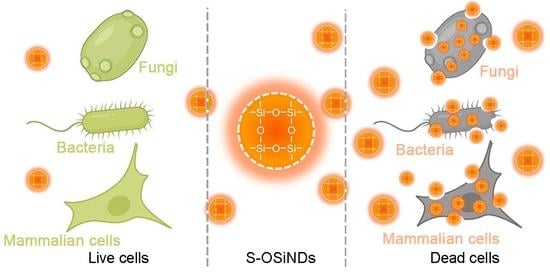
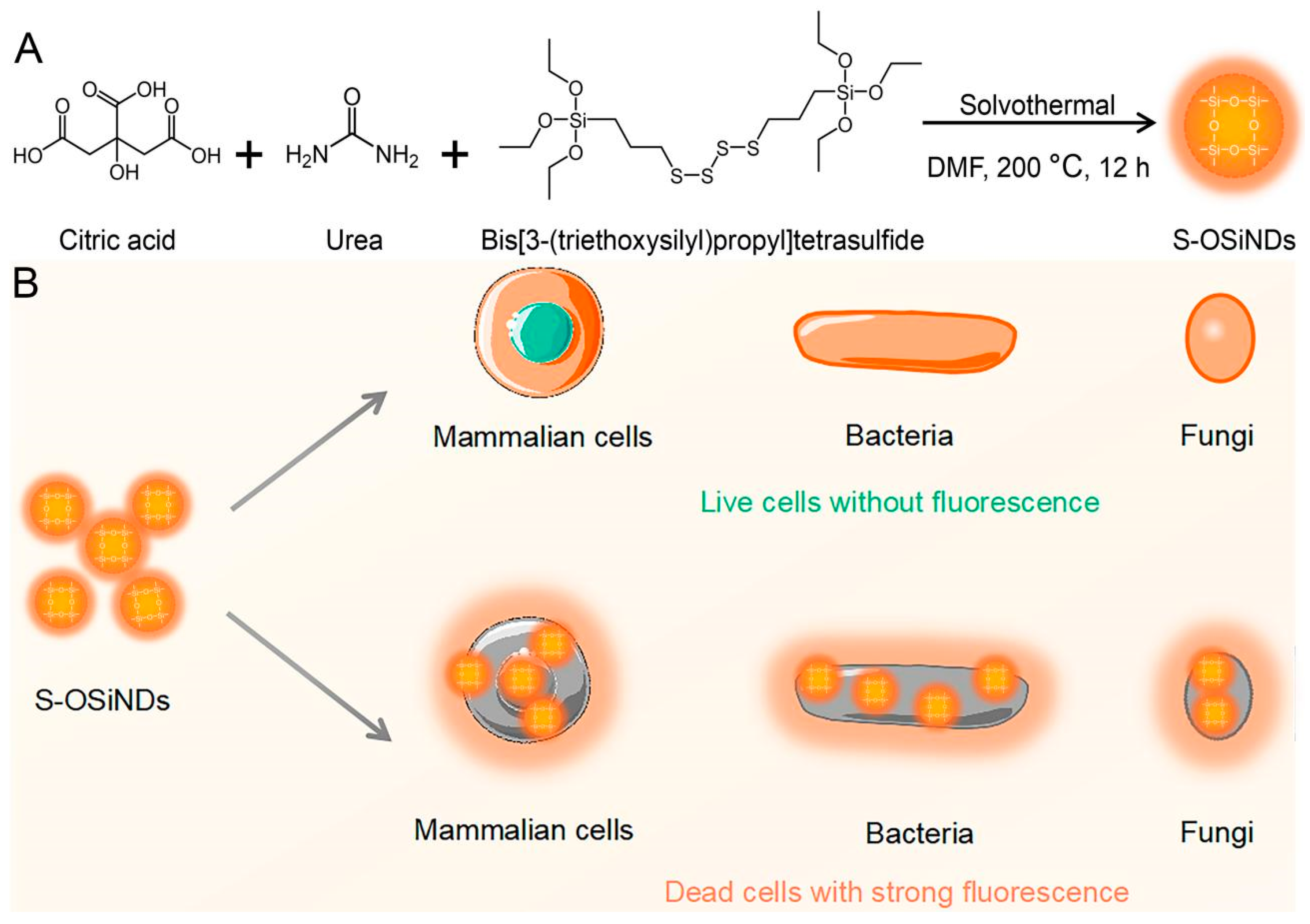
Sulfur-Doped Organosilica Nanodots as a Universal Sensor for Ultrafast Live/Dead Cell Discrimination
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Live/Dead Cells
2.2. Evaluation of the Staining Performance of S-OSiNDs toward Live and Dead Bacteria
2.3. Evaluation of the Staining Performance of S-OSiNDs toward Live and Dead Fungi
2.4. Evaluation of the Staining Performance of S-OSiNDs toward Normal and Cancerous Mammalian Cells
2.5. Comparison between S-OSiNDs and RedDot2 on the Live/Dead Cell Discrimination Performance
3. Results and Discussion
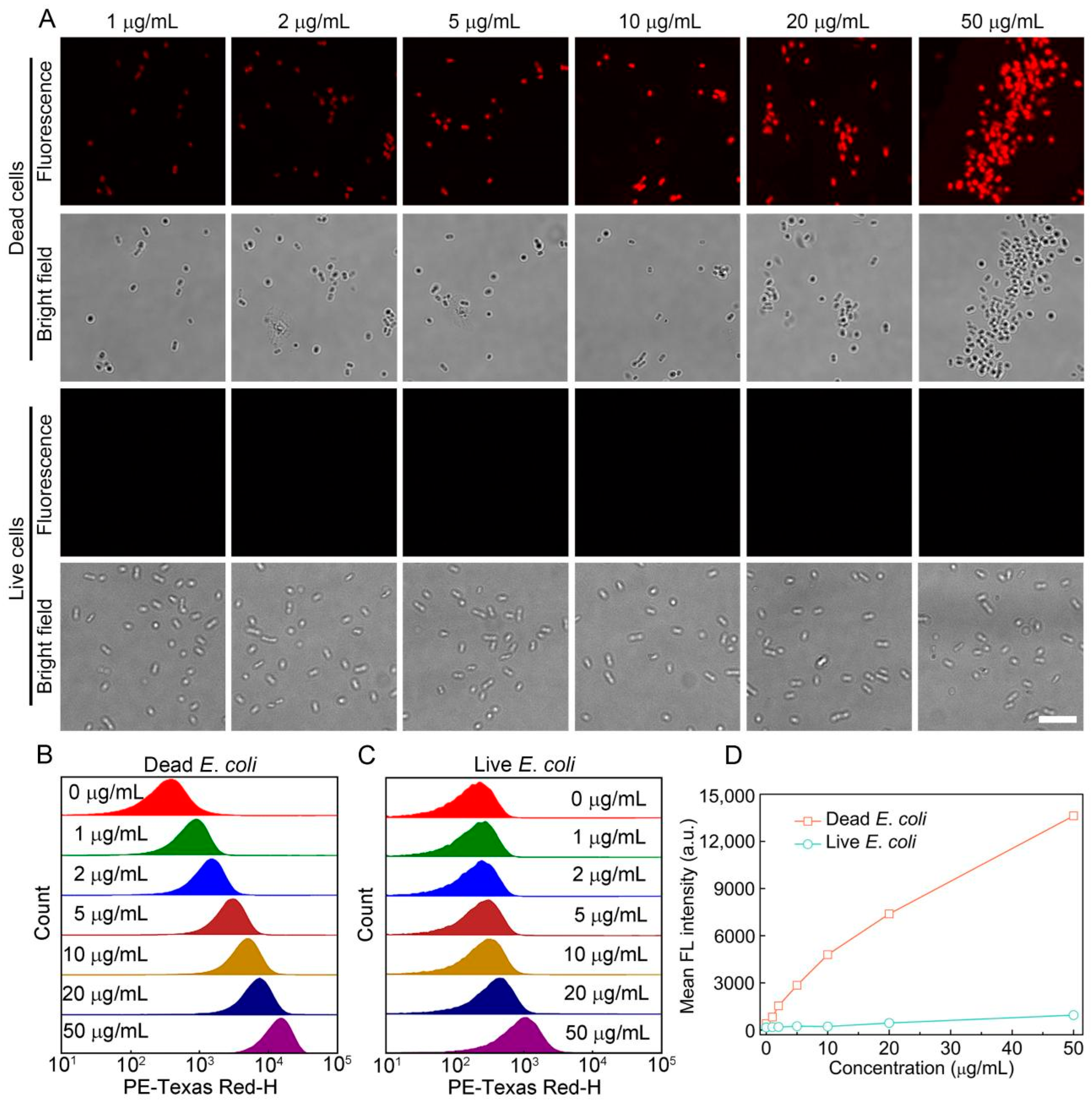
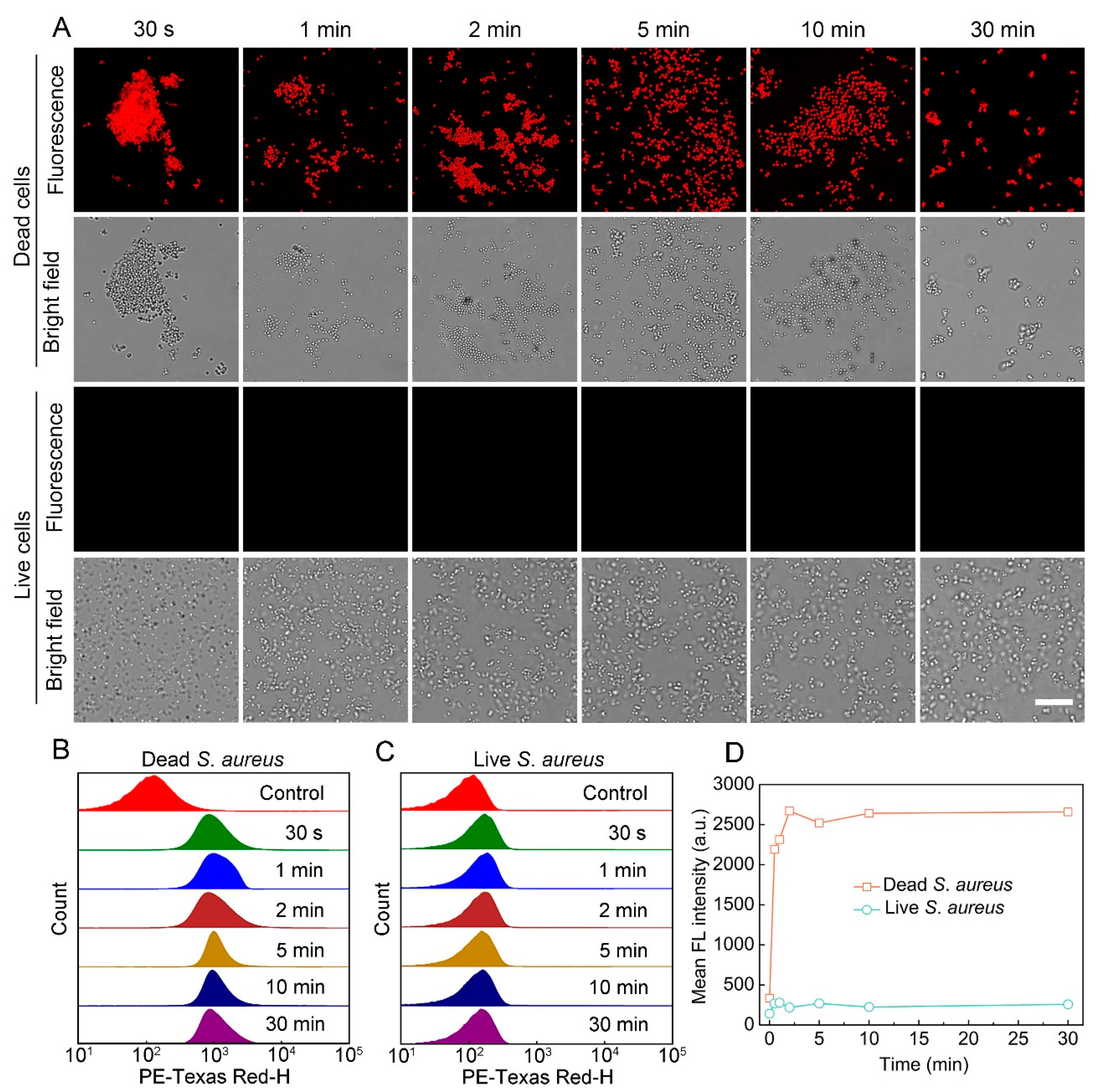
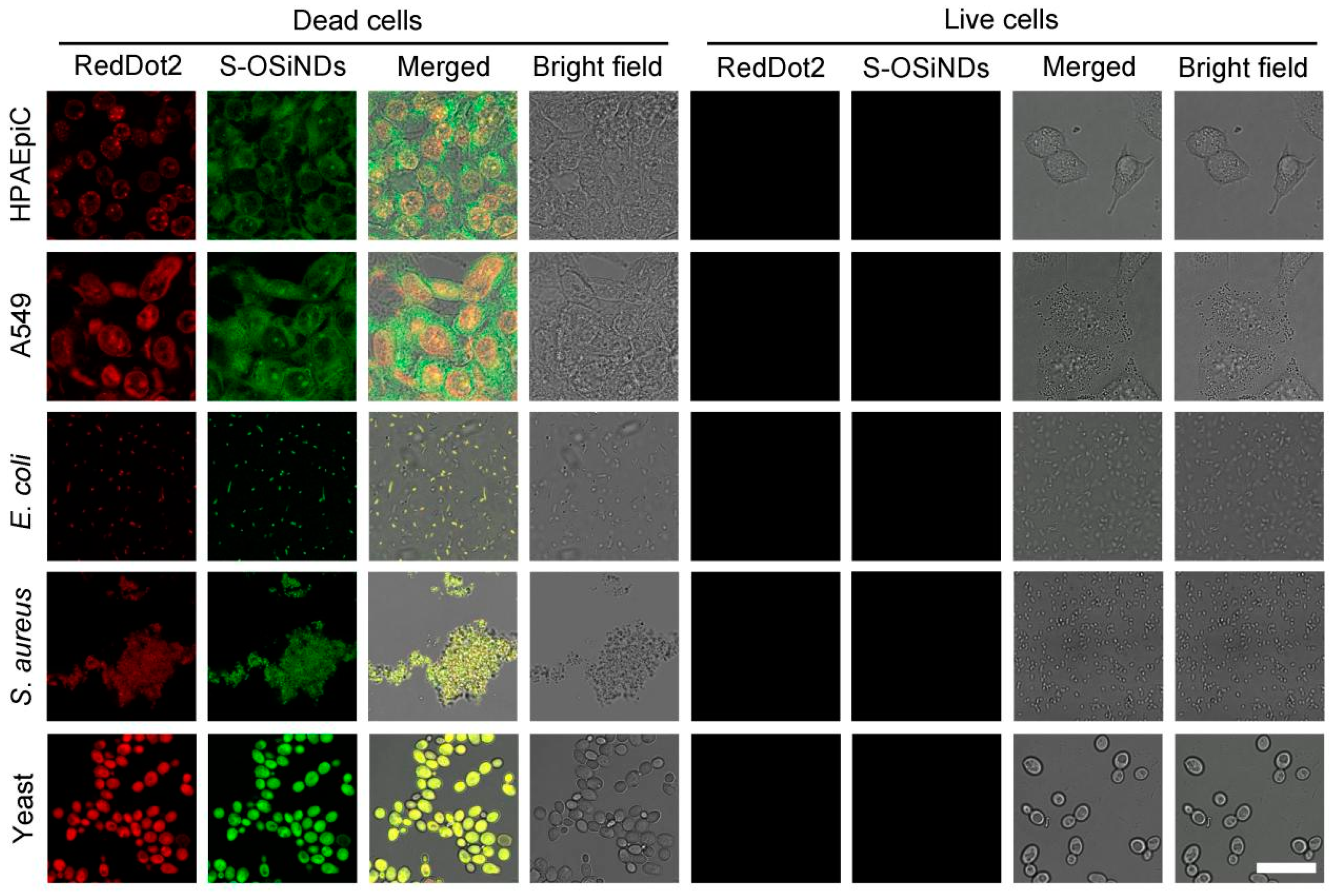
3.1. Staining Performance of S-OSiNDs for Live and Dead Bacteria
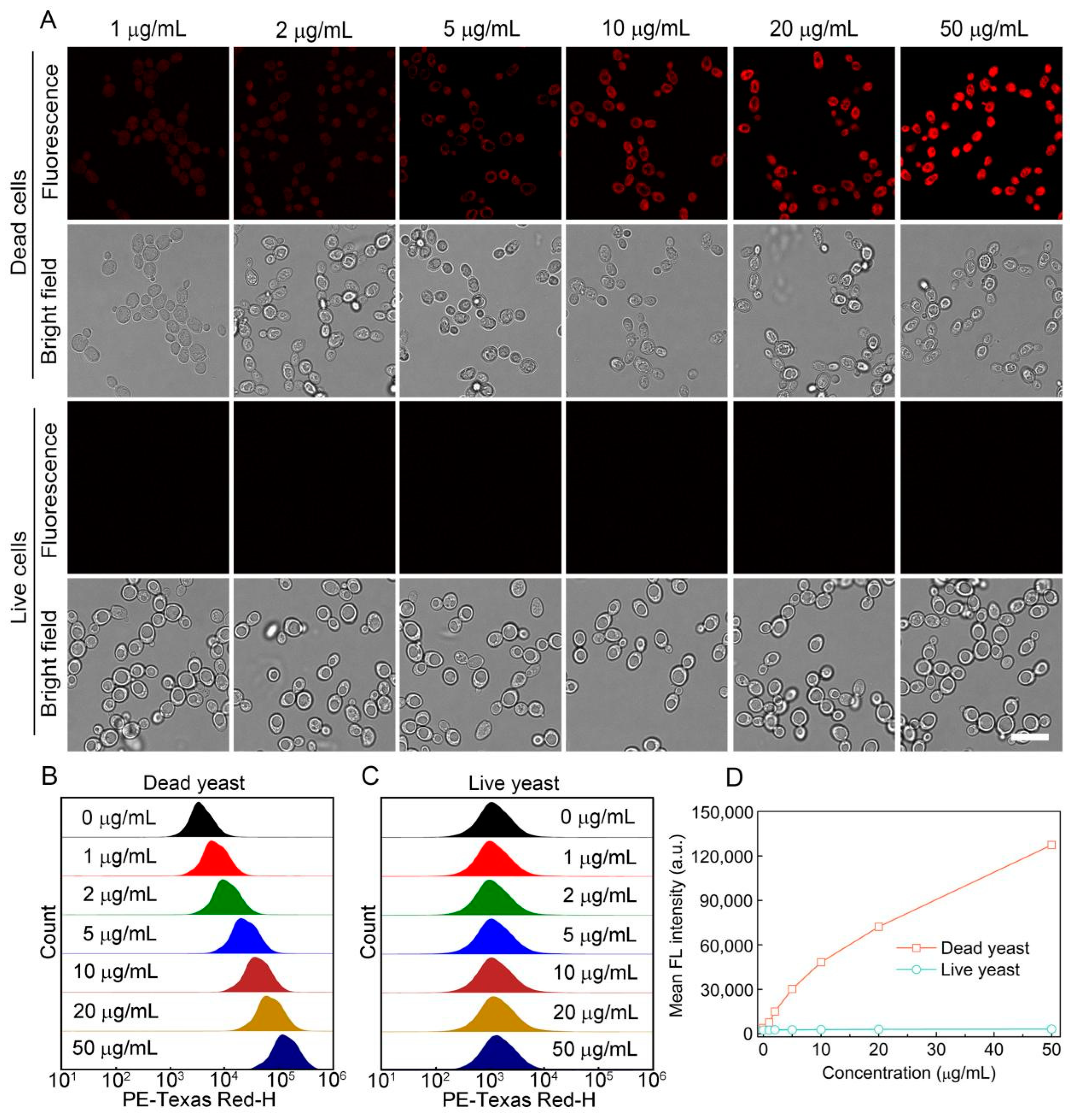
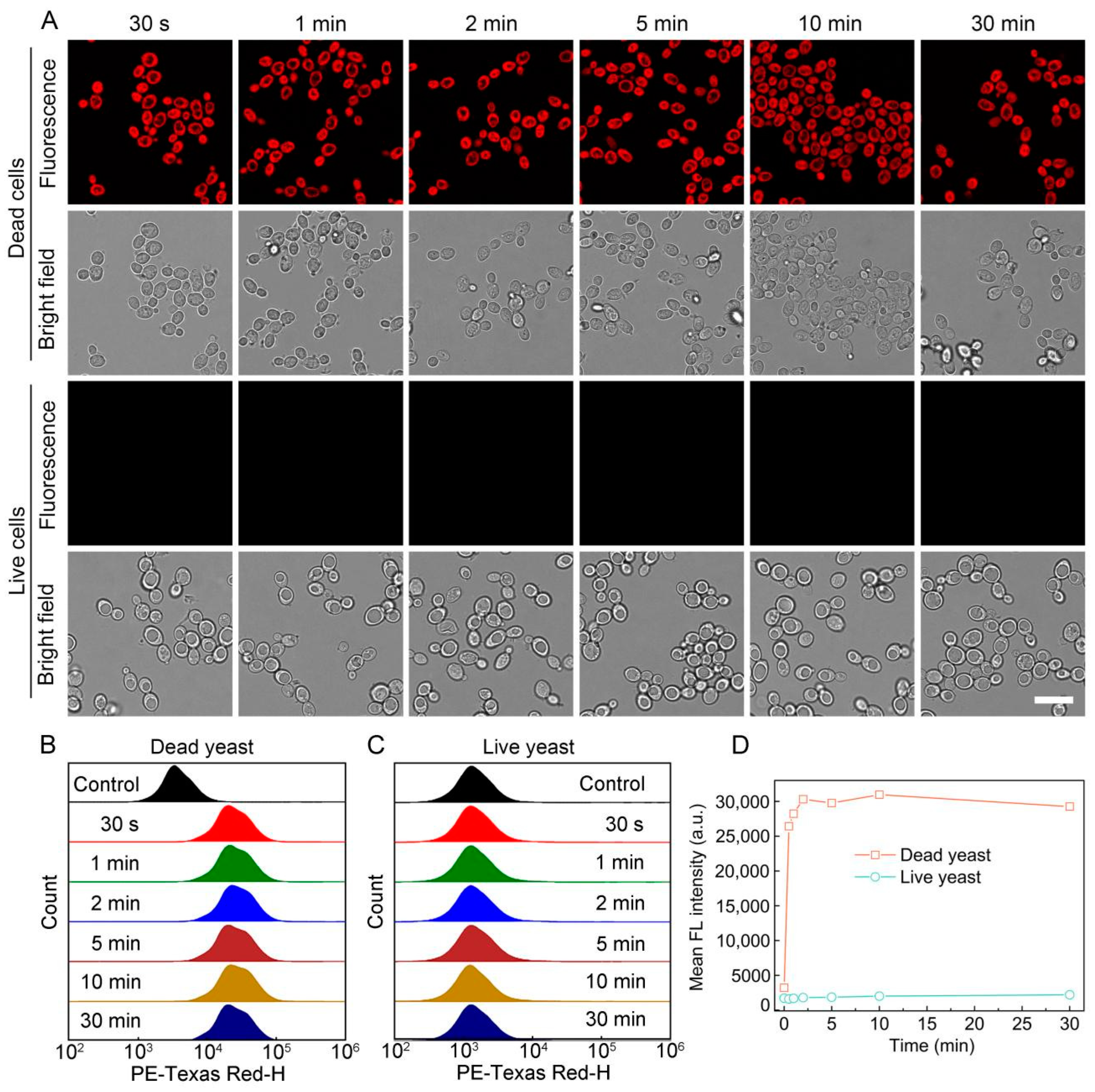
3.2. Staining Performance of S-OSiNDs for Live and Dead Fungi
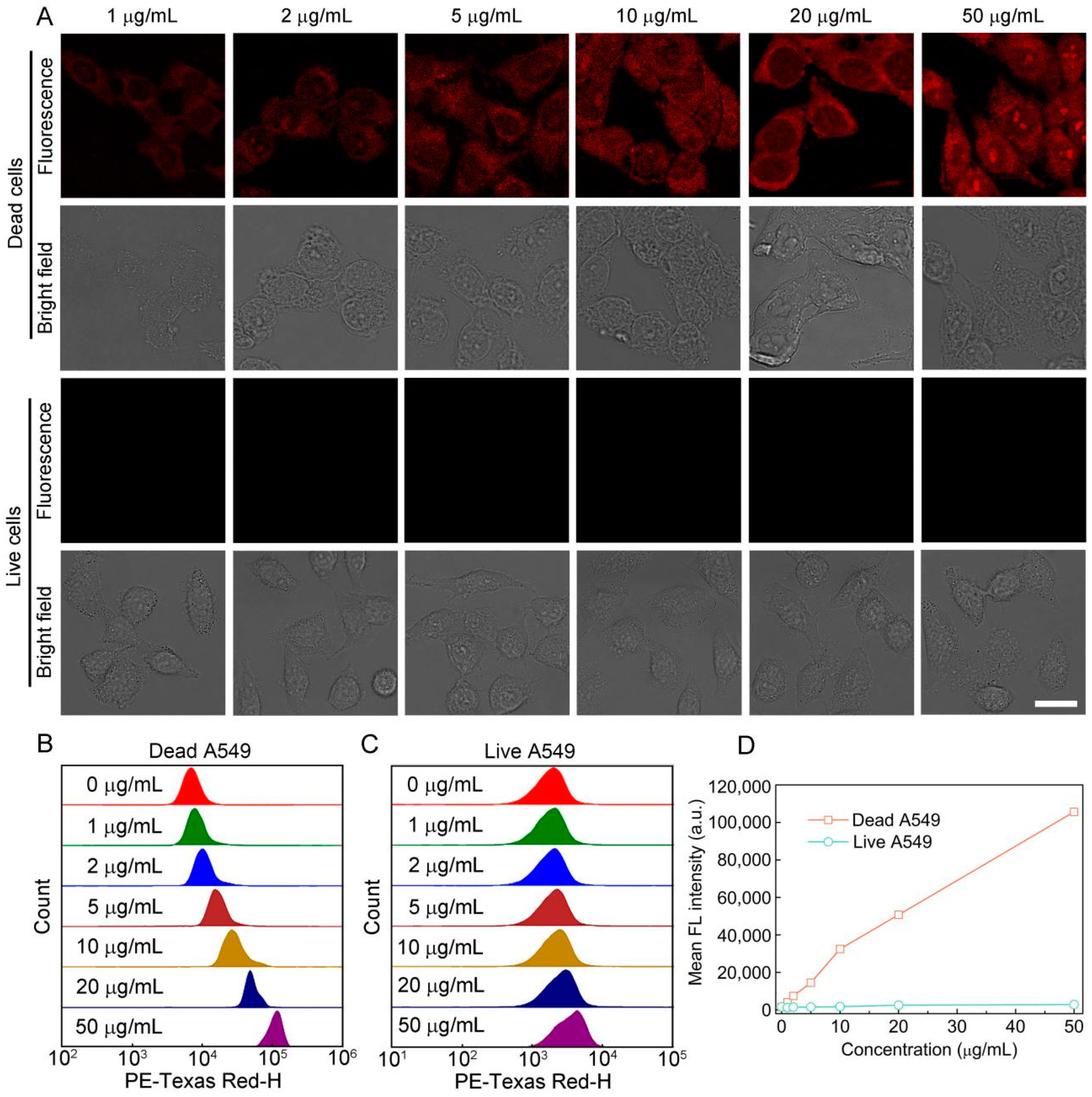
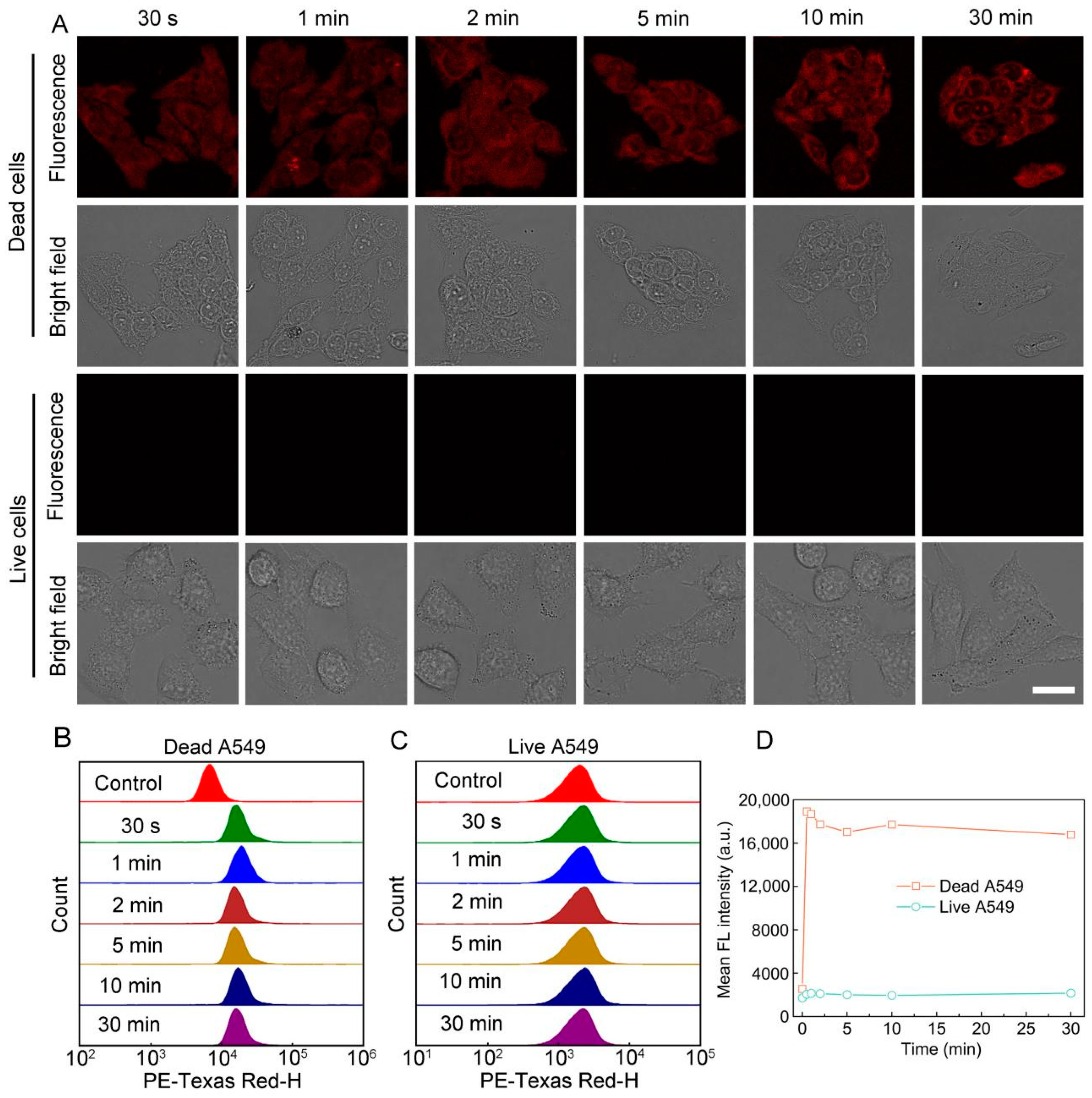
3.3. Staining Performance of S-OSiNDs for Normal and Cancerous Mammalian Cells
3.4. Comparison between S-OSiNDs and RedDot2 on the Discrimination between Live and Dead Cells
3.5. Cytotoxicity Evaluation of S-OSiNDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kepp, O.; Galluzzi, L.; Lipinski, M.; Yuan, J.; Kroemer, G. Cell death assays for drug discovery. Nat. Rev. Drug Discov. 2011, 10, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Emerson, J.B.; Adams, R.I.; Román, C.M.B.; Brooks, B.; Coil, D.A.; Dahlhausen, K.; Ganz, H.H.; Hartmann, E.M.; Hsu, T.; Justice, N.B.; et al. Schrödinger’s microbes: Tools for distinguishing the living from the dead in microbial ecosystems. Microbiome 2017, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Li, C.; Sayed, S.M.; Sun, W.; Lin, F.; Wu, F.G. Superbright orgnaosilica nanodots as a universal sensor for fast discrimination and accurate quantification of live/dead cells. Sens. Actuat B-Chem. 2019, 295, 49–55. [Google Scholar] [CrossRef]
- Hu, X.; Wang, S.; Luo, Q.; Ge, B.; Cheng, Q.; Dong, C.; Xu, J.; Ding, H.; Xu, M.; Tedesco, A.C.; et al. Synthesis of Sn nanocluster@carbon dots for photodynamic therapy application. Chin. Chem. Lett. 2021, 32, 2287–2291. [Google Scholar] [CrossRef]
- Zhou, L.; Qiu, T.; Lv, F.; Liu, L.; Ying, J.; Wang, S. Self-assembled nanomedicines for anticancer and antibacterial applications. Adv. Healthc. Mater. 2018, 7, 1800670. [Google Scholar] [CrossRef]
- Liu, J.; Lu, S.; Tang, Q.; Zhang, K.; Yu, W.; Sun, H.; Yang, B. One-step hydrothermal synthesis of photoluminescent carbon nanodots with selective antibacterial activity against Porphyromonas gingivalis. Nanoscale 2017, 9, 7135–7142. [Google Scholar] [CrossRef]
- Berghe, T.V.; Grootjans, S.; Goossens, V.; Dondelinger, Y.; Krysko, D.V.; Takahashi, N.; Vandenabeele, P. Determination of apoptotic and necrotic cell death in vitro and in vivo. Methods 2013, 61, 117–129. [Google Scholar] [CrossRef]
- Fantner, G.E.; Barbero, R.J.; Gray, D.S.; Belcher, A.M. Kinetics of antimicrobial peptide activity measured on individual bacterial cells using high-speed atomic force microscopy. Nat. Nanotechnol. 2010, 5, 280–285. [Google Scholar] [CrossRef]
- Davis, R.; Deering, A.; Burgula, Y.; Mauer, L.J.; Reuhs, B.L. Differentiation of live, dead and treated cells of Escherichia coli O157:H7 using FT-IR Spectroscopy. J. Appl. Microbiol. 2012, 112, 743–751. [Google Scholar] [CrossRef]
- Krysko, D.V.; Vanden Berghe, T.; D’ Herde, K.; Vandenabeele, P. Apoptosis and necrosis: Detection, discrimination and phagocytosis. Methods 2008, 44, 205–221. [Google Scholar] [CrossRef]
- Tawakoli, P.N.; Al-Ahmad, A.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Comparison of different live/dead stainings for detection and quantification of adherent microorganisms in the initial oral biofilm. Clin. Oral Inv. 2013, 17, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, D.; Ivleva, N.P.; Mircescu, N.E.; Schubert, S.; Niessner, R.; Wieser, A.; Haisch, C. Label-free in situ discrimination of live and dead bacteria by surface-enhanced Raman scattering. Anal. Chem. 2015, 87, 6553–6561. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Dhankhar, D.; Chen, J.; Krishnamoorthi, A.; Cesario, T.C.; Rentzepis, P.M. Identification of live and dead bacteria: A Raman spectroscopic study. IEEE Access 2019, 7, 23549–23559. [Google Scholar] [CrossRef]
- Baymiev, A.K.; Baymiev, A.K.; Kuluev, B.R.; Shvets, K.Y.; Yamidanov, R.S.; Matniyazov, R.T.; Chemeris, D.A.; Zubov, V.V.; Alekseev, Y.I.; Mavzyutov, A.R.; et al. Modern approaches to differentiation of live and dead bacteria using selective amplification of nucleic acids. Microbiology 2020, 89, 13–27. [Google Scholar] [CrossRef]
- Cheng, H.B.; Li, Y.; Tang, B.Z.; Yoon, J. Assembly strategies of organic-based imaging agents for fluorescence and photoacoustic bioimaging applications. Chem. Soc. Rev. 2020, 49, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Chen, J.; Sun, S.; Tang, Z.; Jiang, K.; Song, L.; Wang, Y.; Liu, C.; Lin, H. Fluorescent and photoacoustic bifunctional probe for the detection of ascorbic acid in biological fluids, living cells and in vivo. Nanoscale 2018, 10, 17834–17841. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Wu, S.; Wei, X.; Fu, Y.; Wu, J.; Wang, P.; Zhang, W. Optically tunable fluorescent carbon nanoparticles and their application in fluorometric sensing of copper ions. Nano Res. 2019, 12, 2576–2583. [Google Scholar] [CrossRef]
- Park, S.H.; Kwon, N.; Lee, J.H.; Yoon, J.; Shin, I. Synthetic ratiometric fluorescent probes for detection of ions. Chem. Soc. Rev. 2020, 49, 143–179. [Google Scholar] [CrossRef]
- Wang, H.Y.; Hua, X.W.; Jia, H.R.; Li, C.; Lin, F.; Chen, Z.; Wu, F.G. Universal cell surface imaging for mammalian, fungal, and bacterial cells. ACS Biomater. Sci. Eng. 2016, 2, 987–997. [Google Scholar] [CrossRef]
- Tian, M.; Sun, J.; Tang, Y.; Dong, B.; Lin, W. Discriminating live and dead cells in dual-color mode with a two-photon fluorescent probe based on ESIPT mechanism. Anal. Chem. 2018, 90, 998–1005. [Google Scholar] [CrossRef]
- Zhao, E.; Hong, Y.; Chen, S.; Leung, C.W.T.; Chan, C.Y.K.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Highly fluorescent and photostable probe for long-term bacterial viability assay based on aggregation-induced emission. Adv. Healthc. Mater. 2014, 3, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Santoscoy, M.C.; Jarboe, L.R. Streamlined assessment of membrane permeability and its application to membrane engineering of Escherichia coli for octanoic acid tolerance tetraselmis suecica to evaluate vitality. J. Ind. Microbiol. Biotechnol. 2019, 46, 843–853. [Google Scholar] [CrossRef]
- Roth, B.L.; Poot, M.; Yue, S.T.; Millard, P.J. Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl. Environ. Microbiol. 1997, 63, 2421–2431. [Google Scholar] [CrossRef]
- Krämer, C.E.M.; Wiechert, W.; Kohlheyer, D. Time-resolved, single-cell analysis of induced and programmed cell death via non-invasive propidium iodide and counterstain perfusion. Sci. Rep. 2016, 6, 32104. [Google Scholar] [CrossRef] [PubMed]
- Kaprelyants, A.S.; Kell, D.B. Rapid assessment of bacterial viability and vitality by rhodamine 123 and flow cytometry. J. Appl. Bacteriol. 1992, 72, 410–422. [Google Scholar] [CrossRef]
- Jones, K.H.; Senft, J.A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J. Histochem. Cytochem. 1985, 33, 77–79. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, H.L.; Cullen, J.J. Classification of phytoplankton cells as live or dead using the vital stains fluorescein diacetate and 5-chloromethyl fluorescein diacetate. J. Phycol. 2016, 52, 572–589. [Google Scholar] [CrossRef]
- Guilini, C.; Baehr, C.; Schaeffer, E.; Gizzi, P.; Rufi, F.; Haiech, J.; Weiss, E.; Bonnet, D.; Galzi, J.L. New fluorescein precursors for live bacteria detection. Anal. Chem. 2015, 87, 8858–8866. [Google Scholar] [CrossRef]
- Grieshaber, P.; Lagrèze, W.A.; Noack, C.; Boehringer, D.; Biermann, J. Staining of fluorogold-prelabeled retinal ganglion cells with calcein-AM: A new method for assessing cell vitality. J. Neurosci. Methods 2010, 192, 233–239. [Google Scholar] [CrossRef]
- Abdelkhaliq, A.; van der Zande, M.; Peters, R.J.B.; Bouwmeester, H. Combination of the BeWo b30 placental transport model and the embryonic stem cell test to assess the potential developmental toxicity of silver nanoparticles. Part. Fibre Toxicol. 2020, 17, 11. [Google Scholar] [CrossRef]
- Song, Y.; Li, H.; Lu, F.; Wang, H.; Zhang, M.; Yang, J.; Huang, J.; Huang, H.; Liu, Y.; Kang, Z. Fluorescent carbon dots with highly negative charges as a sensitive probe for real-time monitoring of bacterial viability. J. Mater. Chem. B 2017, 5, 6008–6015. [Google Scholar] [CrossRef]
- Lu, F.; Song, Y.; Huang, H.; Liu, Y.; Fu, Y.; Huang, J.; Li, H.; Qu, H.; Kang, Z. Fluorescent carbon dots with tunable negative charges for bio-imaging in bacterial viability assessment. Carbon 2017, 120, 95–102. [Google Scholar] [CrossRef]
- Hua, X.H.; Bao, Y.W.; Wang, H.Y.; Chen, Z.; Wu, F.G. Bacteria-derived fluorescent carbon dots for microbial live/dead differentiation. Nanoscale 2017, 9, 2150–2161. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.W.; Liu, X.; Jiang, Y.W.; Li, Y.H.; Gao, G.; Zhu, Y.X.; Lin, F.; Wu, F.G. Rose bengal-derived ultrabright sulfur-doped carbon dots for fast discrimination between live and dead cells. Anal. Chem. 2022, 94, 4243–4251. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, W.R.; Li, Y.; Zou, X.; Li, W.; Liang, J.; Zhang, H.; Liu, Y.; Zhang, X.; Hu, C.; et al. Architecting ultra-bright silanized carbon dots by alleviating the spin-orbit coupling effect: A specific fluorescent nanoprobe to label dead cells. Chem. Eng. J. 2022, 428, 131168. [Google Scholar] [CrossRef]
- Yan, C.; Wang, C.; Hou, T.; Guan, P.; Qiao, Y.; Guo, L.; Teng, Y.; Hu, X.; Wu, H. Lasting tracking and rapid discrimination of live Gram-positive bacteria by peptidoglycan-targeting carbon quantum dots. ACS Appl. Mater. Interfaces 2021, 13, 1277–1287. [Google Scholar] [CrossRef]
- Ji, X.; Wang, S.; Luo, Y.; Yuan, X.; Wei, Y.; Zhang, Q.; Qin, K.; Tu, Y. Green synthesis of weissella-derived fluorescence carbon dots for microbial staining, cell imaging and dual sensing of vitamin B12 and hexavalent chromium. Dyes Pigment. 2021, 184, 108818. [Google Scholar] [CrossRef]
- Yuan, X.; Tu, Y.; Chen, W.; Xu, Z.; Wei, Y.; Qin, K.; Zhang, Q.; Xiang, Y.; Zhang, H.; Ji, X. Facile synthesis of carbon dots derived from ampicillin sodium for live/dead microbe differentiation, bioimaging and high selectivity detection of 2, 4-dinitrophenol and Hg(II). Dyes Pigment. 2020, 175, 108187. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, F.G. Ultrasmall silicon nanoparticles for imaging and killing microorganisms: A minireview. ChemNanoMat 2022, 8, e202100369. [Google Scholar] [CrossRef]
- Cui, M.; Li, D.; Du, X.; Li, N.; Rong, Q.; Li, N.; Shui, L.; Zhou, G.; Wang, X.; Brabec, C.J.; et al. A cost-effective, aqueous-solution-processed cathode interlayer based on organosilica nanodots for highly efficient and stable organic solar cells. Adv. Mater. 2020, 32, 2002973. [Google Scholar] [CrossRef]
- Tang, J.; Chu, B.; Wang, J.; Song, B.; Su, Y.; Wang, H.; He, Y. Multifunctional nanoagents for ultrasensitive imaging and photoactive killing of Gram-negative and Gram-positive bacteria. Nat. Commun. 2019, 10, 4057. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Lin, F.; Guo, Y.; Wu, F.G. One-step synthesis of epoxy group-terminated organosilica nanodots: A versatile nanoplatform for imaging and eliminating multidrug-resistant bacteria and their biofilms. Small 2019, 15, 1901647. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Ji, X.; Peng, F.; Zhong, Y.; Chu, B.; Su, Y.; He, Y. Photostable and biocompatible fluorescent silicon nanoparticles for imaging-guided co-delivery of siRNA and doxorubicin to drug-resistant cancer cells. Nano-Micro Lett. 2019, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Guo, Y.; Zhu, Y.X.; Liu, X.; Chen, Z.; Wu, F.G. Smart supramolecular “Trojan horse”-inspired nanogels for realizing light-triggered nuclear drug influx in drug-resistant cancer cells. Adv. Funct. Mater. 2019, 29, 1807772. [Google Scholar] [CrossRef]
- Cui, M.; Liu, S.; Song, B.; Guo, D.; Wang, J.; Hu, G.; Su, Y.; He, Y. Fluorescent silicon nanorods-based nanotheranostic agents for multimodal imaging-guided photothermal therapy. Nano-Micro Lett. 2019, 11, 73. [Google Scholar] [CrossRef]
- Jiang, D.; Pan, Y.; Yao, H.; Sun, J.; Xiong, W.; Li, L.; Zheng, F.; Sun, S.; Zhu, J.J. Synthesis of renal-clearable multicolor fluorescent silicon nanodots for tumor imaging and in vivo H2O2 profiling. Anal. Chem. 2022, 94, 9074–9080. [Google Scholar] [CrossRef]
- Huang, S.; Yu, L.; Su, P.; Wen, T.; Sun, M.; Huang, D.; Wang, X.; Wang, S. Surface enhanced FRET for sensitive and selective detection of doxycycline using organosilicon nanodots as donors. Anal. Chim. Acta 2022, 1197, 339530. [Google Scholar] [CrossRef]
- Chu, B.; Song, B.; Ji, X.; Su, Y.; Wang, H.; He, Y. Fluorescent silicon nanorods-based ratiometric sensors for long-term and real-time measurements of intracellular pH in live cells. Anal. Chem. 2017, 89, 12152–12159. [Google Scholar] [CrossRef]
- Wu, F.G.; Zhang, X.; Kai, S.; Zhang, M.; Wang, H.Y.; Myers, J.N.; Weng, Y.; Liu, P.; Gu, N.; Chen, Z. One-step synthesis of superbright water-soluble silicon nanoparticles with photoluminescence quantum yield exceeding 80%. Adv. Mater. Interfaces 2015, 2, 1500360. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Kai, S.; Wang, H.Y.; Yang, J.; Wu, F.G.; Chen, Z. Highly sensitive and selective detection of dopamine using one-pot synthesized highly photoluminescent silicon nanoparticles. Anal. Chem. 2015, 87, 3360–3365. [Google Scholar] [CrossRef]
- Zeng, J.; Hua, X.W.; Bao, Y.W.; Wu, F.G. Orange-emissive sulfur-doped organosilica nanodots for metal ion/glutathione detection and normal/cancer cell identification. ACS Appl. Nano Mater. 2021, 4, 6083–6092. [Google Scholar] [CrossRef]
- Zhong, Y.; Peng, F.; Bao, F.; Wang, S.; Ji, X.; Yang, L.; Su, Y.; Lee, S.T.; He, Y. Large-scale aqueous synthesis of fluorescent and biocompatible silicon nanoparticles and their use as highly photostable biological probes. J. Am. Chem. Soc. 2013, 135, 8350–8356. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Sun, X.; Wang, S.; Peng, F.; Bao, F.; Su, Y.; Li, Y.; Lee, S.T.; He, Y. Facile, large-quantity synthesis of stable, tunable-color silicon nanoparticles and their application for long-term cellular imaging. ACS Nano 2015, 9, 5958–5967. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Xia, L.Y.; Wang, H.Y.; Chen, Z.; Wu, F.G. One-Step synthesis of ultrasmall and ultrabright organosilica nanodots with 100% photoluminescence quantum yield: Long-term lysosome imaging in living, fixed, and permeabilized cells. Nano Lett. 2018, 18, 1159–1167. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-H.; Zeng, J.; Wang, Z.; Wang, T.-Y.; Wu, S.-Y.; Zhu, X.-Y.; Zhang, X.; Shan, B.-H.; Gao, C.-Z.; Wang, S.-H.; et al. Sulfur-Doped Organosilica Nanodots as a Universal Sensor for Ultrafast Live/Dead Cell Discrimination. Biosensors 2022, 12, 1000. https://doi.org/10.3390/bios12111000
Li Y-H, Zeng J, Wang Z, Wang T-Y, Wu S-Y, Zhu X-Y, Zhang X, Shan B-H, Gao C-Z, Wang S-H, et al. Sulfur-Doped Organosilica Nanodots as a Universal Sensor for Ultrafast Live/Dead Cell Discrimination. Biosensors. 2022; 12(11):1000. https://doi.org/10.3390/bios12111000
Chicago/Turabian StyleLi, Yan-Hong, Jia Zeng, Zihao Wang, Tian-Yu Wang, Shun-Yu Wu, Xiao-Yu Zhu, Xinping Zhang, Bai-Hui Shan, Cheng-Zhe Gao, Shi-Hao Wang, and et al. 2022. "Sulfur-Doped Organosilica Nanodots as a Universal Sensor for Ultrafast Live/Dead Cell Discrimination" Biosensors 12, no. 11: 1000. https://doi.org/10.3390/bios12111000
APA StyleLi, Y.-H., Zeng, J., Wang, Z., Wang, T.-Y., Wu, S.-Y., Zhu, X.-Y., Zhang, X., Shan, B.-H., Gao, C.-Z., Wang, S.-H., & Wu, F.-G. (2022). Sulfur-Doped Organosilica Nanodots as a Universal Sensor for Ultrafast Live/Dead Cell Discrimination. Biosensors, 12(11), 1000. https://doi.org/10.3390/bios12111000