An Innovative Simple Electrochemical Levofloxacin Sensor Assembled from Carbon Paste Enhanced with Nano-Sized Fumed Silica
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. NFS/CPE Preparation
2.3. Instruments
2.4. Real Samples Preparation
3. Results and Discussion
3.1. Characterization of the NFS/CPE
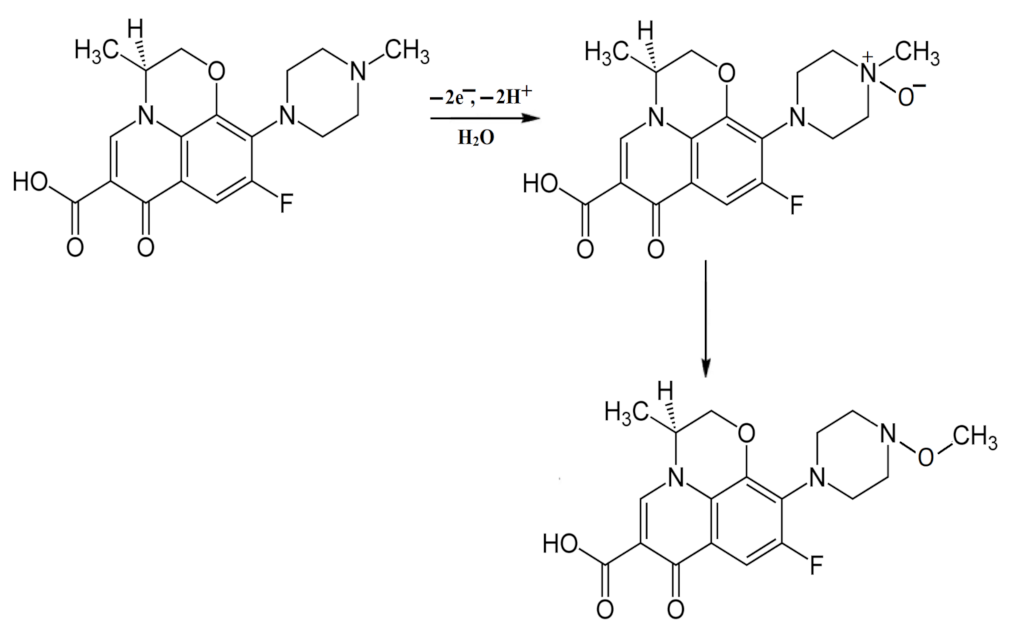
3.2. Electrochemistry of Levofloxacin
3.3. Optimal Settings
3.3.1. Influence of pH
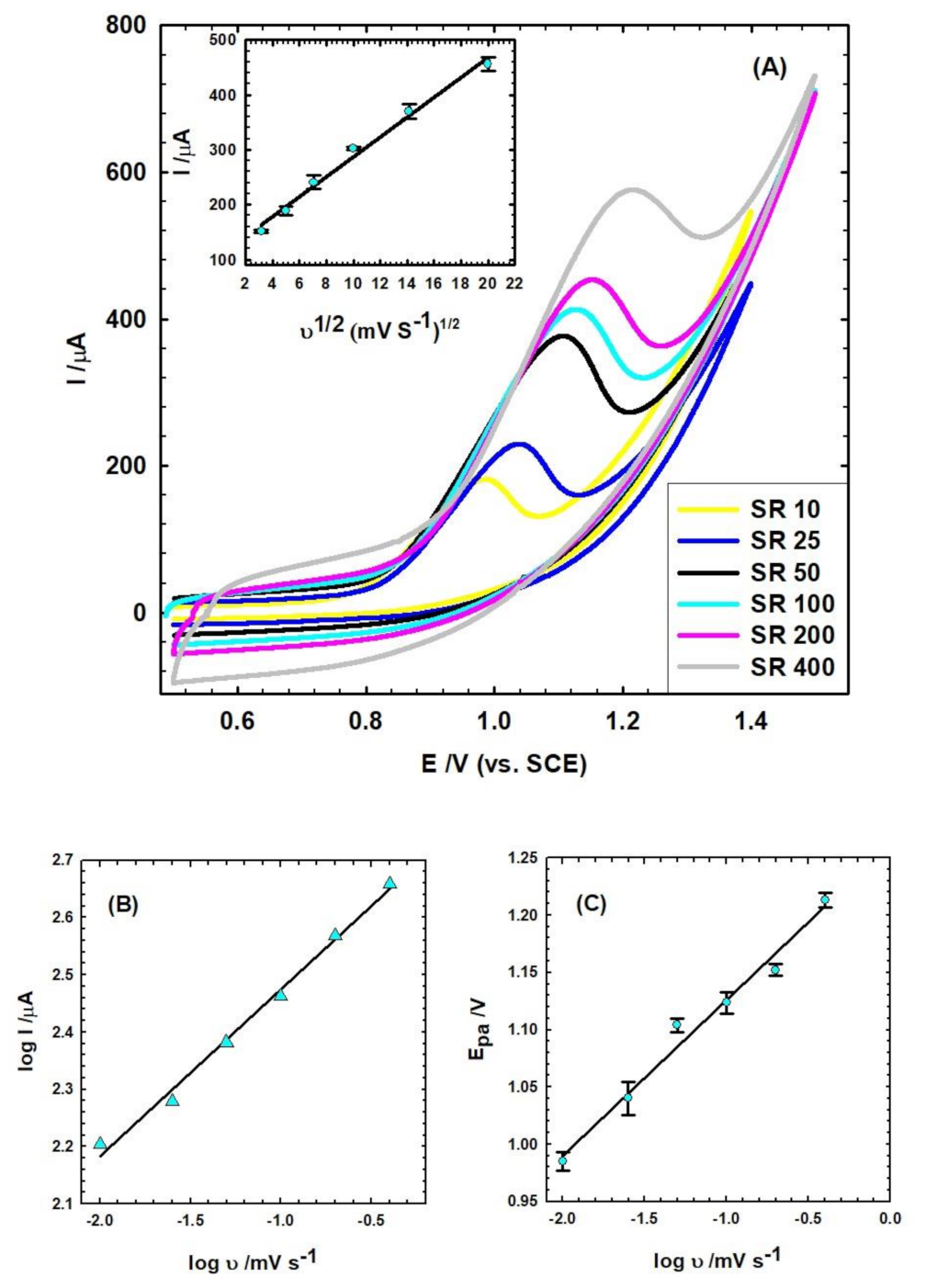
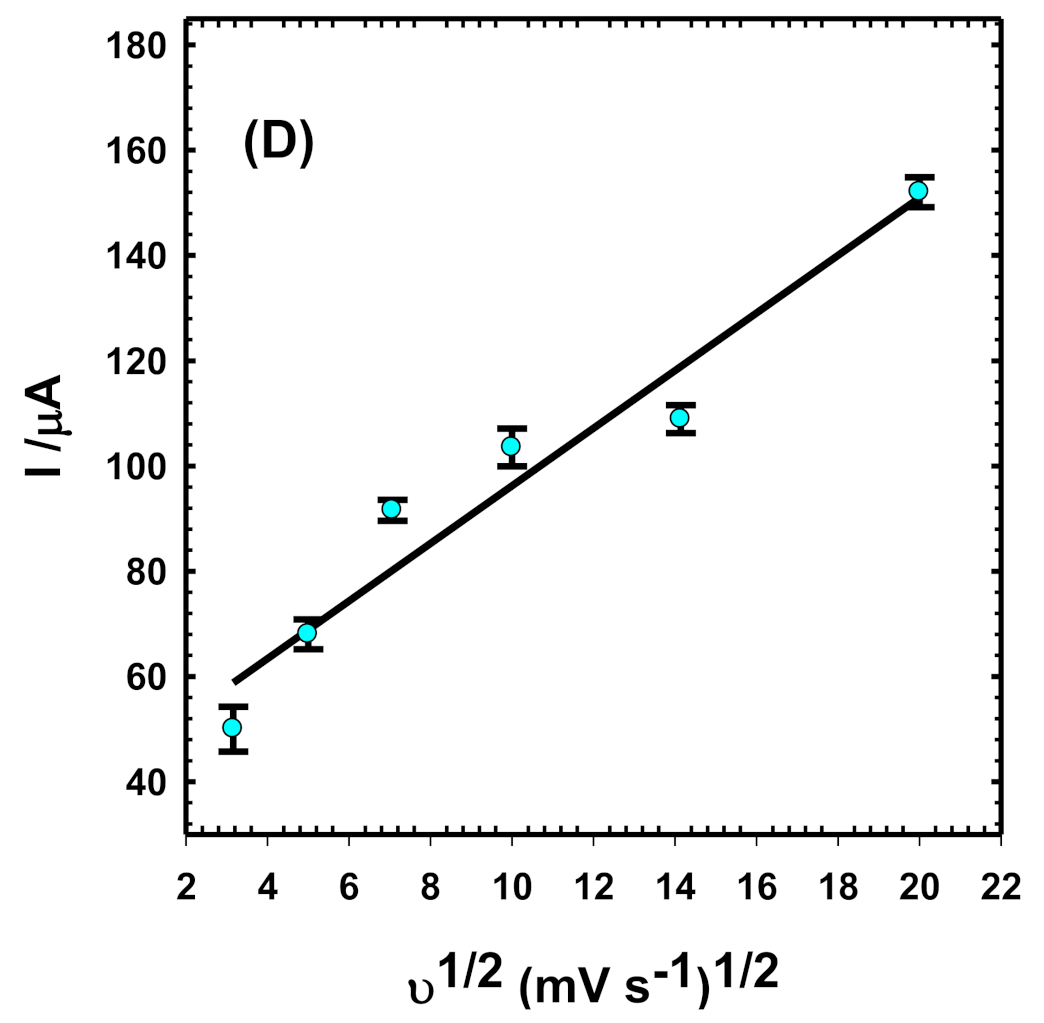
3.3.2. Influence of Scan Rate
3.4. Influence of Accumulation Time
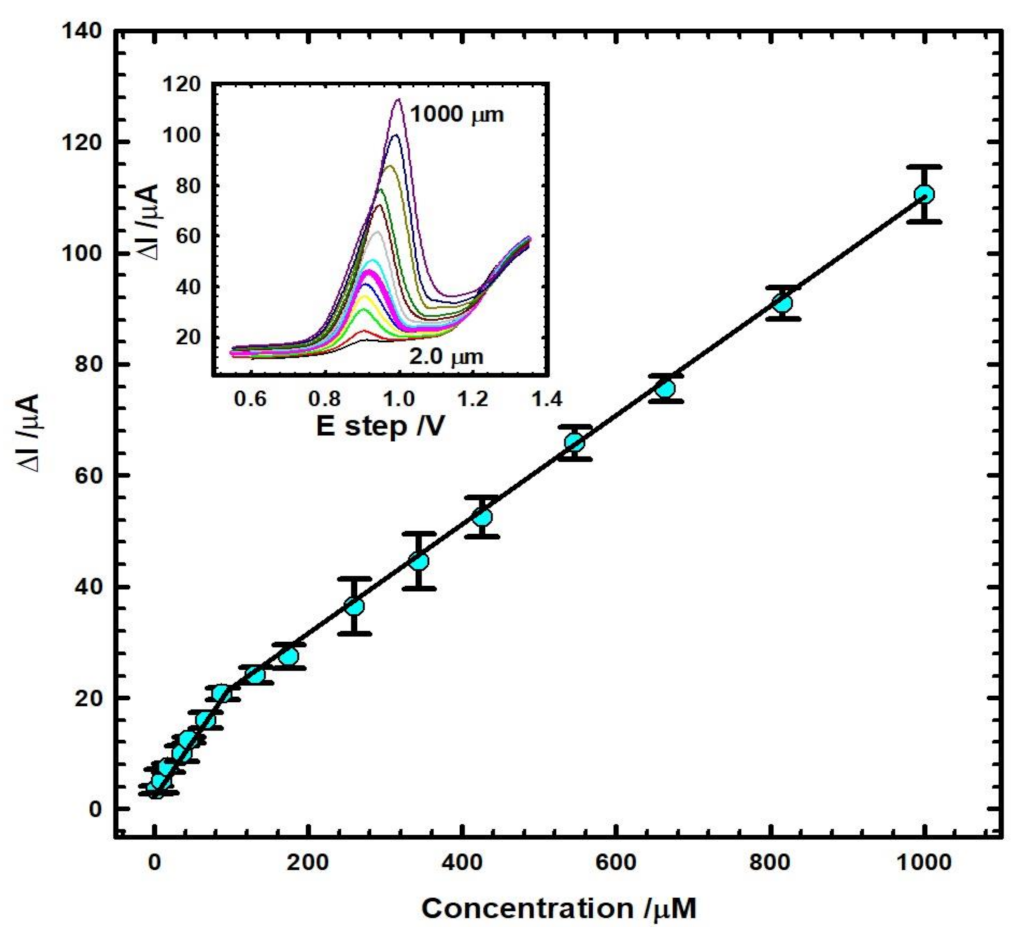
3.5. Calibration Curve and Detection Limit
3.6. Commercial Samples Analysis
3.7. Interference Study, Selectivity, Reproducibility, and Stability of NFS/CPE
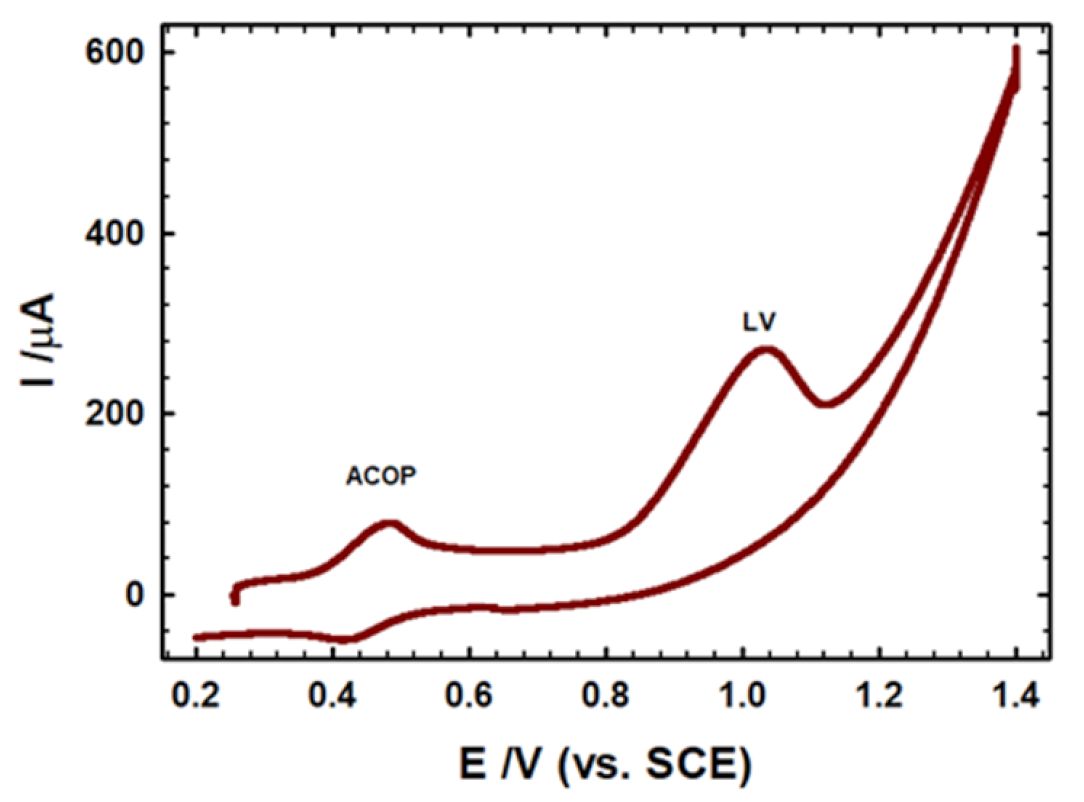
3.8. Instantaneous Determination of LV and Paracetamol
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, L.L.; Cao, W.; Wu, S.C.; Yang, C.; Cheng, J.H. Removal of tetracycline from aqueous solution by MOF/graphite oxide pellets: Preparation, characteristic, adsorption performance and mechanism. Ecotoxicol. Environ. Saf. 2018, 164, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Raoof, J.B.; Teymoori, N.; Khalilzadeh, M.A.; Ojani, R. Synergistic signal amplification based on ionic liquid-ZnO nanoparticle carbon paste electrode for sensitive voltammetric determination of acetaminophen in the presence of NADH. J. Mol. Liq. 2016, 219, 15–20. [Google Scholar] [CrossRef]
- Glavanovic, S.; Glavanovic, M.; Tomisi, V. Simultaneous quantitative determination of paracetamol and tramadol in tablet formulation using UV spectrophotometry and chemometric methods. Spectrochim. Acta Mol. Biomol. Spectrosc. 2016, 157, 258–264. [Google Scholar] [CrossRef]
- Wong, A.; Santos, A.M.; Fatibello-Filho, O. Simultaneous determination of paracetamol and levofloxacin using a glassy carbon electrode modified with carbon black, silver nanoparticles and PEDOT: PSS film. Sens. Actuators B Chem. 2018, 255, 2264–2273. [Google Scholar] [CrossRef]
- Ghanbaria, M.H.; Khoshroo, A.; Sobatid, H.; Ganjalie, M.R.; Rahimi-Nasrabadia, M.; Ahmadi, F. An electrochemical sensor based on poly (L-Cysteine)@ AuNPs@ reduced graphene oxide nanocomposite for determination of levofloxacin. Microchem. J. 2019, 147, 198–206. [Google Scholar] [CrossRef]
- Maleque, M.; Hasan, M.R.; Hossen, F.; Safi, S. Development and validation of a simple UV spectrophotometric method for the determination of levofloxacin both in bulk and marketed dosage formulations. J. Pharm. Anal. 2012, 2, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Zhang, Y.; Li, Q.; Du, X. Selective spectrophotometric determination of paracetamol with sodium nitroprusside in pharmaceutical and biological samples. Anal. Chem. 2011, 66, 215–220. [Google Scholar] [CrossRef]
- Szerkus, O.; Jacyna, J.; Wiczling, P.; Gibas, A.; Sieczkowski, M.; Siluk, D.; Matuszewski, M.; Kaliszan, R.; Markuszewski, M. Ultra-high performance liquid chromatographic determination of levofloxacin in human plasma and prostate tissue with use of experimental design optimization procedures. J. Chromatogr. B 2016, 1029, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Attimarad, M. Simultaneous determination of paracetamol and lornoxicam by RP-HPLC in bulk and tablet formulation. Pharm. Methods 2011, 2, 61–66. [Google Scholar] [CrossRef]
- Shao, X.; Li, Y.; Liu, Y.; Song, Z. Rapid determination of levofloxacin in pharmaceuticals and biological fluids using a new chemiluminescence system. Anal. Chem. 2011, 66, 102–107. [Google Scholar] [CrossRef]
- Dong, Y.; Su, M.; Chen, P.; Sun, H. Chemiluminescence of carbon dots induced by diperiodato-nicklate (IV) in alkaline solution and its application to a quenchometric flow-injection assays of paracetamol, L-cysteine and glutathione. Microchim. Acta 2015, 182, 1071–1077. [Google Scholar] [CrossRef]
- Daneshvar, L.; Rounaghi, G.H.; Tarahomi, S. Voltammetric paracetamol sensor using a gold electrode made from a digital versatile disc chip and modified with a hybrid material consisting of carbon nanotubes and copper nanoparticles. Microchim. Acta 2016, 183, 3001–3007. [Google Scholar] [CrossRef]
- Khoshsafar, H.; Bagheri, H.; Rezaei, M.; Shirzadmehr, A.; Hajian, A.; Sepehri, Z. Magnetic carbon paste electrode modified with a high performance composite based on molecularly imprinted carbon nanotubes for sensitive determination of levofloxacin. J. Electrochem. Soc. 2016, 163, B422–B427. [Google Scholar] [CrossRef]
- Özcan, A.; Hamid, F.; Atılır Özcan, A. Synthesizing of a nanocomposite based on the formation of silver nanoparticles on fumed silica to develop an electrochemical sensor for carbendazim detection. Talanta 2021, 222, 121591. [Google Scholar] [CrossRef] [PubMed]
- Özcan, A.; Ilkbas, S. Poly(pyrrole-3-carboxylic acid)-modified pencil graphite electrode for the determination of serotonin in biological samples by adsorptive stripping voltammetry. Sens. Actuators B Chem. 2015, 215, 518–524. [Google Scholar] [CrossRef]
- Özcan, A.; Ilkbas, S. Preparation of poly(3,4-ethylenedioxythiophene) nanofibers modified pencil graphite electrode and investigation of over-oxidation conditions for the selective and sensitive determination of uric acid in body fluids. Anal. Chim. Acta 2015, 891, 312–320. [Google Scholar] [CrossRef]
- Fekry, A.M. A new simple electrochemical Moxifloxacin Hydrochloride sensor built on carbon paste modified with silver nanoparticles. Biosens. Bioelectron. 2017, 87, 1065–1070. [Google Scholar] [CrossRef]
- Mamdouh, S.; Shehata, M.; Fekry, A.M.; Ameer, M.A. Graphite based sensor amended with fumed silica for electro-detecting Azithromycin. Can. J. Chem. 2022, 100, 589–600. [Google Scholar] [CrossRef]
- Özcan, A.; Gürbüz, M. Development of a modified electrode by using a nanocomposite containing acid-activated multi-walled carbon nanotube and fumed silica for the voltammetric determination of clopyralid. Sens. Actuators B Chem. 2018, 255, 262–267. [Google Scholar] [CrossRef]
- Özcan, A.; Topçuoğulları, D.; Özcan, A.A. Fenitrothion sensing with reduced graphene oxide decorated fumed silica nanocomposite modified glassy carbon electrode. Sensor. Actuator B Chem. 2019, 284, 179–185. [Google Scholar] [CrossRef]
- Rungkitwattanakul, D.; Chaijamorn, W.; Charoensareerat, T.; Charntrakarn, P.; Khamkampud, O.; Rattanaponpasert, N.; Srisawat, N.; Pattharachayakul, S. Optimal levofloxacin dosing regimens in critically ill patients with acute kidney injury receiving continuous renal replacement therapy. J. Crit. Care 2021, 63, 154–160. [Google Scholar] [CrossRef]
- Boumya, W.; Taoufik, N.; Barka, N. Chemically modified carbon-based electrodes for the determination of paracetamol in drugs and biological samples. J. Pharm. Anal. 2021, 11, 138–154. [Google Scholar] [CrossRef] [PubMed]
- Teglia, C.M.; Gutierrez, F.A.; Goicoechea, H.C. Natural deep eutectic solvent: A novelty alternative as multi-walled carbon nanotubes dispersing agent for the determination of paracetamol in urine. Talanta 2022, 242, 123290. [Google Scholar] [CrossRef] [PubMed]
- Prats-Alfonso, E.; Abad, L.; Casan–Pastor, N.; Gonzalo-Ruiz, J.; Baldrich, E. Iridium oxide pH sensor for biomedical applications. Case urea–urease in real urine samples. Biosens. Bioelectron. 2013, 39, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Salama, N.N.; Azab, S.M.; Mohamed, M.A.; Fekry, A.M. A novel methionine/palladium nanoparticle modified carbon paste electrode for simultaneous determination of three antiparkinson drugs. RSC Adv. 2015, 5, 14187–14195. [Google Scholar] [CrossRef]
- Fekry, A.M.; Shehata, M.; Azab, S.M.; Walcarius, A. Voltammetric Detection of Caffeine in Pharmacological and Beverages Samples Based on Simple Nano-Co (II, III) Oxide Modified Carbon Paste Electrode in Aqueous and Micellar Media. Sens. Actuators B Chem. 2020, 302, 127172. [Google Scholar] [CrossRef]
- Abdel-Gawad, S.A.; Fekry, A.M. A novel environmental nano-catalyst of zeolite amended with carbon nanotube/silver nanoparticles decorated carbon paste electrode for electro-oxidation of propylene glycol. Sci. Rep. 2022, 12, 9136. [Google Scholar] [CrossRef]
- Elhakim, H.K.A.; Azab, S.M.; Fekry, A.M. A novel simple biosensor containing silver nanoparticles/propolis (bee glue) for microRNA let-7a determination. Mater. Sci. Eng. C 2018, 92, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Altaf, S.; Zafar, R.; Zaman, W.Q.; Ahmad, S.; Yaqoob, K.; Syed, A.; Khan, A.J.; Bilal, M.; Arshad, M. Removal of levofloxacin from aqueous solution by green synthesized magnetite (Fe3O4) nanoparticles using Moringa olifera: Kinetics and reaction mechanism analysis. Ecotoxicol. Environ. Saf. 2021, 226, 112826. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, V.; Cao, J.; Wang, J.; Wang, X.; Zhou, G.; Berg, A.; Shui, L. High-sensitive electrochemical sensor for determination of Norfloxacin and its metabolism using MWCNT-CPE/pRGO-ANSA/Au. Sens. Actuators B Chem. 2018, 257, 1065–1075. [Google Scholar] [CrossRef]
- Mohamed, M.A.; El-Sherif, A.A. Complex Formation Equilibria Between Zinc(II), Nitrilo-tris(Methyl Phosphonic Acid) and Some Bio-relevant Ligands. The Kinetics and Mechanism for Zinc(II) Ion Promoted Hydrolysis of Glycine Methyl Ester. J. Solut. Chem. 2010, 39, 639–653. [Google Scholar] [CrossRef]
- Jeragh, B.; Al-Wahaib, D.; El-Sherif, A.A.; El-Dissouky, A. Potentiometric and Thermodynamic Studies of Dissociation and Metal Complexation of 4-(3-Hydroxypyridin-2-ylimino)-4-phenylbutan-2-one. J. Chem. Eng. Data 2007, 52, 1609–1614. [Google Scholar] [CrossRef]
- El Azab, N.F.; Mahmoud, A.M.; Trabik, Y.A. Point-of-care diagnostics for therapeutic monitoring of levofloxacin in human plasma utilizing electrochemical sensor mussel-inspired molecularly imprinted copolymer. J. Electroanal. Chem. 2022, 918, 116504. [Google Scholar] [CrossRef]
- Chansud, N.; Longnapa, N.; Bunkoed, O. A nanohybrid magnetic sensing probe for levofloxacin determination integrates porous graphene, selective polymer and grapheme quantum dots. J. Pharm. Biomed. Anal. 2021, 205, 114316. [Google Scholar] [CrossRef]
- Wen, W.; Zhao, D.-M.; Zhang, X.-H.; Xiong, H.-Y.; Wang, S.-F.; Chen, W.; Zhao, Y.-D. One-step fabrication of poly(o-aminophenol)/multi-walled carbon nanotubes composite film modified electrode and its application for levofloxacin determination in pharmaceuticals. Sens. Actuator B Chem. 2012, 174, 202–209. [Google Scholar] [CrossRef]
- Borowiec, J.; Yan, K.; Tin, C.-C.; Zhang, J. Synthesis of PDDA functionalized reduced graphene oxide decorated with gold nanoparticles and its electrochemical response toward levofloxacin. J. Electrochem. Soc. 2015, 162, H164–H169. [Google Scholar] [CrossRef]
- Vinay, M.M.; Arthoba Nayaka, Y.; Yatisha, R.O.; Basavarajappa, K.V.; Manjunatha, P.; Purushothama, H.T. Development of Azure-B modified pencil graphite electrode as an electrochemical sensor for the investigation of Levofloxacin in pharmaceutical and biological samples. Chem. Data Collect. 2020, 28, 100441. [Google Scholar] [CrossRef]
- Radi, A.; El Ries, M.A.; Kandil, S. Electrochemical study of the interaction of levo-floxacin with DNA. Anal. Chim. Acta 2003, 495, 61–67. [Google Scholar] [CrossRef]
- Cesarino, V.; Cesarino, I.; Moraes, F.C.; Machado, S.A.S.; Mascaro, L.H. Carbon na-notubes modified with SnO2 rods for levofloxacin detection. J. Braz. Chem. Soc. 2014, 25, 502–508. [Google Scholar]
- Wang, F.; Zhu, L.; Zhang, J. Electrochemical sensor for levofloxacin based on mo-lecularly imprinted polypyrrole–graphene–gold nanoparticles modified electrode. Sens. Actuators B Chem. 2014, 192, 642–647. [Google Scholar] [CrossRef]
- Hosten, A.O. BUN and creatinine. In Clinica Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Huest, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; Chapter 193. [Google Scholar]




| Modified Electrode | Linear Range (µM) | LOD (µM) | Reference |
|---|---|---|---|
| PoAP/MWCNTs/GCE | 3.0–200 | 1.0 | [35] |
| Au/PDDA/rGO/GCE | 10–800 | 3.9 | [36] |
| Azure-B/PGE | 2–125 | 1.2 | [37] |
| DsDNA/GCE | 0.5–5 | 0.1 | [38] |
| MWCNT-SnO2/GC | 1–9.9 | 0.2 | [39] |
| MIP/G-AuNPs | 1–100 | 0.53 | [40] |
| NFS/CPE | 2–1000 | 0.09 | This work |
| LV Added (µM) | Found (µM) | Recovery (%) | RSD (%) |
|---|---|---|---|
| Human plasma | |||
| 5 | 4.80 | 96.0 | 2.6 |
| 10 | 9.52 | 95.2 | 1.9 |
| 15 | 14.62 | 97.5 | 2.2 |
| Pharmaceutical samples | |||
| 8.88 | 9.81 | 110.47 | 2.3 |
| 17.75 | 19.2 | 108.17 | 1.9 |
| 35.43 | 34.45 | 97.23 | 1.5 |
| 44.25 | 44.38 | 100.29 | 2.1 |
| 88.11 | 88.07 | 99.95 | 1.6 |
| Interfering Material | Relative Sensor Response (%) * | |
|---|---|---|
| 1:1 | 1:2 | |
| Glucose | 97.1 | 96.3 |
| Sucrose | 97.3 | 96.1 |
| Starch | 95.8 | 95.2 |
| Urea | 97.0 | 96.5 |
| Paracetamol | 99.8 | 99.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fekry, A.M. An Innovative Simple Electrochemical Levofloxacin Sensor Assembled from Carbon Paste Enhanced with Nano-Sized Fumed Silica. Biosensors 2022, 12, 906. https://doi.org/10.3390/bios12100906
Fekry AM. An Innovative Simple Electrochemical Levofloxacin Sensor Assembled from Carbon Paste Enhanced with Nano-Sized Fumed Silica. Biosensors. 2022; 12(10):906. https://doi.org/10.3390/bios12100906
Chicago/Turabian StyleFekry, Amany M. 2022. "An Innovative Simple Electrochemical Levofloxacin Sensor Assembled from Carbon Paste Enhanced with Nano-Sized Fumed Silica" Biosensors 12, no. 10: 906. https://doi.org/10.3390/bios12100906
APA StyleFekry, A. M. (2022). An Innovative Simple Electrochemical Levofloxacin Sensor Assembled from Carbon Paste Enhanced with Nano-Sized Fumed Silica. Biosensors, 12(10), 906. https://doi.org/10.3390/bios12100906




