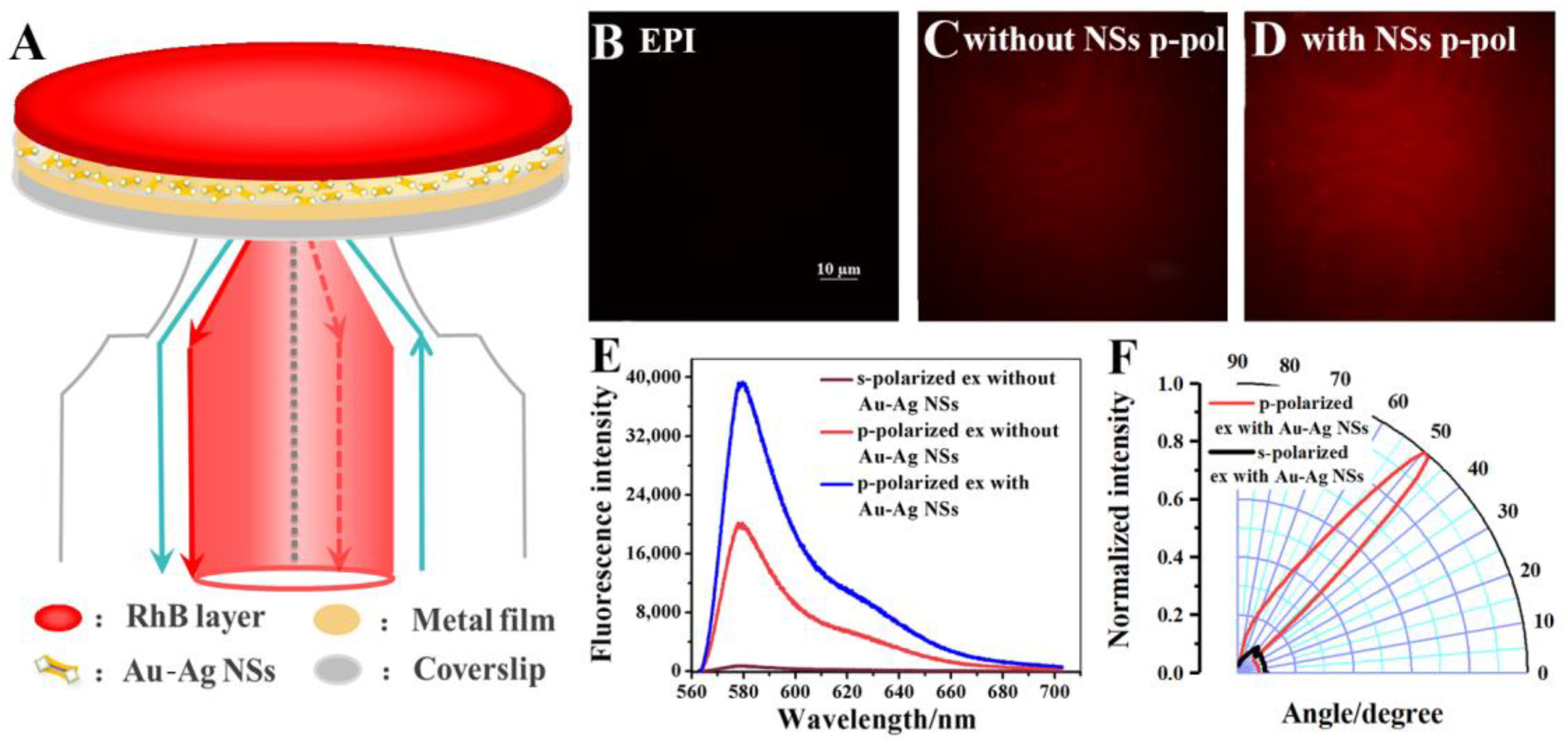
3.1. Au-Ag NS Mediated VANFM with RhB-PMMA Layer as a Model
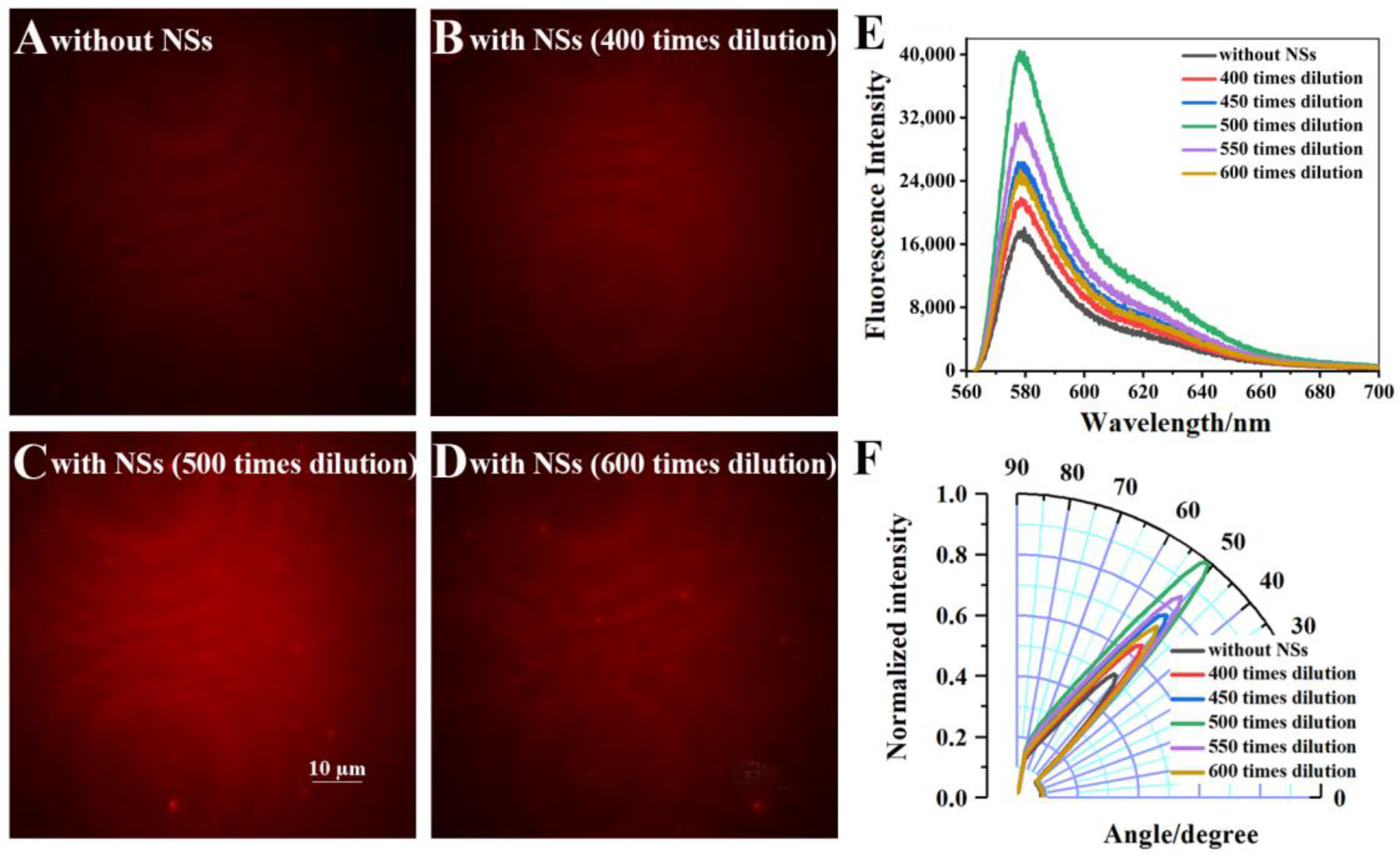
3.2. The Cell Imaging Enhancement by Au-Ag NS through VANFM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lichtman, J.W.; Conchello, J.A. Fluorescence microscopy. Nat. Methods 2005, 2, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Bates, M.; Zhuang, X.W. Super-resolution fluorescence microscopy. Annu. Rev. Biochem. 2009, 78, 993–1016. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Cao, S.H.; Li, Y.Q. Surface plasmon-coupled emission imaging for biological applications. Anal. Bioanal. Chem. 2020, 412, 6085–6100. [Google Scholar] [CrossRef] [PubMed]
- Yuk, J.S.; Guignon, E.F.; Lynes, M.A. Sensitivity enhancement of a grating-based surface plasmon-coupled emission (SPCE) biosensor chip using gold thickness. Chem. Phys. Lett. 2014, 591, 5–9. [Google Scholar] [CrossRef]
- Cao, S.H.; Cai, W.P.; Liu, Q.; Li, Y.Q. Surface plasmon-coupled emission: What can directional fluorescence bring to the analytical sciences? Annu. Rev. Anal. Chem. 2012, 5, 317–336. [Google Scholar] [CrossRef]
- Su, Q.; Jiang, C.; Gou, D.M.; Long, Y. Surface plasmon-assisted fluorescence enhancing and quenching: From theory to application. ACS Appl. Bio Mater. 2021, 4, 4684–4705. [Google Scholar] [CrossRef]
- Bhaskar, S.; Kambhampati, N.S.V.; Ganesh, K.M.; Sharma, P.M.; Srinivasan, V.; Ramamurthy, S.S. Metal-Free, Graphene oxide-based tunable soliton and plasmon engineering for biosensing applications. ACS Appl. Mater. Interfaces 2021, 13, 17046–17061. [Google Scholar] [CrossRef]
- Xie, K.X.; Liu, Q.; Song, X.L.; Huo, R.P.; Shi, X.H.; Liu, Q.L. Amplified fluorescence by hollow-porous plasmonic assembly: A new observation and its application in multiwavelength simultaneous detection. Anal. Chem. 2021, 93, 3671–3676. [Google Scholar] [CrossRef]
- Xie, K.X.; Liu, C.; Liu, Q.; Xiao, X.X.; Li, Z.; Li, M.F. Multiarchitecture-based plasmonic-coupled emission employing gold nanoparticles: An efficient fluorescence modulation and biosensing platform. Langmuir 2021, 37, 11880–11886. [Google Scholar] [CrossRef]
- Xu, L.T.; Chen, M.; Weng, Y.H.; Xie, K.X.; Wang, J.; Cao, S.H.; Li, Y.Q. Label-free fluorescent nanofilm sensor based on surface plasmon coupled emission: In situ monitoring the growth of metal–organic frameworks. Anal. Chem. 2022, 94, 6430–6435. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative decay engineering 3. Surface plasmon-coupled directional emission. Anal. Biochem. 2004, 324, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Yuk, J.S.; McDonagh, C.; MacCraith, B.D. Demonstration of a surface plasmon-coupled emission (SPCE)-based immunoassay in the absence of a spacer layer. Anal. Bioanal. Chem. 2010, 398, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.; Suter, D.; Srinivasan, V.; Venkatesan, K.; Ramamurthy, S.S. Concentration effect in surface plasmon-coupled phosphorescence (SPCP) emission engineering with augmented s-polarization from N-heterocyclic carbene platinum(II) complexes. J. Phys. Chem. C 2021, 125, 16681–16688. [Google Scholar] [CrossRef]
- Tang, W.T.; Chung, E.; Kim, Y.H.; So, P.T.C.; Sheppard, C.J.R. Investigation of the point spread function of surface plasmon-coupled emission microscopy. Opt. Express 2007, 15, 4634–4646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.G.; Yuan, X.C.; Bouhelier, A. Direct image of surface-plasmon-coupled emission by leakage radiation microscopy. Appl. Opt. 2010, 49, 875–879. [Google Scholar] [CrossRef]
- Chen, B.; Wood, A.; Pathak, A.; Mathai, J.; Bok, S.; Zheng, H.; Hamm, S.; Basuray, S.; Grant, S.; Gangopadhyay, K.; et al. Plasmonic gratings with nano-protrusions made by glancing angle deposition for single-molecule super-resolution imaging. Nanoscale 2016, 8, 12189–12201. [Google Scholar] [CrossRef]
- Wood, A.; Mathai, C.J.; Gangopadhyay, K.; Grant, S.; Gangopadhyay, S. Single-molecule surface plasmon-coupled emission with plasmonic gratings. ACS Omega 2017, 2, 2041–2045. [Google Scholar] [CrossRef]
- Chen, M.; Pan, X.H.; Liu, Q.; Huo, S.X.; Cao, S.H.; Zhai, Y.Y.; Zhao, Y.; Li, Y.Q. Variable-angle nanoplasmonic fluorescence microscopy: An axially resolved method for tracking the endocytic pathway. Anal. Chem. 2019, 91, 13658–13664. [Google Scholar] [CrossRef]
- Pan, X.H.; Chen, M.; Cao, S.H.; Xu, Z.Q.; Li, Z.; Li, Y.Q. Plasmon coupling enhanced micro-spectroscopy and imaging for sensitive discrimination of membrane domains of a single cell. Chem. -Eur. J. 2021, 27, 17331–17335. [Google Scholar] [CrossRef]
- Hua, Z.; Yu, T.; Liu, D.; Xianyu, Y. Recent advances in gold nanoparticles-based biosensors for food safety detection. Biosens. Bioelectron. 2021, 179, 113076. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications-a review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Zannotti, M.; Rossi, A.; Giovannetti, R. SERS activity of silver nanosphere, triangular nanoplates, hexagonal nanoplates and quasi-spherical nanoparticles: Effect of shape and morphology. Coatings 2020, 10, 288. [Google Scholar] [CrossRef]
- Bhaskar, S.; Rai, A.; Ganesh, K.M.; Reddy, R.; Reddy, N.; Ramamurthy, S.S. Sericin-based bio-inspired nano-engineering of heterometallic AgAu nanocubes for attomolar mefenamic acid sensing in the mobile phone-based surface plasmon-coupled interface. Langmuir 2022, 38, 12035–12049. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, A.; Asila, V.; Boitel-Aullen, G.; Lam, M.; Salmain, M.; Boujday, S. Silver-based plasmonic nanoparticles for and their use in biosensing. Biosensors 2019, 9, 78. [Google Scholar] [CrossRef]
- Gong, X.Z.; Yang, Y.; Huang, S.M. A novel side-selective galvanic reaction and synthesis of hollow nanoparticles with an alloy core. J. Phys. Chem. C 2010, 114, 18073–18080. [Google Scholar] [CrossRef]
- Huang, J.F.; Zhu, Y.H.; Liu, C.X.; Zhao, Y.F.; Liu, Z.H.; Hedhili, M.N.; Fratalocchi, A.; Han, Y. Fabricating a homogeneously alloyed AuAg shell on Au nanorods to achieve strong, stable, and tunable surface plasmon resonances. Small 2015, 11, 5214–5221. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Tan, J.; Zhang, H.F.; Zhang, G.G.; Liu, F.; Liu, M.C.; Wang, Y.; Zheng, Y.Q. One-pot synthesis of wavy gold-silver alloy nanoplates with tunable elemental compositions: Optical and photothermal properties. J. Alloys Compd. 2021, 889, 161767. [Google Scholar] [CrossRef]
- Tian, Q.H.; Cao, S.Y.; He, G.Y.; Long, Y.T.; Zhou, X.D.; Zhang, J.H.; Xie, J.; Zhao, X.J. Plasmonic Au-Ag alloy nanostars based high sensitivity surface enhanced Raman spectroscopy fiber probes. J. Alloys Compd. 2022, 900, 163345. [Google Scholar] [CrossRef]
- Lei, W.S.; Wang, F.Z.; Pan, X.H.; Lu, B.; Ye, Z.Z. The Au-Ag alloy nanoshuttle-TiO2 nanostructure with enhanced H-2 production under visible light and inactivation analysis. Nanotechnology 2020, 31, 095406. [Google Scholar] [CrossRef]
- Hwang, C.S.H.; Ahn, M.S.; Lee, Y.; Chung, T.; Jeong, K.H. Ag/Au alloyed nanoislands for wafer-level plasmonic color filter arrays. Sci. Rep. 2019, 9, 9082. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.S.; Zhang, X.; Li, K.Y.; Yu, X.F. Optical properties of Au-Ag core/shell nanoshuttles. Opt. Express 2008, 16, 14288–14293. [Google Scholar] [CrossRef] [PubMed]
- Li, D.X.; Zhang, X.F.; Zhu, J.; Wu, C.X.; Zheng, T.R.; Li, C.F.; Cao, M.W. Shuttle-like core-shell gold nanorod@Ag-Au nanostructures: Shape control and electrocatalytic activity for formaldehyde oxidation. Appl. Surf. Sci. 2020, 528, 146935. [Google Scholar] [CrossRef]
- Bai, T.L.; Sun, J.F.; Che, R.C.; Xu, L.N.; Yin, C.Y.; Guo, Z.R.; Gu, N. Controllable preparation of core-shell Au-Ag nanoshuttles with improved refractive index sensitivity and SERS activity. ACS Appl. Mater. Interfaces 2014, 6, 3331–3340. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.J.; Wu, X.C.; Liu, D.F.; Feng, L.L.; Zhang, K.; Chu, W.H.; Zhou, W.Y.; Xie, S.S. Tuning the morphology of gold nanocrystals by switching the growth of {110} facets from restriction to preference. J. Phys. Chem. C 2008, 112, 3203–3208. [Google Scholar] [CrossRef]
- Sako, Y.; Minoguchi, S.; Yanagida, T. Single-molecule imaging of EGFR signalling on the surface of living cells. Nat. Cell Biol. 2000, 2, 168–172. [Google Scholar] [CrossRef]
- Cao, S.H.; Zou, Z.X.; Weng, Y.H.; Cai, W.P.; Liu, Q.; Li, Y.Q. Plasmon-mediated fluorescence with distance independence: From model to a biosensing application. Biosens. Bioelectron. 2014, 58, 258–265. [Google Scholar] [CrossRef]
- Gryczynski, Z.; Borejdo, J.; Calander, N.; Matweva, E.G.; Gryczynski, I. Minimization of detection volume by surface-plasmon-coupled emission. Anal. Biochem. 2006, 356, 125–131. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, K.-X.; Li, Z.; Fang, J.-H.; Cao, S.-H.; Li, Y.-Q. Au-Ag Alloy Nanoshuttle Mediated Surface Plasmon Coupling for Enhanced Fluorescence Imaging. Biosensors 2022, 12, 1014. https://doi.org/10.3390/bios12111014
Xie K-X, Li Z, Fang J-H, Cao S-H, Li Y-Q. Au-Ag Alloy Nanoshuttle Mediated Surface Plasmon Coupling for Enhanced Fluorescence Imaging. Biosensors. 2022; 12(11):1014. https://doi.org/10.3390/bios12111014
Chicago/Turabian StyleXie, Kai-Xin, Zhao Li, Jia-Hua Fang, Shuo-Hui Cao, and Yao-Qun Li. 2022. "Au-Ag Alloy Nanoshuttle Mediated Surface Plasmon Coupling for Enhanced Fluorescence Imaging" Biosensors 12, no. 11: 1014. https://doi.org/10.3390/bios12111014
APA StyleXie, K.-X., Li, Z., Fang, J.-H., Cao, S.-H., & Li, Y.-Q. (2022). Au-Ag Alloy Nanoshuttle Mediated Surface Plasmon Coupling for Enhanced Fluorescence Imaging. Biosensors, 12(11), 1014. https://doi.org/10.3390/bios12111014




