Regenerable ZnO/GaAs Bulk Acoustic Wave Biosensor for Detection of Escherichia coli in “Complex” Biological Medium
Abstract
1. Introduction
2. Materials and Methods
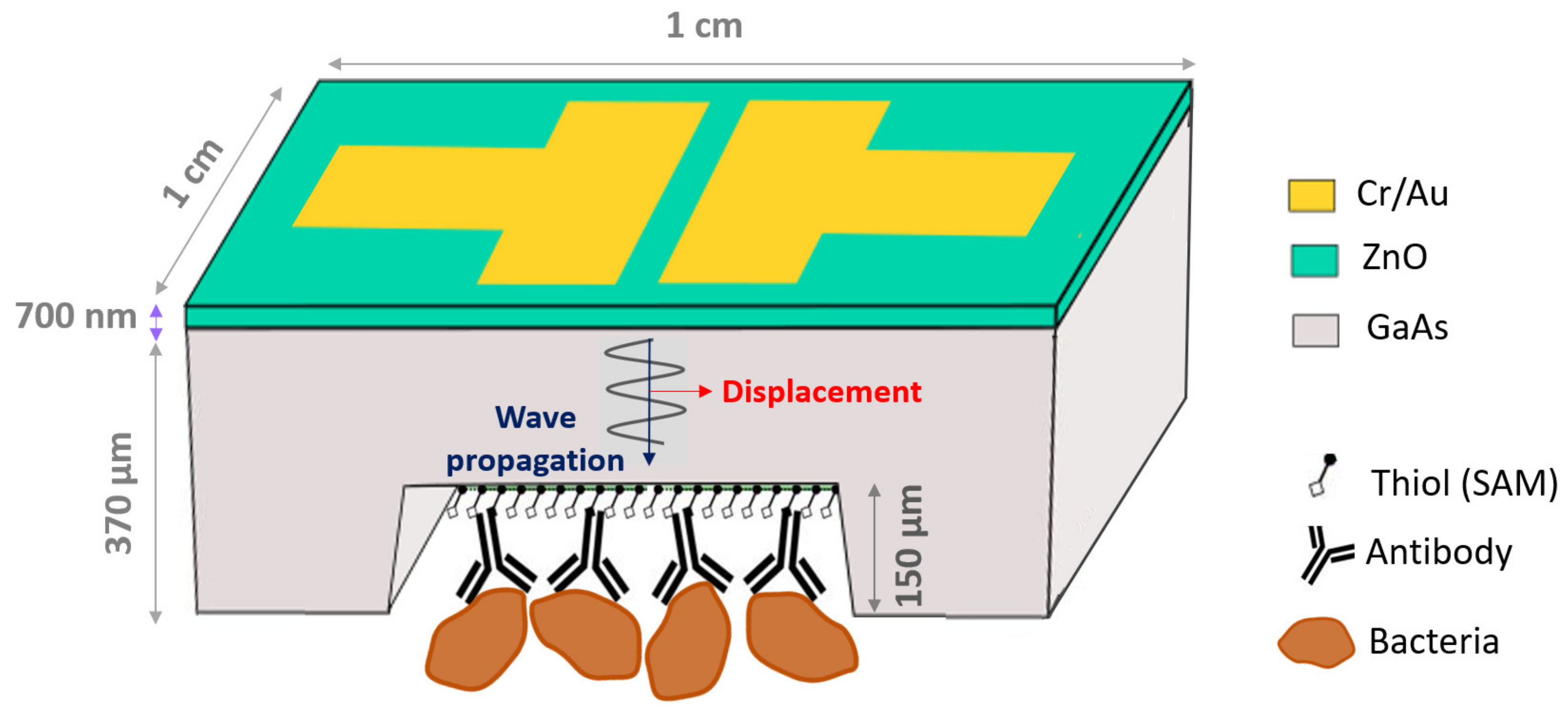
2.1. Design and Operation of the Sensor
2.2. Materials
2.3. Microfabrication of ZnO/GaAs Sensor
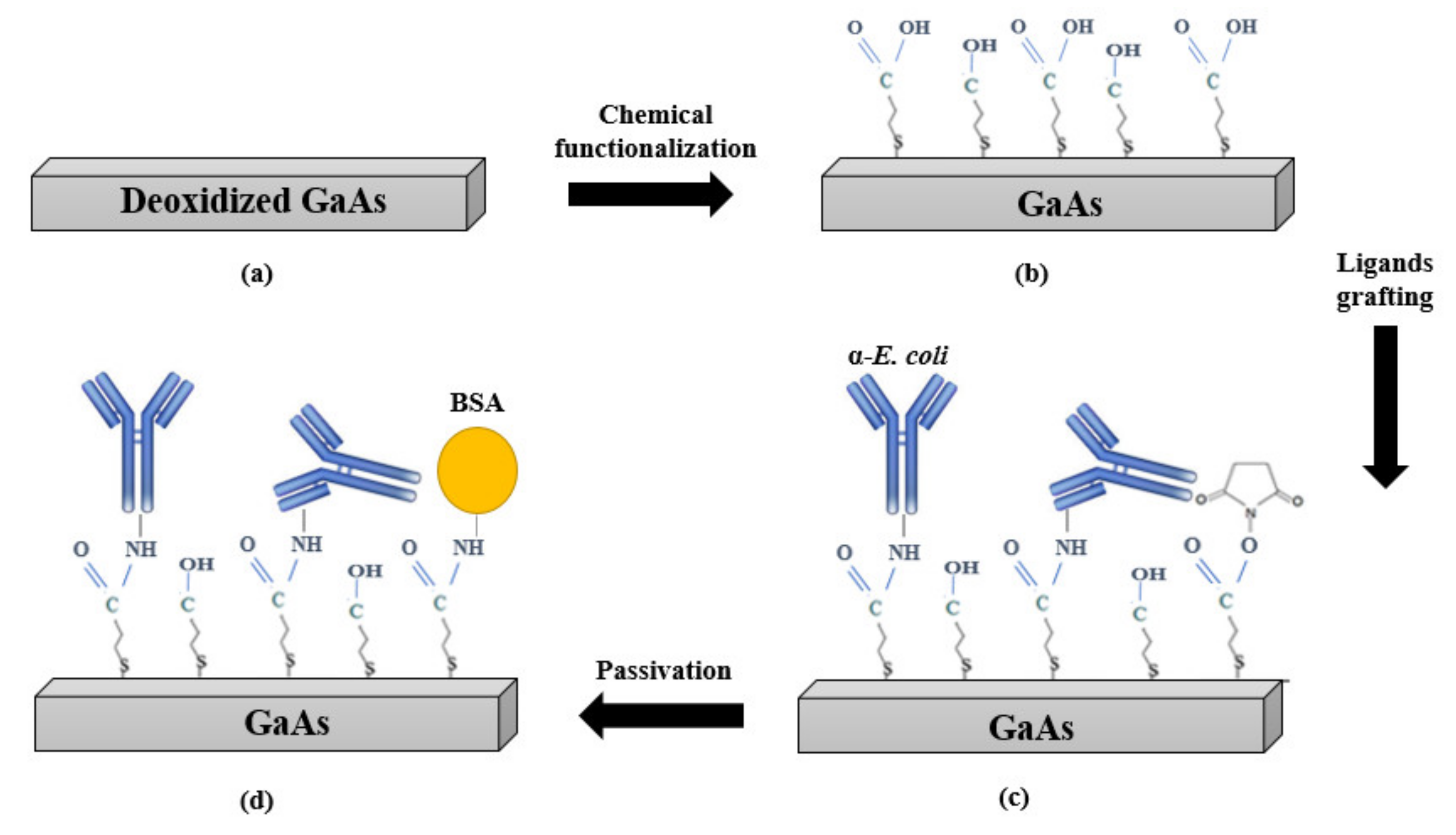
2.4. Biofunctionalization of GaAs Membrane
2.5. Immunocapture of E. coli on the Sensor Surface
2.6. Sensor Specificity
2.7. Regeneration of the Sensor Surface
3. Results and Discussion
3.1. Optimization of the Sensing Architecture on Bulk GaAs
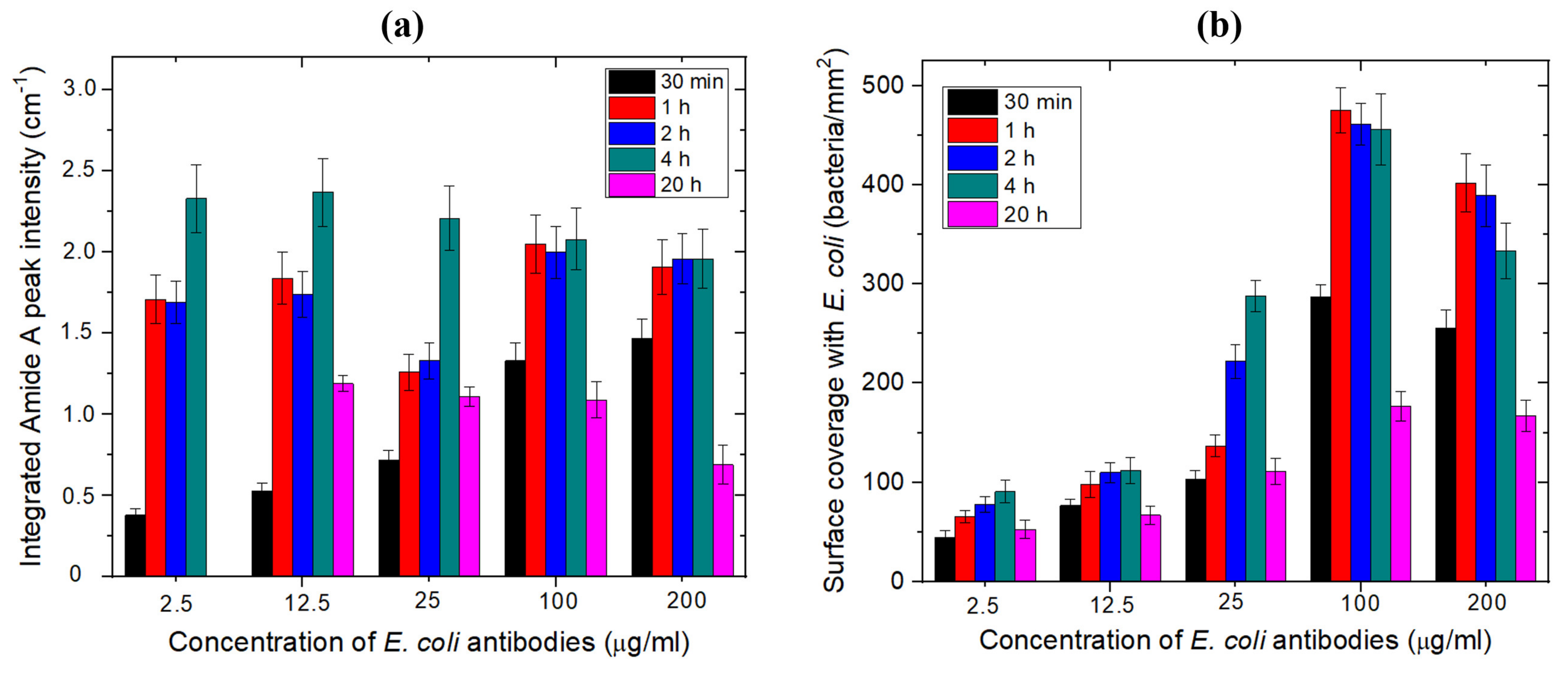
3.1.1. Optimization of the Concentration and Incubation Time for the Immobilization of E. coli Antibodies
3.1.2. Optimization of the Antibody-Grafting Buffer pH
3.1.3. Regeneration Efficiency of the Sensing Structure
3.2. Determination of Antibodies and BSA Grafting Rates Using the ZnO/GaAs BAW Biosensor
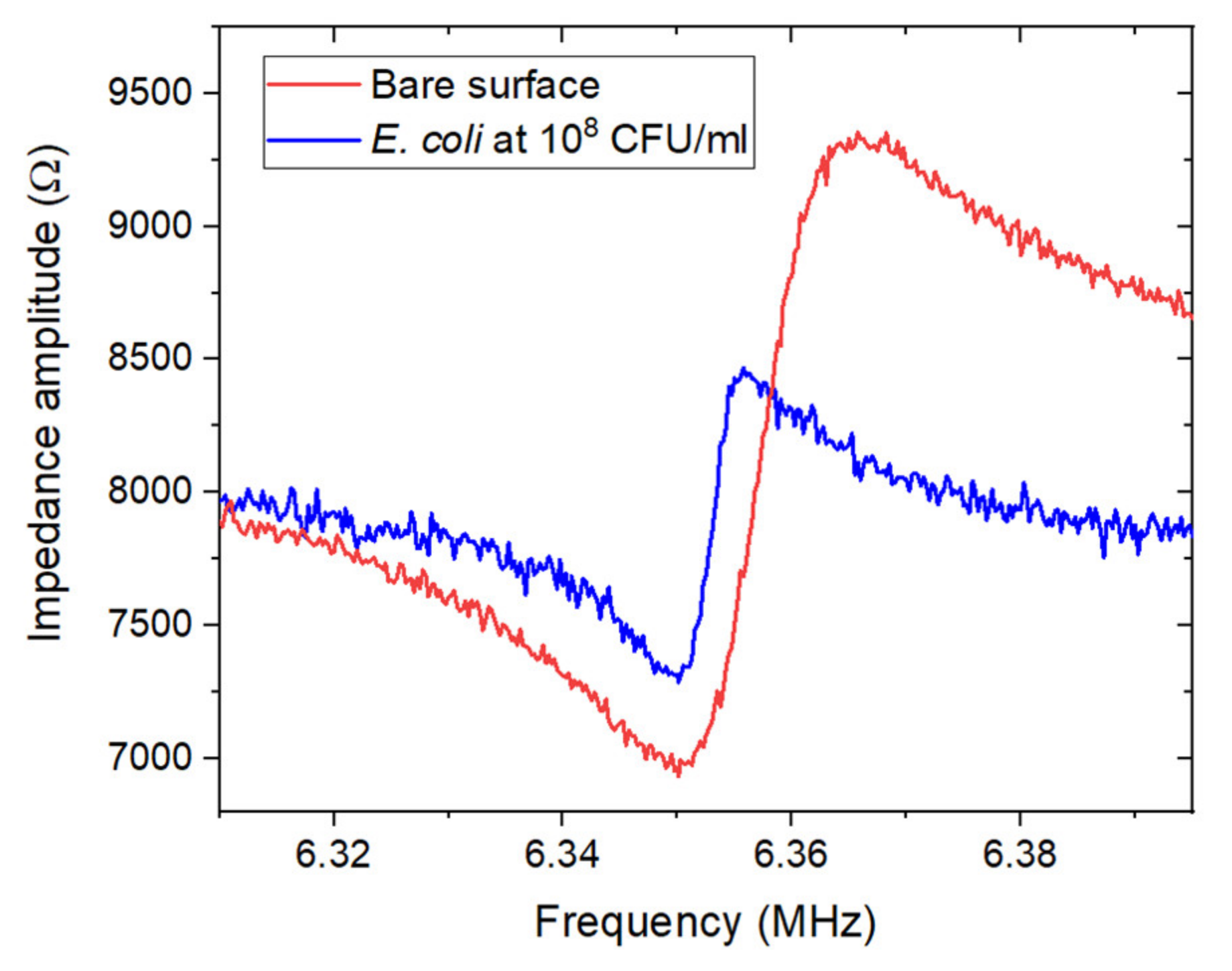
3.3. Detection of E. coli Using the ZnO/GaAs BAW Biosensor
3.3.1. Ideal Medium: Phosphate-Buffered Saline
3.3.2. In the Presence of Bacillus Subtilis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Váradi, L.; Luo, J.L.; Hibbs, D.E.; Perry, J.D.; Anderson, R.J.; Orenga, S.; Groundwater, P.W. Methods for the detection and identification of pathogenic bacteria: Past, present, and future. Chem. Soc. Rev. 2017, 46, 4818–4832. [Google Scholar] [CrossRef]
- Justino, C.; Duarte, A.; Rocha-Santos, T. Recent progress in biosensors for environmental monitoring: A Review. Sensors 2017, 17, 2918. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, S.N.; Rahim, H.A.; Lau, W.-J. Detection of contaminants in water supply: A review on state-of-the-art monitoring technologies and their applications. Sens. Actuators B Chem. 2018, 255, 2657–2689. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, B.; Patil, R.; Dwarakanath, S. A review on detection methods used for foodborne pathogens. Indian J. Med. Res. 2016, 144, 327. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.S.; Kim, S.; Shin, E.; Oh, K.-H.; Kim, Y.; Kim, C.H.; Hwang, M.A.; Jin, C.M.; Na, K.; et al. A waterborne outbreak of multiple diarrhoeagenic Escherichia coli infections associated with drinking water at a school camp. Int. J. Infect. Dis. 2018, 66, 45–50. [Google Scholar] [CrossRef]
- Yang, S.-C.; Lin, C.-H.; Aljuffali, I.A.; Fang, J.-Y. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Decludt, B.; Bouvet, P.; Mariani-Kurkdjian, P.; Grimont, F.; Grimont, P.A.D.; Hubert, B.; Loirat, C. Haemolytic uraemic syndrome and Shiga toxin-producing Escherichia coli infection in children in France. Epidemiol. Infect. 2000, 124, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.; Xie, L.; Ying, Y. Conventional and emerging detection techniques for pathogenic bacteria in food science: A review. Trends Food Sci. Technol. 2018, 81, 61–73. [Google Scholar] [CrossRef]
- Ahmed, A.; Rushworth, J.V.; Hirst, N.A.; Millner, P.A. Biosensors for whole-cell bacterial detection. Clin. Microbiol. Rev. 2014, 27, 631–646. [Google Scholar] [CrossRef]
- Lamanna, L.; Rizzi, F.; Bhethanabotla, V.R.; De Vittorio, M. Conformable surface acoustic wave biosensor for E-coli fabricated on PEN plastic film. Biosens. Bioelectron. 2020, 163, 112164. [Google Scholar] [CrossRef]
- Yuhana Ariffin, E.; Heng, L.Y.; Tan, L.L.; Abd Karim, N.H.; Hasbullah, S.A. A highly sensitive impedimetric DNA biosensor based on hollow silica microspheres for label-free determination of E. coli. Sensors 2020, 20, 1279. [Google Scholar] [CrossRef]
- Mathelié-Guinlet, M.; Cohen-Bouhacina, T.; Gammoudi, I.; Martin, A.; Béven, L.; Delville, M.-H.; Grauby-Heywang, C. Silica nanoparticles-assisted electrochemical biosensor for the rapid, sensitive and specific detection of Escherichia coli. Actuators B Chem. 2019, 292, 314–320. [Google Scholar] [CrossRef]
- Razmi, N.; Hasanzadeh, M.; Willander, M.; Nur, O. Recent progress on the electrochemical biosensing of Escherichia coli O157:H7: Material and methods overview. Biosensors 2020, 10, 54. [Google Scholar] [CrossRef]
- Choinière, S.; Frost, E.H.; Dubowski, J.J. Binding strategies for capturing and growing Escherichia coli on surfaces of biosensing devices. Talanta 2019, 192, 270–277. [Google Scholar] [CrossRef]
- Nazemi, E.; Aithal, S.; Hassen, W.M.; Frost, E.H.; Dubowski, J.J. GaAs/AlGaAs heterostructure based photonic biosensor for rapid detection of Escherichia coli in phosphate buffered saline solution. Sens. Actuators B Chem. 2015, 207, 556–562. [Google Scholar] [CrossRef]
- Zhong, M.; Yang, L.; Yang, H.; Cheng, C.; Deng, W.; Tan, Y.; Xie, Q.; Yao, S. An electrochemical immunobiosensor for ultrasensitive detection of Escherichia coli O157:H7 using CdS quantum dots-encapsulated metal-organic frameworks as signal-amplifying tags. Biosens. Bioelectron. 2019, 126, 493–500. [Google Scholar] [CrossRef]
- Zheng, L.; Cai, G.; Wang, S.; Liao, M.; Li, Y.; Lin, J. A microfluidic colorimetric biosensor for rapid detection of Escherichia coli O157:H7 using gold nanoparticle aggregation and smart phone imaging. Biosens. Bioelectron. 2019, 124–125, 143–149. [Google Scholar] [CrossRef]
- Dhull, N.; Kaur, G.; Jain, P.; Mishra, P.; Singh, D.; Ganju, L.; Gupta, V.; Tomar, M. Label-free amperometric biosensor for Escherichia coli O157:H7 detection. Appl. Surf. Sci. 2019, 495, 143548. [Google Scholar] [CrossRef]
- Van Grinsven, B.; Eersels, K.; Akkermans, O.; Ellermann, S.; Kordek, A.; Peeters, M.; Deschaume, O.; Bartic, C.; Diliën, H.; Steen Redeker, E.; et al. Label-free detection of Escherichia coli based on thermal transport through surface imprinted polymers. ACS Sens. 2016, 1, 1140–1147. [Google Scholar] [CrossRef]
- Eersels, K.; Lieberzeit, P.; Wagner, P. A review on synthetic receptors for bioparticle detection created by surface-imprinting techniques—From principles to applications. ACS Sens. 2016, 1, 1171–1187. [Google Scholar] [CrossRef]
- Coudron, L.; McDonnell, M.B.; Munro, I.; McCluskey, D.K.; Johnston, I.D.; Tan, C.K.L.; Tracey, M.C. Fully integrated digital microfluidics platform for automated immunoassay; A versatile tool for rapid, specific detection of a wide range of pathogens. Biosens. Bioelectron. 2019, 128, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Voiculescu, I.; Nordin, A.N. Acoustic wave based MEMS devices for biosensing applications. Biosens. Bioelectron. 2012, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tellier, C.R.; Huve, G.; Leblois, T.G. Micromachining of GaAs structures with an acidic hydrogen peroxide solution. Sens. Actuators Phys. 2006, 127, 179–193. [Google Scholar] [CrossRef]
- Bienaime, A.; Elie-Caille, C.; Leblois, T. Micro structuration of GaAs surface by wet etching: Towards a specific surface behavior. J. Nanosci. Nanotechnol. 2012, 12, 6855–6863. [Google Scholar] [CrossRef] [PubMed]
- Bienaime, A.; Leblois, T.; Gremaud, N.; Chaudon, M.-J.; Osta, M.; Pecqueur, D.; Ducoroy, P.; Elie-Caille, C. Influence of a thiolate chemical layer on GaAs (100) biofunctionalization: An original approach coupling atomic force microscopy and mass spectrometry methods. Materials 2013, 6, 4946–4966. [Google Scholar] [CrossRef]
- Bienaime, A.; Leblois, T.; Lucchi, G.; Blondeau-Patissier, V.; Ducoroy, P.; Boireau, W.; Elie-Caille, C. Reconstitution of protein monolayer on thiolates functionalized GaAs surface. Int. J. Nanosci. 2012, 11, 1240018. [Google Scholar] [CrossRef]
- Lacour, V.; Moumanis, K.; Hassen, W.M.; Elie-Caille, C.; Leblois, T.; Dubowski, J.J. Formation kinetics of mixed self-assembled monolayers of alkanethiols on GaAs(100). Langmuir 2017, 35, 4415–4427. [Google Scholar] [CrossRef]
- Chawich, J.; Boiteux, P.; Elie-Caille, C.; Leblois, T. Specificity and sensitivity characterization of a gallium arsenide resonant bio-sensor. In Proceedings of the 2018 IEEE Int. Ultrason. Symp. (IUS), Kobe, Japan, 22–25 October 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Sharma, H.; Moumanis, K.; Dubowski, J.J. pH-dependent photocorrosion of GaAs/AlGaAs quantum well microstructures. J. Phys. Chem. C 2016, 120, 26129–26137. [Google Scholar] [CrossRef]
- Lacour, V.; Elie-Caille, C.; Leblois, T.; Dubowski, J.J. Regeneration of a thiolated and antibody functionalized GaAs (001) surface using wet chemical processes. Biointerphases 2016, 11, 019302. [Google Scholar] [CrossRef]
- Azzopardi, C.-L.; Lacour, V.; Manceau, J.-F.; Barthès, M.; Bonnet, D.; Chollet, F.; Leblois, T. A fluidic interface with high flow uniformity for reusable large area resonant biosensors. Micromachines 2017, 8, 308. [Google Scholar] [CrossRef]
- Cerda-Méndez, E.A.; Krizhanovskii, D.; Biermann, K.; Hey, R.; Santos, P.V.; Skolnick, M.S. Effects of the piezoelectric field on the modulation of exciton–polaritons by surface acoustic waves. Superlattices Microstruct. 2011, 49, 233–240. [Google Scholar] [CrossRef]
- Jiao, S.J.; Batista, P.D.; Biermann, K.; Hey, R.; Santos, P.V. Electrical detection of ambipolar acoustic carrier transport by surface acoustic waves. J. Appl. Phys. 2009, 106, 053708. [Google Scholar] [CrossRef]
- Shih, W.-C.; Wu, M.-S. Growth of ZnO films on GaAs substrates with a SiO2 buffer layer by RF planar magnetron sputtering for surface acoustic wave applications. J. Cryst. Growth. 1994, 137, 319–325. [Google Scholar] [CrossRef]
- Ballantine, D.S., Jr.; White, R.M.; Martin, S.J.; Ricco, A.J.; Zellers, E.T.; Frye, G.C.; Wohltjen, H. Acoustic Wave Sensors: Theory, Design, & Physico-Chemical Applications, 1st ed.; Academic Press: Cambridge, MA, USA, 1996. [Google Scholar]
- Thompson, M.; Kipling, A.L.; Duncan-Hewitt, W.C.; Rajaković, L.V.; Čavić-Vlasak, B.A. Thickness-shear-mode acoustic wave sensors in the liquid phase. A review. Analyst 1991, 116, 881–890. [Google Scholar] [CrossRef]
- Chawich, J.; Kuprenaite, S.; Margueron, S.; Boulet, P.; Dubowski, J.J.; Elie-Caille, C.; Leblois, T. Deposition and characterization of ZnO thin films on GaAs and Pt/GaAs substrates. Mater. Chem. Phys. 2020, 247, 122854. [Google Scholar] [CrossRef]
- Bienaime, A.; Liu, L.; Elie-Caille, C.; Leblois, T. Design and microfabrication of a lateral excited gallium arsenide biosensor. Eur. Phys. J.-Appl. Phys. 2012, 57, 21003. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Bandekar, J. Amide modes and protein conformation. Biochim. Biophys. Acta BBA—Protein Struct. Mol. Enzymol. 1992, 1120, 123–143. [Google Scholar] [CrossRef]
- Pei, Z.; Anderson, H.; Myrskog, A.; Dunér, G.; Ingemarsson, B.; Aastrup, T. Optimizing immobilization on two-dimensional carboxyl surface: pH dependence of antibody orientation and antigen binding capacity. Anal. Biochem. 2010, 398, 161–168. [Google Scholar] [CrossRef]
- Djoumerska-Alexieva, I.K.; Dimitrov, J.D.; Voynova, E.N.; Lacroix-Desmazes, S.; Kaveri, S.V.; Vassilev, T.L. Exposure of IgG to an acidic environment results in molecular modifications and in enhanced protective activity in sepsis: Molecular modifications in low pH-exposed IgG. FEBS J. 2010, 277, 3039–3050. [Google Scholar] [CrossRef]
- Lynch, M.; Mosher, C.; Huff, J.; Nettikadan, S.; Johnson, J.; Henderson, E. Functional protein nanoarrays for biomarker profiling. Proteomics 2004, 4, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Jachimska, B.; Tokarczyk, K.; Łapczyńska, M.; Puciul-Malinowska, A.; Zapotoczny, S. Structure of bovine serum albumin adsorbed on silica investigated by quartz crystal microbalance. Colloids Surf. Physicochem. Eng. Asp. 2016, 489, 163–172. [Google Scholar] [CrossRef]
- Kardous, F.; Rouleau, A.; Simon, B.; Yahiaoui, R.; Manceau, J.F.; Boireau, W. Improving immunosensor performances using an acoustic mixer on droplet microarray. Biosens. Bioelectron. 2010, 26, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Neidhardt, F.C. Escherichia coli and Salmonella: Cellular and Molecular Biology, 2nd ed.; ASM Press: Washington, DC, USA, 1996. [Google Scholar]
- Diaspro, A.; Bianchini, P.; Cella Zanacchi, F.; Lanzanò, L.; Vicidomini, G.; Oneto, M.; Pesce, L.; Cainero, I. Fluorescence Microscopy. In Springer Handbook of Microscopy; Hawkes, P.W., Spence, J.C.H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1039–1088. [Google Scholar] [CrossRef]
- Lisle, J.T.; Hamilton, M.A.; Willse, A.R.; McFeters, G.A. Comparison of fluorescence microscopy and solid-phase cytometry methods for counting bacteria in water. Appl. Environ. Microbiol. 2004, 70, 5343–5348. [Google Scholar] [CrossRef]
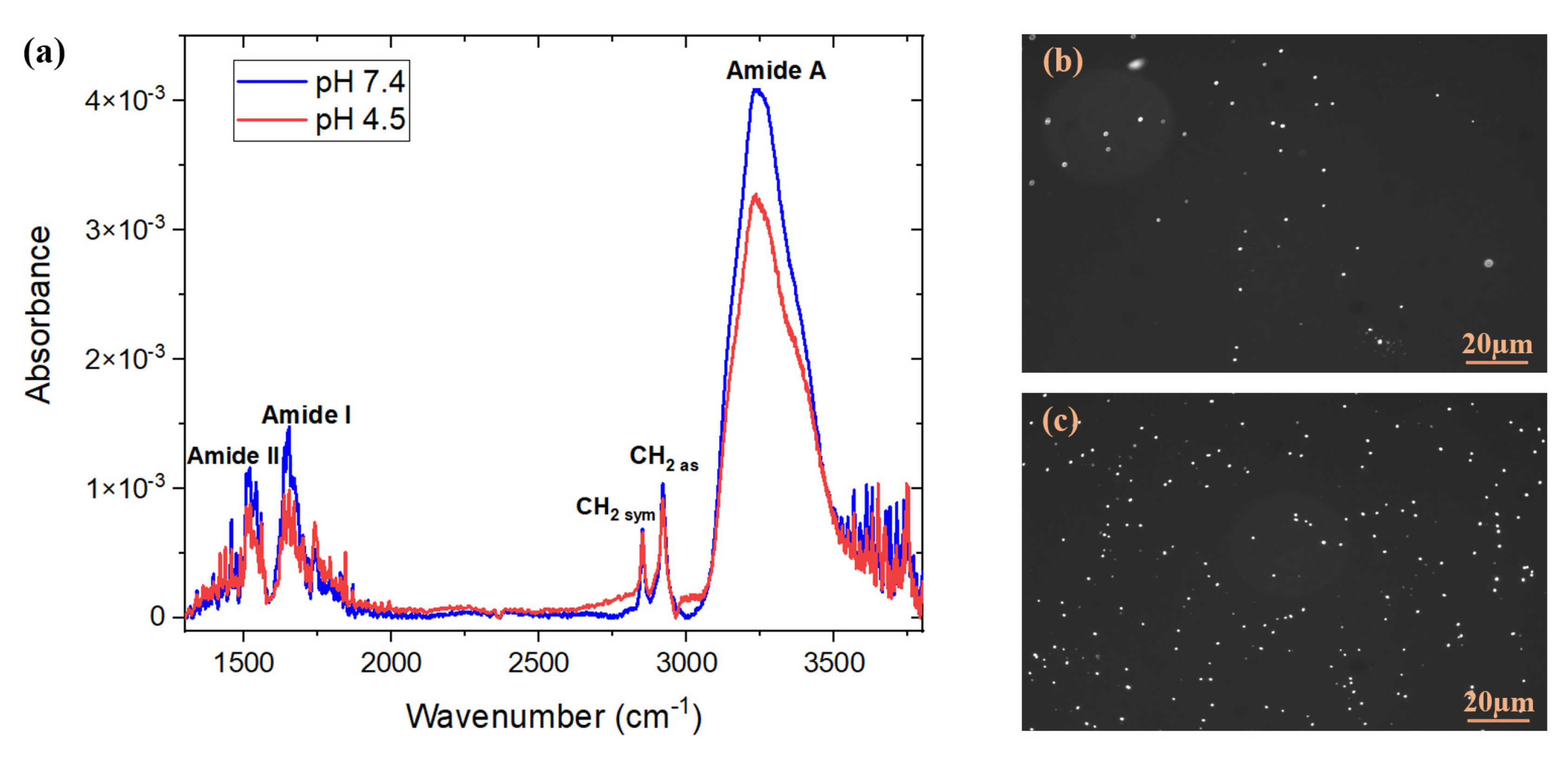
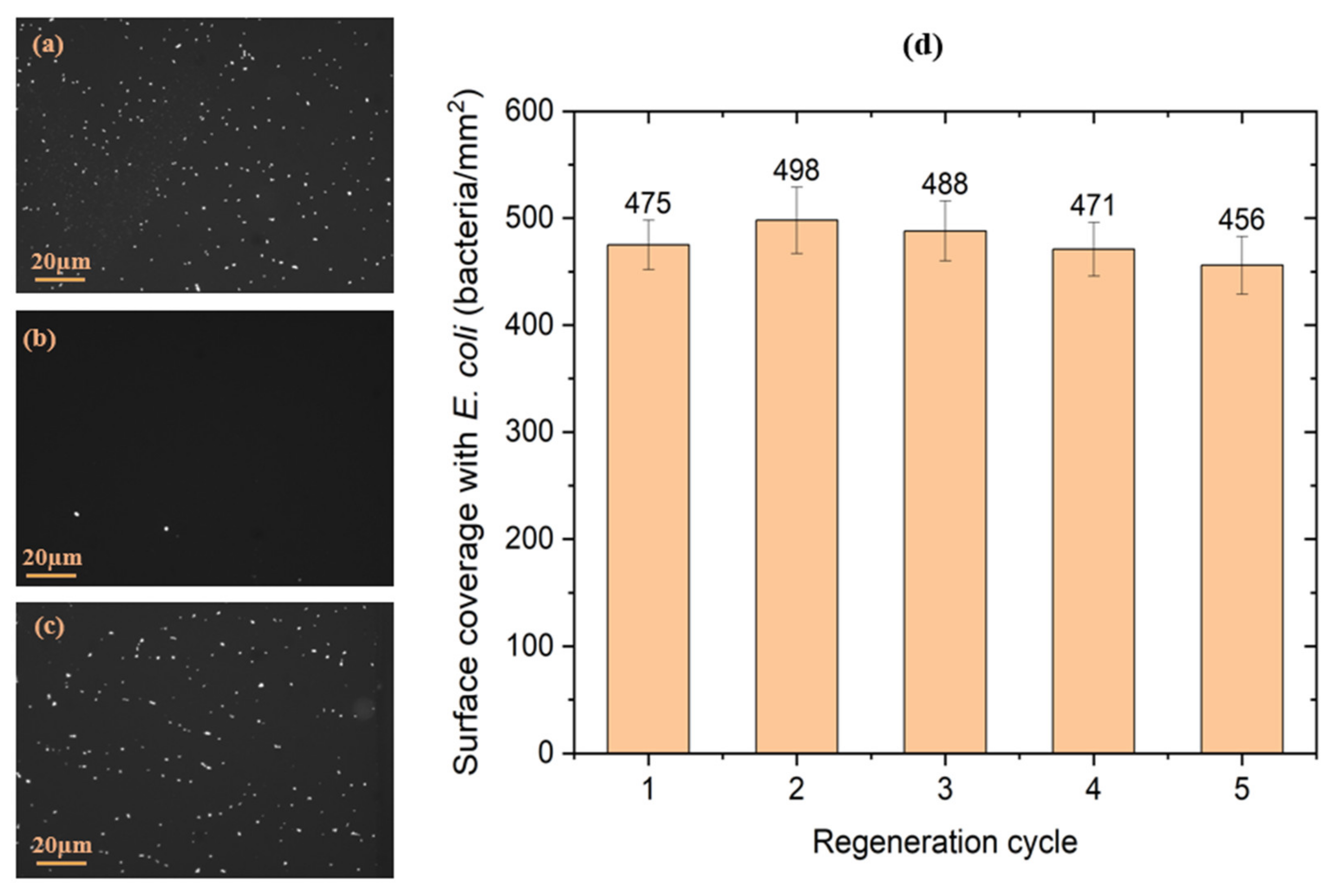

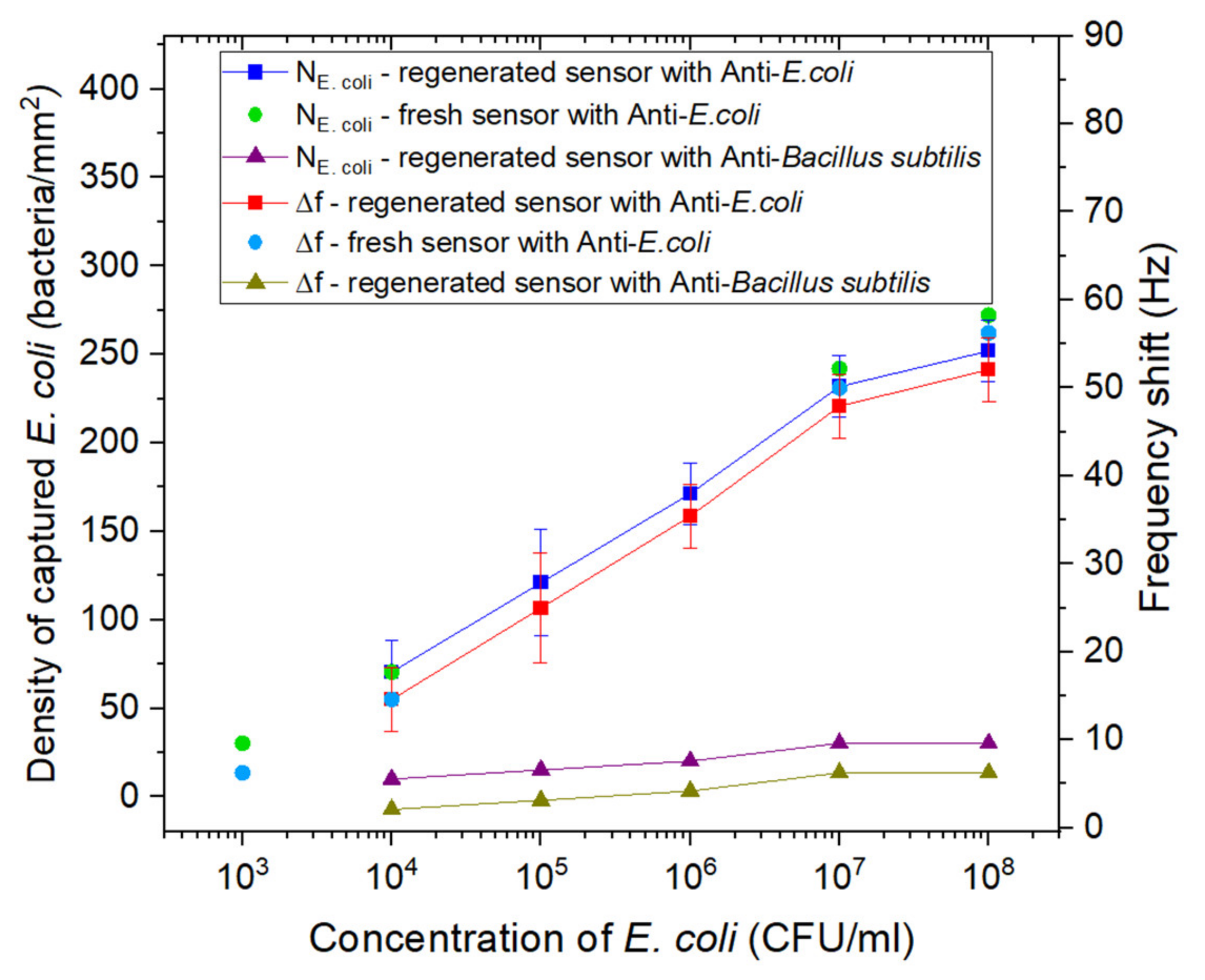
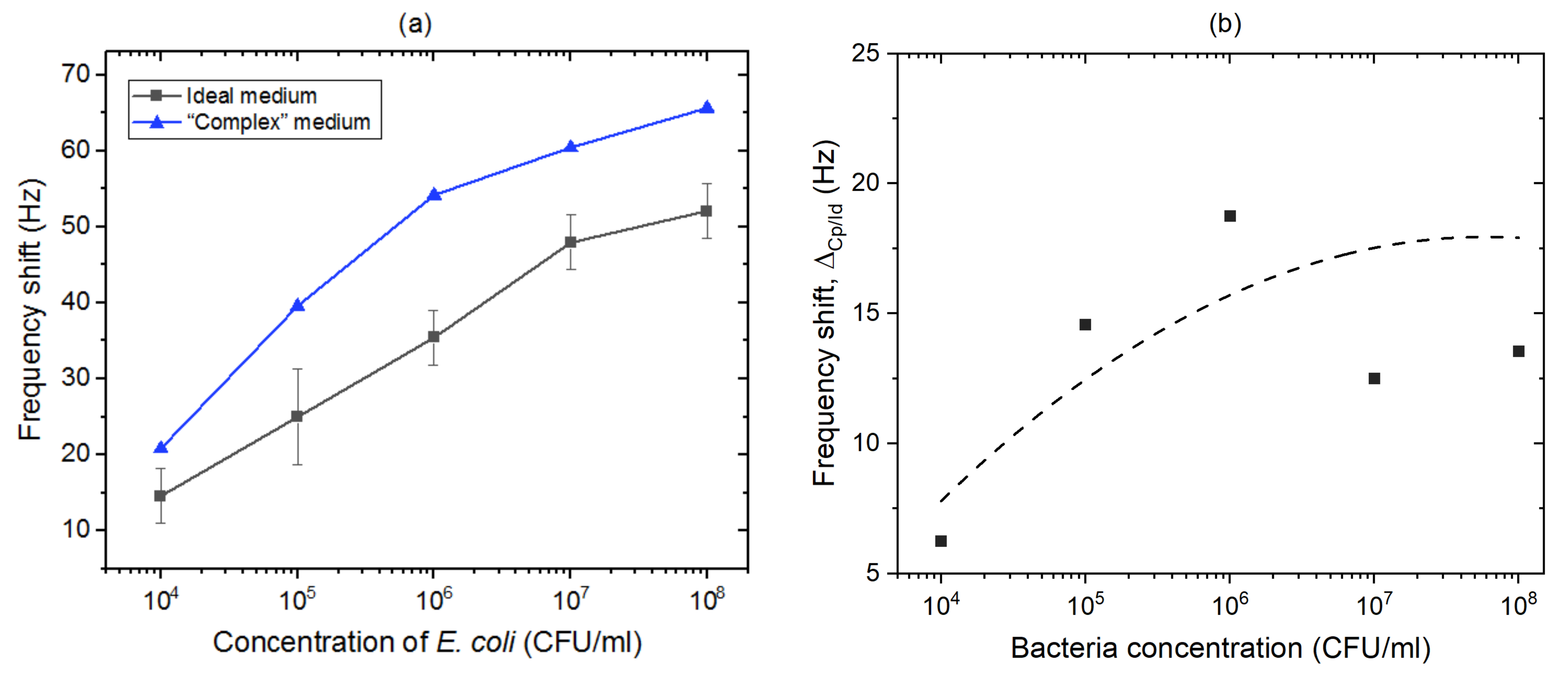
| Stage | Before Regeneration | After Regeneration |
|---|---|---|
| Absorbance | 4.4 × 10−3 ± 2.5 × 10−4 | 5.5 × 10−3 ± 3.8 × 10−4 |
| Integrated absorbance intensity (in the range of 3050 to 3550 cm−1) | 1.53 ± 0.07 | 2.09 ± 0.07 |
| Phase | Entity | ||
|---|---|---|---|
| Theoretical | Experimental | ||
| Antibodies grafting | E. coli antibodies | 39.84 | 27.1 ± 4.5 |
| Passivation | BSA | 136.46 | 59.8 ± 11.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chawich, J.; Hassen, W.M.; Elie-Caille, C.; Leblois, T.; Dubowski, J.J. Regenerable ZnO/GaAs Bulk Acoustic Wave Biosensor for Detection of Escherichia coli in “Complex” Biological Medium. Biosensors 2021, 11, 145. https://doi.org/10.3390/bios11050145
Chawich J, Hassen WM, Elie-Caille C, Leblois T, Dubowski JJ. Regenerable ZnO/GaAs Bulk Acoustic Wave Biosensor for Detection of Escherichia coli in “Complex” Biological Medium. Biosensors. 2021; 11(5):145. https://doi.org/10.3390/bios11050145
Chicago/Turabian StyleChawich, Juliana, Walid M. Hassen, Céline Elie-Caille, Thérèse Leblois, and Jan J. Dubowski. 2021. "Regenerable ZnO/GaAs Bulk Acoustic Wave Biosensor for Detection of Escherichia coli in “Complex” Biological Medium" Biosensors 11, no. 5: 145. https://doi.org/10.3390/bios11050145
APA StyleChawich, J., Hassen, W. M., Elie-Caille, C., Leblois, T., & Dubowski, J. J. (2021). Regenerable ZnO/GaAs Bulk Acoustic Wave Biosensor for Detection of Escherichia coli in “Complex” Biological Medium. Biosensors, 11(5), 145. https://doi.org/10.3390/bios11050145







