Substituent Effects Impact Surface Charge and Aggregation of Thiophenol-Labeled Gold Nanoparticles for SERS Biosensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. AuNP RRM Coating
2.2. AuNP Dynamic Light Scattering Analysis (Zeta-Potential)
2.3. UV-Visible Spectroscopy Analysis
2.4. Nanoparticle Tracking Analysis
2.5. Proof-of-Concept Multiplexed SERS Assay
3. Results
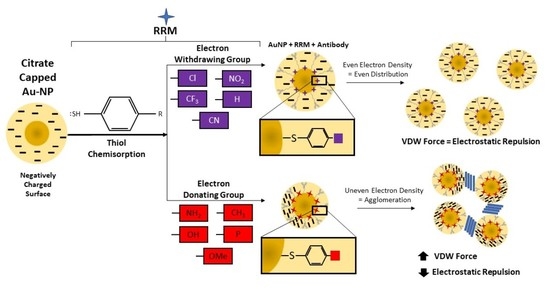
3.1. RRM Effect on AuNP Surface Charge
3.2. RRM Impact on AuNP Aggregation
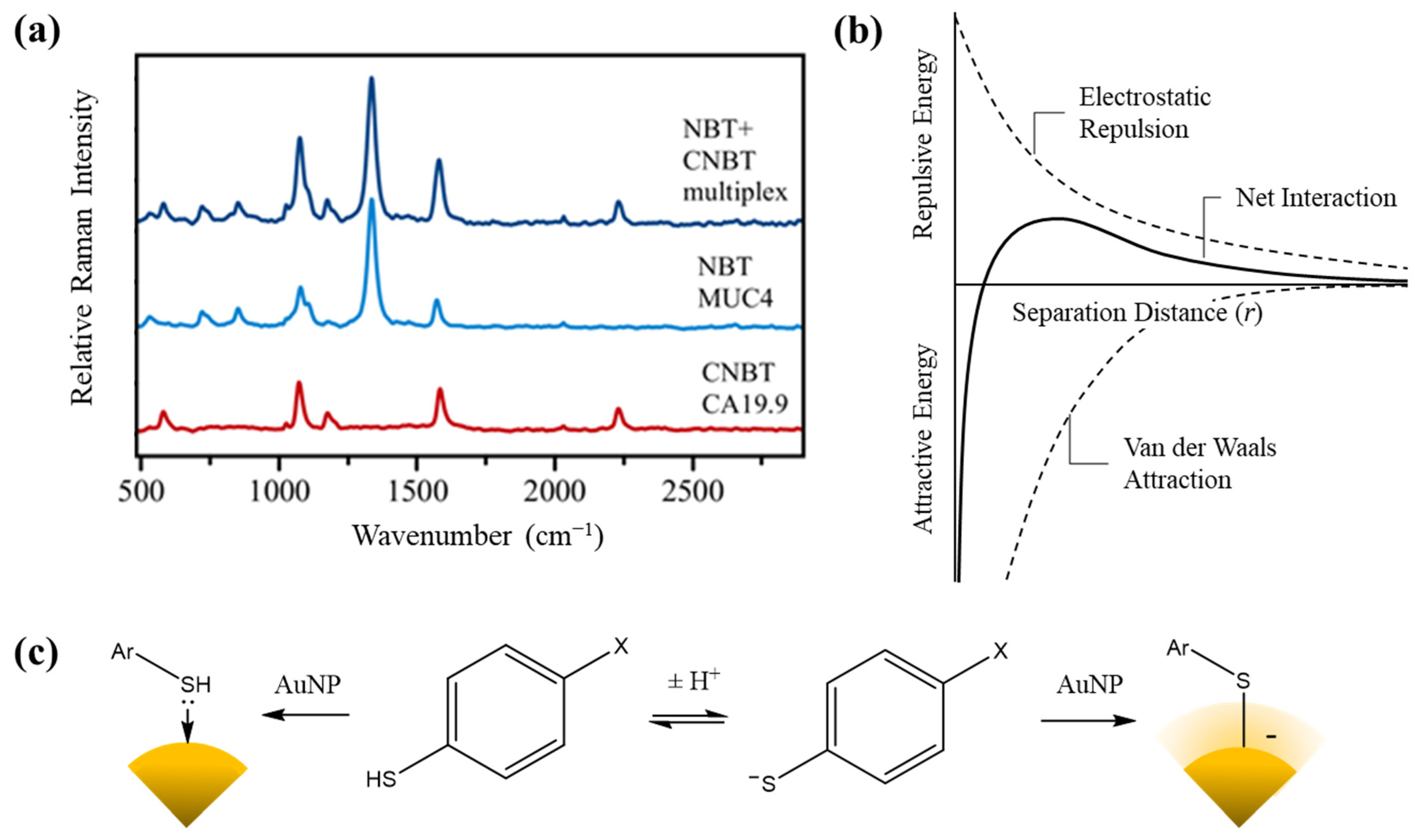
3.3. Proof-of-Concept SERS Multiplexing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pence, I.; Mahadevan-Jansen, A. Clinical instrumentation and applications of Raman spectroscopy. Chem. Soc. Rev. 2016, 45, 1958–1979. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Hutter, T.; Steiner, U.; Mahajan, S. Single molecule SERS and detection of biomolecules with a single gold nanoparticle on a mirror junction. Analyst 2013, 138, 4574–4578. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Hu, Z.; Yu, G.; Yang, D.; Zhao, J. Label and label-free based surface-enhanced Raman scattering for pathogen bacteria detection: A review. Biosens. Bioelectron. 2017, 94, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.; Shao, C.; Huang, Q. Label-free SERS diagnostics of radiation-induced injury via detecting the biomarker Raman signal in the serum and urine bio-samples based on Au-NPs array substrates. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 223, 117282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, L.; Fang, Y.; Zheng, D.; Lin, T.; Wang, H. Label-free exosomal detection and classification in rapid discriminating different cancer types based on specific Raman phenotypes and multivariate statistical analysis. Molecules 2019, 24, 2947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmicheal, J.; Hayashi, C.; Huang, X.; Liu, L.; Lu, Y.; Krasnoslobodtsev, A.; Lushnikov, A.; Kshirsagar, P.G.; Patel, A.; Jain, M.; et al. Label-free characterization of exosome via surface enhanced Raman spectroscopy for the early detection of pancreatic cancer. Nanomedicine 2019, 16, 88–96. [Google Scholar] [CrossRef]
- Wang, Z.; Zong, S.; Wu, L.; Zhu, D.; Cui, Y. SERS-activated platforms for immunoassay: Probes, encoding methods, and applications. Chem. Rev. 2017, 117, 7910–7963. [Google Scholar] [CrossRef]
- Smolsky, J.; Kaur, S.; Hayashi, C.; Batra, S.K.; Krasnoslobodtsev, A.V. Surface-enhanced Raman scattering-based immunoassay technologies for detection of disease biomarkers. Biosensors 2017, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Lipert, R.J.; Jain, M.; Kaur, S.; Chakraboty, S.; Torres, M.P.; Batra, S.K.; Brand, R.E.; Porter, M.D. Detection of the potential pancreatic cancer marker MUC4 in serum using surface-enhanced Raman scattering. Anal. Chem. 2011, 83, 2554–2561. [Google Scholar] [CrossRef] [Green Version]
- Samanta, A.; Das, R.K.; Park, S.J.; Maiti, K.K.; Chang, Y.T. Multiplexing SERS nanotags for the imaging of differentiated mouse embryonic stem cells (mESC) and detection of teratoma in vivo. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 114–124. [Google Scholar] [PubMed]
- Li, L.; Liu, C.; Cao, X.; Tan, L.; Lu, W. Multiplexing determination of cancer-associated biomarkers by surface-enhanced Raman scattering using ordered gold nanohoneycomb arrays. Bioanalysis 2017, 9, 1561–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yan, B.; Chen, L. SERS tags: Novel optical nanoprobes for bioanalysis. Chem. Rev. 2013, 113, 1391–1428. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wang, J.; Hutchison, J.A.; Ma, L.; Zhang, N.; Guo, H.; Hu, Z.; Li, M.; Zhao, Y. Ultrasensitive, multiplex Raman frequency shift immunoassay of liver cancer biomarkers in physiological media. ACS Nano 2016, 10, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tseng, C.-H.; Vickers, T.J.; Mann, C.K.; Schlenoff, J.B. Near-infrared surface-enhanced Raman spectroscopy of chemisorbed compounds on gold colloids. Surf. Sci. 1994, 311, L707–L711. [Google Scholar] [CrossRef]
- Kamińska, A.; Winkler, K.; Kowalska, A.; Witkowska, E.; Szymborski, T.; Janeczek, A.; Waluk, J. SERS-based immunoassay in a microfluidic system for the multiplexed recognition of interleukins from blood plasma: Towards picogram detection. Sci. Rep. 2017, 7, 10656. [Google Scholar] [CrossRef] [PubMed]
- Mir-Simon, I.R.-P.B.; Guerrini, L.; Pazos-Perez, N.; Alvarez-Puebla, R.A. Universal one-pot and scalable synthesis of SERS encoded nanoparticles. Chem. Mater. 2015, 27, 950–958. [Google Scholar] [CrossRef]
- Sun, L.; Sung, K.B.; Dentinger, C.; Lutz, B.; Nguyen, L.; Zhang, J.; Qin, H.; Yamakawa, M.; Cao, M.; Lu, Y.; et al. Composite organic− inorganic nanoparticles as Raman labels for tissue analysis. Nano Lett. 2007, 7, 351–356. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A. Substituent Constants for Correlation Analysis in Chemistry and Biology; Wiley-Interscience: New York, NY, USA, 1979. [Google Scholar]
- Zook, J.M.; Rastogi, V.; Maccuspie, R.I.; Keene, A.M.; Fagan, J. Measuring agglomerate size distribution and dependence of localized surface plasmon resonance absorbance on gold nanoparticle agglomerate size using analytical ultracentrifugation. ACS Nano 2011, 5, 8070–8079. [Google Scholar] [CrossRef]
- Hong, S.; Li, X. Optimal size of gold nanoparticles for surface-enhanced Raman spectroscopy under different conditions. J. Nanomater. 2013, 2013, 790323. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef]
- Singh, A.P.; Chauhan, S.C.; Andrianifahanana, M.; Moniaux, N.; Meza, J.L.; Copin, M.C.; van Seuningen, I.; Hollingsworth, M.A.; Aubert, J.P.; Batra, S.K. MUC4 expression is regulated by cystic fibrosis transmembrane conductance regulator in pancreatic adenocarcinoma cells via transcriptional and post-translational mechanisms. Oncogene 2007, 26, 30–41. [Google Scholar] [CrossRef] [Green Version]
- Farley, A.M.; Braxton, D.R.; Li, J.; Trounson, K.; Sakar-Dey, S.; Nayer, B.; Ikeda, T.; Lau, K.X.; Hardikar, W.; Hasegawa, K.; et al. Antibodies to a CA 19-9 related antigen complex identify SOX9 expressing progenitor cells in human foetal pancreas and pancreatic adenocarcinoma. Sci. Rep. 2019, 9, 2876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derjaguin, B.; Landau, L.D. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Acta Physicochim. USSR 1941, 14, 633–662. [Google Scholar]
- Verwey, E.J.W.; Overbeek, J.T.G. Theory of the stability of lyophobic colloids. J. Phys. Chem. 1947, 51, 631–636. [Google Scholar] [CrossRef] [Green Version]
- Häkkinen, H. The gold–sulfur interface at the nanoscale. Nat. Chem. 2012, 4, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Inkpen, M.; Liu, Z.; Li, H.; Campos, L.; Neaton, J.; Venkataraman, L. Non-chemisorbed gold–sulfur binding prevails in self-assembled monolayers. Nat. Chem. 2019, 11, 351–358. [Google Scholar] [CrossRef]

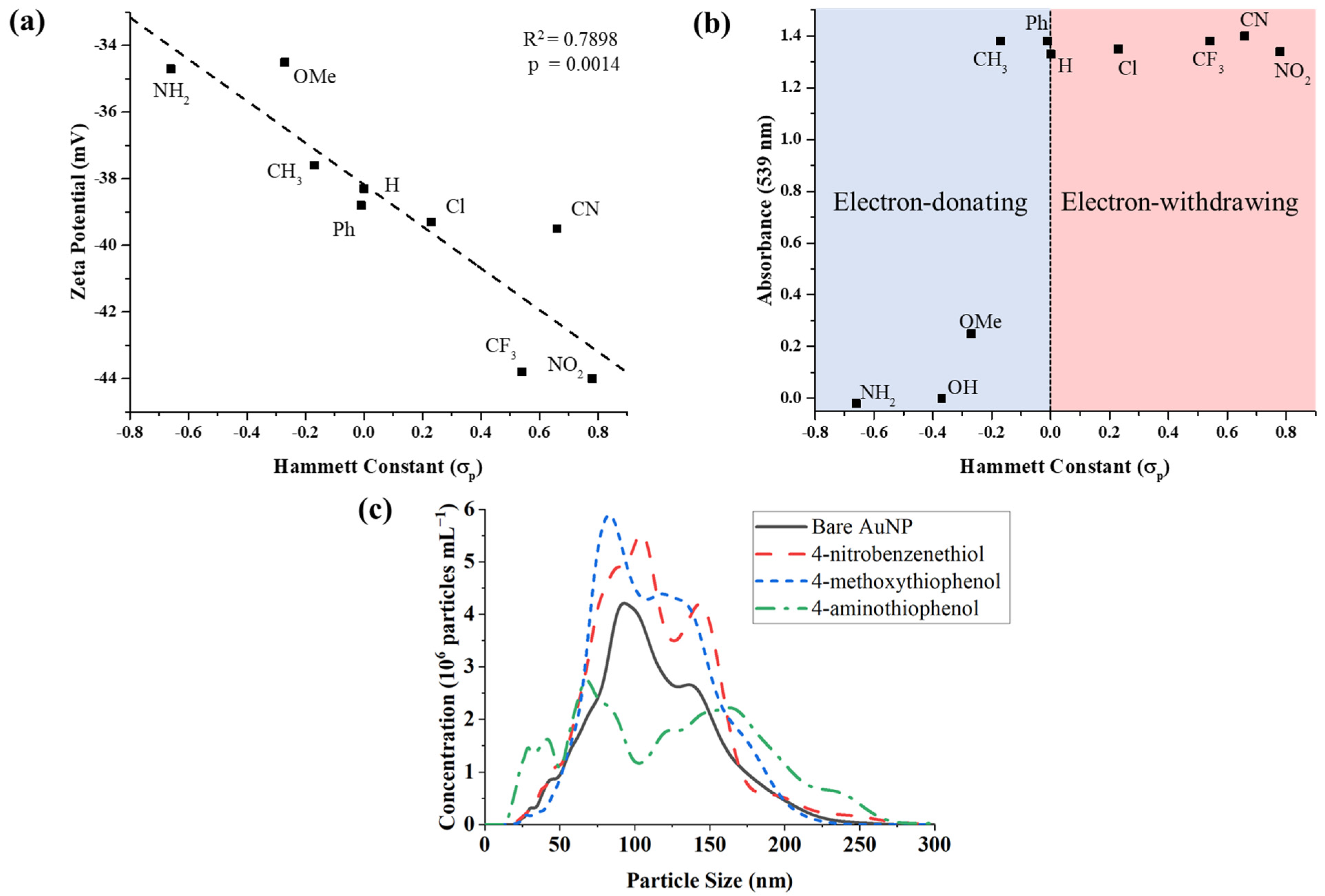
| Reagent | Para Group | Hammett Constant (σp) [18] |
|---|---|---|
| 4-aminothiophenol | NH2 | −0.66 |
| 4-mercaptophenol | OH | −0.37 |
| 5-methoxythiophenol | OMe | −0.27 |
| 4-methylbenzenethiol | CH3 | −0.17 |
| biphenyl-4-thiol | Ph | −0.01 |
| thiophenol | H | 0 |
| 4-chlorothiophenol | Cl | 0.23 |
| 4-(trifluoromethyl) thiophenol | CF3 | 0.54 |
| 4-cyanobenzenethiol (CNBT) | CN | 0.66 |
| 4-nitrobenzenethiol (NBT) | NO2 | 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
File, N.; Carmicheal, J.; Krasnoslobodtsev, A.V.; Japp, N.C.; Souchek, J.J.; Chakravarty, S.; Hollingsworth, M.A.; Sasson, A.A.; Natarajan, G.; Kshirsagar, P.G.; et al. Substituent Effects Impact Surface Charge and Aggregation of Thiophenol-Labeled Gold Nanoparticles for SERS Biosensors. Biosensors 2022, 12, 25. https://doi.org/10.3390/bios12010025
File N, Carmicheal J, Krasnoslobodtsev AV, Japp NC, Souchek JJ, Chakravarty S, Hollingsworth MA, Sasson AA, Natarajan G, Kshirsagar PG, et al. Substituent Effects Impact Surface Charge and Aggregation of Thiophenol-Labeled Gold Nanoparticles for SERS Biosensors. Biosensors. 2022; 12(1):25. https://doi.org/10.3390/bios12010025
Chicago/Turabian StyleFile, Nolan, Joseph Carmicheal, Alexey V. Krasnoslobodtsev, Nicole C. Japp, Joshua J. Souchek, Sudesna Chakravarty, Michael A. Hollingsworth, Aaron A. Sasson, Gopalakrishnan Natarajan, Prakash G. Kshirsagar, and et al. 2022. "Substituent Effects Impact Surface Charge and Aggregation of Thiophenol-Labeled Gold Nanoparticles for SERS Biosensors" Biosensors 12, no. 1: 25. https://doi.org/10.3390/bios12010025
APA StyleFile, N., Carmicheal, J., Krasnoslobodtsev, A. V., Japp, N. C., Souchek, J. J., Chakravarty, S., Hollingsworth, M. A., Sasson, A. A., Natarajan, G., Kshirsagar, P. G., Jain, M., Hayashi, C., Junker, W. M., Kaur, S., & Batra, S. K. (2022). Substituent Effects Impact Surface Charge and Aggregation of Thiophenol-Labeled Gold Nanoparticles for SERS Biosensors. Biosensors, 12(1), 25. https://doi.org/10.3390/bios12010025