Room-Temperature Synthesis of Air-Stable Near-Infrared Emission in FAPbI3 Nanoparticles Embedded in Silica
Abstract
:1. Introduction
2. Materials and Methods
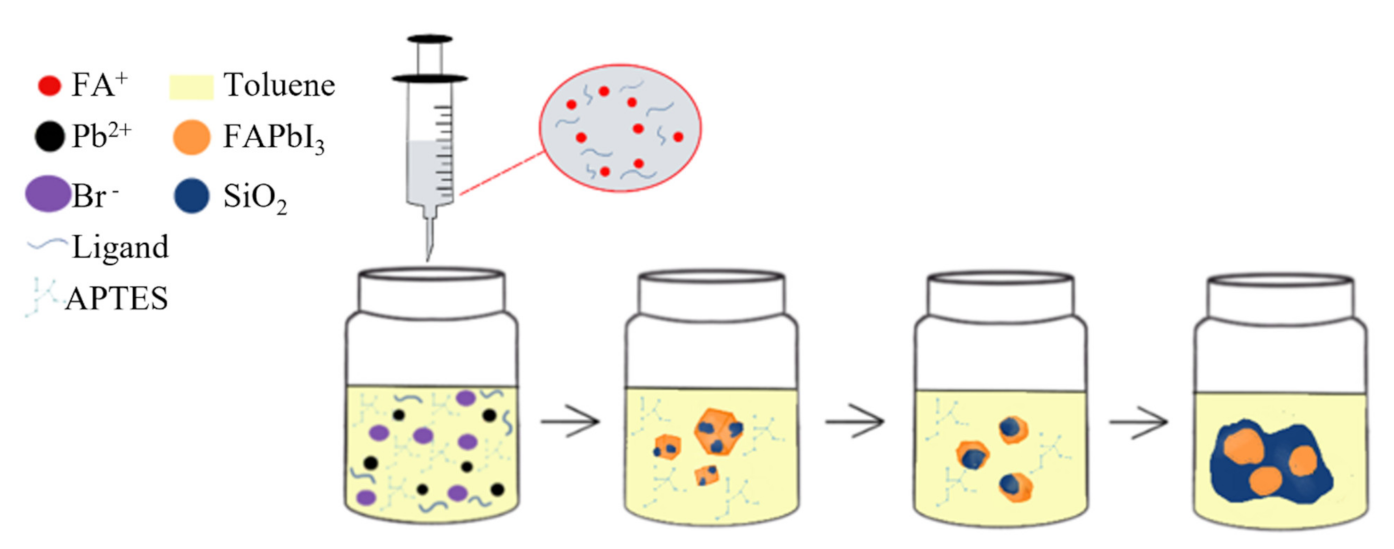
2.1. Air-Synthesis of NIR-FAPbI3 PNPs and PNCs
2.2. Manufacture of NIR-LEDs and Characterization
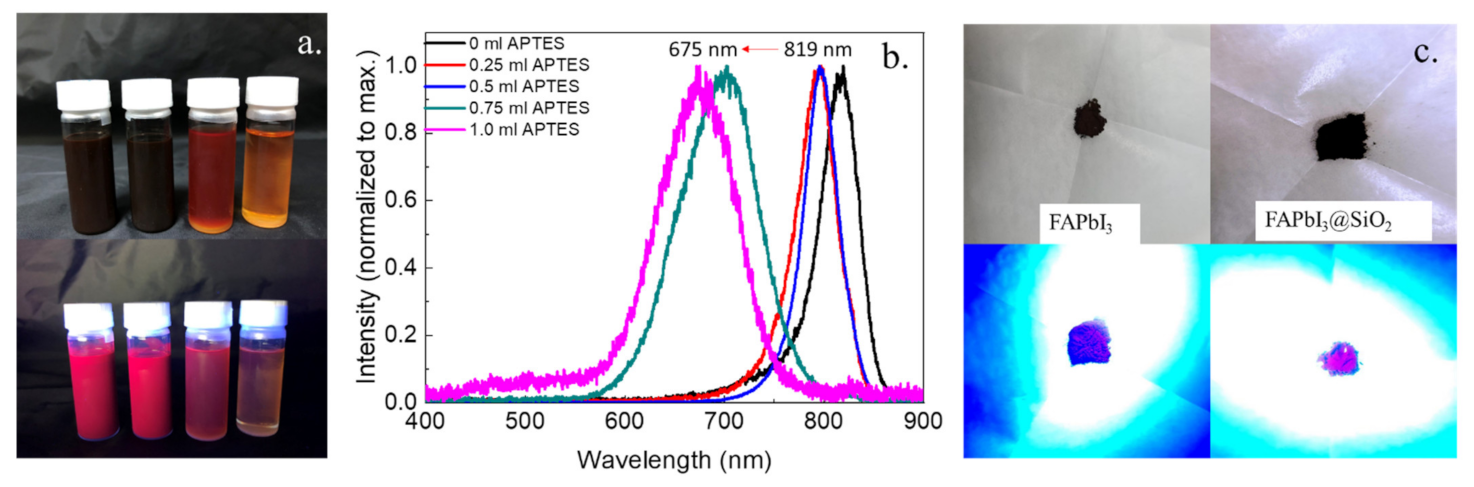
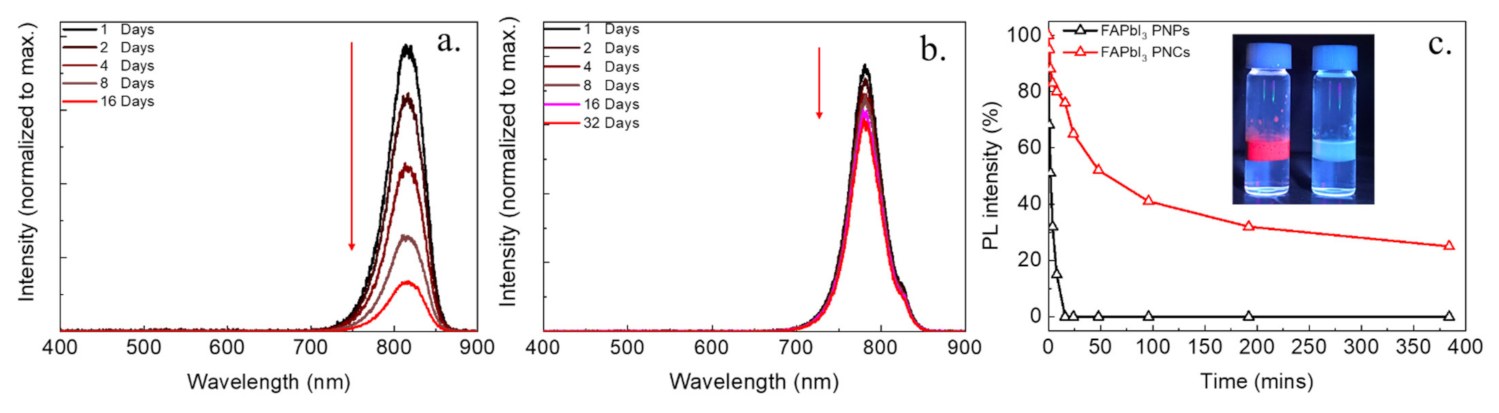
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bekenstein, Y.; Koscher, B.A.; Eaton, S.W.; Yang, P.; Alivisatos, A.P. Highly luminescent colloidal nanoplates of perovskite cesium lead halide and their oriented assemblies. J. Am. Chem. Soc. 2015, 137, 16008–16011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Eaton, S.W.; Yu, Y.; Dou, L.; Yang, P. Solution-phase synthesis of cesium lead halide perovskite nanowires. J. Am. Chem. Soc. 2015, 137, 9230–9233. [Google Scholar] [CrossRef]
- Li, X.; Cao, F.; Yu, D.; Chen, J.; Sun, Z.; Shen, Y.; Zhu, Y.; Wang, L.; Wei, Y.; Wu, Y. All inorganic halide perovskites nanosystem: Synthesis, structural features, optical properties and optoelectronic applications. Small 2017, 13, 1603996. [Google Scholar] [CrossRef]
- Song, J.; Cui, Q.; Li, J.; Xu, J.; Wang, Y.; Xu, L.; Xue, J.; Dong, Y.; Tian, T.; Sun, H. Ultralarge all-inorganic perovskite bulk single crystal for high-performance visible–infrared dual-modal photodetectors. Adv. Opt. Mater. 2017, 5, 1700157. [Google Scholar] [CrossRef]
- Sun, S.; Yuan, D.; Xu, Y.; Wang, A.; Deng, Z. Ligand-mediated synthesis of shape-controlled cesium lead halide perovskite nanocrystals via reprecipitation process at room temperature. ACS Nano 2016, 10, 3648–3657. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, A.; Chulliyil, R.; Ravi, V.K.; Irfanullah, M.; Chowdhury, A.; Nag, A. Colloidal CsPbBr3 perovskite nanocrystals: Luminescence beyond traditional quantum dots. Angew. Chem. 2015, 127, 15644–15648. [Google Scholar] [CrossRef]
- Swarnkar, A.; Ravi, V.K.; Nag, A. Beyond colloidal cesium lead halide perovskite nanocrystals: Analogous metal halides and doping. ACS Energy Lett. 2017, 2, 1089–1098. [Google Scholar] [CrossRef]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum dot–induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramasamy, P.; Lim, D.-H.; Kim, B.; Lee, S.-H.; Lee, M.-S.; Lee, J.-S. All-inorganic cesium lead halide perovskite nanocrystals for photodetector applications. Chem. Commun. 2016, 52, 2067–2070. [Google Scholar] [CrossRef]
- Lv, L.; Xu, Y.; Fang, H.; Luo, W.; Xu, F.; Liu, L.; Wang, B.; Zhang, X.; Yang, D.; Hu, W. Generalized colloidal synthesis of high-quality, two-dimensional cesium lead halide perovskite nanosheets and their applications in photodetectors. Nanoscale 2016, 8, 13589–13596. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.M.; Park, K.; Kim, D.H.; Park, J.; Shojaei, F.; Kang, H.S.; Ahn, J.-P.; Lee, J.W.; Song, J.K. Reversible halide exchange reaction of organometal trihalide perovskite colloidal nanocrystals for full-range band gap tuning. Nano Lett. 2015, 15, 5191–5199. [Google Scholar] [CrossRef] [PubMed]
- Saidaminov, M.I.; Haque, M.A.; Savoie, M.; Abdelhady, A.L.; Cho, N.; Dursun, I.; Buttner, U.; Alarousu, E.; Wu, T.; Bakr, O.M. Perovskite photodetectors operating in both narrowband and broadband regimes. Adv. Mater. 2016, 28, 8144–8149. [Google Scholar] [CrossRef] [PubMed]
- Saidaminov, M.I.; Adinolfi, V.; Comin, R.; Abdelhady, A.L.; Peng, W.; Dursun, I.; Yuan, M.; Hoogland, S.; Sargent, E.H.; Bakr, O.M. Planar-integrated single-crystalline perovskite photodetectors. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Yakunin, S.; Protesescu, L.; Krieg, F.; Bodnarchuk, M.I.; Nedelcu, G.; Humer, M.; De Luca, G.; Fiebig, M.; Heiss, W.; Kovalenko, M.V. Low-threshold amplified spontaneous emission and lasing from colloidal nanocrystals of caesium lead halide perovskites. Nat. Commun. 2015, 6, 1–9. [Google Scholar]
- Fu, Y.; Zhu, H.; Schrader, A.W.; Liang, D.; Ding, Q.; Joshi, P.; Hwang, L.; Zhu, X.; Jin, S. Nanowire lasers of formamidinium lead halide perovskites and their stabilized alloys with improved stability. Nano Lett. 2016, 16, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Colella, S.; Mazzeo, M.; Rizzo, A.; Gigli, G.; Listorti, A. The bright side of perovskites. J. Phys. Chem. Lett. 2016, 7, 4322–4334. [Google Scholar] [CrossRef]
- Aygüler, M.F.; Weber, M.D.; Puscher, B.M.; Medina, D.D.; Docampo, P.; Costa, R.D. Light-emitting electrochemical cells based on hybrid lead halide perovskite nanoparticles. J. Phys. Chem. C 2015, 119, 12047–12054. [Google Scholar] [CrossRef] [Green Version]
- Bade, S.G.R.; Li, J.; Shan, X.; Ling, Y.; Tian, Y.; Dilbeck, T.; Besara, T.; Geske, T.; Gao, H.; Ma, B. Fully printed halide perovskite light-emitting diodes with silver nanowire electrodes. ACS Nano 2016, 10, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Manser, J.S.; Christians, J.A.; Kamat, P.V. Intriguing optoelectronic properties of metal halide perovskites. Chem. Rev. 2016, 116, 12956–13008. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Bertolotti, F.; Masciocchi, N.; Guagliardi, A.; Kovalenko, M.V. Monodisperse formamidinium lead bromide nanocrystals with bright and stable green photoluminescence. J. Am. Chem. Soc. 2016, 138, 14202–14205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 2014, 7, 982–988. [Google Scholar] [CrossRef]
- Song, J.; Hu, W.; Wang, X.-F.; Chen, G.; Tian, W.; Miyasaka, T. HC(NH2)2 PbI3 as a thermally stable absorber for efficient ZnO-based perovskite solar cells. J. Mater. Chem. A 2016, 4, 8435–8443. [Google Scholar] [CrossRef]
- Smecca, E.; Numata, Y.; Deretzis, I.; Pellegrino, G.; Boninelli, S.; Miyasaka, T.; La Magna, A.; Alberti, A. Stability of solution-processed MAPbI3 and FAPbI3 layers. Phys. Chem. Chem. Phys. 2016, 18, 13413–13422. [Google Scholar] [CrossRef]
- Binek, A.; Hanusch, F.C.; Docampo, P.; Bein, T. Stabilization of the trigonal high-temperature phase of formamidinium lead iodide. J. Phys. Chem. Lett. 2015, 6, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Leijtens, T.; Eperon, G.E.; Noel, N.K.; Habisreutinger, S.N.; Petrozza, A.; Snaith, H.J. Stability of metal halide perovskite solar cells. Adv. Energy Mater. 2015, 5, 1500963. [Google Scholar] [CrossRef]
- Chen, L.-C.; Tien, C.-H.; Tseng, Z.-L.; Dong, Y.-S.; Yang, S. Influence of PMMA on all-inorganic halide perovskite CsPbBr3 quantum dots combined with polymer matrix. Materials 2019, 12, 985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Lei, T.; Siron, M.; Zhang, Y.; Yu, S.; Seeler, F.; Dehestani, A.; Quan, L.N.; Schierle-Arndt, K.; Yang, P. Lead-free cesium europium halide perovskite nanocrystals. Nano Lett. 2020, 20, 3734–3739. [Google Scholar] [CrossRef]
- Huang, H.; Chen, B.; Wang, Z.; Hung, T.F.; Susha, A.S.; Zhong, H.; Rogach, A.L. Water resistant CsPbX3 nanocrystals coated with polyhedral oligomeric silsesquioxane and their use as solid state luminophores in all-perovskite white light-emitting devices. Chem. Sci. 2016, 7, 5699–5703. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Shi, Z.-F.; Ma, Z.-Z.; Li, Y.; Li, S.; Wu, D.; Xu, T.-T.; Li, X.-J.; Shan, C.-X.; Du, G.-T. Silica coating enhances the stability of inorganic perovskite nanocrystals for efficient and stable down-conversion in white light-emitting devices. Nanoscale 2018, 10, 20131–20139. [Google Scholar] [CrossRef]
- Tang, X.; Chen, W.; Liu, Z.; Du, J.; Yao, Z.; Huang, Y.; Chen, C.; Yang, Z.; Shi, T.; Hu, W. Ultrathin, core–shell structured SiO2 coated Mn2+-doped perovskite quantum dots for bright white light-emitting diodes. Small 2019, 15, 1900484. [Google Scholar]
- Hu, X.; Zrazhevskiy, P.; Gao, X. Encapsulation of single quantum dots with mesoporous silica. Ann. Biomed. Eng. 2009, 37, 1960–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.C.; Lin, S.Y.; Tang, A.C.; Singh, B.P.; Tong, H.C.; Chen, C.Y.; Lee, Y.C.; Tsai, T.L.; Liu, R.S. Mesoporous silica particles integrated with all-inorganic CsPbBr3 perovskite quantum-dot nanocomposites (MP-PQDs) with high stability and wide color gamut used for backlight display. Angew. Chem. Int. Ed. 2016, 55, 7924–7929. [Google Scholar] [CrossRef]
- Dirin, D.N.; Protesescu, L.; Trummer, D.; Kochetygov, I.V.; Yakunin, S.; Krumeich, F.; Stadie, N.P.; Kovalenko, M.V. Harnessing defect-tolerance at the nanoscale: Highly luminescent lead halide perovskite nanocrystals in mesoporous silica matrixes. Nano Lett. 2016, 16, 5866–5874. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, Y.; Ruan, C.; Yin, C.; Wang, X.; Wang, Y.; Yu, W.W. Efficient and stable white LEDs with silica-coated inorganic perovskite quantum dots. Adv. Mater. 2016, 28, 10088–10094. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shi, T.; Du, J.; Zang, Z.; Yao, Z.; Li, M.; Sun, K.; Hu, W.; Leng, Y.; Tang, X. Highly stable silica-wrapped Mn-doped CsPbCl3 quantum dots for bright white light-emitting devices. ACS Appl. Mater. Interfaces 2018, 10, 43978–43986. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.-L.; Yang, M.; Qin, J.-L.; Teng, F.; Wang, Y.-Q.; Chen, G.-X.; Wang, D.-W.; Peng, H.-S. Ultrastable luminescent organic–inorganic perovskite quantum dots via surface engineering: Coordination of methylammonium bromide and covalent silica encapsulation. ACS Appl. Mater. Interfaces 2018, 10, 42837–42843. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Peng, H.-S.; Zeng, F.-L.; Teng, F.; Qu, Z.; Yang, D.; Wang, Y.-Q.; Chen, G.-X.; Wang, D.-w. In situ silica coating-directed synthesis of orthorhombic methylammonium lead bromide perovskite quantum dots with high stability. J. Colloid Interface Sci. 2018, 509, 32–38. [Google Scholar] [CrossRef]
- Luo, B.; Pu, Y.C.; Lindley, S.A.; Yang, Y.; Lu, L.; Li, Y.; Li, X.; Zhang, J.Z. Organolead halide perovskite nanocrystals: Branched capping ligands control crystal size and stability. Angew. Chem. Int. Ed. 2016, 55, 8864–8868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.M.; Mancini, M.C.; Nie, S. Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Shen, C.; Zhu, Y.; Chen, X.; Bi, Z.; Marimuthu, T.; Xu, G.; Xu, X. Stable Luminescent CsPbI3 Quantum Dots Passivated by (3-Aminopropyl) triethoxysilane. Langmuir 2020, 36, 10210–10217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, A.; Liu, T.; Zhou, L.; Zheng, J.; Zuo, Y.; He, Y.; Li, J. A facile method for preparing Yb3+-doped perovskite nanocrystals with ultra-stable near-infrared light emission. RSC Adv. 2020, 10, 17635–17641. [Google Scholar] [CrossRef]
- Sun, C.; Shen, X.; Zhang, Y.; Wang, Y.; Chen, X.; Ji, C.; Shen, H.; Shi, H.; Wang, Y.; William, W.Y. Highly luminescent, stable, transparent and flexible perovskite quantum dot gels towards light-emitting diodes. Nanotechnology 2017, 28, 365601. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Cao, M.; Hu, H.; Yang, D.; Chen, M.; Li, P.; Wu, L.; Zhang, Q. One-pot synthesis of highly stable CsPbBr3@ SiO2 core–shell nanoparticles. ACS Nano 2018, 12, 8579–8587. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-C.; Chao, L.-W.; Xu, C.-Y.; Hsu, C.-H.; Lee, Y.-T.; Xu, Z.-M.; Lin, C.-C.; Tseng, Z.-L. Room-Temperature Synthesis of Air-Stable Near-Infrared Emission in FAPbI3 Nanoparticles Embedded in Silica. Biosensors 2021, 11, 440. https://doi.org/10.3390/bios11110440
Chen L-C, Chao L-W, Xu C-Y, Hsu C-H, Lee Y-T, Xu Z-M, Lin C-C, Tseng Z-L. Room-Temperature Synthesis of Air-Stable Near-Infrared Emission in FAPbI3 Nanoparticles Embedded in Silica. Biosensors. 2021; 11(11):440. https://doi.org/10.3390/bios11110440
Chicago/Turabian StyleChen, Lung-Chien, Li-Wei Chao, Chen-Yu Xu, Chih-Hung Hsu, Yi-Ting Lee, Zi-Min Xu, Chun-Cheng Lin, and Zong-Liang Tseng. 2021. "Room-Temperature Synthesis of Air-Stable Near-Infrared Emission in FAPbI3 Nanoparticles Embedded in Silica" Biosensors 11, no. 11: 440. https://doi.org/10.3390/bios11110440
APA StyleChen, L.-C., Chao, L.-W., Xu, C.-Y., Hsu, C.-H., Lee, Y.-T., Xu, Z.-M., Lin, C.-C., & Tseng, Z.-L. (2021). Room-Temperature Synthesis of Air-Stable Near-Infrared Emission in FAPbI3 Nanoparticles Embedded in Silica. Biosensors, 11(11), 440. https://doi.org/10.3390/bios11110440





