In Situ LSPR Sensing of Secreted Insulin in Organ-on-Chip
Abstract
1. Introduction
2. Materials and Methods
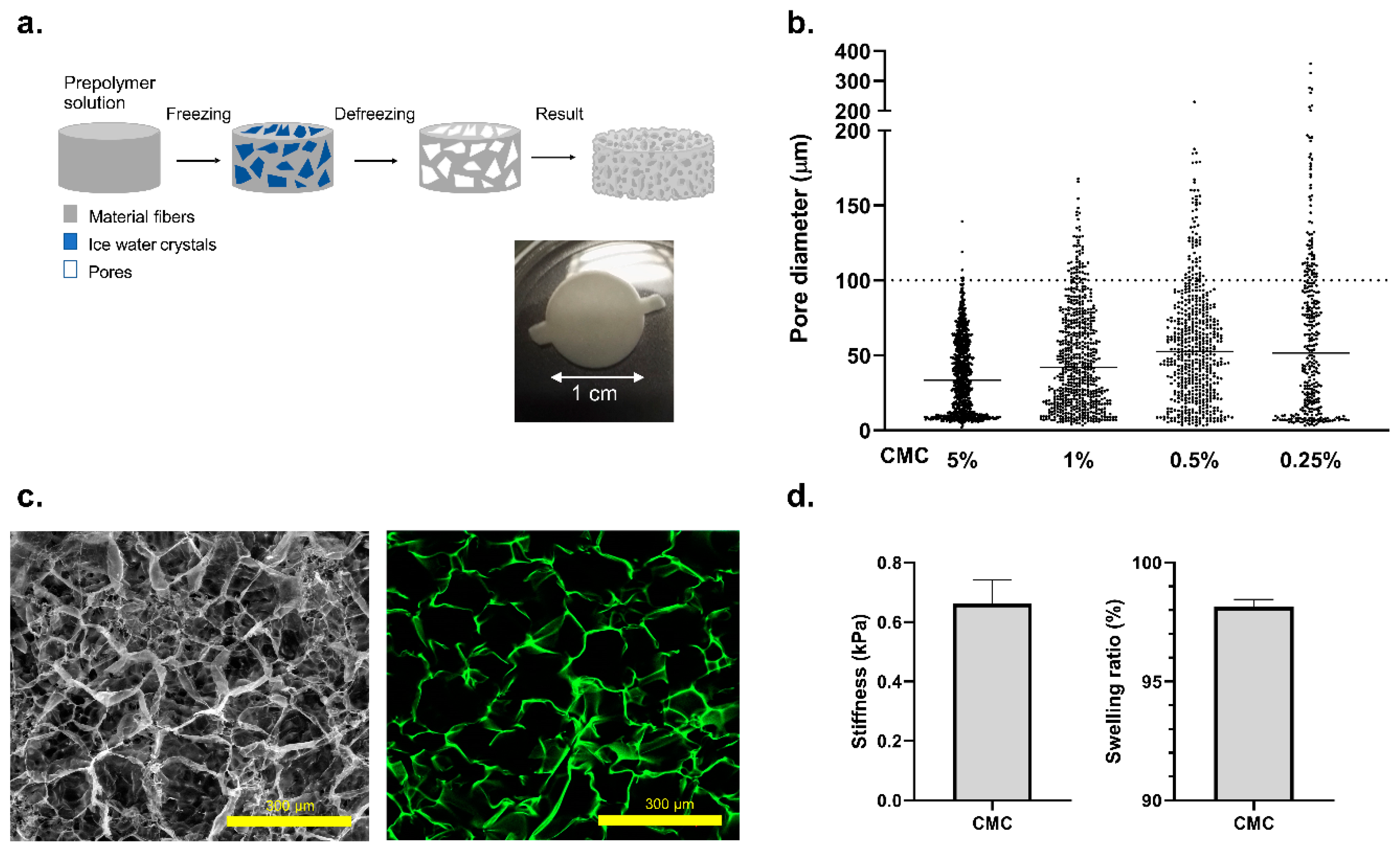
2.1. Carboxymethyl Cellulose (CMC)-Cryogel Fabrication
2.2. Characterization of CMC Cryogels
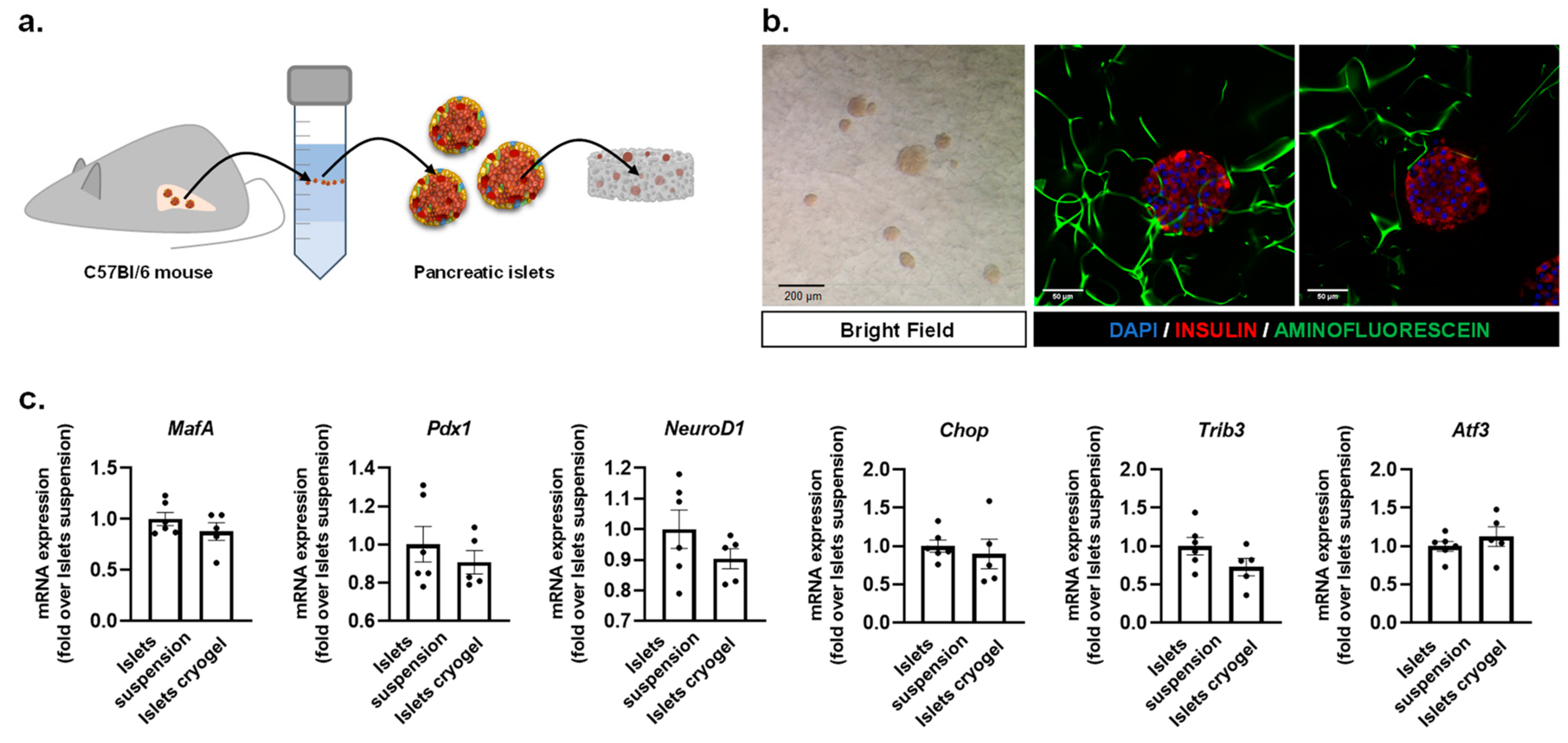
2.3. Mouse Pancreatic Islet Isolation
2.4. Gene Expression Analysis
2.5. Glucose-Stimulated Insulin Secretion (GSIS)
2.6. Immunofluorescence
2.7. Immunoreagents and ELISA Immunoassay Protocol
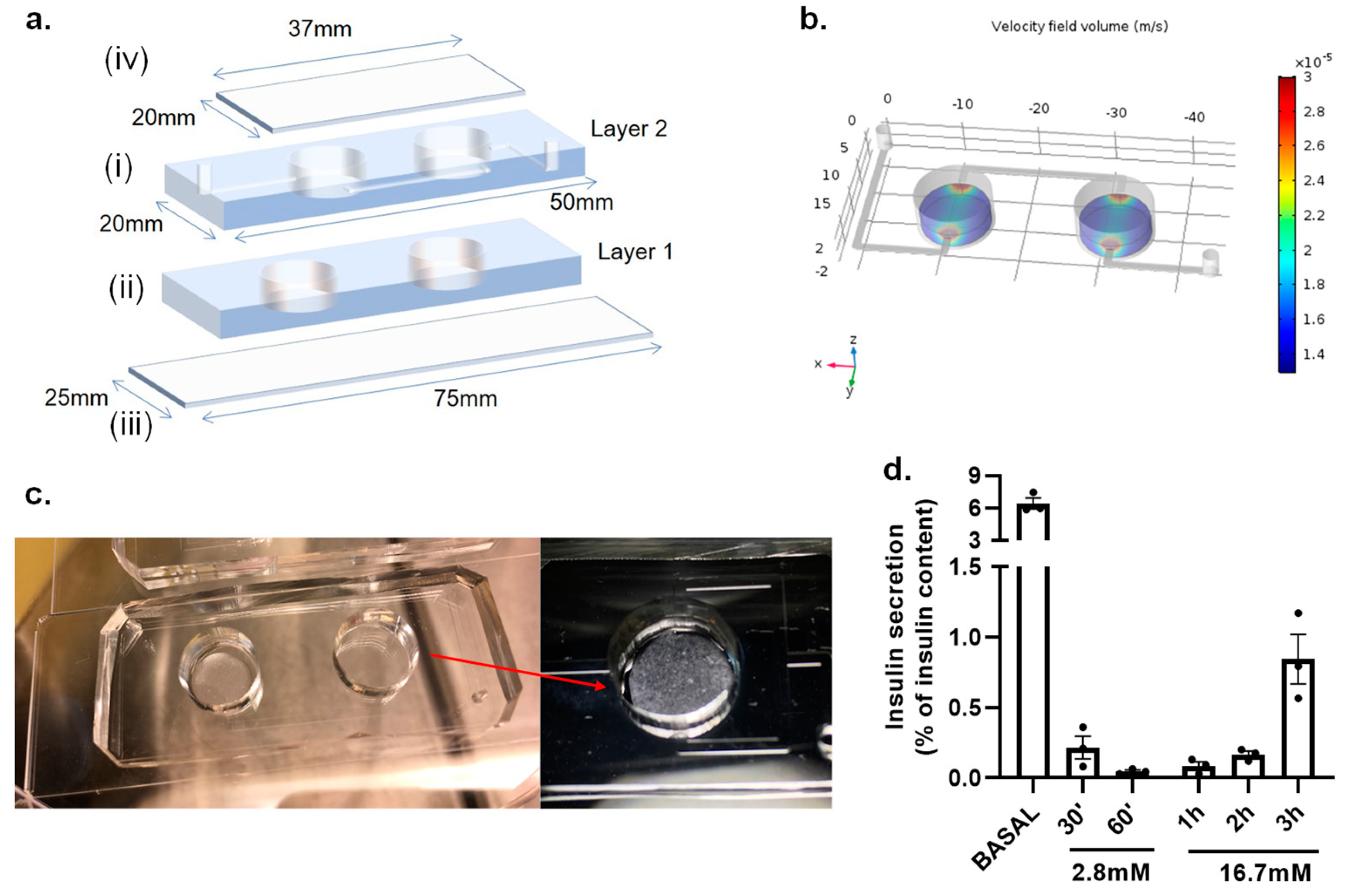
2.8. Fabrication of IOC Microfluidic Platform
2.9. Statistics
3. Results and Discussions
3.1. Fabrication of a 3D Effective Cellulose Matrix to House Pancreatic Islets
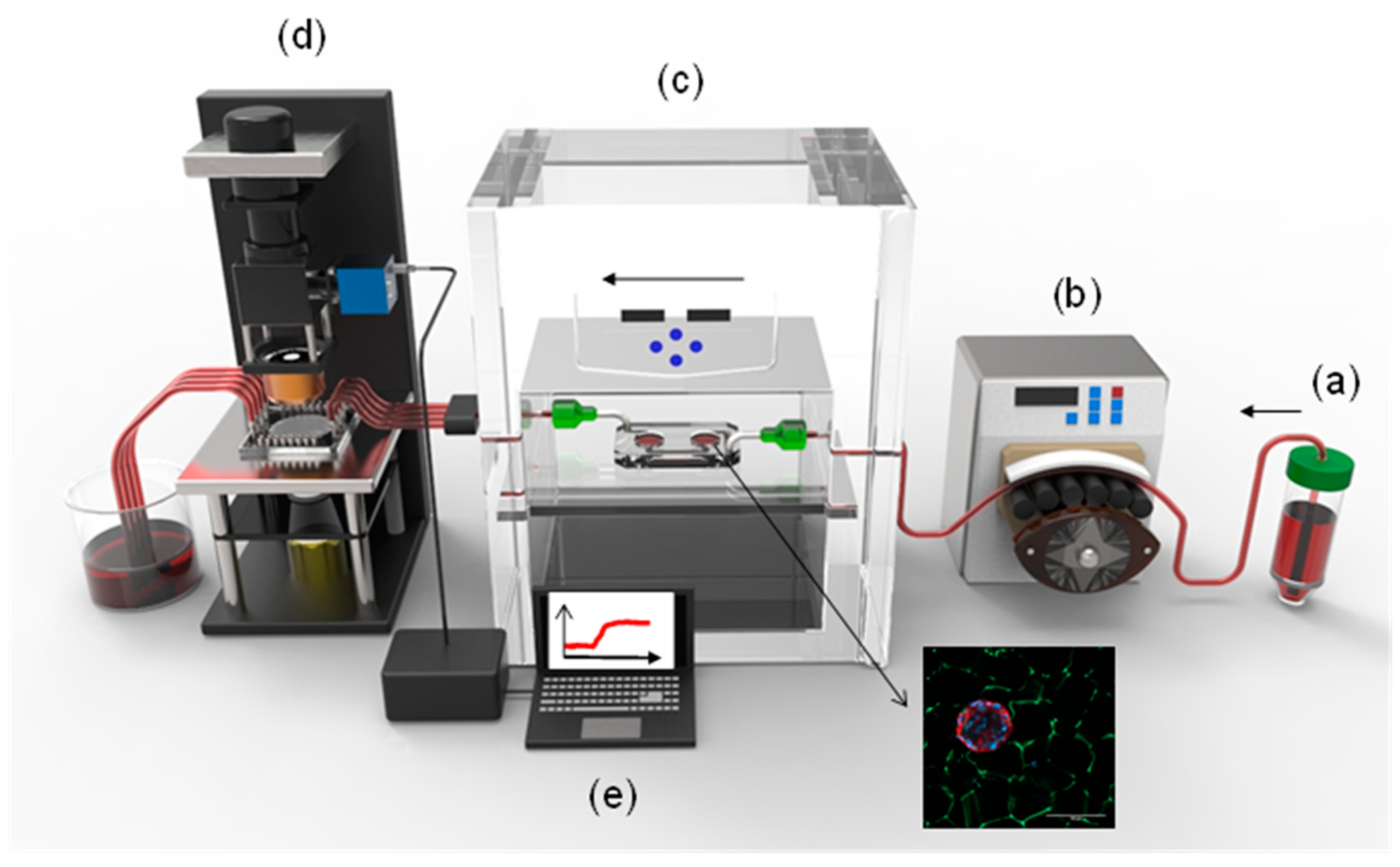
3.2. Islet-on-a-Chip Microfluidic Platform
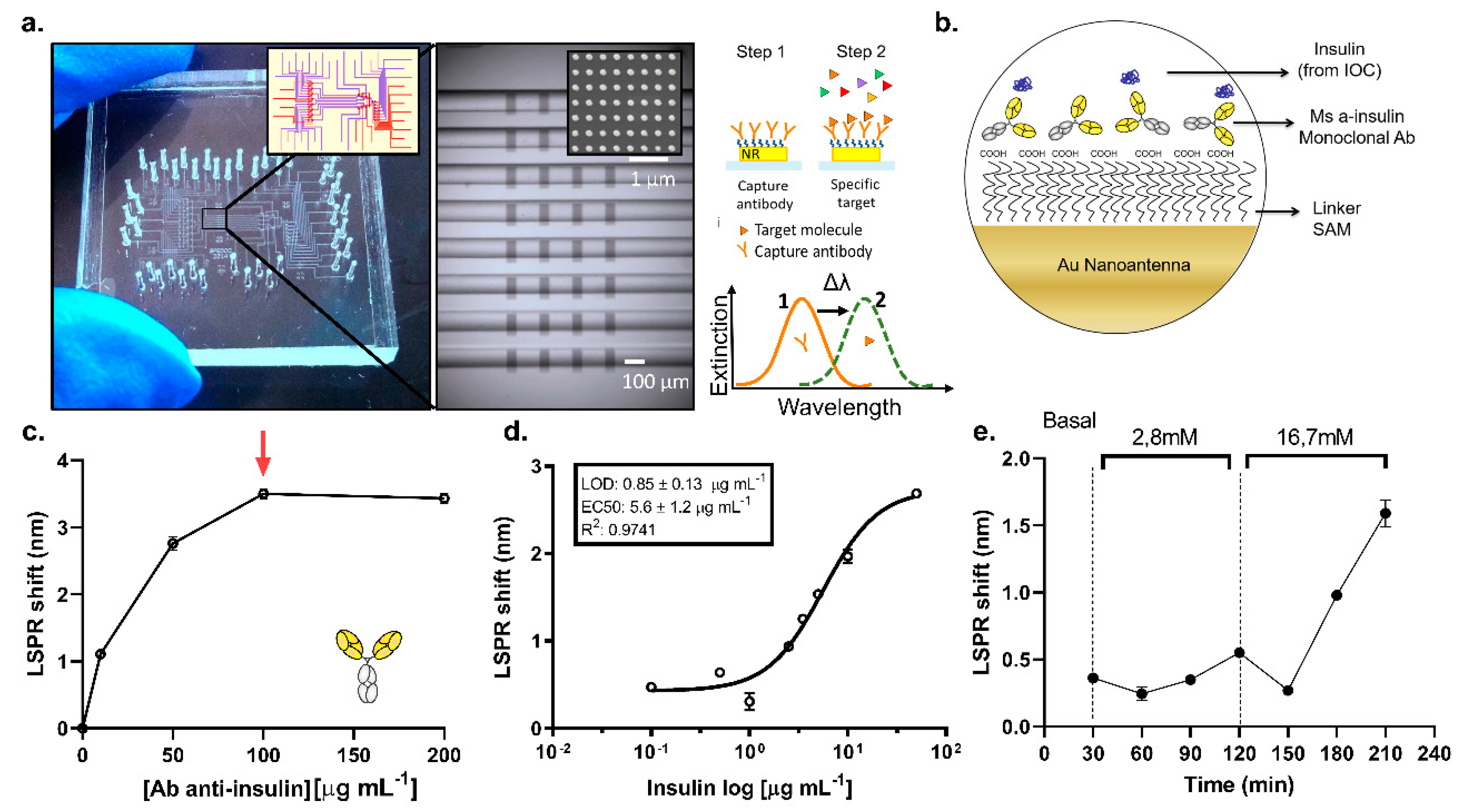
3.3. On-Chip LSPR Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation (IDF). IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; ISBN 9782930229874. [Google Scholar]
- Ortega, M.A.; Fernández-Garibay, X.; Castaño, A.G.; De Chiara, F.; Hernández-Albors, A.; Balaguer-Trias, J.; Ramón-Azcón, J. Muscle-on-a-chip with an on-site multiplexed biosensing system for: In situ monitoring of secreted IL-6 and TNF-α. Lab Chip 2019, 19, 2568–2580. [Google Scholar] [CrossRef]
- Günther, A.; Yasotharan, S.; Vagaon, A.; Lochovsky, C.; Pinto, S.; Yang, J.; Lau, C.; Voigtlaender-Bolz, J.; Bolz, S.S. A microfluidic platform for probing small artery structure and function. Lab Chip 2010, 10, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Van Midwoud, P.M.; Verpoorte, E.; Groothuis, G.M.M. Microfluidic devices for in vitro studies on liver drug metabolism and toxicity. Integr. Biol. 2011, 3, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Beaurivage, C.; Naumovska, E.; Chang, Y.X.; Elstak, E.D.; Nicolas, A.; Wouters, H.; van Moolenbroek, G.; Lanz, H.L.; Trietsch, S.J.; Joore, J.; et al. Development of a gut-on-a-chip model for high throughput disease modeling and drug discovery. Int. J. Mol. Sci. 2019, 20, 5661. [Google Scholar] [CrossRef]
- Castiello, F.R.; Heileman, K.; Tabrizian, M. Microfluidic perfusion systems for secretion fingerprint analysis of pancreatic islets: Applications, challenges and opportunities. Lab Chip 2016, 16, 409–431. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Yuan Hsin, H.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Materne, E.M.; Maschmeyer, I.; Lorenz, A.K.; Horland, R.; Schimek, K.M.S.; Busek, M.; Sonntag, F.; Lauster, R.; Marx, U. The multi-organ chip—A microfluidic platform for long-term multi-tissue coculture. J. Vis. Exp. 2015, 2015, 52526. [Google Scholar] [CrossRef]
- Bauer, S.; Wennberg Huldt, C.; Kanebratt, K.P.; Durieux, I.; Gunne, D.; Andersson, S.; Ewart, L.; Haynes, W.G.; Maschmeyer, I.; Winter, A.; et al. Functional coupling of human pancreatic islets and liver spheroids on-a-chip: Towards a novel human ex vivo type 2 diabetes model. Sci. Rep. 2017, 7, 14620. [Google Scholar] [CrossRef]
- Novak, R.; Ingram, M.; Marquez, S.; Das, D.; Delahanty, A.; Herland, A.; Maoz, B.M.; Jeanty, S.S.F.; Somayaji, M.R.; Burt, M.; et al. Robotic fluidic coupling and interrogation of multiple vascularized organ chips. Nat. Biomed. Eng. 2020. [Google Scholar] [CrossRef]
- Yu, F.; Iliescu, F.S.; Iliescu, C. A comprehensive review on perfusion cell culture systems. Inf. MIDEM 2016, 46, 163–175. [Google Scholar]
- Adam Kratz, S.R.; Höll, G.; Schuller, P.; Ertl, P.; Rothbauer, M. Latest trends in biosensing for microphysiological organs-on-a-chip and body-on-a-chip systems. Biosensors 2019, 9, 110. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Aleman, J.; Shin, S.R.; Kilic, T.; Kim, D.; Mousavi Shaegh, S.A.; Massa, S.; Riahi, R.; Chae, S.; Hu, N.; et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, E2293–E2302. [Google Scholar] [CrossRef]
- Glieberman, A.L.; Pope, B.D.; Zimmerman, J.F.; Liu, Q.; Ferrier, J.P.; Kenty, J.H.R.; Schrell, A.M.; Mukhitov, N.; Shores, K.L.; Tepole, A.B.; et al. Synchronized stimulation and continuous insulin sensing in a microfluidic human Islet on a Chip designed for scalable manufacturing. Lab Chip 2019, 19, 2993–3010. [Google Scholar] [CrossRef]
- Pedraza, E.; Karajić, A.; Raoux, M.; Perrier, R.; Pirog, A.; Lebreton, F.; Arbault, S.; Gaitan, J.; Renaud, S.; Kuhn, A.; et al. Guiding pancreatic beta cells to target electrodes in a whole-cell biosensor for diabetes. Lab Chip 2015, 15, 3880–3890. [Google Scholar] [CrossRef] [PubMed]
- Perrier, R.; Pirog, A.; Ja, M.; Gaitan, J.; Catargi, B.; Renaud, S.; Raoux, M.; Lang, J. Bioelectronic organ-based sensor for microfluidic real-time analysis of the demand in insulin. Biosens. Bioelectron. 2018, 117, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, S.S.; Ortega, M.A.; Sanz, V.; Berthelot, J.; Garcia-Cordero, J.L.; Renger, J.; Maerkl, S.J.; Kreuzer, M.P.; Quidant, R. LSPR chip for parallel, rapid, and sensitive detection of cancer markers in serum. Nano Lett. 2014, 14, 2636–2641. [Google Scholar] [CrossRef] [PubMed]
- Heo, N.S.; Oh, S.Y.; Ryu, M.Y.; Baek, S.H.; Park, T.J.; Choi, C.; Huh, Y.S.; Park, J.P. Affinity peptide-guided plasmonic biosensor for detection of noroviral protein and human norovirus. Biotechnol. Bioprocess Eng. 2019, 24, 318–325. [Google Scholar] [CrossRef]
- Kyriazi, M.-E.; Muskens, O.L.; Kanaras, A.G. DNA: Gold nanoparticles designed for mRNA sensing in cells: Imaging of the gold nanoparticles using two photon photoluminescence spectroscopy. In Proceedings of the Colloidal Nanoparticles for Biomedical Applications XIV 2019, San Francisco, CA, USA, 2–4 February 2019; Volume 10892. [Google Scholar]
- Kaye, S.; Zeng, Z.; Sanders, M.; Chittur, K.; Koelle, P.M.; Lindquist, R.; Manne, U.; Lin, Y.; Wei, J. Label-free detection of DNA hybridization with a compact LSPR-based fiber-optic sensor. Analyst 2017, 142, 1974–1981. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.; Csáki, A.; Jatschka, J.; Fritzsche, W.; Flores, O.; Franco, R.; Pereira, E. Localized surface plasmon resonance (LSPR) biosensing using gold nanotriangles: Detection of DNA hybridization events at room temperature. Analyst 2014, 139, 4964–4973. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.K.; Deitz-McElyea, S.; Johnson, M.; Mali, S.; Korc, M.; Sardar, R. Highly specific plasmonic biosensors for ultrasensitive microRNA detection in plasma from pancreatic cancer patients. Nano Lett. 2014, 14, 6955–6963. [Google Scholar] [CrossRef]
- Yavas, O.; Acimovic, S.S.; Garcia-Guirado, J.; Berthelot, J.; Dobosz, P.; Sanz, V.; Quidant, R. Self-Calibrating On-Chip Localized Surface Plasmon Resonance Sensing for Quantitative and Multiplexed Detection of Cancer Markers in Human Serum. ACS Sens. 2018, 3, 1376–1384. [Google Scholar] [CrossRef]
- Hori, T.; Yamane, K.; Anazawa, T.; Kurosawa, O.; Iwata, H. Compact fluidic system for functional assessment of pancreatic islets. Biomed. Microdevices 2019, 21, 91. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, J.S.; Wang, Y.; Harvat, T.A.; Oberholzer, J.; Eddington, D.T. Microfluidic device for multimodal characterization of pancreatic islets. Lab Chip 2009, 9, 97–106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, D.; Wang, Y.; Mendoza-Elias, J.E.; Adewola, A.F.; Harvat, T.A.; Kinzer, K.; Gutierrez, D.; Qi, M.; Eddington, D.T.; Oberholzer, J. Dual microfluidic perifusion networks for concurrent islet perifusion and optical imaging. Biomed. Microdevices 2012, 14, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.N.; Green, B.J.; Altamentova, S.M.; Rocheleau, J.V. A microfluidic device designed to induce media flow throughout pancreatic islets while limiting shear-induced damage. Lab Chip 2013, 13, 4374–4384. [Google Scholar] [CrossRef]
- Rodríguez-Comas, J.; Moreno-Vedia, J.; Obach, M.; Castano, C.; de Pablo, S.; Alcarraz-Vizan, G.; Diaz-Catalan, D.; Mestre, A.; Horrillo, R.; Costa, M.; et al. Alpha1-antitrypsin ameliorates islet amyloid-induced glucose intolerance and beta-cell dysfunction. Mol. Metab. 2020, 100984. [Google Scholar] [CrossRef]
- Jun, Y.; Lee, J.; Choi, S.; Yang, J.H.; Sander, M.; Chung, S.; Lee, S.-H. In vivo–mimicking microfluidic perfusion culture of pancreatic islet spheroids. Sci. Adv. 2019, 5, eaax4520. [Google Scholar] [CrossRef]
- Folli, F.; La Rosa, S.; Finzi, G.; Davalli, A.M.; Galli, A.; Dick, E.J.; Perego, C.; Mendoza, R.G. Pancreatic islet of Langerhans’ cytoarchitecture and ultrastructure in normal glucose tolerance and in type 2 diabetes mellitus. Diabetes Obes. Metab. 2018, 20, 137–144. [Google Scholar] [CrossRef]
- Röder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef]
- Cabrera, O.; Berman, D.M.; Kenyon, N.S.; Ricordi, C.; Berggren, P.-O.; Caicedo, A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl. Acad. Sci. USA 2006, 103, 2334–2339. [Google Scholar] [CrossRef]
- Dishinger, J.F.; Reid, K.R.; Kennedy, R.T. Quantitative monitoring of insulin secretion from microfluidic chip. Anal. Chem. 2009, 81, 3119–3127. [Google Scholar] [CrossRef]
- Schulze, T.; Mattern, K.; Früh, E.; Hecht, L.; Rustenbeck, I.; Dietzel, A. A 3D microfluidic perfusion system made from glass for multiparametric analysis of stimulus-secretion coupling in pancreatic islets. Biomed. Microdevices 2017, 19, 47. [Google Scholar] [CrossRef]
- Schrell, A.M.; Mukhitov, N.; Yi, L.; Adablah, J.E.; Menezes, J.; Roper, M.G. Online fluorescence anisotropy immunoassays for monitoring insulin secretion from isolated human islets of Langerhans. Anal. Methods 2017, 9, 38–45. [Google Scholar] [CrossRef]
- Misun, P.M.; Yesildag, B.; Forschler, F.; Neelakandhan, A.; Rousset, N.; Biernath, A.; Hierlemann, A.; Frey, O. In vitro platform for studying human insulin release dynamics of single pancreatic islet microtissues at high resolution. Adv. Biosyst. 2020, 4, 1900291. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, L.J.; Rogers, Z.J.; Colombani, T.; Memic, A.; Bencherif, S.A. Injectable Cryogels for Biomedical Applications. Trends Biotechnol. 2020, 418–431. [Google Scholar] [CrossRef]
- García-Lizarribar, A.; Fernández-Garibay, X.; Velasco-Mallorquí, F.; Castaño, A.G.; Samitier, J.; Ramon-Azcon, J. Composite Biomaterials as Long-Lasting Scaffolds for 3D Bioprinting of Highly Aligned Muscle Tissue. Macromol. Biosci. 2018, 18, 1800167. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, R. Functional Human Vascular Network Generated in Photocrosslinkable Gelatin Methacrylate Hydrogels. Adv. Funct. Mater. 2012, 22, 2027–2039. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.; Bae, H.; Hwang, C.M.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2011, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Huber, B.; Borchers, K.; Tovar, G.E.M.; Kluger, P.J. Methacrylated gelatin and mature adipocytes are promising components for adipose tissue engineering. J. Biomater. Appl. 2016, 30, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Marchioli, G.; van Gurp, L.; van Krieken, P.P.; Stamatialis, D.; Engelse, M.; van Blitterswijk, C.A.; Karperien, M.B.J.; de Koning, E.; Alblas, J.; Moroni, L.; et al. Fabrication of three-dimensional bioplotted hydrogel scaffolds for islets of Langerhans transplantation. Biofabrication 2015, 7, 025009. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.M.A.; Ladewig, K.; Haylock, D.N.; McLean, K.M.; O’Connor, A.J. Cryogels for biomedical applications. J. Mater. Chem. B 2013, 1, 2682. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.; Bertera, S.; Olsen, P.; Candiello, J.; Halfter, W.; Uechi, G.; Balasubramani, M.; Johnson, S.; Sicari, B.; Kollar, E.; et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials 2013, 34, 6760–6772. [Google Scholar] [CrossRef] [PubMed]
- Béduer, A.; Braschler, T.; Peric, O.; Fantner, G.E.; Mosser, S.; Fraering, P.C.; Benchérif, S.; Mooney, D.J.; Renaud, P. A compressible scaffold for minimally invasive delivery of large intact neuronal networks. Adv. Healthc. Mater. 2015, 4, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Mallorquí, F.; Rodríguez-Comas, J.; Ramón-Azcón, J. Cellulose-based scaffolds enhance pseudoislets formation and functionality. Biofabrication 2021, in press. [Google Scholar]
- Rodríguez-Comas, J.; Moreno-Asso, A.; Moreno-Vedia, J.; Martín, M.; Castaño, C.; Marzà-Florensa, A.; Bofill-De Ros, X.; Mir-Coll, J.; Montané, J.; Fillat, C.; et al. Stress-induced microRNA-708 impairs β-cell function and growth. Diabetes 2017, 66, 3029–3040. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Svedendahl, M.; Käll, M.; Gunnarsson, L. Dmitriev, a Ultrahigh sensitivity made simple: Nanoplasmonic label-free biosensing with an extremely low limit-of-detection for bacterial and cancer diagnostics. Nanotechnology 2009, 20, 434015. [Google Scholar] [CrossRef]
- Unger, M.A.; Chou, H.P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef]
| Gene | Species | Fw | Rv |
|---|---|---|---|
| MafA | Mouse | CAAGGAGGAGGTCATCCGAC | TCTCCAGAATGTGCCGCTG |
| Pdx1 | Mouse | CCCCAGTTTACAAGCTCGCT | CTCGGTTCCATTCGGGAAAGG |
| NeuroD1 | Mouse | GGATCAATCTTCTCTTCCGGTG | TGCGAATGGCTATCGAAAGAC |
| Ddit3/Chop | Mouse | TCATCCCCAGGAAACGAAGAG | GCTTTGGGATGTGCGTGTG |
| Trib3 | Mouse | CGTGGCACACTGCCACAAG | TCCAGGTTCTCCAGCACCAG |
| Atf3 | Mouse | GTCCGGGCTCAGAATGGAC | CGTGCCACCTCTGCTTAGCT |
| Tbp1 | Mouse | ACCCTTCACCAATGACTCCTATG | ATGATGACTGCAAATCGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, M.A.; Rodríguez-Comas, J.; Yavas, O.; Velasco-Mallorquí, F.; Balaguer-Trias, J.; Parra, V.; Novials, A.; Servitja, J.M.; Quidant, R.; Ramón-Azcón, J. In Situ LSPR Sensing of Secreted Insulin in Organ-on-Chip. Biosensors 2021, 11, 138. https://doi.org/10.3390/bios11050138
Ortega MA, Rodríguez-Comas J, Yavas O, Velasco-Mallorquí F, Balaguer-Trias J, Parra V, Novials A, Servitja JM, Quidant R, Ramón-Azcón J. In Situ LSPR Sensing of Secreted Insulin in Organ-on-Chip. Biosensors. 2021; 11(5):138. https://doi.org/10.3390/bios11050138
Chicago/Turabian StyleOrtega, María A., Júlia Rodríguez-Comas, Ozlem Yavas, Ferran Velasco-Mallorquí, Jordina Balaguer-Trias, Victor Parra, Anna Novials, Joan M. Servitja, Romain Quidant, and Javier Ramón-Azcón. 2021. "In Situ LSPR Sensing of Secreted Insulin in Organ-on-Chip" Biosensors 11, no. 5: 138. https://doi.org/10.3390/bios11050138
APA StyleOrtega, M. A., Rodríguez-Comas, J., Yavas, O., Velasco-Mallorquí, F., Balaguer-Trias, J., Parra, V., Novials, A., Servitja, J. M., Quidant, R., & Ramón-Azcón, J. (2021). In Situ LSPR Sensing of Secreted Insulin in Organ-on-Chip. Biosensors, 11(5), 138. https://doi.org/10.3390/bios11050138






