Abstract
Oxygen (O2) quantification is essential for assessing cell metabolism, and its consumption in cell culture is an important indicator of cell viability. Recent advances in microfluidics have made O2 sensing a crucial feature for organ-on-chip (OOC) devices for various biomedical applications. OOC O2 sensors can be categorized, based on their transducer type, into two main groups, optical and electrochemical. In this review, we provide an overview of on-chip O2 sensors integrated with the OOC devices and evaluate their advantages and disadvantages. Recent innovations in optical O2 sensors integrated with OOCs are discussed in four main categories: (i) basic luminescence-based sensors; (ii) microparticle-based sensors; (iii) nano-enabled sensors; and (iv) commercial probes and portable devices. Furthermore, we discuss recent advancements in electrochemical sensors in five main categories: (i) novel configurations in Clark-type sensors; (ii) novel materials (e.g., polymers, O2 scavenging and passivation materials); (iii) nano-enabled electrochemical sensors; (iv) novel designs and fabrication techniques; and (v) commercial and portable electrochemical readouts. Together, this review provides a comprehensive overview of the current advances in the design, fabrication and application of optical and electrochemical O2 sensors.
1. Introduction
Oxygen (O2) is one of the main components of cellular respiration and energy production [1]. The availability of O2 is a key metric that defines the pathway of adenosine triphosphate (ATP) generation and its resultant metabolites that serve as the living cell’s energy source [2,3]. In a high O2 environment, ATP is synthesized by the phosphorylation of the precursor molecule adenosine diphosphate (ADP). This process, thus called aerobic respiration, requires an adequate level of O2. It consists of the coupling of electron transport and oxidative phosphorylation, where O2 acts as the final electron acceptor from the oxidation of glucose and/or glycogen [4]. In low O2 environments, conversely, ATP is generated at an inefficient but rapid rate via a process called anaerobic glycolysis, where glucose and glycogen are metabolized to pyruvate and lactate in the absence of O2. This pathway is important in the functions of vital organs such as the kidney and retina as well as in tumor formation [5]. O2 availability determines the metabolic pathway that generates energy for cell function and survival and therefore is significantly important to measure for bioassays, cell culture and diagnostic applications.
Precise control of a small amount of fluid is possible inside microfabricated channels of microfluidic technology [6]. Integrated microfluidic chips are capable of performing highly sensitive and low-cost analyses. These platforms can be integrated with new technologies with cell culture/organoid studies at high temporal and spatial resolution. For instance, microfluidic platforms can quantitatively monitor cellular signals and cell secretions using well-developed cell-culture methods on microchips [7]. O2 measurement can be performed using sensors integrated into microfluidic chips for monitoring the metabolism and viability of cell, tissue, and organ [8,9]. Moreover, novel sensors embedded inside implantable microchips have recently been used for real-time in vivo oximetry in human or animal bodies [10]. Sensors can also be used to detect and quantify various analytes in a complex biological environment [11,12,13,14,15,16]. Therefore, a combination of integrated sensors is required for online and non-invasive monitoring of the cell intake, secreted metabolites, and microenvironment status for on-chip microfluidic studies such as OOC and bioreactors [12,13].
On-chip monitoring of O2 is pivotal in OOCs. The low concentration of O2 inside a small chip and its crucial biological role in cell metabolism and function would emphasize the need for its precise and selective quantification in the confined environment of a microfluidic channel or chamber [9,17]. With recent advances in microfluidics-based cell and tissue studies, such as OOC technologies, various sensors have been integrated into chips to monitor the microphysiological parameters of cells [12,18,19,20,21,22,23]. OOCs have the potential to better mimic human organs compared to the traditional in vitro models, and thus they can reduce the need for animal models in interventions such as the studies on drug efficacy and toxicity [24,25,26]. To model the function of many organs, such as the pancreas [27], brain [28,29,30], liver [31], vascular system [32], Gut [33], multiorgan approaches [34] and body-on-the-chip, OOC-integrated O2 sensing techniques have been used to culture cell monolayers, three dimensional (3D) cultures, spheroids, organoids and stem cells. They have also been used to model tumor microenvironment by mimicking the extracellular matrix (ECM) [35] and 3D culture of cancer cells [36,37].
Electrochemical and optical sensors are the main transducers for on-chip O2 monitoring [38], as they are precise, selective, and easy to miniaturize and implement inside chips [18,39]. In addition, micro and nanomaterials along with the innovative designs and polymers, and commercial readout devices are used for signal amplification to overcome the limitation related to the measurement of low O2 levels with electrochemical and optical sensors [13,40,41]. Here, we review the recent innovations in O2 sensors integrated into microfluidic chips, including OOC devices. We have categorized them based on their transducer type into two main sections, namely optical and electrochemical. We also discuss recent innovations and their advantages and disadvantages. Finally, we provide a comprehensive discussion of the current advances in the design, fabrication and application of optical and electrochemical O2 sensors.
Previous reviews have covered related topics such as oxygen control (2016) [9], general optical imaging and sensing (2014) [39], microfluidic OOC sensors (2010) [40], and other microphysiological sensors of OOCs [12,13,18,38]; however, there are no recent publications critically discussing the current advancements in OOC O2 sensing. The present review delves into a critical examination of recent developments in O2 sensors integrated into OOCs devices and provides a comprehensive comparison of their advantages, limitations and required future improvements.
2. Oxygen Sensors in On-Chip Systems
Methods of on-chip O2 measurement can be categorized into two groups of sensors: (i) optical and (ii) electrochemical. Here, we highlight the application of these two types of sensors in on-chip studies, explain their mechanism of action, and discuss their advantages and disadvantages. Table 1 represents the summary of advantages and disadvantages of optical and electrochemical methods for on-chip O2 measurement.

Table 1.
Comparison of chip-based electrochemical and optical O2 sensors.
2.1. Optical Methods
Optical strategies for O2 sensing have been widely used in on-chip systems. Luminescent sensors are the most common type of these sensors that utilize either fluorescence or phosphorescence dyes that respond to O2 molecules as a quencher available inside the chip. Optical methods have been used more than electrochemical methods for on-chip O2 sensing due to their lower limit of detection ranges, lower cost and ease of manufacturing. They have great potential to be used as a portable point-of-need detection and connecting to commercial optical measurement (readout) devices. The advantage of the optical sensing technique is that O2 is not consumed during the optical measurement, unlike other methods. Additionally, optical fibers can measure O2 levels easier without the need of direct contact of the culture media by adding a protecting layer of oxygen diffusing materials, such as PDMS, on the optical fiber. This feature is beneficial for keeping the cell microenvironment intact or when O2 content or resource is limited. Optical O2 measurements can also be simultaneously performed with the measurement of other important parameters, such as pH and cell metabolites (such as lactate, glucose, and amino acids) [9,12,38,39].
In the following sections, we summarize the advancements in optical O2 sensors four main categories based on their type of innovations. Furthermore, to make the comparison easier, Table 2 represents the summary of most recent on-chip optical O2 sensors and their features, applied dyes, and their advantages.

Table 2.
Characteristics of chip-based optical O2 sensors.
2.1.1. Basic Luminescence-Based Sensors
The luminescence-based sensing method relies on quenching of luminescence (fluorescence or phosphorescence) dyes by O2 molecules based on the Stern-Volmer equation [39], which reflects the relationship between the concentration of O2 and fluorescent intensity [52,53]. Dyes are mixed with polymers containing O2 permeable and soluble features to make a sensing layer on chip where the emission readout will be measured following an excitement in specific spectra. The main advantages of this strategy are its high sensitivity and selectivity toward O2, ease of fabrication, high photo-stability (suitable for long-term and continuous O2 monitoring), and short response time (typically less than a few seconds) [52,53,54].
The most popular dyes used for O2 sensing include metal–ligand complexes, particularly metalloporphyrins [39]. These dyes are photostable with sharp, strong luminescent signals that can be excited in the visible spectrum. Moreover, small changes in their structure (peripheral substitution) can alter their chemical and spectroscopic properties, suggesting that they are tunable. Due to the longer lifetime of their excitement, their quenching efficacy is high. Therefore, they have high O2 sensing sensitivity [39,44,55,56]. Few examples of the most used dyes are Platinum(II) tetrakis(pentafluorophenyl)porphyrin (PtTFPP) [57], Platinium (II) octaethylporphyrin (PtOEP) [42], Platinum (II)-meso-tetra(4-fluorophenyl)tetrabenzoporphyrin (PtTPTBPF) [43], Ruthenium-tris(4,7-diphenyl-1,10-phenanthroline) dichloride (Ru(dpp)) [55], and Singlet O2 sensor green (SOSG) [56]. In a few studies, other materials such as polymerized 2-hydroxyethyl methacrylate (HEMA) have been used as a dye [58].
The dyes are mostly embedded into dye-impregnated polymeric layers at the vicinity of the cell chambers during the fabrication of chips. The layers are developed by directly mixing or dissolving the dyes into the solvent or polymer and then forming a film layer inside or at the top of the microfluidic channels and chambers [59]. Impregnated polymers are then patterned into the cell chambers using spin coating [44], knife coating [60], and preheated coating dye solution [41]. Dyes are excited by either light-emitting diode (LEDs) or optical fibers. The signal readout is achieved by using Charge-Coupled Devices (CCD) camera [61], fluorescent microscope imaging [42] (Figure 1A) or via optical fibers [43] (Figure 1B). To quantify the signal in real-time, the collected optical signal is converted into an electrical signal using a designed detection circuitry with output monitoring and data collection components [13,55]. An alternative strategy of ratiometric O2 sensing has been employed in recent studies to improve the readout, defined as the ratio of the O2-sensing dye readout signal or image to the non-O2 sensitive reference dye signal. Figure 1C shows an example of the ratiometric concept, where the standard deviation is proved to be half of the typical imaging intensity-based strategies [61]. In addition, few studies have used phosphorescence for on-chip O2 sensing, using the previously described luminescent dyes with longer excited-state lifetimes [62]. In these studies, the dyes were utilized for phosphorescence sensing of O2 with the same concept used in filmmaking strategies for luminescent methods [62,63,64].
One of the main ways to enhance the sensitivity and stability of the optical O2 measurement is via the fabrication techniques such as solvent-induced fluorophore impregnation (SIFI) method [41]. The method was recently developed which involves the impregnation of PtTFPP dye into the body of the cell culture chip without adding a dye-coating inside the chip. In this strategy, the challenges associated with patterning sensing layers in chambers, such as delamination and formation of cracks, were eliminated [41]. The application of optical fibers in O2 sensing has several advantages including the possibility for online and remote monitoring and miniaturization in O2 sensing devices [65,66]. Additionally, Yang and colleagues developed a capillary optical fiber (COF) with a ring-shaped waveguide used for O2 detection. They coated luminescent dyes on the inner surface of the waveguide in the COF for ratiometric O2 measurement with high sensitivity (high ratio of O2-sensitive dye signal over the control dye) and low response time (less than 7s). However, the main drawbacks of this method are the high cost and the need for special readout devices [67]. In many cases, O2 measurement needs to be performed simultaneously along other important parameters such as pH and cell metabolites to assess cell physiological condition. This can be carried out by several techniques such as using different dyes [43,58], micro-patterning the sensors in various layers of the chip [42], using optical fibers that have several excitation/emission wavelengths corresponding to O2 and pH [43] (Figure 1B).
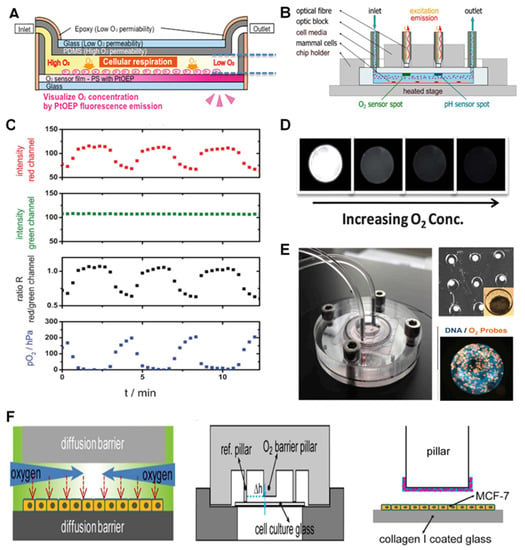
Figure 1.
Basic luminescence-based sensors and microtechnology-based luminescent sensors for O2 monitoring in on-chip studies. (A) The concept of an integrated layer of O2-sensitive dye in an OOC device for liver studies. Reproduced with permission [42], copyright 2019, John Wiley & Sons. (B) Optical fiber-based detection of O2 and pH. Reproduced with permission [43], copyright 2021, Elsevier. (C) The ratiometric concept for optical O2 sensing through comparing the signals from O2-sensitive dyes with that of non-O2 sensitive ones as reference. Reproduced with permission [61], copyright 2013, The Royal Society of Chemistry. (D) PDMS Microbeads containing luminescent dyes were synthesized using microfluidics for O2 sensing. Their performance in an O2 gradient (different concentrations of O2) is represented. Reproduced with permission [68], copyright 2012, The Royal Society of Chemistry. (E) A bioreactor (liver-on-chip) device (left) for the analysis of drug effect on mitochondrial activity of the HepG2/C3A organoids. Each microwell contains an organoid (top right) with integrated microparticles with luminophores for imaging-based O2 sensing (right). Reproduced with permission [69], copyright 2016, National Academy of Sciences. (F) O2 gradient (left) inside the microbioreactor (down right) structure and the O2 barrier and sensing pillars with O2-sensing microbeads (pink circles) (right). Reproduced with permission [45], copyright 2017, Springer Nature.
2.1.2. Microparticle-Based Sensors
Micro-sized materials such as microbeads and microspheres loaded with luminescent dyes have also been used in luminescent O2 sensing inside chip. This method benefits from simplicity of optical O2 detection and add up the advantages of microparticles such as higher surface area with higher interactive surface which enhance the sensitivity, protecting the dye from decay and also lower analyte diffusion distances that enable them to have a faster response. [17,41,67,69,70]. These microparticles are mostly made of polymers such as microparticles of polystyrene (PS), poly (dimethylsiloxane) (PDMS) and silica [65,70]. they have lower analyte diffusion distances that enable them to have a faster response [17,41,67,69,70].
Jiang et al. described a microfluidic-based method to make monodisperse PDMS microbeads containing PtTFPP dye for O2 sensing. After microbead characterization (150 μm diameter), they tested the efficiency of the O2 sensors by spreading them over the bottom of the microfluidic channel of the PMMA chip and exposing them to pure nitrogen (0% O2) and air (21% O2) flow. Similar to the conventional film layers, the phosphorescence effects of the microbeads were quenched by an increase in their O2 content (shown in Figure 1D) [68]. Their higher gas permeability, chemical inertness, nontoxicity, and biocompatibility have made them a more interesting choice for this application. In a relatively similar strategy, PS microbeads (3 µm diameter) with PtTPTBPF dyes were used for the 2D and 3D hydrogel-based cell culture chips which had a sensor spot to connect to a fiber optic and portable commercial FireStingO2 optical O2 m (Pyroscience, Aachen, Germany) [17].
A novel liver-on-chip with O2 monitoring has used tissue-embedded PS microparticles (50 μm diameter) loaded with ruthenium-phenanthroline-based phosphorescence dyes. The system was designed for the study of mitochondrial dysfunction using an organoid of HepG2/C3A cells [69]. Figure 1E shows that each microwell was first loaded with about 100,000 cells and 20 O2-sensing beads. The figure also illustrates the whole bioreactor (a) and its organoids-containing PDMS microwells (b), as well as fluorescent imaging of the organoid incubated overnight with O2-sensitive microbeads (c). The microbeads embedded in the tissue can also be used to assess the cell’s O2 uptake and metabolism rate in real-time measurement. The availability of a continuous O2 uptake measurement over 28 days was mainly due to the bioreactor design, in which the microbeads existed in the trapped cells and are involved in cell migration. This method also guaranteed the absence of any necrosis. The number and location of the microbeads during the experiment, therefore, were fixed over time. In another study with similar microbeads and concepts, O2 monitoring was carried out in a liver-on-chip to test different drugs [47].
Another team designed a microbioreactor for recapitulating intratumor O2 gradients to study the solid tumor microenvironment. The cellular metabolism and physical constraints of a cell layer (MCF-7 cells) between the two diffusion barriers were used in a tumor section-like culture method. The O2 gradient was mimicked without the need for using any O2-gradient making devices [45] (Figure 1F). In order to achieve the gradient, silica microparticles were loaded with an O2-sensitive luminescent dye (tris(4,7-diphenyl-1,10-phenanthroline) ruthenium(II) dichloride), which were glued to the pillar by a PDMS layer inside the microfluidic channels. The fluorescent imaging showed a uniform signal from the O2-sensitive microbeads, which could distinguish different degrees of hypoxia in real-time and perform spatially resolved measurements superior to conventional imaging-based systems in the OOC devices [45]. In another study, microbeads were used to simultaneously detect O2 and pH to study embryonic development inside a single zebrafish embryo culture system. Embryo culture microwells were composed of two sensing hydrogel (Poly(ethylene glycol) diacrylate (PEGDA)) layers. PS microspheres with Pt-porphyrin dyes were used to study the O2 consumption rate (OCR) while dextran microbeads loaded with a (2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF)) dye were applied for sensing the acid extrusion rate (AER). The O2-sensing system was reported to be efficient and capable of monitoring low OCRs of a single early embryo captured in a closed microwell [46].
2.1.3. Nano-Enabled Sensors
The application of nanoparticles (NPs), nanostructures and nanocomposites in sensors has been of great interest over the past decade. This is mainly because of wide range of source materials, shapes and surface functionalization options which increase the flexibility of choosing the suitable nanoparticle for the sensing strategy of each study. The smaller size and/or pores of such materials increase their aspect ratio as well as their active surface, thereby improving their reactivity to a greater degree compared to micro-sized materials. The presence of functionalized groups for attachment to different surfaces, linkers and molecules on their surface expands their features and applications. The unique optical and plasmonic properties of NPs has great potential for enabling rapid and sensitive O2 optical sensing. Moreover, these NPs make the dyes more soluble and protect them from direct contact with the solutions which protect the dye from decay and also from potentially interfering molecules inside the chips and improve the selectivity and storage ability of the chip O2 sensor [71,72,73,74,75]. NPs based on polymers such as poly (methyl methacrylate) (PMMA), PS, poly(styrene-block-vinylpyrrolidone), poly(styrene-co-maleic anhydride), (poly(fluorene-alt-benzothiadiazole), are among the most commonly used types in microfluidic devices [65,70].
The application of NPs for O2 sensing in cells has been studied by the synthesis of PMMA NPs loaded with Pt(II) Octaethylporphine (PtOEP) dye and surface modification with Poly L-lysine (PLL), which makes the system biocompatible and easy to enter the living cells due to their positive charges. These NPs are efficient in sensing dissolved O2 (DO) concentrations as low as 0–43 ppm [71]. The NP-based O2 sensing has also been applied in microfluidic chips for bacterial growth monitoring. They benefited from PS NPs with platinum (II) 5, 10, 15, 20-meso-tetraphenyltetrabenzoporphyrin (PtTPTBP) dyes for rapid and simple monitoring of the metabolic activity of bacteria for 72 h [72]. In another study, Horka and colleagues used poly(styrene-block-vinylpyrrolidone) NPs with PtTPTBPF dye to monitor the metabolic activity of the bacteria via phosphorescence lifetime-based measurement strategies for O2 sensing inside a microfluidic droplet [49].
In the O2 sensors developed by Lasave and colleagues, polymer NPs (about 25 to 35 nm) were physically adsorbed on the silica microparticles at the bottom of the glass chip (Figure 2A). The comparison between glass chips with and without the microparticles as the NPs bed for O2 sensing showed that the chips with microparticles perform better compared to bare glass [51]. They showed that the sensors were stable with fast response and capable of using detection dyes (pH-sensitive dyes). The sensors could be used inside the microfluidic channels, even closed ones, by introducing the NPs. This method, however, is limited to glass chips (not plastic-based devices).
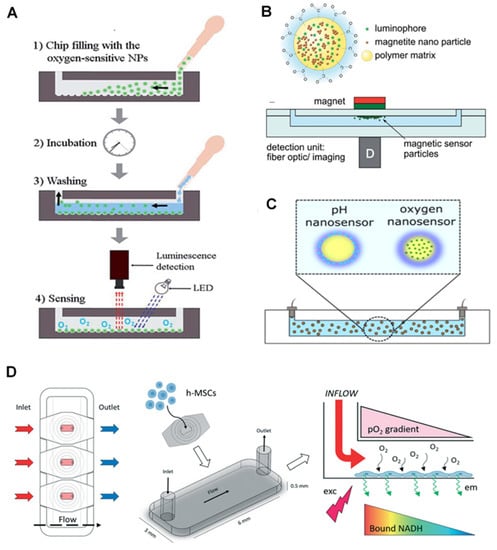
Figure 2.
Application of nanoparticles for O2 sensors in on-chips. (A) Nanoparticles are introduced into the channels to attach to the microbeads inside the chip and form an O2-sensitive layer. Reproduced with permission [51], copyright 2015, The Royal Society of Chemistry. (B) Magnetic NPs with luminophore come together in a spot in the chip parallel to where the outside magnet is located. Reproduced with permission [76], copyright 2014, The Royal Society of Chemistry. (C) Sensitive dyes in core-shell NPs for simultaneous detection of O2 and pH. Reproduced with permission [76], copyright 2014, The Royal Society of Chemistry. (D) Chip-based stem cell culture to monitor O2 gradient via optical luminescent sensing layers. Reproduced with permission [48], copyright 2021, The Royal Society of Chemistry.
Magnetic NPs can also be used for chip-based O2 sensing. For instance, magnetic NPs and PtTFPP dyes (luminophore dyes) were merged into the poly(styrene-co-maleic anhydride) (PSMA) polymer to form a magnetic nano-complex. They were brought together via an external magnet over the chip’s sensing spot, while the imaging or optical fibers were used as the readout [51]. The sensing spots can measure not only the other O2 level but also the temperature and pH.
Core-shell poly(styrene-block-vinylpyrrolidone) magnetic NPs, with a PS core and hydrophilic polyvinylpyrrolidone shell, were used for simultaneous detection of O2 and pH. PtTPTBPF as an O2-sensitive dye is incorporated in the core, where the pH indicator and the reference dye are applied on the shell (Figure 2B,C) [76]. Based on the reported results, a mixture of NPs loaded with O2, and pH dyes performed better than those containing both dyes. This strategy allows simultaneous detection of the two parameters and is easy, reproducible, and stable in different circumstances. Scientists also introduced a perivascular niche-on-a-chip device, in which the intracellular levels of O2 were monitored in human mesenchymal stem cells (h-MSCs) using polyfluorene NPs (poly(fluorene-alt-benzothiadiazole)) covalently conjugated with dyes (platinum(II) meso-bis(pentafluorophenyl)bis(4-bromophenyl)porphyrin (PtTFPPBr2)). They used NAD(P)H fluorescence signal as a reference in a fluorescence lifetime imaging microscopy (FLIM) system (Figure 2D). The applied NPs are reported to have a high-intensity fluorescent signal with high cell uptake levels, resulting in the chip-based real-time assay of intracellular O2 levels. These NPs can also be easily applied to closed chips [48].
2.1.4. Commercial Probes and Portable Devices
Commercial and/or portable modules have been used for on-chip monitoring of O2 levels in embryo, cell and tissue cultures [17,77]. While the benefits of portable O2 sensing in microfluidic devices are well known, in OOCs, portability can bring additional features such as compactness and miniaturization of the sensing module, an automated, simple working platform with their own software, and multiplex detection of factors such as pH, CO2, and temperature besides O2. In this regard, few companies have introduced commercial portable Universal Serial Bus (USB)-based optical readout systems such as FireStingO2 (PyroScience, Aachen, Germany) [17,77], PiccolO2 device (PyroScience, Germany) [49,50] or portable detection modules such as VisiSens device (PreSens, Regensburg, Germany) [77]. These optical readout modules can be mounted on (Figure 3A) or connected to (Figure 3B) the sensing spot on channel/chamber or implemented in a modulated box (Figure 3C).
For Example, Ehgartner et al. introduced sensor spots integrated with silicon-glass microreactors connected with commercial optical readout devices [78,79]. Figure 3A represents the chip and its sensing spots (left) as well as the commercial picolo2 readout with a USB port (right) which can be connected to the computer for further analysis. The chip was designed to measure the O2 level and to monitor the enzymatic activity of D-amino acid oxidase and glucose oxidase as model enzymes. Seven cell culture chambers and four O2 sensing spots were read by a simple USB O2 m (Piccolo2) connected to an optic fiber or by a four-channel optical O2 m (FireStingO2). The chip was compatible with two famous luminescent O2-sensitive dyes, with two for PdTPTBPF and the other two for PtTPTBPF. The method, therefore, was concluded to be accurate with an automated and inexpensive production line and compatible with commercial readout devices. In another example, but for monitoring the metabolism of bacteria in droplets, Horka et al., also used Piccolo2 device enabling the non-invasive contactless measurement through the wall of a tubing as it is shown in the Figure 3B [49].
Zhu et al. introduced a microbioreactor for monitoring metabolic activities of zebrafish embryos with a sensor foil integrated with O2 monitoring using a Fluorescence Ratiometric Imaging (FRIM) system [77]. An O2-sensing foil was integrated into an embryo trapping chamber in a PMMA chip. A portable USB-embedded commercial ViviSens detector was used for fluorescent imaging. A calibration curve was plotted before the embryo culturing for chip-based O2 measurements. The method was proved to provide a real-time and non-invasive measurement for the embryos. Other advantages were the possibility to use commercial O2 sensing devices and software, such as a small integrating and portable detector with USB port, and the capability of measuring O2 gradient.
In another development, Wang and colleagues developed a portable detector for O2 detection using a PDMS chip and a PtTFPP film [79,80]. The detector (Figure 3C), comprised of a photomultiplier (PMT) counter (with a Charge Coupled Device (CCD) camera and an imaging spectrograph) connected to a smart-phone application and was able to detect DO levels with an LOD of 0.01 mg/L (0.37 µM) and short response time of 22 s. The simplicity, low-cost production, handling of the device, high sensitivity, short response time and portability are among important features which make this detector a desirable device for on-chip O2 sensing, especially in medical applications.
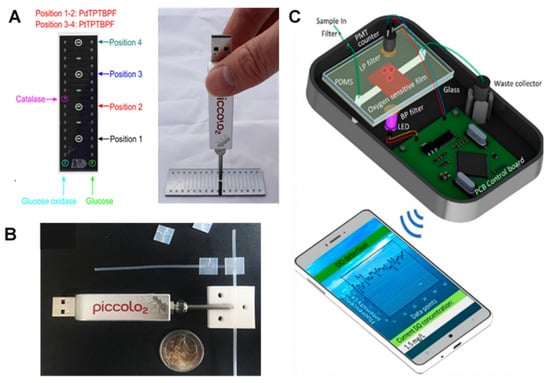
Figure 3.
Commercial and portable optical readout systems for on-chip O2 monitoring. (A) A chip (left) designed for commercial optical readout devices (right). Reproduced with permission [78], copyright 2016, Elsevier. (B) chip-based bacteria study with O2 measurement through the wall of tubing using a commercial readout device. Reproduced with permission [49], copyright 2016, American Chemical Society. (C) A portable handheld photodetector device connected to a mobile app for chip-based O2 monitoring. Reproduced with permission [80], copyright 2021, MDPI.
2.2. Electrochemical Methods
Electrochemical (EC) sensing is a common technique to measure low concentrations of DO, often in microfluidic applications, with high sensitivity [81,82]. Being introduced over two decades ago, it is believed that certain shortcomings of optical measurement techniques are resolved by using EC sensing, such as the need for time-lapse bright field and fluorescence microscopy in combination with various staining techniques as well as a collection of supernatants and cellular samples traditionally used in the OCCs. However, the direct integration of EC-based DO sensors in OOC systems is not common because of the complexity, multi-step and high-temperature fabrication process and risk of material incompatibility, all of which increase the complexity of the whole system as well as the overall probability of failure [12]. In the following subsections, we review several innovations to overcome such difficulties in EC sensors and categorized into five main innovation groups: (i) novel configurations in Clark-type sensors; (ii) novel materials (e.g., polymers, O2 scavenging and passivation materials); (iii) nano-enabled electrochemical sensors; (iv) novel designs and fabrication techniques; and (v) commercial and portable electrochemical readouts. Table 3 summarizes the components, advantages and LOD of the selected EC sensors for the measurement of DO.

Table 3.
Characteristics of chip-based electrochemical O2 sensors.
2.2.1. Novel Configurations in Clark-Type Sensors
The Clark-type configuration has transcended the main limitations of the optical sensors, such as high cost and complicated fabrication process. The system normally consists of an electrode covered with a thin layer of electrolyte solution and a thin polymer membrane enabling selective permeation of O2 to the immersed electrode. The membrane also separates the electrolyte from the specimen, preventing cross-sensitivity and resulting in a short turnaround time. However, the need for mechanical agitation, circulation of the sample or immersion of the sensor in the sample (which requires large amounts of sample and reagents) are the main drawbacks of such sensors [81,97]. Apart from the high risk of mechanical failure in the thin gas permeable membrane, such sensors are not compatible with continuous monitoring, automation and high-throughput measurements [98].
Reducing the size of the sensor and/or limiting the measurement time is necessary to reduce O2 consumption by the EC sensor and to prevent possible endangerment of the biological sample. This is important for the measurement of the kinetics of O2 variation in samples and for monitoring the cell growth. Therefore, using a micro total analysis system (μTAS) has overcome some of these challenges. O2-plasma bonding was one of the first methods used in miniaturized Clark O2 sensors [83]. Unlike the field-assisted bonding technique, which requires high electric voltage (kV) and temperature (250 °C) to combine the glass and silicon substrates, this simple and low-temperature fabrication process enabled the integration of several semiconductor elements and polymer-material structures in an ordinary laboratory environment [99]. The use of thick-film technology with the SU8 photoresist and extremely thin O2 separation membrane resulted in a low response time (6.8 s). However, the sensor was not suitable for long-term measurements.
In a more recent attempt, a low temperature co-fired ceramic (LTCC)-based microfluidic Clark-type O2 sensor was used for real-time monitoring of localized DO [84]. The advantages outlined for the LTCC materials include hermeticity and mechanical durability, high scalable prototyping/manufacturing, and the ability to directly integrate both electronic and microfluidic with a compact 3D package [100,101,102]. The EC sensor consisted of a solid proton conductive electrolyte that facilitated the fabrication process and improved shelf life. The solid-state proton conductive matrix (PCM) membrane (Nafion 117 membrane) was used as a support to lower the risk of mechanical failure of the PDMS O2 permeable membrane (OPM). Also, the microfabricated electrodes and continuous flow of the sample through the microchannel could reduce the O2 depletion risk.
Clark-type O2 microsensors also promise zero analyte consumption if the feedback mode (Ross principle) is successfully implemented [103]. According to this principle, a suitable sensor configuration allows for the compensation of O2 consumption at the working electrode (WE) through evolving O2 at the counter electrode (CE) while maintaining the sensor pH. This is confirmed by showing O2 formation as the only oxidation reaction at the CE. Another example is a microsensor with Pt as WE was fabricated on a glass chip using thin film technology. The use of a poly-2-hydroxyethyl methacrylate (pHEMA) hydrogel layer containing buffer solution as an electrolyte and PDMS as a gas-permeable membrane showed promising results in monitoring cell respiration in cultures (Figure 4A) [85]. Thanks to the dried-out hydrogel layer, the microsensors could be stored dry and activated by immersion into aqueous analytes. As a result, the measurement in the gas atmosphere, even with ambient humidity, was possible only for less than one hour. Chronoamperometric protocols to renew the electrode surface through the formation of PtO and subsequent removal before O2 measurement was applied to enable long-term stability, for more than one week, without any need for recalibration.
In order to simplify and speed up the prototyping process, a confined microfluidic cell culture system was developed using a microscopic indium tin oxide (ITO) slide with planar Pt sensors at the bottom of a plate for the measurement of acidification, O2 consumption, and cell adhesion [104]. The structure of the slide, along with the gas permeability of the PDMS lid, ensured 100% air saturation in the culture medium, desirable for the cells but responsible for possible bubble formation. Using an ultrashort pulse laser, the fabrication process of such amperometric Clark O2 sensors took about three minutes per chip. This not only improved the precision of the chip but also prevented possible exposure to toxic chemicals or adverse effects in the cell culture.
In a clinical application of a multi-analyte OOC, dynamic measurement of DO was performed in low volumes of urine using Clark-type microsensors with a high sensitivity of 3.60 ± 0.2 nA mg L−1 [86]. The multi-sensor platform was composed of three different polymeric layers: a 127 mm thick polyimide film (Kapton® 500HN, DuPont Co., Wilmington, DE, USA incorporating the gold microelectrode array, a 175 mm structured Polyethylene terephthalate (PET)-two-side adhesive sheet (AR8939) defining the microfluidic manifold, and a 188 mm cyclo-olefin polymer (COP) film (Zeonex ZF14-188, purchased from Ibidi, Gräfelfing, Germany) as the cover. The use of flexible polyimide Kapton®, besides its thermal and mechanical stability, high chemical resistance, and low dielectric constants, ensured the platform was cost beneficial.
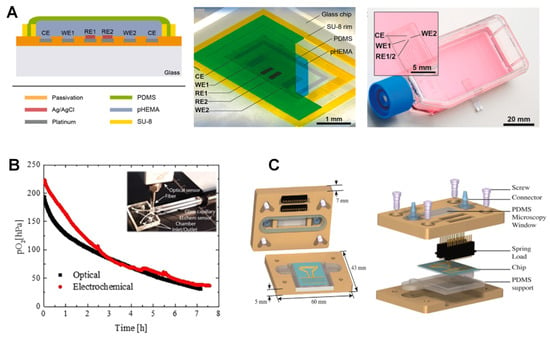
Figure 4.
Electrochemical sensors for on-chip O2 measurement. (A) cross-section and top view of the Clark-type O2 monitoring microsensor via chronoamperometric sensing protocols in cell culture and organ-on-chip systems. Reproduced with permission [85], copyright 2020, Elsevier. (B) microfluidic cell culture chip with intra-channel parallel electrochemical and optical O2 measurement using commercial optical and electrochemical readout devices showing agreement between the results (red and black curves). Reproduced with permission [105], copyright 2019, American Chemical Society. (C) Components and cross-section of the microfluidic chip with electrochemical detection electrode arrays for a distinct set of three-electrode for O2 and the electrode array for Na+, K+ and pH measurement. Reproduced with permission [106], copyright 2015, Elsevier.
2.2.2. Novel Materials: Polymers, O2 Scavenging and Passivation Materials
As mentioned in the previous section, polymer membranes are an important part of EC-based O2 sensors. This is because O2 is diffused to the reaction chamber, where it is consumed, while biological cells and other organics are prevented from passing through. PDMS is the most common polymer used to construct the microfluidic channels and the sensor support because of its characteristics, such as biocompatibility, gas permeability, mechanical flexibility, and optical transparency.
In an integrated microelectrochemical reactor, an EC sensor with incorporated Pt interdigitated array (IDA) as WE, a Pt CE, and Ag pseudo-reference electrodes (RE) were used. The WE was fully immersed in a liquid electrolyte confined in the channels [107]. The O2 reduction reaction (ORR) was used in this platform for both pH tuning and fluid actuating reactions. In this regard, a microelectrochemical cell design inherent to membrane-covered Clark-type sensors was used. Unlike common polymers that are less permeable to O2 than the electrolyte, a highly permeable PDMS membrane, capable of supporting significant current densities, was used. This simple change enabled remarkable pH gradients capable of initiating and/or sustaining marked fluid displacements within the microfluidic channel system with low applied potentials (0.1 V) and no need for additional electrochemically active components. Due to the high O2 permeability of PDMS, the microfluidic cell supported significantly higher current densities in the ORR compared to those measured in conventional (quiescent) EC cells with similar electrode areas. This results in achieving more stable ORR currents. Another advantage of this system is the absence of bubbles. Using membranes thinner than 0.1 mm allows for a higher diffusion rate with limited ORR current densities. The significant O2 mass transfer in the PDMS structures of the microfluidic cell helped generate transient chronoamperometric waveforms while sustaining the steady-state mass transfer limited the ORR current densities.
As another example, a multi-sensor glass chip with a PDMS imprinted microfluidic channel grid was developed to characterize cellular behavior [87]. The microfluidic manifold was fabricated based on the Haversian bone-canal system to ensure a homogeneous flow. They resembled a Clark-type amperometric sensor without any O2-selective membrane. The glass-wafer technology ensured the microscopic observability of the on-chip cell culture along with online monitoring of the physiological parameters. On the other hand, glass is less costly compared with silicon-sensor technology [88,108,109,110]. Circular 78.5 μm2 Pt electrodes, as O2 sensors, led to a sensitivity of 100 pA per each 1% increase in saturated air (21% O2). The sensor worked for 80h during cell culture, and the fluctuations noted in the current rates were possibly due to large gas bubble formations.
Although PDMS is the most common polymer used in this regard, other polymers have also been tested. PTFE has higher permeability and faster O2 diffusion rates compared to PDMS, which results in O2 depletion in the channel [111]. Adjusting the electrolyte flow rate and the channel length can ensure the presence of sufficient O2 in the biomass chamber and thus the reliability of the results. However, PDMS-based respirometers allow shorter analysis times, especially when thicker membranes are needed or the sample is rich in organic matter. Using gas permeable membranes such as PDMS alone has limitations such as loss of vapor which changes the fluid composition of the microfluidic cultivation chambers and may lead to reoxygenation from the ambient air. Other materials benefit from diffusion and mixing deoxygenated fluids, chemicals, electrolytic, photocatalytic, and biological O2 depleting liquids such as sodium sulphite and pyrogallol [112]. These materials, however, can interfere with the cellular processes and metabolic pathways. Using an external gas supply with nitrogen to equilibrate the O2 concentrations is another alternative, even though it is less accurate [113].
Functional microfluidic materials with intrinsic O2 scavenging properties allow for long-term cell cultivation under reduced O2 concentrations. These are cheap substitutes for bulky and expensive hypoxic chambers. Adjusting the temperature and curing time during the process of fabrication and altering the microfluidic layout and the surface area-to-volume aspect ratios of the channels, as well as modifying the flow rates during cell cultivation, can help fine-tune the O2 scavenging rates and address specific biological issues. In an example of a miniaturized OOC with embedded O2 sensors, an off-stoichiometric thiol-one epoxy polymer was integrated into a functional biochip to efficiently remove DO to below 1.0 hPa. In a clinical application, this system was used to study acute ischemic stroke using a murine blood−brain barrier (BBB) model (Figure 4B) [105]. In this system, anaerobe bacteria were cultivated under ambient air until efficient germination of pathogens was achieved. They compared two parallel O2-sensing methods: an opto-chemical O2-sensing based on luminescence lifetime measurement using an O2 indicator luminescent dye (PtTPTBPF) and an EC sensor directly detecting DO levels by oxidation at the electrode surface measured by a commercial readout OX-NP Clark-type sensor (Unisense, Denmark). Both sensors had similar time-dependent O2 depletion profiles, resulting in O2 tension of 30.76 and 37.58 hPa after 7 h, respectively. They showed similar DO removal from about 200 to 30 hPa within 7 h in a 25 μL microchannel volume. O2-sensitive microbeads, however, were selected as the best options because of their smaller footprint and ease of integration within the microchannels.
Passivation materials are also used to not only define the electrode surface but also ensure their long-term EC stability. In an attempt to develop a low-cost, easy-to-use, reproducible and portable microfluidic device, a specific B33 glass substrate was used [91]. The idea was to initiate and monitor microalgae photosynthesis activity while electrochemically assessing the microalgal O2 production rate during artificial night/day cycles. The ElecCell (Electrochemical microcell) technological platform, with fully integrated EC microcells (titanium/platinum (Ti/Pt) as the working microelectrode, the counter and the sub-structure of the pseudo-reference) was developed using double physical vapor deposition (PVD). A silicon nitride (SiNx) wafer-level was deposited for passivation using low-temperature PVD. The microfluidic system was fabricated through laminating dry films to overcome the need for any micropumps or other fluid actuation devices.
In another scalable microfluidic platform developed for continuous monitoring of biofilm proliferation and activity under shear stress conditions, a double layer of PVD deposited silicon oxide and silicon nitride (Si3N4) was applied for passivation purposes (Figure 4C) [106]. The combination of two interdigitated microelectrodes (IDuEs) and punctual electrodes helped with the measurement of DO, K+, Na+ and pH. The optimized IDuEs permitted sensitive and reliable label-free monitoring of Staphylococcus aureus V329 growth. The four-electrode system eliminated possible electrode-electrolyte effects, improving sensitivity and providing morphological and structural information necessary for assessing the bacterial growth on the electrode. A combination of polycarbonate and PDMS was used to fabricate the upper and lower lids, respectively. The novel platform could thus be integrated into multiple environments, allowing simultaneous optical microscopy and impedance spectroscopy measurements.
2.2.3. Nano-Enabled Electrochemical Sensors
Nano-enabled sensors are those types use nanomaterials or nanolayers of materials in order to enhance the sensitivity of the oxygen measurement and electrical and mechanical properties of the used materials. Nafion is a permeable membrane that enhances the electrodes’ shelf life and their sensitivity due to its anti-fouling properties. In an example of miniaturized cell-based biochips for toxicological analysis during cell growth and development, EC amperometric sensors were integrated at the inlet and outlet microchannels of a PDMS cell chamber to perform real-time continuous glucose and O2 monitoring [89]. Two arrays of thin-film gold microelectrodes (0.16 and 0.016 mm2 for glucose and O2, respectively) were incorporated in a biocompatible PDMS microfluidic cell culture device. Nafion-modified O2 sensors could measure O2 concentrations from 237 to 50 mmol L−1 with a sensitivity of 0.50 ± 0.05 nA mol−1O2 L−1). The integrated Au/Nafion electrodes were concluded to be suitable for DO monitoring in dynamic flow conditions with high stability and short response time. The flow rate, however, needed to be controlled due to the high O2 permeation through the PDMS.
A lab-on-a-tube (LoT) integrated with spirally-rolled pressure, temperature, O2 and glucose microsensors was developed for multimodal real-time neuromonitoring as well as draining CSF in patients with traumatic brain injury [90]. The approach was reported to be less invasive and cheaper compared with traditional techniques. The temperature and DO sensors helped adjust the output, reducing errors commonly seen in in-vivo biosensors. DO sensors, the focus of the current review were fabricated on 25 mm thick Kapton film patterned with Ti/Ir/Au and finally parylene layers. The Au layer on the reference electrode was selectively etched, and iridium oxide (IrOx), a promising material for in-vivo reference electrodes, was grown anodically in a 0.7 M Na2HPO4 solution. Nafion and silicone membranes were deposited as a polymer electrolyte and O2 permeable layer, respectively. With a sensitivity of 12.89 nA mmHg−1, DO sensors could continuously monitor parentage of O2 of 152 mmHg (air-saturated) and 38 mmHg (5% O2) for at least five days with less than 9% sensitivity error.
2.2.4. Novel Designs and Fabrication Techniques
The use of an ultra-microelectrode array (UMEA) for ultra-short (<5 ms) measurements is another novel technique to monitor DO concentrations in any solution [92]. As an example, the sensor consisted of Pt electrodes recessed in a glass substrate with oxide-nitride-oxide (ONO) as the insulating material. Such sensor was reported to have a high sensitivity of 0.49 nAs−0.5/mg/L with a drastically (about 10 times) low OCR, valuable for in situ assessment of the microtissues’ respiratory activity. One of the main limiting factors for the lifetime application of such sensors is the need for repeated recalibration. This drawback has been addressed by arranging the cell cultures and sensors in a multi-well system in a way that they come in contact with each other at certain times and then separated at others such that the measurement would have no impact on the cell growth [93]. The bottom side of the cultivation wells, made of 0.1–0.3 mm thick Al2O3 membrane with interconnecting pores (200–300 nm), was connected to the sensor area using a special coupling mechanism. The planar multi-sensor chip was made of screen-printed electrodes (SPEs) on the Al2O3 substrate, with those dedicated to O2 measurement covered with pHEMA and PUR membranes using dispensing technology. The gap between the two modules enabled the repeated flow of the analyte-containing liquid through a biocompatible nano-porous sterile membrane, resulting in continuous pH, glucose and O2 measurement during the cultivation phase. The system could be reutilized after sterilization with gamma-rays.
Inkjet printing (IJP) is a novel technique used for sensor fabrication. The main advantage of this technique is its compatibility with delicate substrates that cannot withstand high temperatures due to the possibility of drop-on-demand material deposition. Moreover, the direct writing approach without masks reduces the overall cost and fabrication time considerably and facilitates iterative design changes. Multiple sensors are integrated into an extremely thin, porous, and delicate membrane inside the OOC in such applications. The DO sensors are printed along the microfluidic channel allowing local online monitoring of O2 concentrations. A primer biocompatible dielectric layer (SU-8) is commonly used to seal the porosity of the membrane at defined areas, build a uniform deposition of conductive inks, accurate definition of the electrode area, and prevent possible short circuits [114]. In one example, DO sensors were fabricated using IJP in ExoLiver, a liver-on-a-chip device for real-time monitoring of the cell culture, O2 gradient, and OCR [94]. ExoLiver, a modular bioreactor consisting of two plates separated by a porous membrane, mimics the liver sinusoid system. The 300 μm DO sensors had a negligible O2 consumption of about 2.94 × 10 − 8 mg s−1 per sensor, therefore not affecting the viability of the cell culture. They were calibrated by polarization at −650 mV, an optimal reduction potential value for determining DO concentrations without interfering with the electro-active medium. The sensor had a sensitivity of 28 ± 1 nA L mg−1 (range, 0–9 mg L−1) with a turnaround time of the 60s. A max of 17.5% gradient measured between the system inflow and the outflow could be due to the metabolic activity of the sinusoidal hepatocytes and cell consumption along with the bioreactor’s lower plate. This is of great value as the O2 gradient has a critical regulatory role, as it is directly linked with the metabolic zonation, morphology and xenobiotic transformation in the hepatocytes.
In another clinical application, the multi-analyte microphysiometer (MAMP), a modified Cytosensor Microphysiometer combined with additional amperometric glucose, lactate, and O2 sensors, enabled real-time measurement of changes caused by the metabolism of cells immobilized in a microfluidic chamber [115]. The unique combination allowed for the monitoring of both aerobic and anaerobic respiration. The main benefits of the planar SPEs include simple fabrication, versatility, high reproducibility, and low cost, all resulting in a simplified microfluidic chamber. A Dimatix materials inkjet printer was used to deposit the enzyme and polymer films on Pt SPEs to fabricate the glucose, lactate, and O2 sensors. This process guaranteed the homogenous coating of the electrode surface and its reproducibility. Low concentrations of BSA-PB solution instead of BSA also reduced the risk of bubble formation. A newer generation of MAMP was later developed consisting of five modifiable Pt electrodes along with an Ag/AgCl quasi-reference, designed to measure glucose, lactate, O2, and pH simultaneously in a single microfluidic channel [116]. Reproducible surface modification was again performed using IJP. The O2 sensor was modified with 2.5% Nafion. The highly adaptable system could act as a microphysiometry platform with a lifetime of up to 6 weeks.
Optical transparency of the OOC chip is obligatory for ongoing monitoring of cells using phase-contrast microscopy. This is while most of the existing microphysiometers, i.e., the Cytosensor® and Bionas Discovery™ 2500 system, are silicon chips [117,118]. To overcome these shortcomings, an optically transparent multi-parametric microphysiometer was developed for continuous dynamic measurement of pH, O2, lactate and glucose in T98G human tumor cell cultures [88]. The biocompatible glass-based chip was composed of EC microsensors fabricated using a hybrid thin film and laminate technology. Microfluidics enabled controlled medium exchange and substance exposure to low volumes (<10 μL) with low flow rates (2 μL/min), reducing shear stress on the cells. The sensors were located upstream in the inlet channel (control), inside the cell cultivation area and downstream in the outlet channel. In order to separate the biosensors from the cell culture area, protect the cells from hydrogen peroxide exposure and prevent O2 depletion during cultivation, the fluidic channels and electrodes were fabricated using a permanent epoxy resist and were partly covered with a laminated polyimide film. The amperometric reduction of DO at the thin-film Pt-based circular electrodes of the O2 sensors resulted in a sensitivity of −0.735 μA μM−1 cm−2 (±0.013). The sensor, however, showed a 10% decrease in sensitivity when first entered into the cell culture medium, possibly due to protein adsorption on the electrode. The O2 sensors were reportedly stable for the long-term with linear behavior and negligible O2 consumption (<3%).
2.2.5. Commercial and Portable Electrochemical Readouts
Commercial products have been used in EC methods for the measurement of O2 inside the chips. For example, the liver-on-a-chip device developed by Moya and colleagues in 2018 [94] and the blood–brain barrier (BBB) model developed by Sticker and colleagues in 2019 [105] have used the OX-NP Clark-type commercial EC readout device by Unisense Co., Denmark. A modular portable method was developed for cell culture monitoring [93]. The Bionas Analysis System 2500 (Bionas GmbH, Warnemünde, Germany, www.bionas.de, accessed on 20 October 2021) was used for in-vitro, non-invasive and parallel measurement of metabolic parameters of respiration, acidification and cell adhesion in time-frames ranging from minutes to days [119]. The Clark-type sensors detected O2 mediated current (charge transfer rate) with a sensitivity of 0.12 pA·s−1 ± 0.21. The use of the Koester coating protocol helped ensure comparable conditions for cell growth on different surfaces [120]. The inhomogeneity in cell distribution and growth over the entire chip surface, in practice, resulted in variations in values measured by the same sensors of the chip.
Another miniaturized EC respirometer monitored DO concentrations in water samples semi-continuously, unlike traditional expensive biochemical O2 demand (BOD) methods [121]. The device was composed of a double-flow cell, reaction chamber, housing the sample and microbial mixture, and an electrolyte chamber, separated by a thin membrane from the bioreactor to provide sufficient flexibility to perform measurements in a wide range of organic matter concentrations. In another attempt, a novel encapsulation design and a membrane barrier material, the Intelligent Mobile Lab for In-Vitro Diagnostics (IMOLA-IVD), was used for cellular microphysiometry [96]. Extracellular acidification (EAR) and O2 uptake (OUR) rates were passively monitored using a modular, label-free EC platform. A three-electrode membrane-free Clark cell was used to measure the DO levels. Above and under the 3D multicellular spheroids, the integrated layers allowed fluidic contact between the spheroids in microwells and the BioChip sensors while preventing any washout from medium perfusion.
The 3D cellular models mimicked the native environment of the tissue; however, their increased size and geometry, as well as limited access to O2 and nutrients due to restricted diffusion into the scaffolds, were the main limiting factors [122]. Multicellular spheroids incorporated with OOC platforms are a promising solution. EC microsensors with a small cross-section can be inserted directly into a microtiter plate well containing a single spheroid, microtissue or organoid. Due to their high sensitivity, excellent selectivity and defined zero-point, such EC sensors can measure small changes in O2 concentrations in the microtiter plate caused by the spheroid metabolism [95].
3. Conclusions and Future Perspectives
In this review, we discussed recent advancements of oxygen sensors in on-chip systems and categorized them in two main groups: optical and electrochemical methods. We also discussed recent research and novelties in each section with schematic Figures and an overview Tables. The optical methods are reported to be more sensitive, easier to operate and cheaper compared to the EC methods. In most cases, they do not consume O2 during the process. In addition, they are compatible with commercially available luminescent dyes and optical readout devices, even fluorescent microscope which is convenient and available in most cell and tissue process centers. These sensors can be used for contactless monitoring by adding a sensing spot outside the chip readout or optical fiber, making the handling and sterilizing of the chip simpler. In addition, they do not need recalibration or experience decay over time. Compared with EC methods, therefore, these techniques are more commonly used in OOC applications. This is especially important for low concentrations of the sample when the stability and reusability of the sensor and its remaining intact are crucial. On the other hand, the EC methods have a shorter response time, and in most cases, a higher sensitivity than the previous group. In most cases, they can be used as label-free sensors, which again reduces the cost of sensor fabrication compared with optical ones. However, their integration in the chip is relatively expensive, complicated, and sometimes requires special relatively expensive instruments (Potentiostat/Galvanostat) and skilled operators.
In the future, the use of novel materials, fabrication techniques, and chemical/physical surface modifications can help facilitate the fabrication steps, reducing the price and required specialty, making the chip-integrated O2 sensors more affordable. Although there are few examples of micro- and nanomaterials used for the chip-based O2 sensors of either type, the field is rapidly progressing. It is expected that a variety of materials with various properties to help with O2 sensing will be used for these sensors in the near future to improve their sensitivity. This is mainly because these materials are believed to increase the active surface area, enhance the stability of the surface and increase the glow of the dyes. For instance, graphene and quantum dots can be considered as potential materials due to their exceptional optical and electrochemical properties. Micro- and nanostructures of novel metal oxides are other examples of such materials because of their catalytic activity. In addition, quantum dots (QDs) are photostable and their excitement spectra are broad, while their emission spectra are size-tunable, which makes them suitable for optical sensors. Further, using a smartphone to quantify the emitted signal can significantly simplify the measurement process. Artificial intelligence and innovations in image and signal processing help enhance the sensitivity and specificity of O2 sensors. Additionally, using 3D printing to manufacture the sensors can result in flexible, rapid and low-cost designs and integration of sensors in OOCs, and improve the sensitivity of the O2 sensors inside the chips. Three-dimensional printing techniques can also be used to build customizable optical holders and modules that can be integrated with mobile phones or other portable detectors.
Author Contributions
M.A. (Mostafa Azimzadeh), P.K., M.A. (Meitham Amereh) and N.T.; writing—original draft preparation, M.A. (Mohsen Akbari) and M.H.; editing and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to acknowledge the support received by the National Sciences and Engineering Research Council of Canada (NSERC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akram, M. Mini-review on Glycolysis and Cancer. J. Cancer Educ. 2013, 28, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, N.K.; Nguyen, M.K.; Darch, M.A.; Nakaoka, H.J.; Cousineau, D.; ten Hoeve, J.; Graeber, T.G.; Schuelke, M.; Maltepe, E.; Kampmann, M.; et al. Defining the ATPome reveals cross-optimization of metabolic pathways. Nat. Commun. 2020, 11, 4319. [Google Scholar] [CrossRef]
- Salway, J.G. Metabolism at a Glance; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Beckner, M.E.; Stracke, M.L.; Liotta, L.A.; Schiffmann, E. Glycolysis as primary energy source in tumor cell chemotaxis. J. Natl. Cancer Inst. 1990, 82, 1836–1840. [Google Scholar] [CrossRef]
- Xiong, B.; Ren, K.; Shu, Y.; Chen, Y.; Shen, B.; Wu, H. Recent Developments in Microfluidics for Cell Studies. Adv. Mater. 2014, 26, 5525–5532. [Google Scholar] [CrossRef]
- Spiller, D.G.; Wood, C.D.; Rand, D.A.; White, M.R.H. Measurement of single-cell dynamics. Nature 2010, 465, 736–745. [Google Scholar] [CrossRef]
- McLean, I.C.; Schwerdtfeger, L.A.; Tobet, S.A.; Henry, C.S. Powering ex vivo tissue models in microfluidic systems. Lab Chip 2018, 18, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Oomen, P.E.; Skolimowski, M.D.; Verpoorte, E. Implementing oxygen control in chip-based cell and tissue culture systems. Lab Chip 2016, 16, 3394–3414. [Google Scholar] [CrossRef]
- Kmiec, M.M.; Tse, D.; Mast, J.M.; Ahmad, R.; Kuppusamy, P. Implantable microchip containing oxygen-sensing paramagnetic crystals for long-term, repeated, and multisite in vivo oximetry. Biomed. Microdevices 2019, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Moradi, V.; Akbari, M.; Wild, P. A fluorescence-based pH sensor with microfluidic mixing and fiber optic detection for wide range pH measurements. Sens. Actuators A Phys. 2019, 297, 111507. [Google Scholar] [CrossRef]
- Ferrari, E.; Palma, C.; Vesentini, S.; Occhetta, P.; Rasponi, M. Integrating Biosensors in Organs-on-Chip Devices: A Perspective on Current Strategies to Monitor Microphysiological Systems. Biosensors 2020, 10, 110. [Google Scholar] [CrossRef]
- Kieninger, J.; Weltin, A.; Flamm, H.; Urban, G.A. Microsensor systems for cell metabolism—From 2D culture to organ-on-chip. Lab Chip 2018, 18, 1274–1291. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Cruz, J.; Nair, S.; Manjarrez-Hernandez, A.; Gavilanes-Parra, S.; Ascanio, G.; Escobedo, C. Cost-effective flow-through nanohole array-based biosensing platform for the label-free detection of uropathogenic E. coli in real time. Biosens. Bioelectron. 2018, 106, 105–110. [Google Scholar] [CrossRef]
- Rodoplu, D.; Chang, C.-S.; Kao, C.-Y.; Hsu, C.-H. A simple magnetic-assisted microfluidic method for rapid detection and phenotypic characterization of ultralow concentrations of bacteria. Talanta 2021, 230, 122291. [Google Scholar] [CrossRef]
- Guo, S.; Stevens Corey, A.; Vance Tyler, D.R.; Olijve Luuk, L.C.; Graham Laurie, A.; Campbell Robert, L.; Yazdi Saeed, R.; Escobedo, C.; Bar-Dolev, M.; Yashunsky, V.; et al. Structure of a 1.5-MDa adhesin that binds its Antarctic bacterium to diatoms and ice. Sci. Adv. 2017, 3, e1701440. [Google Scholar] [CrossRef] [Green Version]
- Zirath, H.; Rothbauer, M.; Spitz, S.; Bachmann, B.; Jordan, C.; Müller, B.; Ehgartner, J.; Priglinger, E.; Mühleder, S.; Redl, H.; et al. Every Breath You Take: Non-invasive Real-Time Oxygen Biosensing in Two- and Three-Dimensional Microfluidic Cell Models. Front. Physiol. 2018, 9, 815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics Integrated Biosensors: A Leading Technology towards Lab-on-a-Chip and Sensing Applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Soler, M.; Szydzik, C.; Khoshmanesh, K.; Schmidt, J.; Coukos, G.; Mitchell, A.; Altug, H. Single Cell Analysis: Label-Free Optofluidic Nanobiosensor Enables Real-Time Analysis of Single-Cell Cytokine Secretion. Small 2018, 14, 1870119. [Google Scholar] [CrossRef]
- Errico, V.; Ninno, A.D.; Bertani, F.R.; Businaro, L.; Bisegna, P.; Caselli, F. Mitigating positional dependence in coplanar electrode Coulter-type microfluidic devices. Sens. Actuators B Chem. 2017, 247, 580–586. [Google Scholar] [CrossRef]
- Li, X.; Soler, M.; Szydzik, C.; Khoshmanesh, K.; Schmidt, J.; Coukos, G.; Mitchell, A.; Altug, H. Label-Free Optofluidic Nanobiosensor Enables Real-Time Analysis of Single-Cell Cytokine Secretion. Small 2018, 14, 1800698. [Google Scholar] [CrossRef]
- Podwin, A.; Lizanets, D.; Przystupski, D.; Kubicki, W.; Śniadek, P.; Kulbacka, J.; Wymysłowski, A.; Walczak, R.; Dziuban, J.A. Lab-on-Chip Platform for Culturing and Dynamic Evaluation of Cells Development. Micromachines 2020, 11, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, Y.R.F.; Bürgel, S.C.; Misun, P.M.; Hierlemann, A.; Frey, O. Electrical Impedance Spectroscopy for Microtissue Spheroid Analysis in Hanging-Drop Networks. ACS Sens. 2016, 1, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Rezaei Kolahchi, A.; Khadem Mohtaram, N.; Pezeshgi Modarres, H.; Mohammadi, M.H.; Geraili, A.; Jafari, P.; Akbari, M.; Sanati-Nezhad, A. Microfluidic-Based Multi-Organ Platforms for Drug Discovery. Micromachines 2016, 7, 162. [Google Scholar] [CrossRef]
- Lee, S.H.; Hong, S.; Song, J.; Cho, B.; Han, E.J.; Kondapavulur, S.; Kim, D.; Lee, L.P. Microphysiological Analysis Platform of Pancreatic Islet β-Cell Spheroids. Adv. Healthc. Mater. 2018, 7, 1701111. [Google Scholar] [CrossRef]
- Järvinen, P.; Bonabi, A.; Jokinen, V.; Sikanen, T. Simultaneous Culturing of Cell Monolayers and Spheroids on a Single Microfluidic Device for Bridging the Gap between 2D and 3D Cell Assays in Drug Research. Adv. Funct. Mater. 2020, 30, 2000479. [Google Scholar] [CrossRef]
- Zbinden, A.; Marzi, J.; Schlünder, K.; Probst, C.; Urbanczyk, M.; Black, S.; Brauchle, E.M.; Layland, S.L.; Kraushaar, U.; Duffy, G.; et al. Non-invasive marker-independent high content analysis of a microphysiological human pancreas-on-a-chip model. Matrix Biol. 2020, 85, 205–220. [Google Scholar] [CrossRef]
- Saleheen, A.; Acharyya, D.; Prosser, R.A.; Baker, C.A. A microfluidic bubble perfusion device for brain slice culture. Anal. Methods 2021, 13, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Meng, T.; Yang, J.; Hu, N.; Zhao, H.; Tian, T. Three-dimensional in vitro tissue culture models of brain organoids. Exp. Neurol. 2021, 339, 113619. [Google Scholar] [CrossRef]
- Samiei, E.; Seyfoori, A.; Toyota, B.; Ghavami, S.; Akbari, M. Investigating Programmed Cell Death and Tumor Invasion in a Three-Dimensional (3D) Microfluidic Model of Glioblastoma. Int. J. Mol. Sci. 2020, 21, 3162. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Deng, P.; Tao, T.; Liu, H.; Wu, S.; Chen, W.; Qin, J. Modeling Human Nonalcoholic Fatty Liver Disease (NAFLD) with an Organoids-on-a-Chip System. ACS Biomater. Sci. Eng. 2020, 6, 5734–5743. [Google Scholar] [CrossRef]
- Tronolone, J.J.; Jain, A. Engineering New Microvascular Networks On-Chip: Ingredients, Assembly, and Best Practices. Adv. Funct. Mater. 2021, 31, 2007199. [Google Scholar] [CrossRef] [PubMed]
- Marrero, D.; Pujol-Vila, F.; Vera, D.; Gabriel, G.; Illa, X.; Elizalde-Torrent, A.; Alvarez, M.; Villa, R. Gut-on-a-chip: Mimicking and monitoring the human intestine. Biosens. Bioelectron. 2021, 181, 113156. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, B.; Sun, Y.; Dai, B.; Fu, Y.; Zhang, Y.; Wang, Y.; Yang, Z.; Sun, Z.; Zhuang, S.; et al. An Oxygen-Concentration-Controllable Multiorgan Microfluidic Platform for Studying Hypoxia-Induced Lung Cancer-Liver Metastasis and Screening Drugs. ACS Sens. 2021, 6, 823–832. [Google Scholar] [CrossRef]
- Valente, K.P.; Thind, S.S.; Akbari, M.; Suleman, A.; Brolo, A.G. Collagen Type I-Gelatin Methacryloyl Composites: Mimicking the Tumor Microenvironment. ACS Biomater. Sci. Eng. 2019, 5, 2887–2898. [Google Scholar] [CrossRef]
- Marconi, A.; Quadri, M.; Saltari, A.; Pincelli, C. Progress in melanoma modelling in vitro. Exp. Dermatol. 2018, 27, 578–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fetah, K.L.; DiPardo, B.J.; Kongadzem, E.-M.; Tomlinson, J.S.; Elzagheid, A.; Elmusrati, M.; Khademhosseini, A.; Ashammakhi, N. Cancer Modeling-on-a-Chip with Future Artificial Intelligence Integration. Small 2019, 15, 1901985. [Google Scholar] [CrossRef]
- Clarke, G.A.; Hartse, B.X.; Niaraki Asli, A.E.; Taghavimehr, M.; Hashemi, N.; Abbasi Shirsavar, M.; Montazami, R.; Alimoradi, N.; Nasirian, V.; Ouedraogo, L.J.; et al. Advancement of Sensor Integrated Organ-on-Chip Devices. Sensors 2021, 21, 1367. [Google Scholar] [CrossRef]
- Wang, X.-D.; Wolfbeis, O.S. Optical methods for sensing and imaging oxygen: Materials, spectroscopies and applications. Chem. Soc. Rev. 2014, 43, 3666–3761. [Google Scholar] [CrossRef] [Green Version]
- Grist, S.M.; Chrostowski, L.; Cheung, K.C. Optical Oxygen Sensors for Applications in Microfluidic Cell Culture. Sensors 2010, 10, 9286–9316. [Google Scholar] [CrossRef] [Green Version]
- Grate, J.W.; Liu, B.; Kelly, R.T.; Anheier, N.C.; Schmidt, T.M. Microfluidic Sensors with Impregnated Fluorophores for Simultaneous Imaging of Spatial Structure and Chemical Oxygen Gradients. ACS Sens. 2019, 4, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Safitri, A.R.; Danoy, M.; Maekawa, T.; Kinoshita, H.; Shinohara, M.; Sakai, Y.; Fujii, T.; Leclerc, E. Investigation of the hepatic respiration and liver zonation on rat hepatocytes using an integrated oxygen biosensor in a microscale device. Biotechnol. Prog. 2019, 35, e2854. [Google Scholar] [CrossRef]
- Müller, B.; Sulzer, P.; Walch, M.; Zirath, H.; Buryška, T.; Rothbauer, M.; Ertl, P.; Mayr, T. Measurement of respiration and acidification rates of mammalian cells in thermoplastic microfluidic devices. Sens. Actuators B Chem. 2021, 334, 129664. [Google Scholar] [CrossRef]
- Orcheston-Findlay, L.; Hashemi, A.; Garrill, A.; Nock, V. A microfluidic gradient generator to simulate the oxygen microenvironment in cancer cell culture. Microelectron. Eng. 2018, 195, 107–113. [Google Scholar] [CrossRef]
- Ando, Y.; Ta, H.P.; Yen, D.P.; Lee, S.-S.; Raola, S.; Shen, K. A Microdevice Platform Recapitulating Hypoxic Tumor Microenvironments. Sci. Rep. 2017, 7, 15233. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-H.; Huang, K.-S.; Liou, Y.-M. Simultaneous monitoring of oxygen consumption and acidification rates of a single zebrafish embryo during embryonic development within a microfluidic device. Microfluid. Nanofluid. 2016, 21, 3. [Google Scholar] [CrossRef]
- Ehrlich, A.; Tsytkin-Kirschenzweig, S.; Ioannidis, K.; Ayyash, M.; Riu, A.; Note, R.; Ouedraogo, G.; Vanfleteren, J.; Cohen, M.; Nahmias, Y. Microphysiological flux balance platform unravels the dynamics of drug induced steatosis. Lab Chip 2018, 18, 2510–2522. [Google Scholar] [CrossRef]
- Perottoni, S.; Neto, N.G.B.; Di Nitto, C.; Dmitriev, R.I.; Raimondi, M.T.; Monaghan, M.G. Intracellular label-free detection of mesenchymal stem cell metabolism within a perivascular niche-on-a-chip. Lab Chip 2021, 21, 1395–1408. [Google Scholar] [CrossRef]
- Horka, M.; Sun, S.; Ruszczak, A.; Garstecki, P.; Mayr, T. Lifetime of Phosphorescence from Nanoparticles Yields Accurate Measurement of Concentration of Oxygen in Microdroplets, Allowing One To Monitor the Metabolism of Bacteria. Anal. Chem. 2016, 88, 12006–12012. [Google Scholar] [CrossRef] [PubMed]
- Ehgartner, J.; Strobl, M.; Bolivar, J.M.; Rabl, D.; Rothbauer, M.; Ertl, P.; Borisov, S.M.; Mayr, T. Simultaneous Determination of Oxygen and pH Inside Microfluidic Devices Using Core-Shell Nanosensors. Anal. Chem. 2016, 88, 9796–9804. [Google Scholar] [CrossRef] [PubMed]
- Lasave, L.C.; Borisov, S.M.; Ehgartner, J.; Mayr, T. Quick and simple integration of optical oxygen sensors into glass-based microfluidic devices. RSC Adv. 2015, 5, 70808–70816. [Google Scholar] [CrossRef]
- Qiu, W.; Nagl, S. Automated Miniaturized Digital Microfluidic Antimicrobial Susceptibility Test Using a Chip-Integrated Optical Oxygen Sensor. ACS Sens. 2021, 6, 1147–1156. [Google Scholar] [CrossRef]
- Gitlin, L.; Hoera, C.; Meier, R.J.; Nagl, S.; Belder, D. Micro flow reactor chips with integrated luminescent chemosensors for spatially resolved on-line chemical reaction monitoring. Lab Chip 2013, 13, 4134–4141. [Google Scholar] [CrossRef]
- Grist, S.M.; Oyunerdene, N.; Flueckiger, J.; Kim, J.; Wong, P.C.; Chrostowski, L.; Cheung, K.C. Fabrication and laser patterning of polystyrene optical oxygen sensor films for lab-on-a-chip applications. Analyst 2014, 139, 5718–5727. [Google Scholar] [CrossRef] [PubMed]
- Shaegh, S.A.M.; Ferrari, F.D.; Zhang, Y.S.; Nabavinia, M.; Mohammad, N.B.; Ryan, J.; Pourmand, A.; Laukaitis, E.; Sadeghian, R.B.; Nadhman, A.; et al. A microfluidic optical platform for real-time monitoring of pH and oxygen in microfluidic bioreactors and organ-on-chip devices. Biomicrofluidics 2016, 10, 044111. [Google Scholar] [CrossRef]
- Yoon, H.K.; Lou, X.; Chen, Y.-C.; Koo Lee, Y.-E.; Yoon, E.; Kopelman, R. Nanophotosensitizers Engineered to Generate a Tunable Mix of Reactive Oxygen Species, for Optimizing Photodynamic Therapy, Using a Microfluidic Device. Chem. Mater. 2014, 26, 1592–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeiffer, S.A.; Borisov, S.M.; Nagl, S. In-line monitoring of pH and oxygen during enzymatic reactions in off-the-shelf all-glass microreactors using integrated luminescent microsensors. Microchim. Acta 2017, 184, 621–626. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, X.; Su, F.; Tian, Y.; Ashili, S.; Holl, M.R.; Meldrum, D.R. Micro-patterning and characterization of PHEMA-co-PAM-based optical chemical sensors for lab-on-a-chip applications. Sens. Actuators B Chem. 2012, 173, 817–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelgawad, M.; Freire, S.L.S.; Yang, H.; Wheeler, A.R. All-terrain droplet actuation. Lab Chip 2008, 8, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Lehner, P.; Staudinger, C.; Borisov, S.M.; Regensburger, J.; Klimant, I. Intrinsic Artefacts in Optical Oxygen Sensors—How Reliable are our Measurements? Chem.—Eur. J. 2015, 21, 3978–3986. [Google Scholar] [CrossRef] [PubMed]
- Ungerböck, B.; Charwat, V.; Ertl, P.; Mayr, T. Microfluidic oxygen imaging using integrated optical sensor layers and a color camera. Lab Chip 2013, 13, 1593–1601. [Google Scholar] [CrossRef] [Green Version]
- Bunge, F.; van den Driesche, S.; Waespy, M.; Radtke, A.; Belge, G.; Kelm, S.; Waite, A.M.; Mirastschijski, U.; Vellekoop, M.J. Microfluidic oxygen sensor system as a tool to monitor the metabolism of mammalian cells. Sens. Actuators B Chem. 2019, 289, 24–31. [Google Scholar] [CrossRef]
- Rivera, K.R.; Pozdin, V.A.; Young, A.T.; Erb, P.D.; Wisniewski, N.A.; Magness, S.T.; Daniele, M. Integrated phosphorescence-based photonic biosensor (iPOB) for monitoring oxygen levels in 3D cell culture systems. Biosens. Bioelectron. 2019, 123, 131–140. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, L.; Luo, T.; Hong, L.; Peng, X.; Austin, R.H.; Qu, J. A platinum-porphine/poly(perfluoroether) film oxygen tension sensor for noninvasive local monitoring of cellular oxygen metabolism using phosphorescence lifetime imaging. Sens. Actuators B Chem. 2018, 269, 88–95. [Google Scholar] [CrossRef]
- Mazetyte-Stasinskiene, R.; Köhler, J.M. Sensor Micro and Nanoparticles for Microfluidic Application. Appl. Sci. 2020, 10, 8353. [Google Scholar] [CrossRef]
- Li, B.; Zhao, Y.; Zhang, Y.; Zhang, A.; Li, X.; Gu, J.; Xi, S.; Zhou, G. Functionalized Micro Structured Optical Fibers and Devices for Sensing Applications: A Review. J. Lightw. Technol. 2021, 39, 3812–3823. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, Y.; Luo, S.; Liu, Y.; Yuan, L. Microfluidic in-fiber oxygen sensor derivates from a capillary optical fiber with a ring-shaped waveguide. Sens. Actuators B Chem. 2013, 182, 571–575. [Google Scholar] [CrossRef]
- Jiang, K.; Thomas, P.C.; Forry, S.P.; DeVoe, D.L.; Raghavan, S.R. Microfluidic synthesis of monodisperse PDMS microbeads as discrete oxygen sensors. Soft Matter 2012, 8, 923–926. [Google Scholar] [CrossRef]
- Bavli, D.; Prill, S.; Ezra, E.; Levy, G.; Cohen, M.; Vinken, M.; Vanfleteren, J.; Jaeger, M.; Nahmias, Y. Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction. Proc. Natl. Acad. Sci. USA 2016, 113, E2231–E2240. [Google Scholar] [CrossRef] [Green Version]
- Tanumihardja, E.; Olthuis, W.; Van den Berg, A. Ruthenium Oxide Nanorods as Potentiometric pH Sensor for Organs-On-Chip Purposes. Sensors 2018, 18, 2901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-H.; Peng, H.-S.; Chang, Z.; Hou, L.-L.; You, F.-T.; Teng, F.; Song, H.-W.; Dong, B. Synthesis of ratiometric fluorescent nanoparticles for sensing oxygen. Microchim. Acta 2012, 178, 147–152. [Google Scholar] [CrossRef]
- Cao, J.; Nagl, S.; Kothe, E.; Köhler, J.M. Oxygen sensor nanoparticles for monitoring bacterial growth and characterization of dose-response functions in microfluidic screenings. Microchim. Acta 2015, 182, 385–394. [Google Scholar] [CrossRef]
- Li, Y.-C.E.; Lee, I.-C. The Current Trends of Biosensors in Tissue Engineering. Biosensors 2020, 10, 88. [Google Scholar] [CrossRef]
- Yoon, J.; Shin, M.; Lee, T.; Choi, J.-W. Highly Sensitive Biosensors Based on Biomolecules and Functional Nanomaterials Depending on the Types of Nanomaterials: A Perspective Review. Materials 2020, 13, 299. [Google Scholar] [CrossRef] [Green Version]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Ungerböck, B.; Fellinger, S.; Sulzer, P.; Abel, T.; Mayr, T. Magnetic optical sensor particles: A flexible analytical tool for microfluidic devices. Analyst 2014, 139, 2551–2559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F.; Baker, D.; Skommer, J.; Sewell, M.; Wlodkowic, D. Real-time 2D visualization of metabolic activities in zebrafish embryos using a microfluidic technology. Cytom. Part A 2015, 87, 446–450. [Google Scholar] [CrossRef]
- Ehgartner, J.; Sulzer, P.; Burger, T.; Kasjanow, A.; Bouwes, D.; Krühne, U.; Klimant, I.; Mayr, T. Online analysis of oxygen inside silicon-glass microreactors with integrated optical sensors. Sens. Actuators B Chem. 2016, 228, 748–757. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Koivisto, O.; Liu, C.; Zhou, J.; Miihkinen, M.; Jacquemet, G.; Wang, D.; Rosenholm, J.M.; Shu, Y.; Zhang, H. Effective Delivery of the CRISPR/Cas9 System Enabled by Functionalized Mesoporous Silica Nanoparticles for GFP-Tagged Paxillin Knock-In. Adv. Ther. 2021, 4. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Zhu, J.; Hu, X.; Yang, Y. A Phosphorescence Quenching-Based Intelligent Dissolved Oxygen Sensor on an Optofluidic Platform. Micromachines 2021, 12, 281. [Google Scholar] [CrossRef]
- Park, J.; Nam, H.; Ahn, S.Y.; Pak, Y.K.; Pak, J.J. A reservoir-type oxygen sensor with 2 × 3 array for measuring cellular respiration levels. Sens. Actuators B Chem. 2013, 176, 913–920. [Google Scholar] [CrossRef]
- Mestres, P.; Morguet, A. The Bionas technology for anticancer drug screening. Expert Opin. Drug Discov. 2009, 4, 785–797. [Google Scholar] [CrossRef]
- Wu, C.-C.; Yasukawa, T.; Shiku, H.; Matsue, T. Fabrication of miniature Clark oxygen sensor integrated with microstructure. Sens. Actuators B Chem. 2005, 110, 342–349. [Google Scholar] [CrossRef]
- Luo, J.; Dziubla, T.; Eitel, R. A low temperature co-fired ceramic based microfluidic Clark-type oxygen sensor for real-time oxygen sensing. Sens. Actuators B Chem. 2017, 240, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Liebisch, F.; Weltin, A.; Marzioch, J.; Urban, G.A.; Kieninger, J. Zero-consumption Clark-type microsensor for oxygen monitoring in cell culture and organ-on-chip systems. Sens. Actuators B Chem. 2020, 322, 128652. [Google Scholar] [CrossRef]
- Moya, A.; Illa, X.; Gimenez, I.; Lazo-Fernandez, Y.; Villa, R.; Errachid, A.; Gabriel, G. Miniaturized multiparametric flexible platform for the simultaneous monitoring of ionic: Application in real urine. Sens. Actuators B Chem. 2018, 255, 2861–2870. [Google Scholar] [CrossRef] [Green Version]
- Bonk, S.M.; Stubbe, M.; Buehler, S.M.; Tautorat, C.; Baumann, W.; Klinkenberg, E.D.; Gimsa, J. Design and Characterization of a Sensorized Microfluidic Cell-Culture System with Electro-Thermal Micro-Pumps and Sensors for Cell Adhesion, Oxygen, and pH on a Glass Chip. Biosensors 2015, 5, 513–536. [Google Scholar] [CrossRef] [Green Version]
- Weltin, A.; Slotwinski, K.; Kieninger, J.; Moser, I.; Jobst, G.; Wego, M.; Ehret, R.; Urban, G.A. Cell culture monitoring for drug screening and cancer research: A transparent, microfluidic, multi-sensor microsystem. Lab Chip 2014, 14, 138–146. [Google Scholar] [CrossRef]
- Pereira Rodrigues, N.; Sakai, Y.; Fujii, T. Cell-based microfluidic biochip for the electrochemical real-time monitoring of glucose and oxygen. Sens. Actuators B Chem. 2008, 132, 608–613. [Google Scholar] [CrossRef]
- Li, C.; Wu, P.-M.; Jung, W.; Ahn, C.H.; Shutter, L.A.; Narayan, R.K. A novel lab-on-a-tube for multimodality neuromonitoring of patients with traumatic brain injury (TBI). Lab Chip 2009, 9, 1988–1990. [Google Scholar] [CrossRef] [PubMed]
- Sekli Belaïdi, F.; Salvagnac, L.; Assié Souleille, S.; Blatché, M.C.; Bedel-Pereira, E.; Séguy, I.; Temple-Boyer, P.; Launay, J. Accurate physiological monitoring using lab-on-a-chip platform for aquatic micro-organisms growth and optimized culture. Sens. Actuators B Chem. 2020, 321, 128492. [Google Scholar] [CrossRef]
- Van Rossem, F.; Bomer, J.G.; de Boer, H.L.; Abbas, Y.; de Weerd, E.; van den Berg, A.; Le Gac, S. Sensing oxygen at the millisecond time-scale using an ultra-microelectrode array (UMEA). Sens. Actuators B Chem. 2017, 238, 1008–1016. [Google Scholar] [CrossRef]
- Vonau, W.; Gerlach, F.; Herrmann, S. Conception of a new technique in cell cultivation using a lab-on-chip aided miniaturised device with calibratable electrochemical sensors. Microchim. Acta 2010, 171, 451–456. [Google Scholar] [CrossRef]
- Moya, A.; Ortega-Ribera, M.; Guimerà, X.; Sowade, E.; Zea, M.; Illa, X.; Ramon, E.; Villa, R.; Gracia-Sancho, J.; Gabriel, G. Online oxygen monitoring using integrated inkjet-printed sensors in a liver-on-a-chip system. Lab Chip 2018, 18, 2023–2035. [Google Scholar] [CrossRef] [Green Version]
- Weltin, A.; Hammer, S.; Noor, F.; Kaminski, Y.; Kieninger, J.; Urban, G.A. Accessing 3D microtissue metabolism: Lactate and oxygen monitoring in hepatocyte spheroids. Biosens. Bioelectron. 2017, 87, 941–948. [Google Scholar] [CrossRef]
- Alexander, F., Jr.; Eggert, S.; Wiest, J. A novel lab-on-a-chip platform for spheroid metabolism monitoring. Cytotechnology 2018, 70, 375–386. [Google Scholar] [CrossRef]
- Yang, Z.; Suzuki, H.; Sasaki, S.; Karube, I. Disposable sensor for biochemical oxygen demand. Appl. Microbiol. Biotechnol. 1996, 46, 10–14. [Google Scholar] [CrossRef]
- Mehta, G.; Mehta, K.; Sud, D.; Song, J.W.; Bersano-Begey, T.; Futai, N.; Heo, Y.S.; Mycek, M.A.; Linderman, J.J.; Takayama, S. Quantitative measurement and control of oxygen levels in microfluidic poly(dimethylsiloxane) bioreactors during cell culture. Biomed. Microdevices 2007, 9, 123–134. [Google Scholar] [CrossRef]
- Suzuki, H.; Sugama, A.; Kojima, N. Micromachined Clark oxygen electrode. Sens. Actuators B Chem. 1993, 10, 91–98. [Google Scholar] [CrossRef]
- Gongora-Rubio, M.R.; Espinoza-Vallejos, P.; Sola-Laguna, L.; Santiago-Avilés, J.J. Overview of low temperature co-fired ceramics tape technology for meso-system technology (MsST). Sens. Actuators A Phys. 2001, 89, 222–241. [Google Scholar] [CrossRef]
- Jurków, D.; Maeder, T.; Dąbrowski, A.; Zarnik, M.S.; Belavič, D.; Bartsch, H.; Müller, J. Overview on low temperature co-fired ceramic sensors. Sens. Actuators A Phys. 2015, 233, 125–146. [Google Scholar] [CrossRef]
- Brandenburg, A.; Wappler, E.; Kita, J.; Moos, R. Miniaturized ceramic DSC device with strain gauge-based mass detection—First steps to realize a fully integrated DSC/TGA device. Sens. Actuators A Phys. 2016, 241, 145–151. [Google Scholar] [CrossRef]
- Ross, J.J.W. Method and Apparatus for Electrolytically Determining a Species in a Fluid. U.S. Patent 3,260,656, 12 July 1966. [Google Scholar]
- Bonk, S.M.; Oldorf, P.; Peters, R.; Baumann, W.; Gimsa, J. Fast Prototyping of Sensorized Cell Culture Chips and Microfluidic Systems with Ultrashort Laser Pulses. Micromachines 2015, 6, 364–374. [Google Scholar] [CrossRef] [Green Version]
- Sticker, D.; Rothbauer, M.; Ehgartner, J.; Steininger, C.; Liske, O.; Liska, R.; Neuhaus, W.; Mayr, T.; Haraldsson, T.; Kutter, J.P.; et al. Oxygen Management at the Microscale: A Functional Biochip Material with Long-Lasting and Tunable Oxygen Scavenging Properties for Cell Culture Applications. ACS Appl. Mater. Interfaces 2019, 11, 9730–9739. [Google Scholar] [CrossRef]
- Estrada-Leypon, O.; Moya, A.; Guimera, A.; Gabriel, G.; Agut, M.; Sanchez, B.; Borros, S. Simultaneous monitoring of Staphylococcus aureus growth in a multi-parametric microfluidic platform using microscopy and impedance spectroscopy. Bioelectrochemistry 2015, 105, 56–64. [Google Scholar] [CrossRef]
- Mitrovski, S.M.; Nuzzo, R.G. An electrochemically driven poly(dimethylsiloxane) microfluidic actuator: Oxygen sensing and programmable flows and pH gradients. Lab Chip 2005, 5, 634–645. [Google Scholar] [CrossRef]
- Furlani, D.; Li, W.; Pittermann, E.; Klopsch, C.; Wang, L.; Knopp, A.; Jungebluth, P.; Thedinga, E.; Havenstein, C.; Westien, I.; et al. A Transformed Cell Population Derived from Cultured Mesenchymal Stem Cells has no Functional Effect after Transplantation into the Injured Heart. Cell Transplant. 2009, 18, 319–332. [Google Scholar] [CrossRef] [Green Version]
- Thedinga, E.; Kob, A.; Holst, H.; Keuer, A.; Drechsler, S.; Niendorf, R.; Baumann, W.; Freund, I.; Lehmann, M.; Ehret, R. Online monitoring of cell metabolism for studying pharmacodynamic effects. Toxicol. Appl. Pharmacol. 2007, 220, 33–44. [Google Scholar] [CrossRef]
- Thedinga, E.; Ullrich, A.; Drechsler, S.; Niendorf, R.; Kob, A.; Runge, D.; Keuer, A.; Freund, I.; Lehmann, M.; Ehret, R. In vitro system for the prediction of hepatotoxic effects in primary hepatocytes. Altex 2007, 24, 22–34. [Google Scholar] [CrossRef]
- Yao, J.; Guan, Y.; Park, Y.; Choi, Y.E.; Kim, H.S.; Park, J. Optimization of PTFE Coating on PDMS Surfaces for Inhibition of Hydrophobic Molecule Absorption for Increased Optical Detection Sensitivity. Sensors 2021, 21, 1754. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.; Wang, Y.; Wang, J.C.; Tu, Q.; Liu, R.; Wang, J. Construction of oxygen and chemical concentration gradients in a single microfluidic device for studying tumor cell-drug interactions in a dynamic hypoxia microenvironment. Lab Chip 2013, 13, 695–705. [Google Scholar] [CrossRef]
- Lo, J.F.; Sinkala, E.; Eddington, D.T. Oxygen gradients for open well cellular cultures via microfluidic substrates. Lab Chip 2010, 10, 2394–2401. [Google Scholar] [CrossRef] [Green Version]
- Moya, A.; Sowade, E.; del Campo, F.J.; Mitra, K.Y.; Ramon, E.; Villa, R.; Baumann, R.R.; Gabriel, G. All-inkjet-printed dissolved oxygen sensors on flexible plastic substrates. Org. Electron. 2016, 39, 168–176. [Google Scholar] [CrossRef]
- Eklund, S.E.; Taylor, D.; Kozlov, E.; Prokop, A.; Cliffel, D.E. A Microphysiometer for Simultaneous Measurement of Changes in Extracellular Glucose, Lactate, Oxygen, and Acidification Rate. Anal. Chem. 2004, 76, 519–527. [Google Scholar] [CrossRef]
- McKenzie, J.R.; Cognata, A.C.; Davis, A.N.; Wikswo, J.P.; Cliffel, D.E. Real-Time Monitoring of Cellular Bioenergetics with a Multianalyte Screen-Printed Electrode. Anal. Chem. 2015, 87, 7857–7864. [Google Scholar] [CrossRef] [Green Version]
- Hafner, F. Cytosensor Microphysiometer: Technology and recent applications. Biosens. Bioelectron. 2000, 15, 149–158. [Google Scholar] [CrossRef]
- Lehmann, M.; Baumann, W.; Brischwein, M.; Ehret, R.; Kraus, M.; Schwinde, A.; Bitzenhofer, M.; Freund, I.; Wolf, B. Non-invasive measurement of cell membrane associated proton gradients by ion-sensitive field effect transistor arrays for microphysiological and bioelectronical applications. Biosens. Bioelectron. 2000, 15, 117–124. [Google Scholar] [CrossRef]
- Buehler, S.M.; Stubbe, M.; Gimsa, U.; Baumann, W.; Gimsa, J. A decrease of intracellular ATP is compensated by increased respiration and acidification at sub-lethal parathion concentrations in murine embryonic neuronal cells: Measurements in metabolic cell-culture chips. Toxicol. Lett. 2011, 207, 182–190. [Google Scholar] [CrossRef]
- Koester, P.J.; Buehler, S.M.; Stubbe, M.; Tautorat, C.; Niendorf, M.; Baumann, W.; Gimsa, J. Modular glass chip system measuring the electric activity and adhesion of neuronal cells—Application and drug testing with sodium valproic acid. Lab Chip 2010, 10, 1579–1586. [Google Scholar] [CrossRef]
- Torrents, A.; Mas, J.; Muñoz, F.X.; del Campo, F.J. Design of a microfluidic respirometer for semi-continuous amperometric short time biochemical oxygen demand (BODst) analysis. Biochem. Eng. J. 2012, 66, 27–37. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).