Dual Optical Nanosensor Based on Ormosil Nanoparticles for Monitoring O2 and pH
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Instruments
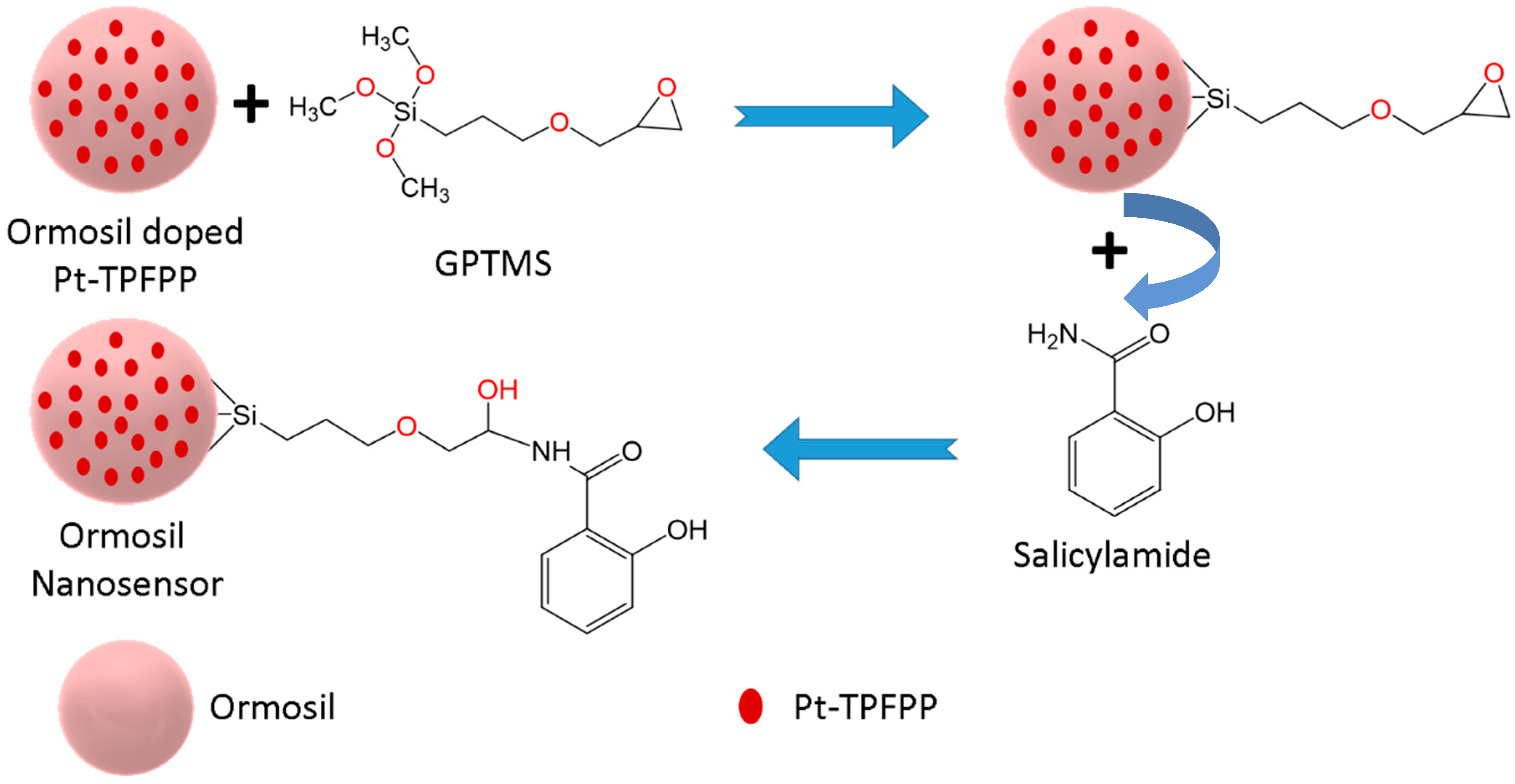
2.3. One-Pot Synthesis of Ormosil Nanosensor
2.4. UV-Vis and Fluorescence Measurements for Salicylamide and Ormosil Nanosensor
3. Results
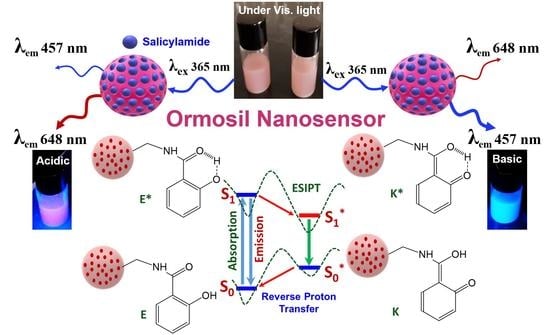
3.1. Choice of Materials and Method of Preparation
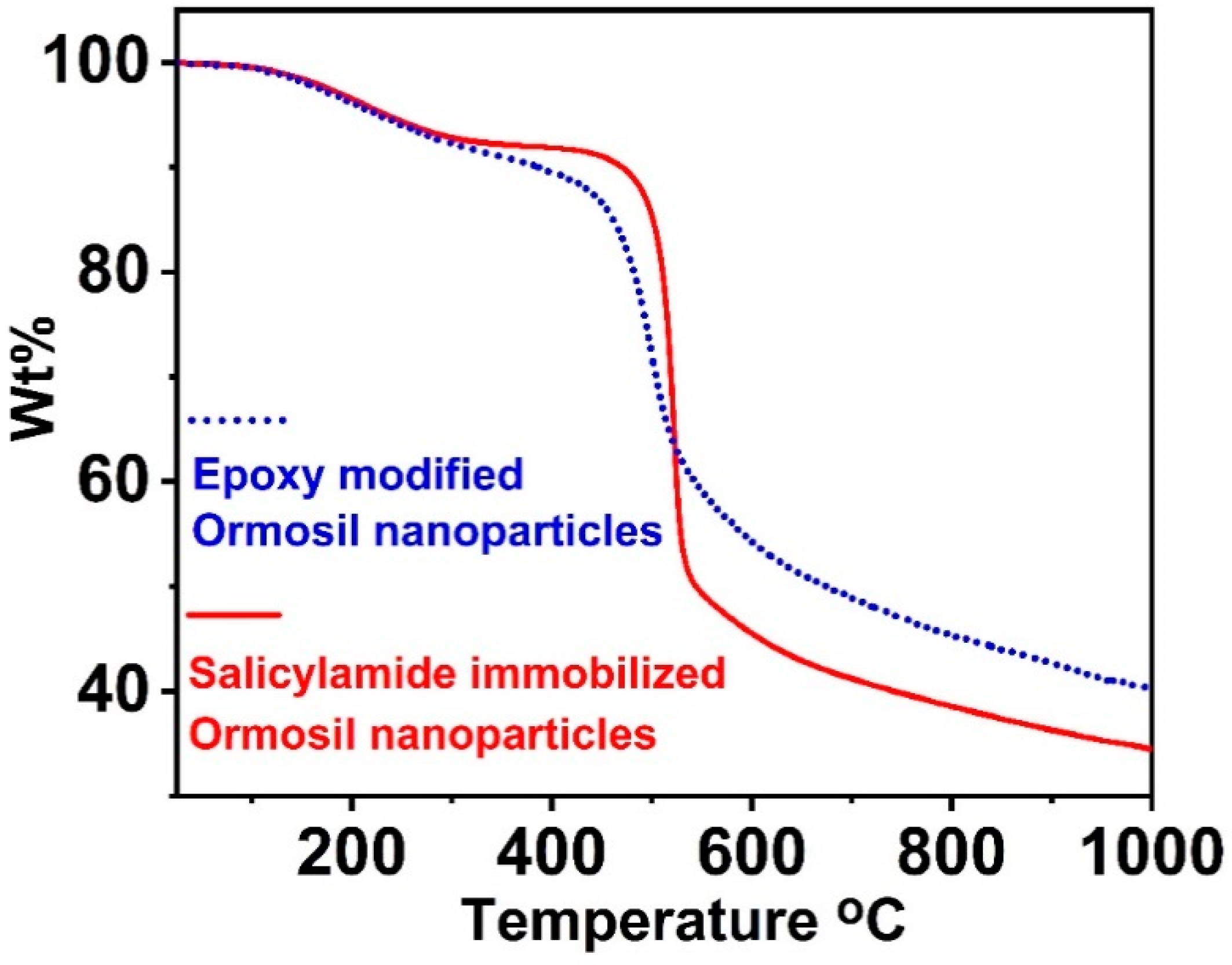
3.2. Structural Characterization of the Ormosil Nanosensor
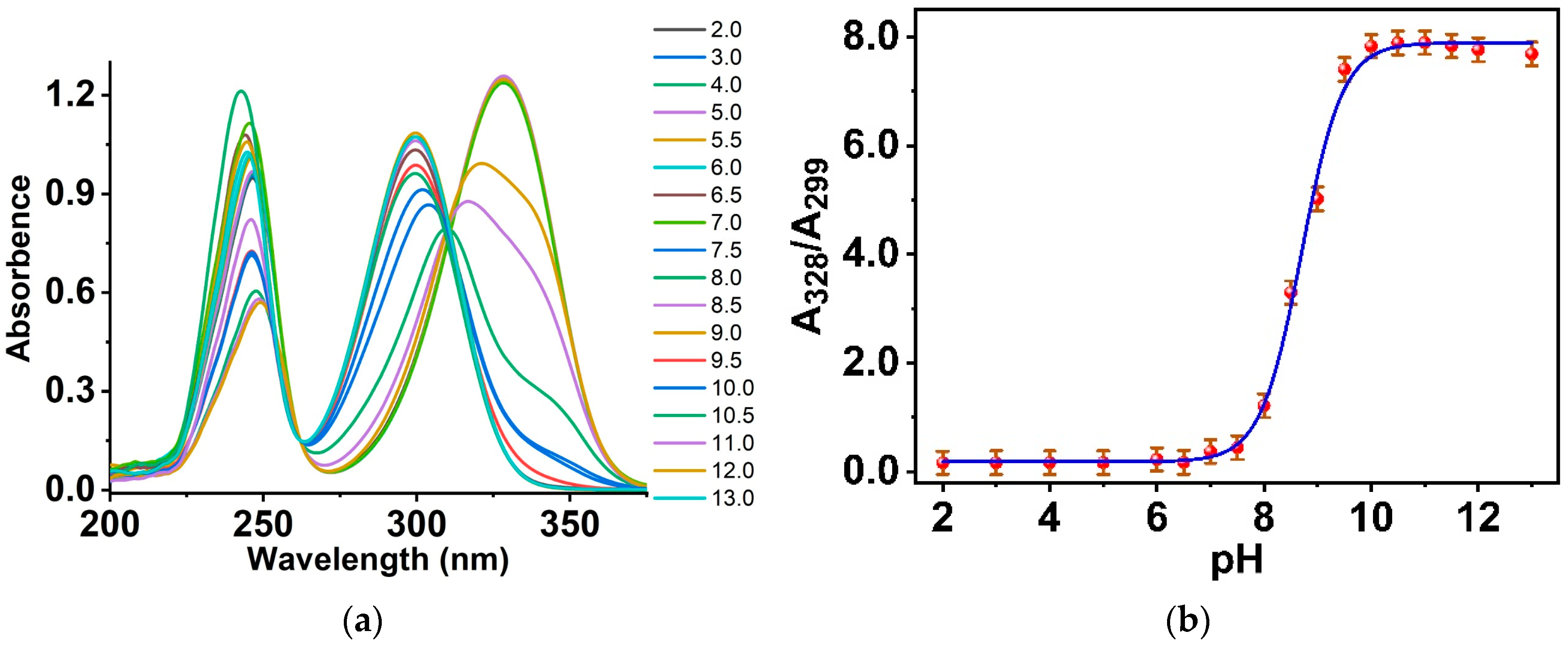
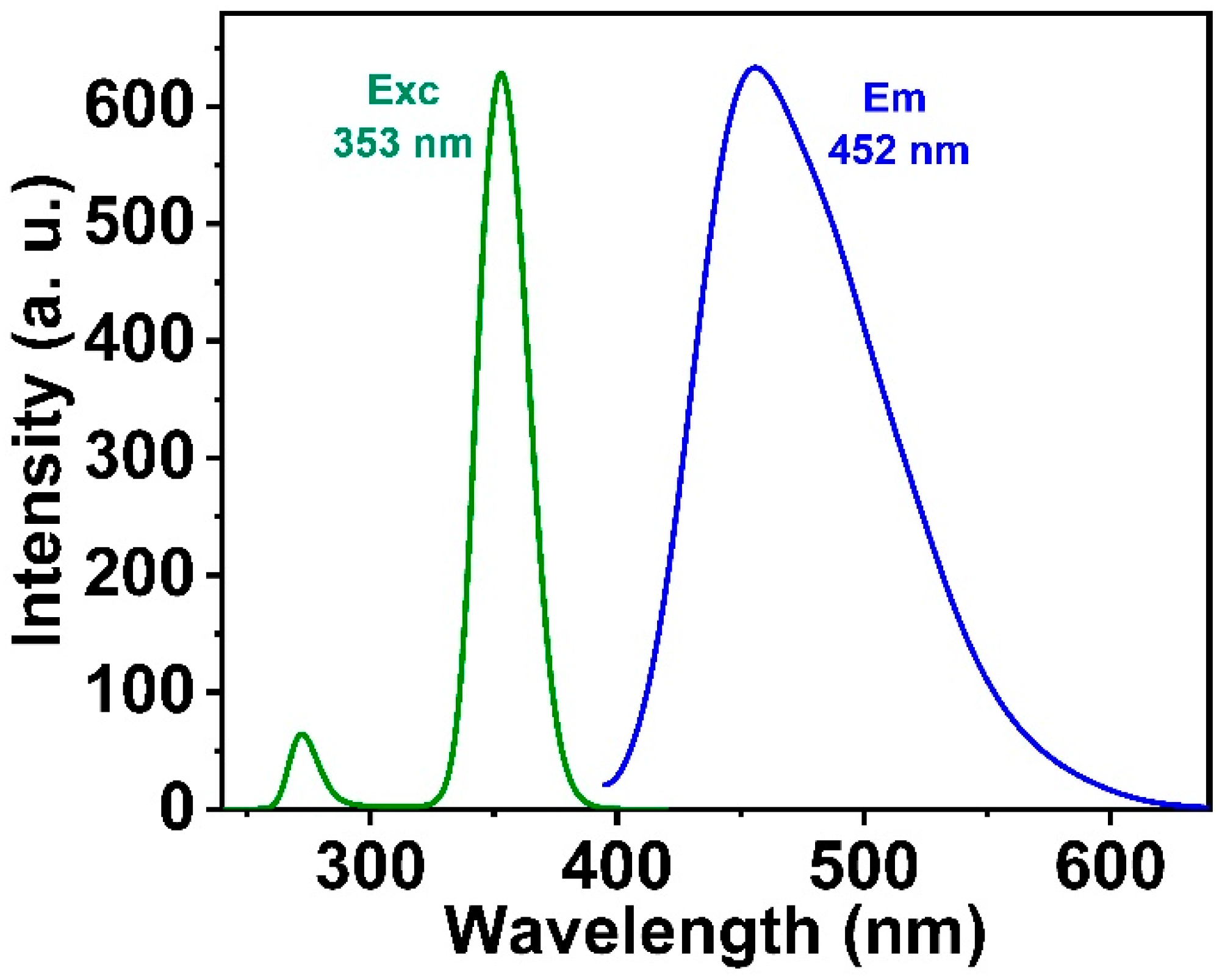
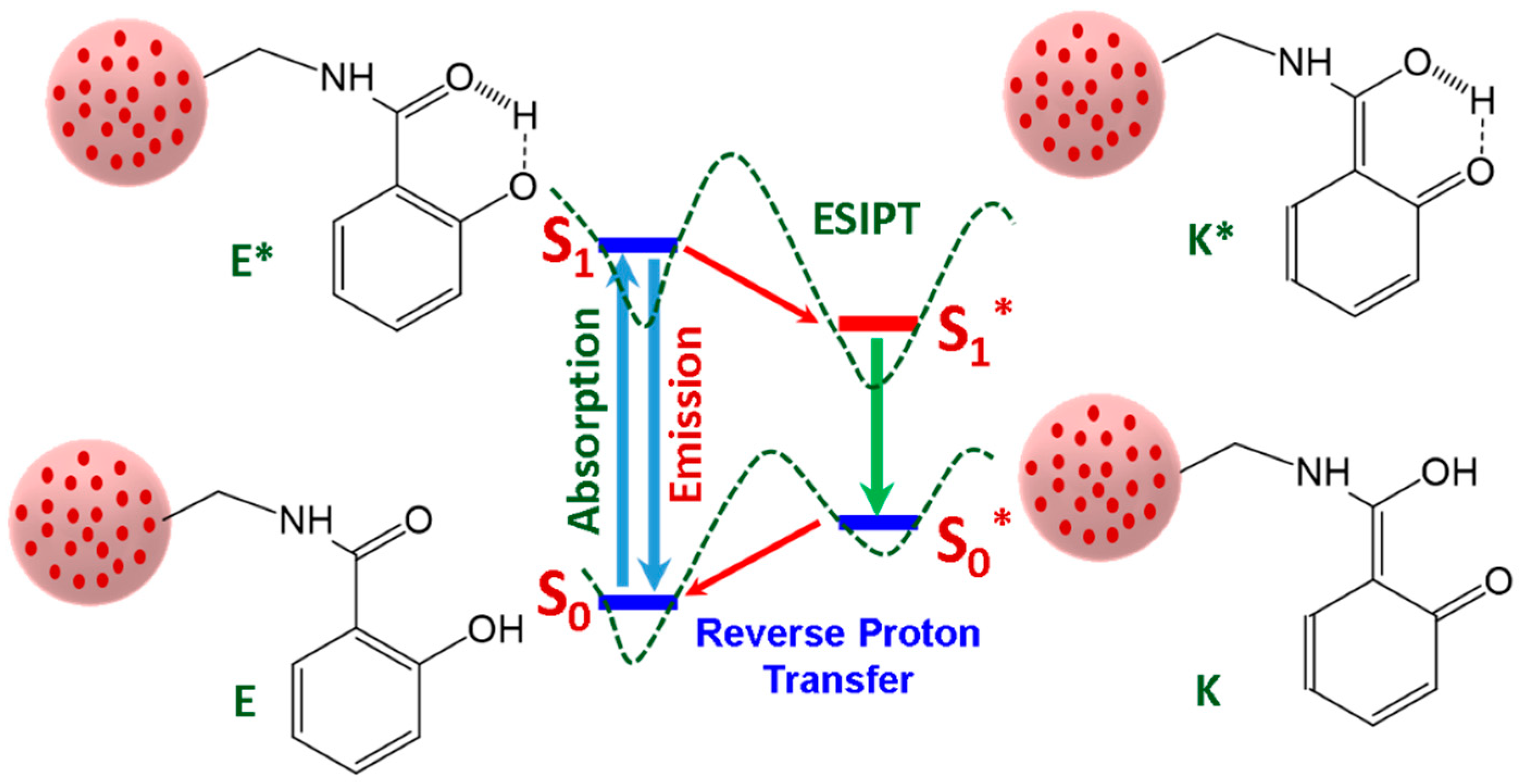
3.3. Optical Properties of Salicylamide and the Ormosil Nanosensor
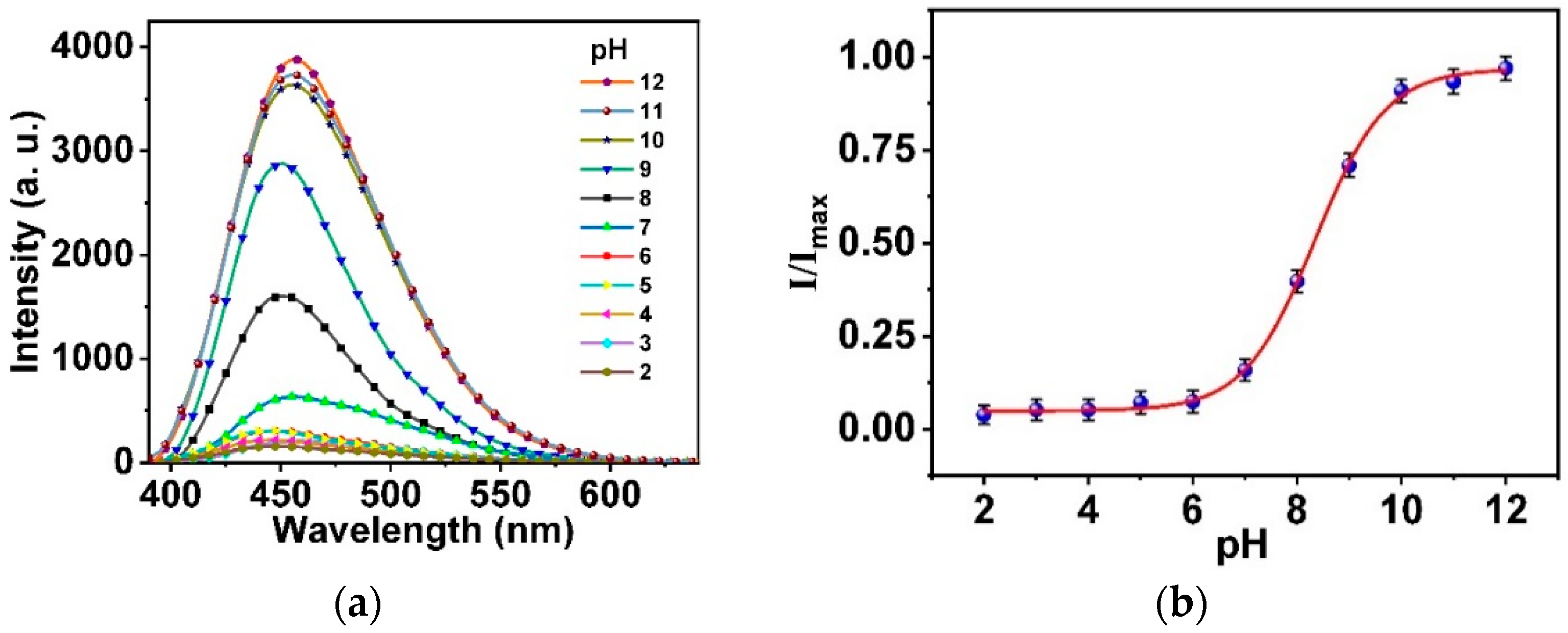
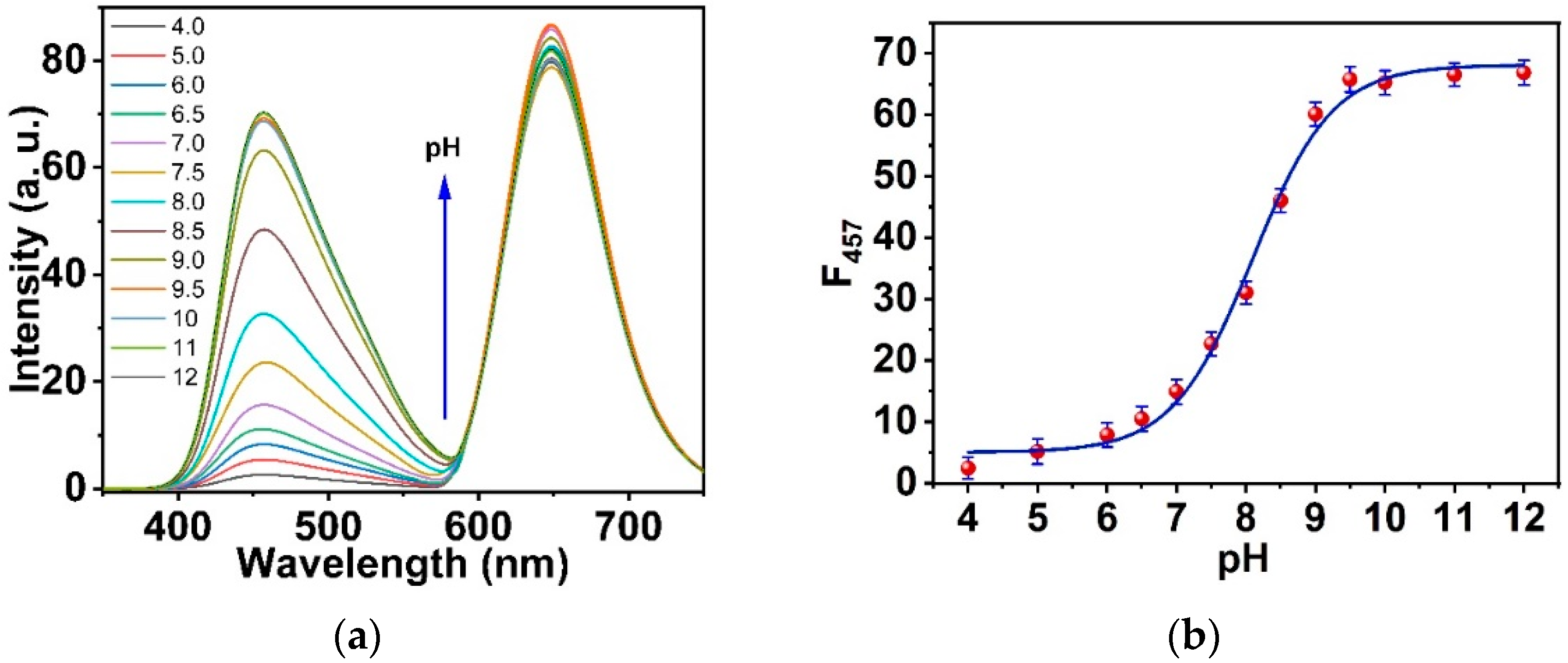
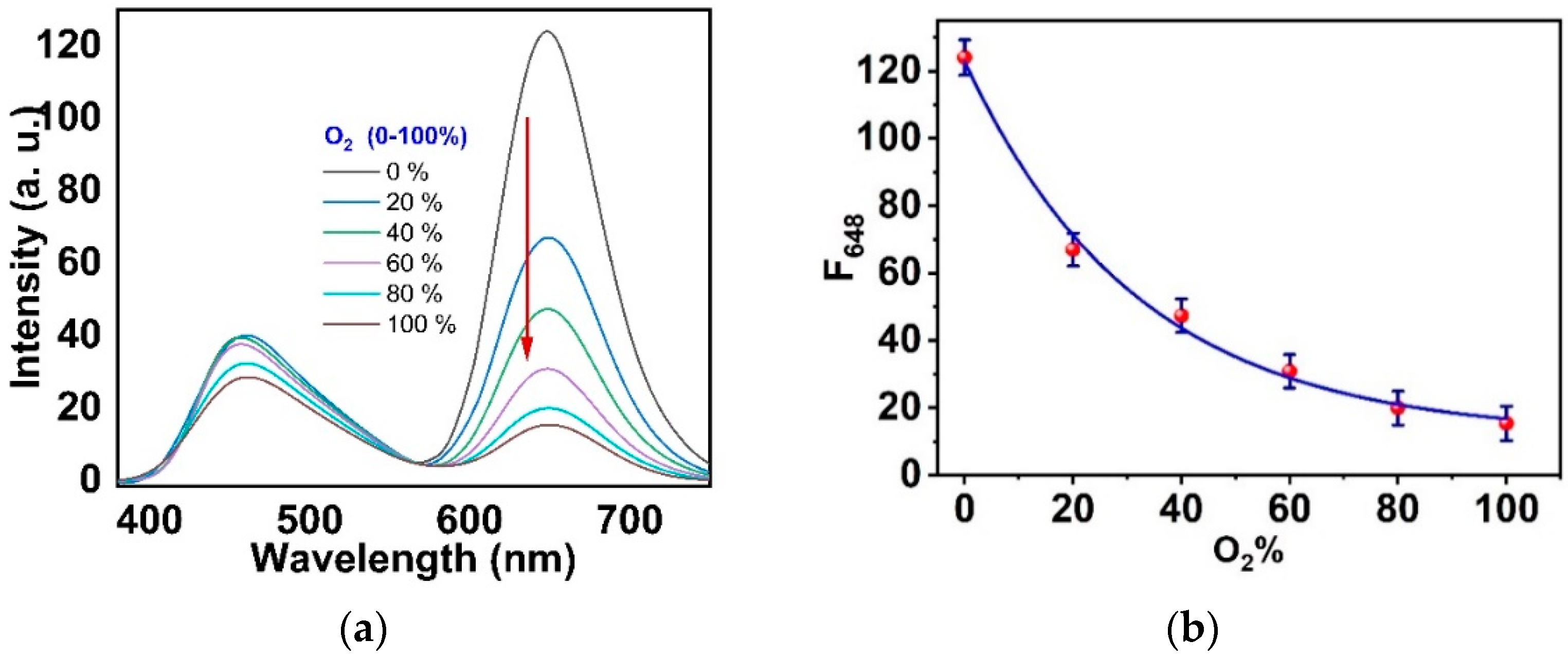
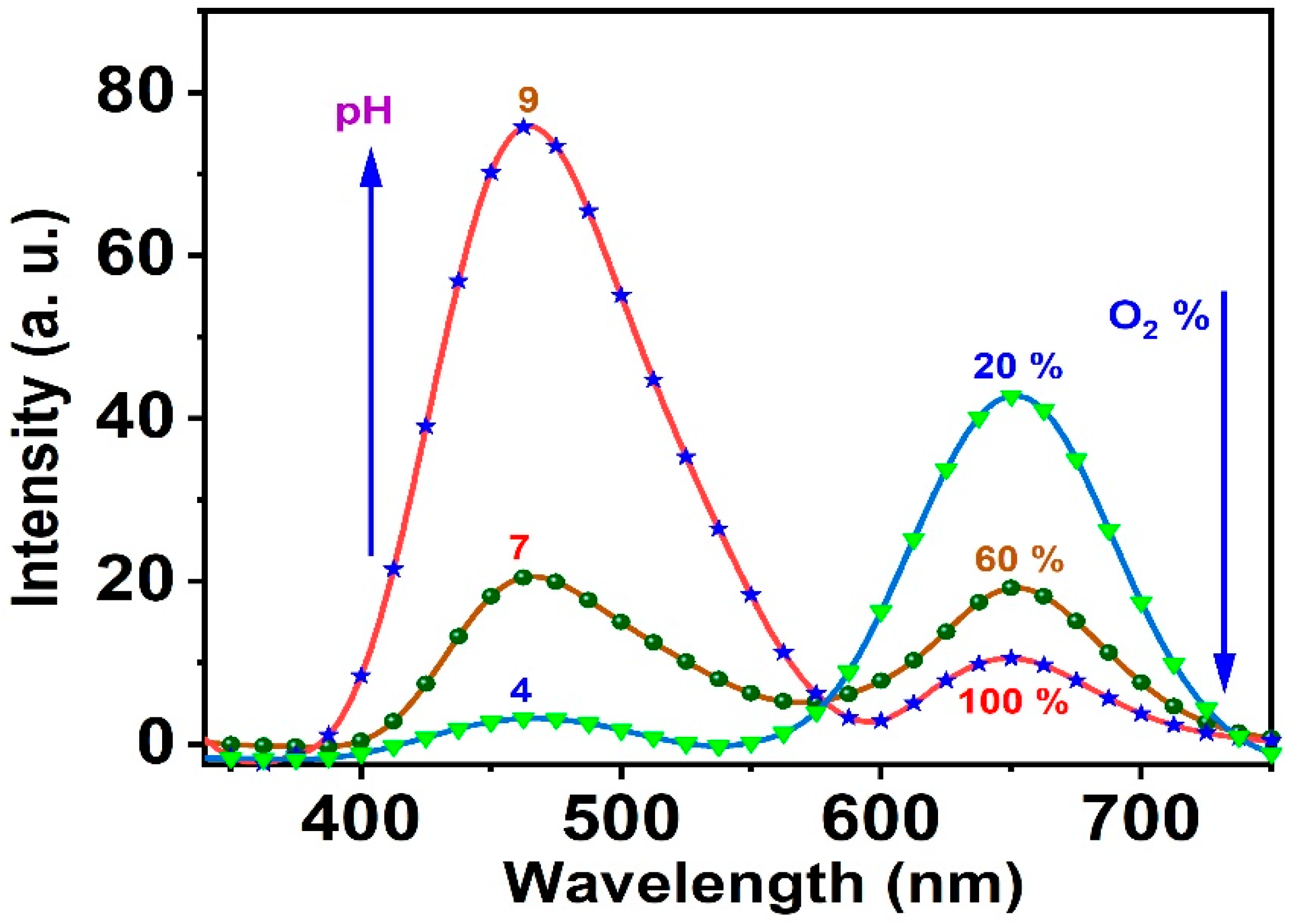
3.4. Response, Response Time, and Reversibility of the Ormosil Nanosensor
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reinders, Y.; Meier, R.J.; Liebsch, G.; Pohl, F.; Schreml, S.; Prantl, L.; Haubner, F. Imaging of pH and pO2 gives insight in molecular processes of irradiated cells. Exp. Dermatol. 2019, 28, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Lin, Z.; Zhang, Z.; Wang, X. Active-Targeting Polymeric Dual Nanosensor for Ratiometrically Measuring Proton and Oxygen Concentrations in Mitochondria. Anal. Chem. 2021, 93, 8291–8299. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Stolwijk, J.A.; Sun, L.N.; Wegener, J.; Wolfbeis, O.S. A nanogel for ratiometric fluorescent sensing of intracellular pH values. Angew. Chem. 2010, 122, 4342–4345. [Google Scholar] [CrossRef]
- Steinegger, A.; Wolfbeis, O.S.; Borisov, S.M. Optical sensing and imaging of pH values: Spectroscopies, materials, and applications. Chem. Rev. 2020, 120, 12357–12489. [Google Scholar] [CrossRef]
- Saleh, S.M.; Elkady, E.M.; Ali, R.; Alminderej, F.; Mohamed, T.A. Novel chemical sensor for detection Ca (II) ions based on ferutinin. Spectrochim. Acta A 2018, 205, 264–268. [Google Scholar] [CrossRef]
- Ali, R.; Elshaarawy, R.F.; Saleh, S.M. Turn-on ratiometric fluorescence sensor film for ammonia based on salicylaldehyde-ionic liquid. J. Environ. Chem. Eng. 2017, 5, 4813–4818. [Google Scholar] [CrossRef]
- Corsi, M.; Paghi, A.; Mariani, S.; Golinelli, G.; Debrassi, A.; Egri, G.; Leo, G.; Vandini, E.; Vilella, A.; Dähne, L.; et al. Bioresorbable Nanostructured Chemical Sensor for Monitoring of pH Level In Vivo. Adv. Sci. 2022, 9, 2202062. [Google Scholar] [CrossRef]
- Yu, K.-K.; Li, K.; Hou, J.-T.; Yang, J.; Xie, Y.-M.; Yu, X.-Q. Rhodamine based pH-sensitive ‘intelligent’ polymers as lysosome targeting probes and their imaging applications in vivo. Polym. Chem. 2014, 5, 5804–5812. [Google Scholar] [CrossRef]
- Shen, S.-L.; Chen, X.-P.; Zhang, X.-F.; Miao, J.-Y.; Zhao, B.-X. A rhodamine B-based lysosomal pH probe. J. Mater. Chem. B 2015, 3, 919–925. [Google Scholar] [CrossRef]
- Ali, R.; Alminderej, F.M.; Messaoudi, S.; Saleh, S.M. Ratiometric ultrasensitive optical chemisensor film based antibiotic drug for Al (III) and Cu (II) detection. Talanta 2021, 221, 121412. [Google Scholar] [CrossRef]
- Saleh, S.M.; Ali, R.; Wolfbeis, O.S. New silica and polystyrene nanoparticles labeled with longwave absorbing and fluorescent chameleon dyes. Microchim. Acta 2011, 174, 429–434. [Google Scholar] [CrossRef]
- Brinker, C.J.; Sherer, G.W. Sol–Gel Science, The Principle and Chemistry of Sol–Gel Processing; Academic Press: Cambridge, MA, USA, 1989. [Google Scholar]
- Saleh, S.; Younis, A.; Ali, R.; Elkady, E. Phenol removal from aqueous solution using amino modified silica nanoparticles. Korean J. Chem. Eng. 2019, 36, 529–539. [Google Scholar] [CrossRef]
- Ali, R.; Saleh, S.M.; Elshaarawy, R.F.M. Turn-on pH nano-fluorosensor based on imidazolium salicylaldehyde ionic liquid-labeled silica nanoparticles. RSC Adv. 2016, 6, 86965–86975. [Google Scholar] [CrossRef]
- del Monte, F.; Ferrer, M.L.; Levy, D. Probing the chemical environment at the porous cage of ormosils through the fluorescence of oxazine 1. J. Mater. Chem. 2001, 11, 1745–1751. [Google Scholar] [CrossRef]
- Kim, S.; Pudavar, H.E.; Prasad, P.N. Dye-concentrated organically modified silica nanoparticles as a ratiometric fluorescent pH probe by one-and two-photon excitation. Chem. Commun. 2006, 19, 2071–2073. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.Y.; Kim, J.S.; Kim, H.Y.; Ha, J.M.; Kim, Y.H.; Koo, S.M. One-pot synthesis and surface modifications of organically modified silica (ORMOSIL) particles having multiple functional groups. J. Colloid Interface Sci. 2012, 367, 67–73. [Google Scholar] [CrossRef]
- Sharma, R.K.; Das, S.; Maitra, A. Surface modified ormosil nanoparticles. J. Colloid Interface Sci. 2004, 277, 342–346. [Google Scholar] [CrossRef]
- Babhair, S.A.; Al-Badr, A.A.; Aboul-Enein, H.Y. Salicylamide. In Analytical Profiles of Drug Substances; Academic Press: Cambridge, MA, USA, 1984; Volume 13, pp. 521–551. [Google Scholar]
- Kushkevych, I.; Kollar, P.; Suchy, P.; Parak, T.; Pauk, K.; Imramovsky, A. Activity of selected salicylamides against intestinal sulfate-reducing bacteria. Neuro Endocrinol. Lett. 2015, 36, 106–113. [Google Scholar]
- Nishiya, T.; Yamauchi, S.; Hirota, N.; Baba, M.; Hanazaki, I. Fluorescence studies of intramolecularly hydrogen-bonded o-hydroxyacetophenone, salicylamide, and related molecules. J. Phys. Chem. A 1986, 90, 5730–5735. [Google Scholar] [CrossRef]
- Woolfe, G.J.; Thistlethwaite, P.J. Excited-state prototropic reactivity in salicylamide and salicylanilide. J. Am. Chem. Soc. 1980, 102, 6917–6923. [Google Scholar] [CrossRef]
- Chu, C.S.; Lo, Y.L.; Sung, T.W. Enhanced oxygen sensing properties of Pt (II) complex and dye entrapped core–shell silica nanoparticles embedded in sol–gel matrix. Talanta 2010, 82, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Klimant, I. Luminescent nanobeads for optical sensing and imaging of dissolved oxygen. Microchim. Acta 2009, 164, 7–15. [Google Scholar] [CrossRef]
- Wu, S.; Fan, Z.; Wang, W.; Fan, H.; Mei, Z.; Sun, D.; Cheng, X.; Zhao, X.; Tian, Y. Microfabricable ratiometric gaseous oxygen sensors based on inorganic perovskite nanocrystals and PtTFPP. Sens. Actuators B Chem. 2018, 271, 104–109. [Google Scholar] [CrossRef]
- Basu, B.J. Optical oxygen sensing based on luminescence quenching of platinum porphyrin dyes doped in ormosil coating. Sens. Actuators B Chem. 2007, 123, 568–577. [Google Scholar] [CrossRef]
- Mills, A.; Lepre, A. Controlling the response characteristics of luminescent porphyrin plastic film sensors for oxygen. Anal. Chem. 1997, 69, 4653–4659. [Google Scholar] [CrossRef]
- Schröder, C.R.; Polerecky, L.; Klimant, I. Time-resolved pH/pO2 mapping with luminescent hybrid sensors. Anal. Chem. 2007, 79, 60–70. [Google Scholar] [CrossRef]
- Borchert, N.B.; Ponomarev, G.V.; Kerry, J.P.; Papkovsky, D.B. O2/pH multisensor based on one phosphorescent dye. Anal. Chem. 2011, 83, 18–22. [Google Scholar] [CrossRef]
- Meier, R.J.; Schreml, S.; Wang, X.; Landthaler, M.; Babilas, P.; Wolfbeis, O.S. Simultaneous photographing of oxygen and pH in vivo using sensor films. Angew. Chem. Int. Ed. 2011, 50, 10893–10896. [Google Scholar] [CrossRef]
- Lu, H.; Jin, Y.; Tian, Y.; Zhang, W.; Holl, M.R.; Meldrum, D.R. New ratiometric optical oxygen and pH dual sensors with three emission colors for measuring photosynthetic activity in cyanobacteria. J. Mater. Chem. 2011, 21, 19293–19301. [Google Scholar] [CrossRef]
- Zou, X.; Pan, T.; Chen, L.; Tian, Y.; Zhang, W. Luminescence materials for pH and oxygen sensing in microbial cells–structures, optical properties, and biological applications. Crit. Rev. Biotechnol. 2017, 37, 723–738. [Google Scholar] [CrossRef]
- Wang, X.; Stolwijk, J.A.; Lang, T.; Sperber, M.; Meier, R.J.; Wegener, J.; Wolfbeis, O.S. Ultra-small, highly stable, and sensitive dual nanosensors for imaging intracellular oxygen and pH in cytosol. J. Am. Chem. Soc. 2012, 134, 17011–17014. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.-E.L.; Cao, Y.; Kopelman, R.; Koo, S.M.; Brasuel, M.; Philbert, M.A. Real-time measurements of dissolved oxygen inside live cells by organically modified silicate fluorescent nanosensors. Anal. Chem. 2004, 76, 2498–2505. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.U.; Park, J.K.; Kim, S.H. Material and rheological properties of (glycidoxypropyl) trimethoxysilane modified colloidal silica coatings. Korea Aust. Rheol. J. 2004, 16, 175–182. [Google Scholar]
- Roy, I.; Kumar, P.; Kumar, R.; Ohulchanskyy, T.Y.; Yong, K.T.; Prasad, P.N. Ormosil nanoparticles as a sustained-release drug delivery vehicle. RSC Adv. 2014, 4, 53498–53504. [Google Scholar] [CrossRef]
- Meng, Q.; Han, T.; Wang, G.; Zheng, N.; Cao, C.; Xie, S. Preparation of a natural dye doped Ormosil coating for the detection of formaldehyde in the optical gas sensor. Sens. Actuators B Chem. 2014, 196, 238–244. [Google Scholar] [CrossRef]
- Saleh, S.M.; Ali, R.; Hirsch, T.; Wolfbeis, O.S. Detection of biotin–avidin affinity binding by exploiting a self-referenced system composed of upconverting luminescent nanoparticles and gold nanoparticles. J. Nanoparticle Res. 2011, 13, 4603–4611. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrieres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Catalan, J.; Toribio, F.; Acuna, A.U. Intramolecular hydrogen bonding and fluorescence of salicylaldehyde, salicylamide, and o-hydroxyacetophenone in gas and condensed phase. J. Phys. Chem. A 1982, 86, 303–306. [Google Scholar] [CrossRef]
- Chou, P.T.; Chiou, C.S.; Yu, W.S.; Wu, G.R.; Wei, T.H. Studies of the triplet state of the proton-transfer tautomer in salicylaldehydes. Chem. Phys. Lett. 2003, 370, 747–755. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, R. Dual Optical Nanosensor Based on Ormosil Nanoparticles for Monitoring O2 and pH. Biosensors 2022, 12, 1011. https://doi.org/10.3390/bios12111011
Ali R. Dual Optical Nanosensor Based on Ormosil Nanoparticles for Monitoring O2 and pH. Biosensors. 2022; 12(11):1011. https://doi.org/10.3390/bios12111011
Chicago/Turabian StyleAli, Reham. 2022. "Dual Optical Nanosensor Based on Ormosil Nanoparticles for Monitoring O2 and pH" Biosensors 12, no. 11: 1011. https://doi.org/10.3390/bios12111011
APA StyleAli, R. (2022). Dual Optical Nanosensor Based on Ormosil Nanoparticles for Monitoring O2 and pH. Biosensors, 12(11), 1011. https://doi.org/10.3390/bios12111011