Graphene-Based Electrochemical Biosensors for Breast Cancer Detection
Abstract
1. Introduction
2. Biomarkers of BC
3. Graphene and Its Derivatives for the Electrochemical Sensing of BC Biomarkers
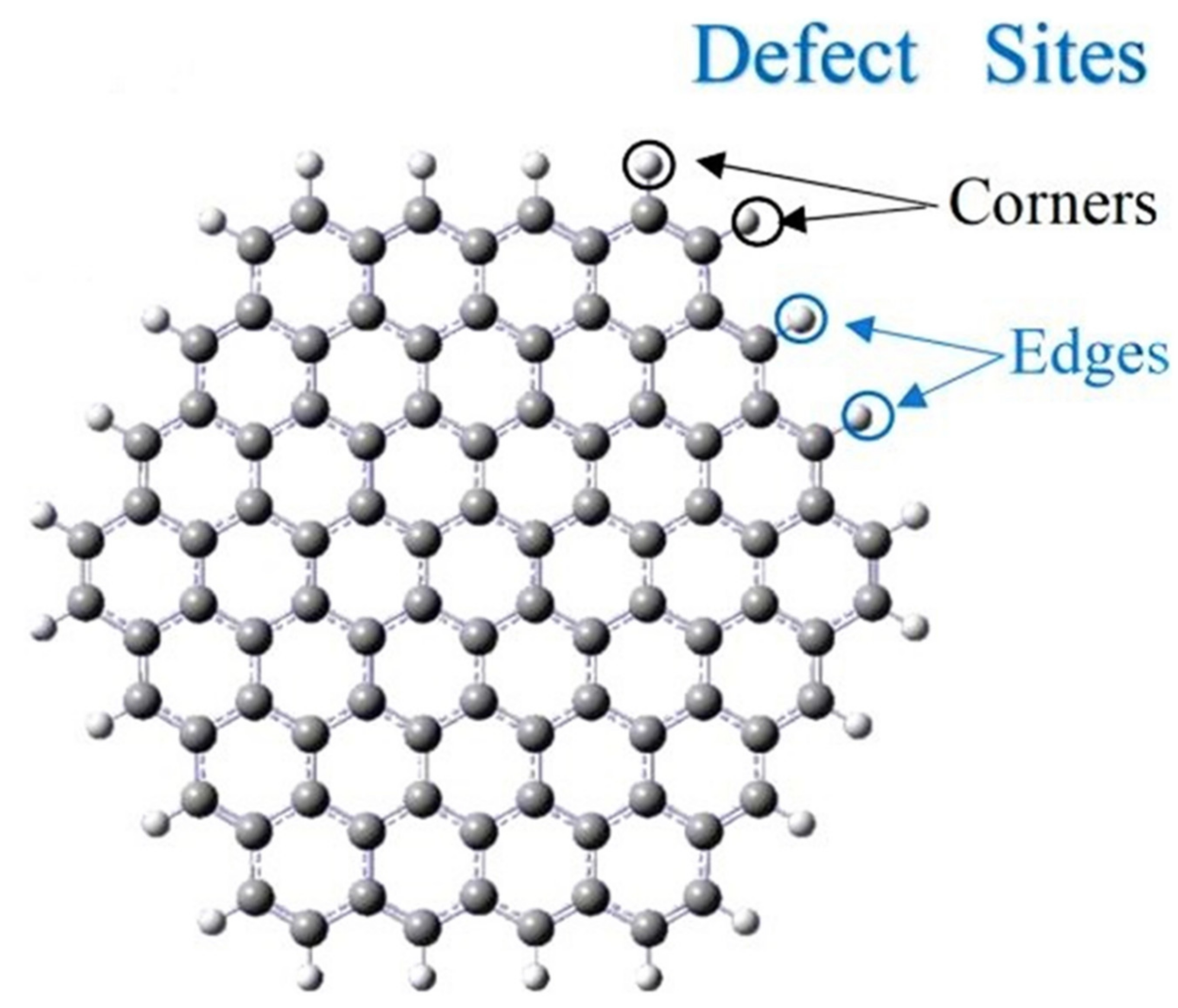
3.1. Electrochemical Biosensors Based on Pristine Graphene
| Electrode | Detection Technique | Target | LR | LOD | Ref. |
|---|---|---|---|---|---|
| Gr/poly-L-lysine | DPV | miRNA | - | 1 fM | [72] |
| Gr foam/TiO2 nanofibers | EIS | ErbB2 | 1 fM–0.1 μM | - | [73] |
| Gr/Anti-CA 15-3/GCE | DPV | CA 15-3 | 0.1–20 U/mL | 0.012 U/mL | [74] |
| Gr/AuNPs/PPY | DPV | miRNA-21 | 1 fM–1 nM | 0.02 fM | [75] |
| Gr/Herceptin/GCE | DPV | HER2 | - | - | [6] |
| Gr/DNA/AuNPs/GCE | CA | BRCA1 | 1 fM–1 nM | 1 fM | [76] |
| N2-doped Gr/AgNPs/PANI a | DPV | HER2 | 10–5 × 106 cells.mL−1 | 2 cells.mL−1 | [77] |
| N2-doped Gr/AgNPs/PANI | DPV | miRNA-21 | 10 fM–10 μM | 0.2 fM | [78] |
| 3DGrH/AuNPs | DPV | CA 15-3 | 10–2–150 U.mL–1 | 11.2 × 10–2 U.mL–1 | [79] |
| Gr/Au nanorods/GCE | DPV | CEA | 5 pg.mL−1–50 ng.mL−1 | 1.5 pg.mL−1 | [80] |
| Gr aerogels/SIL | DPV | BRCA1 | - | 3 pM | [81] |
| Gr/meso-SiO2/PET b | CA | HER2 | - | 0.6 × 10−15 M | [82] |
| amine-functionalized Gr/GCE | DPV | miRNA-155 | 3 × 10−11–10−9 M | 1.25 × 10−11 M | [83] |
| Gr/dNCs c | CA | H2O2 from MCF 7 | 1 pM–10 μM | 1 pM | [84] |
| Gr/AuNCs d/MWCNTs e/ Ab1 f/GCE | DPV | MCF 7 cells | 102–106 cells.mL−1 | 80 cells.mL−1 | [85] |
3.2. Electrochemical Biosensors Based on Graphene Oxide (GO)
3.3. Electrochemical Biosensors Based on Reduced Graphene Oxide (rGO)
3.4. Electrochemical Biosensors Based on Graphene Quantum Dot (GQD)
4. Summary and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Sui, L.; Huang, J.; Miao, L.; Nie, Y.; Wang, K.; Yang, Z.; Huang, Q.; Gong, X.; Nan, Y.; et al. MoS2-based nanocomposites for cancer diagnosis and therapy. Bioact. Mater. 2021, 6, 4209–4242. [Google Scholar] [CrossRef] [PubMed]
- Torul, H.; Yarali, E.; Eksin, E.; Ganguly, A.; Benson, J.; Tamer, U.; Papakonstantinou, P.; Erdem, A. based electrochemical biosensors for voltammetric detection of miRNA biomarkers using reduced graphene oxide or MoS2 nanosheets decorated with gold nanoparticle electrodes. Biosensors 2021, 11, 236. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, J.; Guo, Z.; Liu, Y.; Chen, H.; Chen, Z.; He, N. Applications of Aptamer-Bound Nanomaterials in Cancer Therapy. Biosensors 2021, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Pourmadadi, M.; Yazdian, F.; Ghorbanian, S.; Shamsabadipour, A.; Khandel, E.; Rashedi, H.; Rahdar, A.; Díez-Pascual, A.M. Construction of Aptamer-Based Nanobiosensor for Breast Cancer Biomarkers Detection Utilizing g-C3N4/Magnetic Nano-Structure. Biosensors 2022, 12, 921. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Wang, Y.; Zhang, J.; Huang, K.; Wang, X.; Jiang, H. Aptamer Embedded Arch-Cruciform DNA Assemblies on 2-D VS2 Scaffolds for Sensitive Detection of Breast Cancer Cells. Biosensors 2021, 11, 378. [Google Scholar] [CrossRef]
- Rahimzadeh, Z.; Naghib, S.M.; Askari, E.; Molaabasi, F.; Sadr, A.; Zare, Y.; Afsharpad, M.; Rhee, K.Y. A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer. Nanotechnol. Rev. 2021, 10, 744–753. [Google Scholar] [CrossRef]
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer in Women: Burden and TrendsGlobal Cancer in Women: Burden and Trends. Cancer Epidemiol. Biomark. Prev. 2017, 26, 444–457. [Google Scholar] [CrossRef]
- El Aamri, M.; Yammouri, G.; Mohammadi, H.; Amine, A.; Korri-Youssoufi, H. Electrochemical biosensors for detection of microRNA as a cancer biomarker: Pros and cons. Biosensors 2020, 10, 186. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Bakhshpour, M.; Pişkin, A.K.; Denizli, A. Microfluidic Systems for Cancer Diagnosis and Applications. Micromachines 2021, 12, 1349. [Google Scholar] [CrossRef]
- Gupta, N.; Renugopalakrishnan, V.; Liepmann, D.; Paulmurugan, R.; Malhotra, B.D. Cell-based biosensors: Recent trends, challenges and future perspectives. Biosens. Bioelectron. 2019, 141, 111435. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. Effect of contact resistance on the electrical conductivity of polymer graphene nanocomposites to optimize the biosensors detecting breast cancer cells. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour-Haratbar, A.; Zare, Y.; Rhee, K.Y. Electrochemical biosensors based on polymer nanocomposites for detecting breast cancer: Recent progress and future prospects. Adv. Colloid Interface Sci. 2022, 102795. [Google Scholar] [CrossRef] [PubMed]
- Zare, Y.; Rhee, K.Y. Electrical conductivity of graphene-containing composites by the conduction and volume share of networked interphase and the properties of tunnels applicable in breast cancer sensors. J. Mater. Sci. 2022, 1–12. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y.; Hui, D. Predicting of electrical conductivity for graphene-filled products by tunneling mechanism and interphase piece to enhance the performance of breast cancer biosensors. Eur. Phys. J. Plus 2022, 137, 980. [Google Scholar] [CrossRef]
- Mittal, S.; Kaur, H.; Gautam, N.; Mantha, A.K. Biosensors for breast cancer diagnosis: A review of bioreceptors, biotransducers and signal amplification strategies. Biosens. Bioelectron. 2017, 88, 217–231. [Google Scholar] [CrossRef]
- Hwang, H.S.; Jeong, J.W.; Kim, Y.A.; Chang, M. Carbon nanomaterials as versatile platforms for biosensing applications. Micromachines 2020, 11, 814. [Google Scholar] [CrossRef]
- Novodchuk, I.; Bajcsy, M.; Yavuz, M. Graphene-based field effect transistor biosensors for breast cancer detection: A review on biosensing strategies. Carbon N. Y. 2021, 172, 431–453. [Google Scholar] [CrossRef]
- Anchidin-Norocel, L.; Savage, W.K.; Gutt, G.; Amariei, S. Development, Optimization, Characterization, and Application of Electrochemical Biosensors for Detecting Nickel Ions in Food. Biosensors 2021, 11, 519. [Google Scholar] [CrossRef]
- Rafat, N.; Satoh, P.; Worden, R.M. Electrochemical biosensor for markers of neurological esterase inhibition. Biosensors 2021, 11, 459. [Google Scholar] [CrossRef]
- Mohammadpour-Haratbar, A.; Mazinani, S.; Sharif, F.; Bazargan, A.M. Improving Nonenzymatic Biosensing Performance of Electrospun Carbon Nanofibers decorated with Ni/Co Particles via Oxidation. Appl. Biochem. Biotechnol. 2022, 194, 6, 2542. [Google Scholar] [CrossRef]
- Mohammadpour-Haratbar, A.; Mosallanejad, B.; Zare, Y.; Rhee, K.Y.; Park, S.-J. Co3O4 nanoparticles embedded in electrospun carbon nanofibers as free-standing nanocomposite electrodes as highly sensitive enzyme-free glucose biosensors. Rev. Adv. Mater. Sci. 2022, 61, 744–755. [Google Scholar] [CrossRef]
- Mohammadpour-Haratbar, A.; Zare, Y.; Rhee, K.Y. Development of a theoretical model for estimating the electrical conductivity of a polymeric system reinforced with silver nanowires applicable for the biosensing of breast cancer cells. J. Mater. Res. Technol. 2022, 18, 4894–4902. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. An innovative model for conductivity of graphene-based system by networked nano-sheets, interphase and tunneling zone. Sci. Rep. 2022, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Mittal, S.K.; Choudhary, A.; Dang, R.K. Graphene: A two dimensional super material for sensor applications. Mater. Today Proc. 2021, 43, 203–208. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y.; Park, S.J. Advancement of the Power-Law Model and Its Percolation Exponent for the Electrical Conductivity of a Graphene-Containing System as a Component in the Biosensing of Breast Cancer. Polymers 2022, 14, 3057. [Google Scholar] [CrossRef] [PubMed]
- Taniselass, S.; Arshad, M.K.M.; Gopinath, S.C.B. Graphene-based electrochemical biosensors for monitoring noncommunicable disease biomarkers. Biosens. Bioelectron. 2019, 130, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Naghib, S.M.; Behzad, F.; Rahmanian, M.; Zare, Y.; Rhee, K.Y. A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination. Nanotechnol. Rev. 2020, 9, 760–767. [Google Scholar] [CrossRef]
- Ranjan, P.; Yadav, S.; Sadique, M.A.; Khan, R.; Chaurasia, J.P.; Srivastava, A.K. Functional Ionic Liquids Decorated Carbon Hybrid Nanomaterials for the Electrochemical Biosensors. Biosensors 2021, 11, 414. [Google Scholar] [CrossRef]
- Pandikumar, A.; How, G.T.S.; See, T.P.; Omar, F.S.; Jayabal, S.; Kamali, K.Z.; Yusoff, N.; Jamil, A.; Ramaraj, R.; John, S.A.; et al. Graphene and its nanocomposite material based electrochemical sensor platform for dopamine. Rsc Adv. 2014, 4, 63296–63323. [Google Scholar] [CrossRef]
- Yuan, W.; Zhou, Y.; Li, Y.; Li, C.; Peng, H.; Zhang, J.; Liu, Z.; Dai, L.; Shi, G. The edge-and basal-plane-specific electrochemistry of a single-layer graphene sheet. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef]
- Wu, S.; He, Q.; Tan, C.; Wang, Y.; Zhang, H. Graphene-based electrochemical sensors. Small 2013, 9, 1160–1172. [Google Scholar] [CrossRef] [PubMed]
- Brownson, D.A.C.; Banks, C.E. Graphene electrochemistry: An overview of potential applications. Analyst 2010, 135, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Pang, J.; Li, Y.; Sun, B.; Ibarlucea, B.; Liu, X.; Gemming, T.; Cheng, Q.; Zhang, S.; Liu, H.; et al. Graphene biodevices for early disease diagnosis based on biomarker detection. ACS Sens. 2021, 6, 3841–3881. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, K.; Kumar, K.; Venkatesu, P.; Masram, D.T. Protein immobilization on graphene oxide or reduced graphene oxide surface and their applications: Influence over activity, structural and thermal stability of protein. Adv. Colloid Interface Sci. 2021, 289, 102367. [Google Scholar] [CrossRef]
- Gosai, A.; Khondakar, K.R.; Ma, X.; Ali, M.A. Application of Functionalized Graphene Oxide Based Biosensors for Health Monitoring: Simple Graphene Derivatives to 3D Printed Platforms. Biosensors 2021, 11, 384. [Google Scholar] [CrossRef]
- Tabish, T.A.; Hayat, H.; Abbas, A.; Narayan, R.J. Graphene quantum dot--based electrochemical biosensing for early cancer detection. Curr. Opin. Electrochem. 2021, 30, 100786. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Jiang, Y.; Wang, Y.; Chen, X. Graphene and graphene-based nanomaterials: The promising materials for bright future of electroanalytical chemistry. Analyst 2011, 136, 4631–4640. [Google Scholar] [CrossRef]
- Pumera, M. Graphene-based nanomaterials and their electrochemistry. Chem. Soc. Rev. 2010, 39, 4146–4157. [Google Scholar] [CrossRef]
- Tung, T.T.; Nine, M.J.; Krebsz, M.; Pasinszki, T.; Coghlan, C.J.; Tran, D.N.H.; Losic, D. Recent advances in sensing applications of graphene assemblies and their composites. Adv. Funct. Mater. 2017, 27, 1702891. [Google Scholar] [CrossRef]
- Bai, J.; Chen, C.; Zheng, J.; Guo, C. Regulation of ferroelectric polarization and reduced graphene oxide (RGO) synergistically promoting photocatalytic performance of Bi3TiNbO9. Mater. Today Phys. 2022, 24, 100691. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.S.; Navarrete, F.; Sala, F.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Biomarkers in psychiatry: Concept, definition, types and relevance to the clinical reality. Front. Psychiatry 2020, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K.; Ferner, R.E. Biomarkers—A general review. Curr. Protoc. Pharmacol. 2017, 76, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Verma, M. Cancer biomarkers: Are we ready for the prime time? Cancers 2010, 2, 190–208. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Qu, X. Cancer biomarker detection: Recent achievements and challenges. Chem. Soc. Rev. 2015, 44, 2963–2997. [Google Scholar] [CrossRef]
- Khanmohammadi, A.; Aghaie, A.; Vahedi, E.; Qazvini, A.; Ghanei, M.; Afkhami, A.; Hajian, A.; Bagheri, H. Electrochemical biosensors for the detection of lung cancer biomarkers: A review. Talanta 2020, 206, 120251. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Zhou, X.; Yuan, M.; Qu, D.; Zheng, Y.; Vishinkin, R.; Khatib, M.; Wu, W.; Haick, H. Disease detection with molecular biomarkers: From chemistry of body fluids to nature-inspired chemical sensors. Chem. Rev. 2019, 119, 11761–11817. [Google Scholar] [CrossRef]
- Steckl, A.J.; Ray, P. Stress biomarkers in biological fluids and their point-of-use detection. ACS Sens. 2018, 3, 2025–2044. [Google Scholar] [CrossRef]
- Mauriz, E. Low-fouling substrates for plasmonic sensing of circulating biomarkers in biological fluids. Biosensors 2020, 10, 63. [Google Scholar] [CrossRef]
- Heneghan, H.M.; Miller, N.; Lowery, A.J.; Sweeney, K.J.; Kerin, M.J. MicroRNAs as novel biomarkers for breast cancer. J. Oncol. 2009, 2010. [Google Scholar] [CrossRef]
- Rashid, S.; Nawaz, M.H.; ur Rehman, I.; Hayat, A.; Marty, J.L. Dopamine/mucin-1 functionalized electro-active carbon nanotubes as a probe for direct competitive electrochemical immunosensing of breast cancer biomarker. Sens. Actuators B Chem. 2021, 330, 129351. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Tagi, S.; Solhi, E.; Mokhtarzadeh, A.; Shadjou, N.; Eftekhari, A.; Mahboob, S. An innovative immunosensor for ultrasensitive detection of breast cancer specific carbohydrate (CA 15-3) in unprocessed human plasma and MCF-7 breast cancer cell lysates using gold nanospear electrochemically assembled onto thiolated graphene quantum dots. Int. J. Biol. Macromol. 2018, 114, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, I.M.; Tian, Y.; Anjum, S.; Hanif, S.; Hosseini, M.; Lou, B.; Xu, G. Comprehensive review on the electrochemical biosensors of different breast cancer biomarkers. Sens. Actuators B Chem. 2022, 131944. [Google Scholar] [CrossRef]
- Sadeghi, M.; Kashanian, S.; Naghib, S.M.; Haghiralsadat, F.; Tofighi, D. An efficient electrochemical biosensor based on pencil graphite electrode mediated by 2D functionalized graphene oxide to detect HER2 breast cancer biomarker. Int. J. Electrochem. Sci. 2022, 17, 1–15. [Google Scholar] [CrossRef]
- Wang, L. Early diagnosis of breast cancer. Sensors 2017, 17, 1572. [Google Scholar] [CrossRef]
- Topkaya, S.N.; Azimzadeh, M.; Ozsoz, M. Electrochemical biosensors for cancer biomarkers detection: Recent advances and challenges. Electroanalysis 2016, 28, 1402–1419. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S. Graphene and its derivative-based sensing materials for analytical devices. J. Mater. Chem. 2011, 21, 18503–18516. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, S.-J.; Choi, J.-W. Electrical property of graphene and its application to electrochemical biosensing. Nanomaterials 2019, 9, 297. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, T.; Zhang, X. Graphene-based biosensors for detection of biomarkers. Micromachines 2020, 11, 60. [Google Scholar] [CrossRef]
- Pumera, M. Graphene in biosensing. Mater. Today 2011, 14, 308–315. [Google Scholar] [CrossRef]
- Gómez-Navarro, C.; Meyer, J.C.; Sundaram, R.S.; Chuvilin, A.; Kurasch, S.; Burghard, M.; Kern, K.; Kaiser, U. Atomic Structure of Reduced Graphene Oxide. Nano Lett. 2010, 10, 1144–1148. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jena, A.; Meng, F.; Wen, S.; Ma, J.; Li, X.; Li, W. Indirect electron-phonon interaction leading to significant reduction of thermal conductivity in graphene. Mater. Today Phys. 2021, 18, 100315. [Google Scholar] [CrossRef]
- Alhamoud, Y.; Li, Y.; Zhou, H.; Al-Wazer, R.; Gong, Y.; Zhi, S.; Yang, D. Label-free and highly-sensitive detection of Ochratoxin A using one-pot synthesized reduced graphene oxide/gold nanoparticles-based impedimetric aptasensor. Biosensors 2021, 11, 87. [Google Scholar] [CrossRef]
- Waiwinya, W.; Putnin, T.; Pimalai, D.; Chawjiraphan, W.; Sathirapongsasuti, N.; Japrung, D. Immobilization-free electrochemical sensor coupled with a graphene-oxide-based aptasensor for glycated albumin detection. Biosensors 2021, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Rehman, O.; Zhuang, H.; Muhamed Ali, A.; Ibrahim, A.; Li, Z. Validation of miRNAs as breast cancer biomarkers with a machine learning approach. Cancers 2019, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Rafiee-Pour, H.-A.; Behpour, M.; Keshavarz, M. A novel label-free electrochemical miRNA biosensor using methylene blue as redox indicator: Application to breast cancer biomarker miRNA-21. Biosens. Bioelectron. 2016, 77, 202–207. [Google Scholar] [CrossRef]
- Wang, H.; Peng, R.; Wang, J.; Qin, Z.; Xue, L. Circulating microRNAs as potential cancer biomarkers: The advantage and disadvantage. Clin. Epigenetics 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Tan, W.; Liu, B.; Qu, S.; Liang, G.; Luo, W.; Gong, C. MicroRNAs and cancer: Key paradigms in molecular therapy. Oncol. Lett. 2018, 15, 2735–2742. [Google Scholar] [CrossRef]
- Zhang, J.; Hun, X. Electrochemical determination of miRNA-155 using molybdenum carbide nanosheets and colloidal gold modified electrode coupled with mismatched catalytic hairpin assembly strategy. Microchem. J. 2019, 150, 104095. [Google Scholar] [CrossRef]
- Iorio, M.V.; Ferracin, M.; Liu, C.-G.; Veronese, A.; Spizzo, R.; Sabbioni, S.; Magri, E.; Pedriali, M.; Fabbri, M.; Campiglio, M.; et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005, 65, 7065–7070. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, C.; Wang, C.; Chu, Y.; Wang, S.; Sun, M.Y.; Ji, H.; Gao, Y.; Wang, Y.; Han, Y.; et al. Poly-l-lysine-modified graphene field-effect transistor biosensors for ultrasensitive breast cancer miRNAs and SARS-CoV-2 RNA detection. Anal. Chem. 2022, 94, 1626–1636. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Mondal, K.; Jiao, Y.; Oren, S.; Xu, Z.; Sharma, A.; Dong, L. Microfluidic immuno-biochip for detection of breast cancer biomarkers using hierarchical composite of porous graphene and titanium dioxide nanofibers. ACS Appl. Mater. Interfaces 2016, 8, 20570–20582. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, J.; Li, S.; Turner, A.P.F. Electrochemical immunosensor with N-doped graphene-modified electrode for label-free detection of the breast cancer biomarker CA 15-3. Biosens. Bioelectron. 2013, 43, 25–29. [Google Scholar] [CrossRef]
- Pothipor, C.; Aroonyadet, N.; Bamrungsap, S.; Jakmunee, J.; Ounnunkad, K. A highly sensitive electrochemical microRNA-21 biosensor based on intercalating methylene blue signal amplification and a highly dispersed gold nanoparticles/graphene/polypyrrole composite. Analyst 2021, 146, 2679–2688. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Sandhyarani, N. Graphene-DNA electrochemical sensor for the sensitive detection of BRCA1 gene. Sens. Actuators B Chem. 2014, 204, 777–782. [Google Scholar] [CrossRef]
- Salahandish, R.; Ghaffarinejad, A.; Naghib, S.M.; Majidzadeh-A, K.; Zargartalebi, H.; Sanati-Nezhad, A. Nano-biosensor for highly sensitive detection of HER2 positive breast cancer. Biosens. Bioelectron. 2018, 117, 104–111. [Google Scholar] [CrossRef]
- Salahandish, R.; Ghaffarinejad, A.; Omidinia, E.; Zargartalebi, H.; Majidzadeh-A, K.; Naghib, S.M.; Sanati-Nezhad, A. Label-free ultrasensitive detection of breast cancer miRNA-21 biomarker employing electrochemical nano-genosensor based on sandwiched AgNPs in PANI and N-doped graphene. Biosens. Bioelectron. 2018, 120, 129–136. [Google Scholar] [CrossRef]
- Shekari, Z.; Zare, H.R.; Falahati, A. Dual assaying of breast cancer biomarkers by using a sandwich--type electrochemical aptasensor based on a gold nanoparticles--3D graphene hydrogel nanocomposite and redox probes labeled aptamers. Sens. Actuators B Chem. 2021, 332, 129515. [Google Scholar] [CrossRef]
- Wen, W.; Huang, J.-Y.; Bao, T.; Zhou, J.; Xia, H.-X.; Zhang, X.-H.; Wang, S.-F.; Zhao, Y.-D. Increased electrocatalyzed performance through hairpin oligonucleotide aptamer-functionalized gold nanorods labels and graphene-streptavidin nanomatrix: Highly selective and sensitive electrochemical biosensor of carcinoembryonic antigen. Biosens. Bioelectron. 2016, 83, 142–148. [Google Scholar] [CrossRef]
- Kazerooni, H.; Nassernejad, B. A novel biosensor nanomaterial for the ultraselective and ultrasensitive electrochemical diagnosis of the breast cancer-related BRCA1 gene. Anal. Methods 2016, 8, 3069–3074. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Zou, X.; Wu, S.; Wan, D.; Cao, A.; Liao, L.; Yuan, Q.; Duan, X. Ultrafine graphene nanomesh with large on/off ratio for high-performance flexible biosensors. Adv. Funct. Mater. 2017, 27, 1604096. [Google Scholar] [CrossRef]
- Salimi, A.; Kavosi, B.; Navaee, A. Amine-functionalized graphene as an effective electrochemical platform toward easily miRNA hybridization detection. Measurement 2019, 143, 191–198. [Google Scholar] [CrossRef]
- Del Real Mata, C.; Moakhar, R.S.; Hosseini, I.I.; Jalali, M.; Mahshid, S. A nanostructured microfluidic device for plasmon-assisted electrochemical detection of hydrogen peroxide released from cancer cells. Nanoscale 2021, 13, 14316–14329. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fu, Y.; Su, H.; Mao, L.; Chen, M. Sensitive detection of MCF-7 human breast cancer cells by using a novel DNA-labeled sandwich electrochemical biosensor. Biosens. Bioelectron. 2018, 122, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cao, Z.; Liu, R.; Liu, L.; Li, H.; Li, X.; Chen, Y.; Lu, C.; Liu, Y. AuNPs as an important inorganic nanoparticle applied in drug carrier systems. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4222–4233. [Google Scholar] [CrossRef] [PubMed]
- Işın, D.; Eksin, E.; Erdem, A. Graphene-Oxide and Ionic Liquid Modified Electrodes for Electrochemical Sensing of Breast Cancer 1 Gene. Biosensors 2022, 12, 95. [Google Scholar] [CrossRef]
- Tutt, A.; Ashworth, A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol. Med. 2002, 8, 571–576. [Google Scholar] [CrossRef]
- Rosen, E.M.; Fan, S.; Pestell, R.G.; Goldberg, I.D. BRCA1 gene in breast cancer. J. Cell. Physiol. 2003, 196, 19–41. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Bobenrieth, A.; De Winter, J.; Gerbaux, P.; Raquez, J.-M.; Dubois, P. A supramolecular approach toward organo-dispersible graphene and its straightforward polymer nanocomposites. J. Mater. Chem. 2012, 22, 18124–18126. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Sun, Y.; Tan, Y.-Z.; Yang, S.; Feng, X.; Müllen, K. Three-dimensional graphene-based macro-and mesoporous frameworks for high-performance electrochemical capacitive energy storage. J. Am. Chem. Soc. 2012, 134, 19532–19535. [Google Scholar] [CrossRef] [PubMed]
- Worsley, M.A.; Pauzauskie, P.J.; Olson, T.Y.; Biener, J.; Satcher, J.H., Jr.; Baumann, T.F. Synthesis of graphene aerogel with high electrical conductivity. J. Am. Chem. Soc. 2010, 132, 14067–14069. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, T.; Pourhassan-Moghaddam, M.; Shirdel, B.; Baradaran, B.; Morales-Narváez, E.; Golmohammadi, H. On-site detection of carcinoembryonic antigen in human serum. Biosensors 2021, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-W.; Oh, J.-W.; Kim, J.-H.; Park, S.H.; Kim, K.-S.; Kim, J.H.; Lee, K.S. Preoperative CA 15-3 and CEA serum levels as predictor for breast cancer outcomes. Ann. Oncol. 2008, 19, 675–681. [Google Scholar] [CrossRef]
- Marrelli, D.; Roviello, F.; De Stefano, A.; Farnetani, M.; Garosi, L.; Messano, A.; Pinto, E. Prognostic significance of CEA, CA 19-9 and CA 72-4 preoperative serum levels in gastric carcinoma. Oncology 1999, 57, 55–62. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, B.-T. Surface plasmon resonance biosensor based on graphene oxide/silver coated polymer cladding silica fiber. Sens. Actuators B Chem. 2018, 275, 332–338. [Google Scholar] [CrossRef]
- Tao, Y.; Qian, Y.; Li, Y.; Hao, J.; Xu, T.; Li, W.; Jiang, Q.; Luo, Y.; Yang, J. High-performance and long-term thermal management material of MIL-101Cr@ GO. Mater. Today Phys. 2022, 22, 100572. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Sun, Y.; Ding, C.; Lin, Y.; Sun, W.; Luo, C. A novel ionic liquid functionalized graphene oxide supported gold nanoparticle composite film for sensitive electrochemical detection of dopamine. RSC Adv. 2017, 7, 2315–2322. [Google Scholar] [CrossRef]
- Albishri, H.M.; Abd El-Hady, D. Hyphenation of enzyme/graphene oxide-ionic liquid/glassy carbon biosensors with anodic differential pulse stripping voltammetry for reliable determination of choline and acetylcholine in human serum. Talanta 2019, 200, 107–114. [Google Scholar] [CrossRef]
- Milosavljevic, V.; Mitrevska, K.; Adam, V. Benefits of oxidation and size reduction of graphene/graphene oxide nanoparticles in biosensing application: Classification of graphene/graphene oxide nanoparticles. Sens. Actuators B Chem. 2022, 353, 131122. [Google Scholar] [CrossRef]
- Pothipor, C.; Bamrungsap, S.; Jakmunee, J.; Ounnunkad, K. A gold nanoparticle-dye/poly (3-aminobenzylamine)/two dimensional MoSe2/graphene oxide electrode towards label-free electrochemical biosensor for simultaneous dual-mode detection of cancer antigen 15-3 and microRNA-21. Colloids Surf. B Biointerfaces 2022, 210, 112260. [Google Scholar] [CrossRef] [PubMed]
- Benvidi, A.; Abbasi, Z.; Tezerjani, M.D.; Banaei, M.; Zare, H.R.; Molahosseini, H.; Jahanbani, S. A highly selective DNA sensor based on graphene oxide-silk fibroin composite and AuNPs as a probe oligonucleotide immobilization platform. Acta Chim. Slov. 2018, 65, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Azimzadeh, M.; Rahaie, M.; Nasirizadeh, N.; Ashtari, K.; Naderi-Manesh, H. An electrochemical nanobiosensor for plasma miRNA-155, based on graphene oxide and gold nanorod, for early detection of breast cancer. Biosens. Bioelectron. 2016, 77, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Bharti, A.; Rana, S.; Dahiya, D.; Agnihotri, N.; Prabhakar, N. An electrochemical aptasensor for analysis of MUC1 using gold platinum bimetallic nanoparticles deposited carboxylated graphene oxide. Anal. Chim. Acta 2020, 1097, 186–195. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Nikokar, I.; Zokaei, M.; Bozorgzadeh, E. Design, development and evaluation of microRNA-199a-5p detecting electrochemical nanobiosensor with diagnostic application in Triple Negative Breast Cancer. Talanta 2018, 189, 592–598. [Google Scholar] [CrossRef]
- Gupta, P.; Bharti, A.; Kaur, N.; Singh, S.; Prabhakar, N. An electrochemical aptasensor based on gold nanoparticles and graphene oxide doped poly (3, 4-ethylenedioxythiophene) nanocomposite for detection of MUC1. J. Electroanal. Chem. 2018, 813, 102–108. [Google Scholar] [CrossRef]
- Saeed, A.A.; Sánchez, J.L.A.; O’Sullivan, C.K.; Abbas, M.N. DNA biosensors based on gold nanoparticles-modified graphene oxide for the detection of breast cancer biomarkers for early diagnosis. Bioelectrochemistry 2017, 118, 91–99. [Google Scholar] [CrossRef]
- Wang, K.; He, M.-Q.; Zhai, F.-H.; He, R.-H.; Yu, Y.-L. A novel electrochemical biosensor based on polyadenine modified aptamer for label-free and ultrasensitive detection of human breast cancer cells. Talanta 2017, 166, 87–92. [Google Scholar] [CrossRef]
- Yan, M.; Sun, G.; Liu, F.; Lu, J.; Yu, J.; Song, X. An aptasensor for sensitive detection of human breast cancer cells by using porous GO/Au composites and porous PtFe alloy as effective sensing platform and signal amplification labels. Anal. Chim. Acta 2013, 798, 33–39. [Google Scholar] [CrossRef]
- Shuai, H.-L.; Huang, K.-J.; Zhang, W.-J.; Cao, X.; Jia, M.-P. Sandwich-type microRNA biosensor based on magnesium oxide nanoflower and graphene oxide--gold nanoparticles hybrids coupling with enzyme signal amplification. Sens. Actuators B Chem. 2017, 243, 403–411. [Google Scholar] [CrossRef]
- Park, S.; Singh, A.; Kim, S.; Yang, H. Electroreduction-based electrochemical-enzymatic redox cycling for the detection of cancer antigen 15-3 using graphene oxide-modified indium--tin oxide electrodes. Anal. Chem. 2014, 86, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Wei, K.-C.; Liao, S.; Huang, C.-Y.; Sun, C.-L.; Wu, P.-J.; Lu, Y.-J.; Yang, H.-W.; Ma, C.-C.M. A reusable magnetic graphene oxide-modified biosensor for vascular endothelial growth factor detection in cancer diagnosis. Biosens. Bioelectron. 2015, 67, 431–437. [Google Scholar] [CrossRef]
- Ranjan, P.; Abubakar Sadique, M.; Yadav, S.; Khan, R. An Electrochemical Immunosensor Based on Gold-Graphene Oxide Nanocomposites with Ionic Liquid for Detecting the Breast Cancer CD44 Biomarker. ACS Appl. Mater. Interfaces 2022, 14, 20802–20812. [Google Scholar] [CrossRef] [PubMed]
- Narang, J.; Mishra, A.; Pilloton, R.; Wadhwa, S.; Pundir, C.S.; Khanuja, M. Development of MoSe2 nano-urchins as a sensing platform for a selective bio-capturing of Escherichia coli shiga toxin DNA. Biosensors 2018, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Brayman, M.; Thathiah, A.; Carson, D.D. MUC1: A multifunctional cell surface component of reproductive tissue epithelia. Reprod. Biol. Endocrinol. 2004, 2, 1–9. [Google Scholar] [CrossRef]
- Nath, S.; Mukherjee, P. MUC1: A multifaceted oncoproteihn with a key role in cancer progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef]
- Hu, R.; Wen, W.; Wang, Q.; Xiong, H.; Zhang, X.; Gu, H.; Wang, S. Novel electrochemical aptamer biosensor based on an enzyme--gold nanoparticle dual label for the ultrasensitive detection of epithelial tumour marker MUC1. Biosens. Bioelectron. 2014, 53, 384–389. [Google Scholar] [CrossRef]
- Zaretsky, J.Z.; Barnea, I.; Aylon, Y.; Gorivodsky, M.; Wreschner, D.H.; Keydar, I. MUC1 gene overexpressed in breast cancer: Structure and transcriptional activity of the MUC1 promoter and role of estrogen receptor alpha (ER$α$) in regulation of the MUC1 gene expression. Mol. Cancer 2006, 5, 1–14. [Google Scholar] [CrossRef]
- Nawaz, M.A.H.; Rauf, S.; Catanante, G.; Nawaz, M.H.; Nunes, G.; Louis Marty, J.; Hayat, A. One step assembly of thin films of carbon nanotubes on screen printed interface for electrochemical aptasensing of breast cancer biomarker. Sensors 2016, 16, 1651. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Willander, M.; Sharma, J.G.; Malhotra, B.D. A solution processed carbon nanotube modified conducting paper sensor for cancer detection. J. Mater. Chem. B 2015, 3, 9305–9314. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-S.; Xu, J.-K.; Lu, L.-M.; Zhu, X.-F.; Wang, W.-M.; Yang, T.-T.; Zhang, K.-X.; Yu, Y.-F. A label-free electrochemical immunosensor for carcinoembryonic antigen detection on a graphene platform doped with poly (3, 4-ethylenedioxythiophene)/Au nanoparticles. RSC Adv. 2015, 5, 86910–86918. [Google Scholar] [CrossRef]
- Jain, U.; Gupta, S.; Chauhan, N. Construction of an amperometric glycated hemoglobin biosensor based on Au--Pt bimetallic nanoparticles and poly (indole-5-carboxylic acid) modified Au electrode. Int. J. Biol. Macromol. 2017, 105, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Thakur, H.; Kumar, R.; Prabhakar, N. An electrochemical sensor modified with poly (3, 4-ethylenedioxythiophene)-wrapped multi-walled carbon nanotubes for enzyme inhibition-based determination of organophosphates. Microchim. Acta 2016, 183, 2307–2315. [Google Scholar] [CrossRef]
- Thakur, H.; Kaur, N.; Sareen, D.; Prabhakar, N. Electrochemical determination of M. tuberculosis antigen based on Poly (3, 4-ethylenedioxythiophene) and functionalized carbon nanotubes hybrid platform. Talanta 2017, 171, 115–123. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Y.; Wang, L.; Liang, Z.; Li, D.; Xu, X.; Chen, Y.; Yang, X.; Zhang, H.; Niu, H. Self-crosslinkable chitosan-hyaluronic acid dialdehyde nanoparticles for CD44-targeted siRNA delivery to treat bladder cancer. Bioact. Mater. 2021, 6, 433–446. [Google Scholar] [CrossRef]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef]
- Xu, H.; Niu, M.; Yuan, X.; Wu, K.; Liu, A. CD44 as a tumor biomarker and therapeutic target. Exp. Hematol. Oncol. 2020, 9, 1–14. [Google Scholar] [CrossRef]
- Inoue, K.; Fry, E.A. Aberrant splicing of estrogen receptor, HER2, and CD44 genes in breast cancer. Genet. Epigenet. 2015, 7, GEG--S35500. [Google Scholar] [CrossRef]
- McFarlane, S.; Coulter, J.A.; Tibbits, P.; O’Grady, A.; McFarlane, C.; Montgomery, N.; Hill, A.; McCarthy, H.O.; Young, L.S.; Kay, E.W.; et al. CD44 increases the efficiency of distant metastasis of breast cancer. Oncotarget 2015, 6, 11465. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanizamani, F.; Timur, S. Ionic Liquids from Biocompatibility and Electrochemical Aspects toward Applying in Biosensing Devices. Anal. Chem. 2018, 90, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Sun, X.; Han, B. Synthesis of functional nanomaterials in ionic liquids. Adv. Mater. 2016, 28, 1011–1030. [Google Scholar] [CrossRef] [PubMed]
- Aldroubi, S.; Brun, N.; Malham, I.B.; Mehdi, A. When graphene meets ionic liquids: A good match for the design of functional materials. Nanoscale 2021, 13, 2750–2779. [Google Scholar] [CrossRef] [PubMed]
- Abo-Hamad, A.; AlSaadi, M.A.; Hayyan, M.; Juneidi, I.; Hashim, M.A. Ionic liquid-carbon nanomaterial hybrids for electrochemical sensor applications: A review. Electrochim. Acta 2016, 193, 321–343. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon N. Y. 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Azizighannad, S.; Mitra, S. Stepwise reduction of graphene oxide (GO) and its effects on chemical and colloidal properties. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Hallfors, N.G.; Al Junaibi, S.A.; Liao, K.; Ismail, M.; Isakovic, A.F. Reduced Graphene oxide for the design of electrocardiogram sensors: Current status and perspectives. In The IoT Physical Layer; Springer: Berlin/Heidelberg, Germany, 2019; pp. 3–11. [Google Scholar]
- Rostamabadi, P.F.; Heydari-Bafrooei, E. Impedimetric aptasensing of the breast cancer biomarker HER2 using a glassy carbon electrode modified with gold nanoparticles in a composite consisting of electrochemically reduced graphene oxide and single-walled carbon nanotubes. Microchim. Acta 2019, 186, 1–9. [Google Scholar] [CrossRef]
- Pimalai, D.; Putnin, T.; Waiwinya, W.; Chotsuwan, C.; Aroonyadet, N.; Japrung, D. Development of electrochemical biosensors for simultaneous multiplex detection of microRNA for breast cancer screening. Microchim. Acta 2021, 188, 1–10. [Google Scholar] [CrossRef]
- Jozghorbani, M.; Fathi, M.; Kazemi, S.H.; Alinejadian, N. Determination of carcinoembryonic antigen as a tumor marker using a novel graphene-based label-free electrochemical immunosensor. Anal. Biochem. 2021, 613, 114017. [Google Scholar] [CrossRef]
- Augustine, S.; Kumar, P.; Malhotra, B.D. Amine-functionalized MoO3@ RGO nanohybrid-based biosensor for breast cancer detection. ACS Appl. Bio Mater. 2019, 2, 5366–5378. [Google Scholar] [CrossRef] [PubMed]
- Amani, J.; Khoshroo, A.; Rahimi-Nasrabadi, M. Electrochemical immunosensor for the breast cancer marker CA 15--3 based on the catalytic activity of a CuS/reduced graphene oxide nanocomposite towards the electrooxidation of catechol. Microchim. Acta 2018, 185, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Benvidi, A.; Firouzabadi, A.D.; Moshtaghiun, S.M.; Mazloum-Ardakani, M.; Tezerjani, M.D. Ultrasensitive DNA sensor based on gold nanoparticles/reduced graphene oxide/glassy carbon electrode. Anal. Biochem. 2015, 484, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Benvidi, A.; Tezerjani, M.D.; Jahanbani, S.; Ardakani, M.M.; Moshtaghioun, S.M. Comparison of impedimetric detection of DNA hybridization on the various biosensors based on modified glassy carbon electrodes with PANHS and nanomaterials of RGO and MWCNTs. Talanta 2016, 147, 621–627. [Google Scholar] [CrossRef]
- Cheng, F.-F.; He, T.-T.; Miao, H.-T.; Shi, J.-J.; Jiang, L.-P.; Zhu, J.-J. Electron transfer mediated electrochemical biosensor for microRNAs detection based on metal ion functionalized titanium phosphate nanospheres at attomole level. ACS Appl. Mater. Interfaces 2015, 7, 2979–2985. [Google Scholar] [CrossRef]
- Dong, W.; Ren, Y.; Bai, Z.; Yang, Y.; Wang, Z.; Zhang, C.; Chen, Q. Trimetallic AuPtPd nanocomposites platform on graphene: Applied to electrochemical detection and breast cancer diagnosis. Talanta 2018, 189, 79–85. [Google Scholar] [CrossRef]
- Dong, W.; Ren, Y.; Bai, Z.; Yang, Y.; Chen, Q. Fabrication of hexahedral Au-Pd/graphene nanocomposites biosensor and its application in cancer cell H2O2 detection. Bioelectrochemistry 2019, 128, 274–282. [Google Scholar] [CrossRef]
- Shafiei, F.; Saberi, R.S.; Mehrgardi, M.A. A label-free electrochemical aptasensor for breast cancer cell detection based on a reduced graphene oxide-chitosan-gold nanoparticle composite. Bioelectrochemistry 2021, 140, 107807. [Google Scholar] [CrossRef]
- Safavipour, M.; Kharaziha, M.; Amjadi, E.; Karimzadeh, F.; Allafchian, A. TiO2 nanotubes/reduced GO nanoparticles for sensitive detection of breast cancer cells and photothermal performance. Talanta 2020, 208, 120369. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Salimian, R. Ultrasensitive detection of cancer biomarkers using conducting polymer/electrochemically reduced graphene oxide-based biosensor: Application toward BRCA1 sensing. Sens. Actuators B Chem. 2018, 266, 160–169. [Google Scholar] [CrossRef]
- Tian, L.; Qi, J.; Qian, K.; Oderinde, O.; Liu, Q.; Yao, C.; Song, W.; Wang, Y. Copper (II) oxide nanozyme based electrochemical cytosensor for high sensitive detection of circulating tumor cells in breast cancer. J. Electroanal. Chem. 2018, 812, 1–9. [Google Scholar] [CrossRef]
- Tian, L.; Qi, J.; Qian, K.; Oderinde, O.; Cai, Y.; Yao, C.; Song, W.; Wang, Y. An ultrasensitive electrochemical cytosensor based on the magnetic field assisted binanozymes synergistic catalysis of Fe3O4 nanozyme and reduced graphene oxide/molybdenum disulfide nanozyme. Sens. Actuators B Chem. 2018, 260, 676–684. [Google Scholar] [CrossRef]
- Xia, Y.-M.; Li, M.-Y.; Chen, C.-L.; Xia, M.; Zhang, W.; Gao, W.-W. Employing Label-free Electrochemical Biosensor Based on 3D-Reduced Graphene Oxide and Polyaniline Nanofibers for Ultrasensitive Detection of Breast Cancer BRCA1 Biomarker. Electroanalysis 2020, 32, 2045–2055. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Solhi, E.; Jafari, M.; Mokhtarzadeh, A.; Soleymani, J.; Jouyban, A.; Mahboob, S. Ultrasensitive immunoassay of tumor protein CA 15.3 in MCF-7 breast cancer cell lysates and unprocessed human plasma using gold nanoparticles doped on the structure of mesoporous silica. Int. J. Biol. Macromol. 2018, 120, 2493–2508. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, F.; Liang, Q.; Wang, Z. A three-dimensional graphene-based ratiometric signal amplification aptasensor for MUC1 detection. Biosens. Bioelectron. 2018, 120, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Low, S.S.; Pan, Y.; Ji, D.; Li, Y.; Lu, Y.; He, Y.; Chen, Q.; Liu, Q. Smartphone-based portable electrochemical biosensing system for detection of circulating microRNA-21 in saliva as a proof-of-concept. Sens. Actuators B Chem. 2020, 308, 127718. [Google Scholar]
- Hassanpour, S.; Hasanzadeh, M.; Saadati, A.; Shadjou, N.; Soleymani, J.; Jouyban, A. A novel paper based immunoassay of breast cancer specific carbohydrate (CA 15.3) using silver nanoparticles-reduced graphene oxide nano-ink technology: A new platform to construction of microfluidic paper-based analytical devices ($μ$PADs) towards biomedi. Microchem. J. 2019, 146, 345–358. [Google Scholar] [CrossRef]
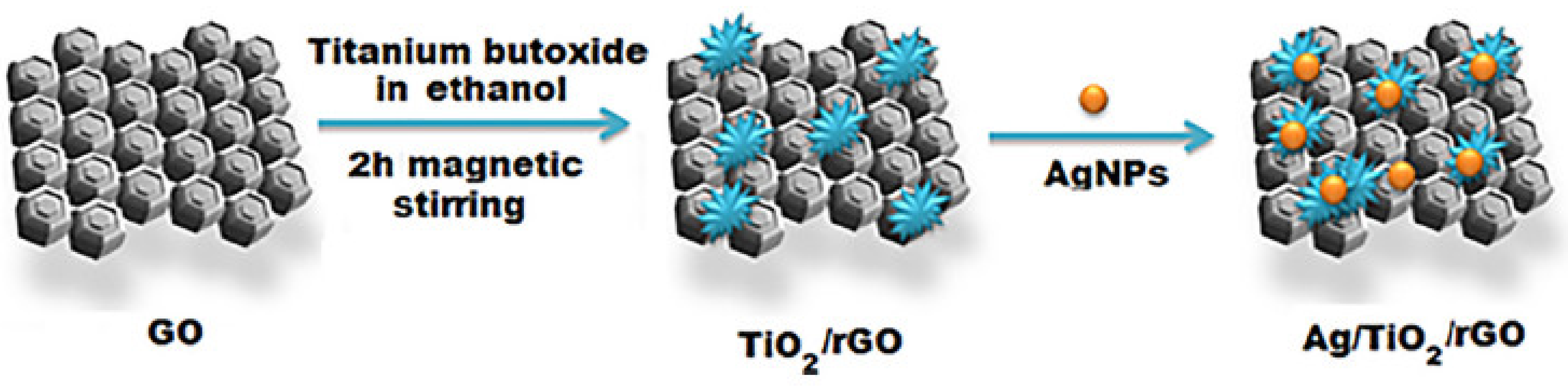
- Shawky, A.M.; El-Tohamy, M. Signal amplification strategy of label-free ultrasenstive electrochemical immunosensor based ternary Ag/TiO2/rGO nanocomposites for detecting breast cancer biomarker CA 15-3. Mater. Chem. Phys. 2021, 272, 124983. [Google Scholar] [CrossRef]
- Li, Y.; Huan, K.; Deng, D.; Tang, L.; Wang, J.; Luo, L. Facile synthesis of ZnMn2O4@ rGO microspheres for ultrasensitive electrochemical detection of hydrogen peroxide from human breast cancer cells. ACS Appl. Mater. Interfaces 2019, 12, 3430–3437. [Google Scholar] [CrossRef]
- Tabasi, A.; Noorbakhsh, A.; Sharifi, E. Reduced graphene oxide-chitosan-aptamer interface as new platform for ultrasensitive detection of human epidermal growth factor receptor 2. Biosens. Bioelectron. 2017, 95, 117–123. [Google Scholar] [CrossRef]
- Hurvitz, S.; McCann, K. HER2-Positive Breast Cancer; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Hung, M.-C.; Matin, A.; Zhang, Y.; Xing, X.; Sorgi, F.; Huang, L.; Yu, D. HER-2/neu-targeting gene therapy-a review. Gene 1995, 159, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; McRae, L.; Firby, C.J.; Al-Hussein, M.; Elezzabi, A.Y. Nanohybridization of molybdenum oxide with tungsten molybdenum oxide nanowires for solution-processed fully reversible switching of energy storing smart windows. Nano Energy 2018, 47, 130–139. [Google Scholar] [CrossRef]
- De Castro, I.A.; Datta, R.S.; Ou, J.Z.; Castellanos-Gomez, A.; Sriram, S.; Daeneke, T.; Kalantar-zadeh, K. Molybdenum oxides--from fundamentals to functionality. Adv. Mater. 2017, 29, 1701619. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fu, Z.; Yan, F.; Ju, H. Biomedical and clinical applications of immunoassays and immunosensors for tumor markers. TrAC Trends Anal. Chem. 2007, 26, 679–688. [Google Scholar] [CrossRef]
- Xie, Y.; Carbone, L.; Nobile, C.; Grillo, V.; D’Agostino, S.; Della Sala, F.; Giannini, C.; Altamura, D.; Oelsner, C.; Kryschi, C.; et al. Metallic-like stoichiometric copper sulfide nanocrystals: Phase-and shape-selective synthesis, near-infrared surface plasmon resonance properties, and their modeling. ACS Nano 2013, 7, 7352–7369. [Google Scholar] [CrossRef]
- Huang, C.; Zhu, M.; Kang, L.; Li, X.; Dai, B. Active carbon supported TiO2--AuCl3/AC catalyst with excellent stability for acetylene hydrochlorination reaction. Chem. Eng. J. 2014, 242, 69–75. [Google Scholar] [CrossRef]
- Alexandre, J.; Batteux, F.; Nicco, C.; Chéreau, C.; Laurent, A.; Guillevin, L.; Weill, B.; Goldwasser, F. Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. Int. J. Cancer 2006, 119, 41–48. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Gao, C.; Tian, Y.; Zhang, R.; Jing, J.; Zhang, X. Endoplasmic reticulum-directed ratiometric fluorescent probe for quantitive detection of basal H2O2. Anal. Chem. 2017, 89, 12945–12950. [Google Scholar] [CrossRef]
- Li, Z.; Xin, Y.; Zhang, Z. New photocathodic analysis platform with quasi-core/shell-structured TiO2@ Cu2O for sensitive detection of H2O2 release from living cells. Anal. Chem. 2015, 87, 10491–10497. [Google Scholar] [CrossRef]
- Liu, F.; Bing, T.; Shangguan, D.; Zhao, M.; Shao, N. Ratiometric fluorescent biosensing of hydrogen peroxide and hydroxyl radical in living cells with lysozyme--silver nanoclusters: Lysozyme as stabilizing ligand and fluorescence signal unit. Anal. Chem. 2016, 88, 10631–10638. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Nouws, H.P.A.; Delerue-Matos, C. Voltammetric immunosensor to track a major peanut allergen (Ara h 1) in food products employing quantum dot labels. Biosensors 2021, 11, 426. [Google Scholar] [CrossRef]
- Díaz-Álvarez, M.; Martín-Esteban, A. Molecularly imprinted polymer-quantum dot materials in optical sensors: An overview of their synthesis and applications. Biosensors 2021, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Umrao, S.; Sharma, P.; Bansal, A.; Sinha, R.; Singh, R.K.; Srivastava, A. Multi-layered graphene quantum dots derived photodegradation mechanism of methylene blue. RSC Adv. 2015, 5, 51790–51798. [Google Scholar] [CrossRef]
- Nashruddin, S.N.A.; Abdullah, J.; Mohammad Haniff, M.A.S.; Mat Zaid, M.H.; Choon, O.P.; Mohd Razip Wee, M.F. Label free glucose electrochemical biosensor based on poly (3, 4-ethylenedioxy thiophene): Polystyrene sulfonate/titanium carbide/graphene quantum dots. Biosensors 2021, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Tang, L.; Teng, K.S.; Lau, S.P. Graphene quantum dots from chemistry to applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- Liu, Z.; Tan, L.; Hou, P.-P.; Jin, X.-J.; Li, M.-C.; Zhou, Q.-Y.; Liao, P.; Zeng, Z.; Deng, S.; Dai, G.-P. One-pot synthesis of single-component graphene quantum dots for stable and bright white luminescence films as a phosphor. Opt. Mater. 2022, 127, 112368. [Google Scholar] [CrossRef]
- Zhao, C.; Song, X.; Liu, Y.; Fu, Y.; Ye, L.; Wang, N.; Wang, F.; Li, L.; Mohammadniaei, M.; Zhang, M.; et al. Synthesis of graphene quantum dots and their applications in drug delivery. J. Nanobiotechnol. 2020, 18, 1–32. [Google Scholar] [CrossRef]
- Du, Y.; Guo, S. Chemically doped fluorescent carbon and graphene quantum dots for bioimaging, sensor, catalytic and photoelectronic applications. Nanoscale 2016, 8, 2532–2543. [Google Scholar] [CrossRef]
- Ghaffarkhah, A.; Hosseini, E.; Kamkar, M.; Sehat, A.A.; Dordanihaghighi, S.; Allahbakhsh, A.; van der Kuur, C.; Arjmand, M. Synthesis, applications, and prospects of graphene quantum dots: A comprehensive review. Small 2022, 18, 2102683. [Google Scholar] [CrossRef]
- Younis, M.R.; He, G.; Lin, J.; Huang, P. Recent advances on graphene quantum dots for bioimaging applications. Front. Chem. 2020, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.L.; Dega, N.K.; Lu, S.-M.; Huang, Y.-F.; Doong, R. Ultrasensitive detection of breast cancer cells with a lectin-based electrochemical sensor using N-doped graphene quantum dots as the sensing probe. Sens. Actuators B Chem. 2022, 368, 132233. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Tagi, S.; Solhi, E.; Shadjou, N.; Jouyban, A.; Mokhtarzadeh, A. Immunosensing of breast cancer prognostic marker in adenocarcinoma cell lysates and unprocessed human plasma samples using gold nanostructure coated on organic substrate. Int. J. Biol. Macromol. 2018, 118, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Pothipor, C.; Jakmunee, J.; Bamrungsap, S.; Ounnunkad, K. An electrochemical biosensor for simultaneous detection of breast cancer clinically related microRNAs based on a gold nanoparticles/graphene quantum dots/graphene oxide film. Analyst 2021, 146, 4000–4009. [Google Scholar] [CrossRef]
- Farshchi, F.; Saadati, A.; Fathi, N.; Hasanzadeh, M.; Samiei, M. Flexible paper-based label-free electrochemical biosensor for the monitoring of miRNA-21 using core--shell Ag@ Au/GQD nano-ink: A new platform for the accurate and rapid analysis by low cost lab-on-paper technology. Anal. Methods 2021, 13, 1286–1294. [Google Scholar] [CrossRef]
- Hwang, M.T.; Heiranian, M.; Kim, Y.; You, S.; Leem, J.; Taqieddin, A.; Faramarzi, V.; Jing, Y.; Park, I.; van der Zande, A.M.; et al. Ultrasensitive detection of nucleic acids using deformed graphene channel field effect biosensors. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Xuan, X.; Kim, J.Y.; Hui, X.; Das, P.S.; Yoon, H.S.; Park, J.-Y. A highly stretchable and conductive 3D porous graphene metal nanocomposite based electrochemical-physiological hybrid biosensor. Biosens. Bioelectron. 2018, 120, 160–167. [Google Scholar] [CrossRef]
- Cao, W.; He, L.; Cao, W.; Huang, X.; Jia, K.; Dai, J. Recent progress of graphene oxide as a potential vaccine carrier and adjuvant. Acta Biomater. 2020, 112, 14–28. [Google Scholar] [CrossRef]



| Electrode | Detection Technique | Target | LR | LOD | Ref. |
|---|---|---|---|---|---|
| GO/P3ABA/2D-MoSe2/ AuNPs | DPV | CA 15-3 miRNA-21 | - | 0.14 U.mL−1 1.2 fM | [102] |
| GO/MCH a/DNA/AuNPs/ SF b/GCE | EIS | BRCA1 | 10–16–10–8 M | 3.3 × 10–17 M | [103] |
| GO/GNR c/GCE | DPV | miRNA-155 | 2 fM–8 pM | 0.6 fM | [104] |
| CGO/Au-Pt BNPs/FTO | DPV | MUC1 | 1 fM–100 nM | 0.79 fM | [105] |
| GO/GNR/GCE | EIS | miRNA-199a-5p | 15 Fm–148 pM | 4.5 fM | [106] |
| GO/AuNPs/PEDOT/FTO | DPV | MUC1 | 3.13 aM–31.25 nM | 0.031 fM | [107] |
| GO/DNA/AuNPs/GCE | CA | HER2 | - | 0.23 nM | [108] |
| Apt/GO/AuNPs/GCE | DPV | MCF-7 cells | 10–105 cells.mL−1 | 8 cells.mL−1 | [109] |
| GO/AuNPs/PtFe alloy/thionine/GCE | DPV | MCF-7 cells | 100–5 × 107cells.mL−1 | 38 cells.mL−1 | [110] |
| GO-IL-PGEs d | DPV | BRCA1 | - | 251 nM | [87] |
| GO/AuNPs/MgO nanoflower | DPV | miRNA-21 | 0.1–100 fM | 0.05 fM | [111] |
| GO/ITO | - | CA 15-3 | - | 0.1 U/mL | [112] |
| MGO/Avastin/Au electrode | DPV | VEGF | 31.25–2000 pg.mL−1 | 31.25 pg.mL−1 | [113] |
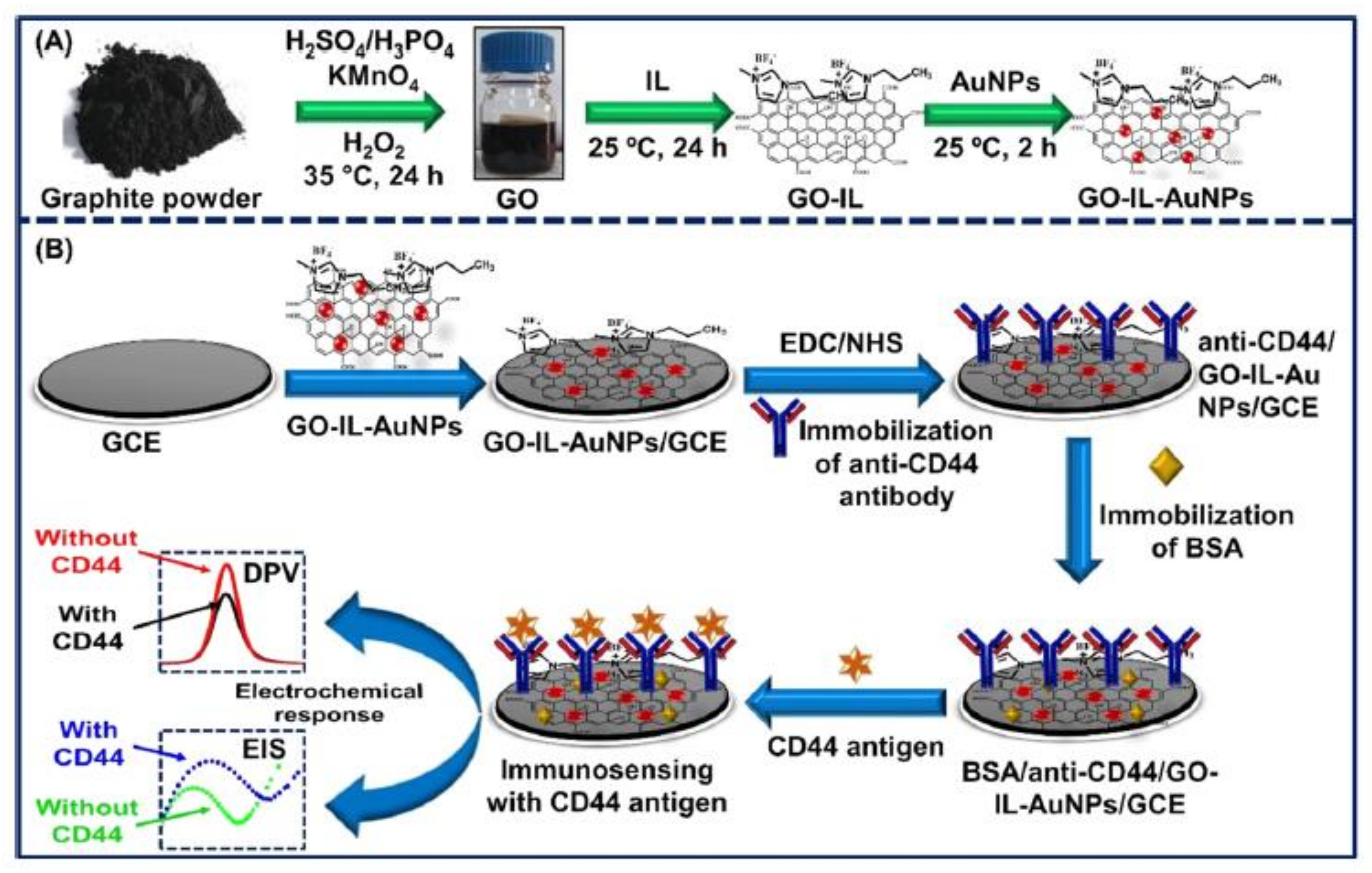
| GO/IL/AuNPs/GCE | DPV | CD44 | 5 fg.mL−1–50 μg.mL−1 | 2 fg.mL−1 | [114] |
| Electrode | Detection Technique | Target | LR | LOD | Ref. |
|---|---|---|---|---|---|
| rGO/SWCNT/AuNPs/GCE | EIS | HER2 | 0.1 pg.mL−1–1 ng.mL−1 | 50 fg.mL−1 | [139] |
| rGO/P2ABA a/AuNPs/ SPCE b | DPV | miRNA-155 miRNA-21 miRNA-16 | 1 fM to 10 nM 1 fM to 10 nM 1 fM to 10 nM | 0.98 fM 3.58 fM 0.25 fM | [140] |
| rGO/anti-CEA/GCE | EIS | CEA | 0.1–5 ng.mL−1 | 0.05 ng.mL−1 | [141] |
| rGO/APTES c/MoO3/ITO | DPV | HER2 | 0.001−500 ng.mL−1 | 0.001 ng.mL−1 | [142] |
| rGO/CuS/SPGE | DPV | CA15-3 | 1–150 U.mL−1 | 0.3 U.mL−1 | [143] |
| rGO/AuNPs/GCE | EIS | BRCA1 | 3 × 10−20–10−12 M 10−12–10−7 M | 10−20 M | [144] |
| rGO/DNA/PANHS d/GCE | EIS | BRCA1 | 10−18–10−10 M | 3.5 × 10−19 M | [145] |
| rGO/AuNPs/PE | DPV | miRNA-21 miRNA-155 | 37.5 nM–150 nM 33.8 nM–135.3 nM | 12 nM 25.7 nM | [2] |
| rGO/TiP-Cd2 + e-SA f/Au NPs/GCE | SWV | miRNA-21 | 1 aM–10 pM | 0.76 aM | [146] |
| rGO/trimetallic AuPtPd NPs/GCE | CA | H2O2 from MDA-MB-231 cells | 0.005–6.5 mM | 2 nM | [147] |
| rGO/Au-Pd NPs/GCE | CA | H2O2 from BC cells | 0.005–3500 μM | 0.004 μM | [148] |
| rGO/CS g/AuNPs/GCE | EIS | MCF-7 cells | 10–106 cells.mL−1 | 4 cells.mL−1 | [149] |
| rGO/TiO2 nanotube/MUC1 Apt. | EIS | MCF-7 cells | 103–107 cells.mL−1 | 40 cells.mL−1 | [150] |
| rGO/PP3CA h/GCE | DPV EIS | BRCA1 | 10−14–10−8M | 3 × 10−15 M | [151] |
| rGO/Au NPs/CuO NPs/GCE | DPV | MCF-7 cells | 50–104 cells.mL−1 | 27 cells.mL−1 | [152] |
| rGO/MoS2/Fe3O4 NPs/GCE | DPV | MCF-7 cells | 15–45 cells.mL−1 | 6 cells.mL−1 | [153] |
| 3D-rGO/PANI/GCE | DPV | BRCA1 | 10−15–10−7 M | 3.01 × 10−16 M | [154] |
| rGO/Au NPs/PDA i/MSNs j/ GCE | SWV | CA 15-3 | 0.002–125 U/mL | 0.002 U/mL | [155] |
| rGO/Au NPs/GCE | ACV | MUC1 | 1 pM–1 μM | 0.25 pM | [156] |
| rGO/Au NPs | DPV | miRNA-21 | 10−12–10−4 M | 1 pM | [157] |
| rGO/Ag NPs/Au NPs | CA | CA 15-3 | 15–125 U/mL | - | [158] |
| rGO/Ag NPs/TiO2 | CA | CA 15-3 | 0.1–300 U/mL | 0.07 U/mL | [159] |
| rGO/ZnMn2O4 | CA | H2O2 from MCF-7 | 0.03–6000 μM | 0.012 μM | [160] |
| rGO/CS/GCE | DPV | HER2 | 0.5–2 ng.mL−1 2–75 ng.mL−1 | 0.22 ng.mL−1 | [161] |
| Electrode | Detection Technique | Target | LR | LOD | Ref. |
|---|---|---|---|---|---|
| NGQDs/PHA-L/SPE | LSV | MCF-7 cells | 5–106 cells.mL−1 | 1 cells.mL−1 | [184] |
| GQDs/CysA a/Au NSs/ BSA b/GCE | SWV | CA 15-3 | 0.3–1 U/mL and 2–250 U/mL | 0.011 U/mL | [185] |
| GQDs/AuNPs/GO/SPCE | SWV | miRNA-21 miRNA-155 miRNA-210 | 0.001–1000 pM 0.001–1000 pM 0.001–1000 pM | 0.04 fM 0.33 fM 0.28 fM | [186] |
| GQD/Ag@Au core–shell | CA | miRNA-21 | 5 pM–5 mM | - | [187] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadpour-Haratbar, A.; Boraei, S.B.A.; Zare, Y.; Rhee, K.Y.; Park, S.-J. Graphene-Based Electrochemical Biosensors for Breast Cancer Detection. Biosensors 2023, 13, 80. https://doi.org/10.3390/bios13010080
Mohammadpour-Haratbar A, Boraei SBA, Zare Y, Rhee KY, Park S-J. Graphene-Based Electrochemical Biosensors for Breast Cancer Detection. Biosensors. 2023; 13(1):80. https://doi.org/10.3390/bios13010080
Chicago/Turabian StyleMohammadpour-Haratbar, Ali, Seyyed Behnam Abdollahi Boraei, Yasser Zare, Kyong Yop Rhee, and Soo-Jin Park. 2023. "Graphene-Based Electrochemical Biosensors for Breast Cancer Detection" Biosensors 13, no. 1: 80. https://doi.org/10.3390/bios13010080
APA StyleMohammadpour-Haratbar, A., Boraei, S. B. A., Zare, Y., Rhee, K. Y., & Park, S.-J. (2023). Graphene-Based Electrochemical Biosensors for Breast Cancer Detection. Biosensors, 13(1), 80. https://doi.org/10.3390/bios13010080






