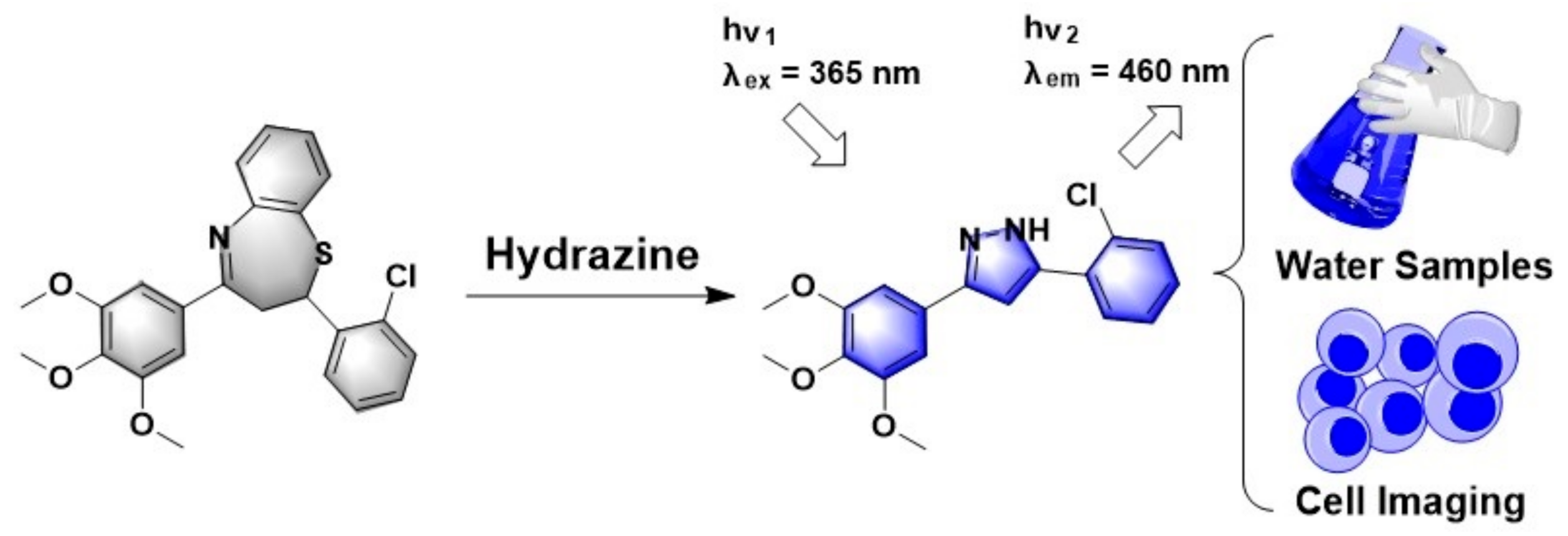
A Novel Fluorescent Probe for Selective Detection of Hydrazine and Its Application in Imaging
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Water Sample Pretreatment
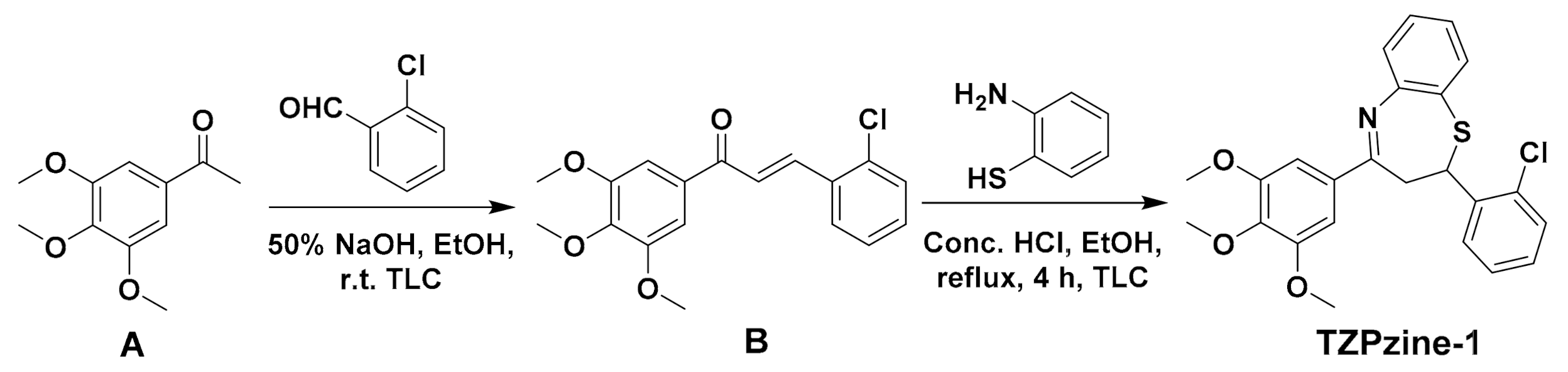
2.3. Synthesis of the Probe TZPzine-1
3. Results and Discussion
3.1. Synthesis of the Probe TZPzine-1
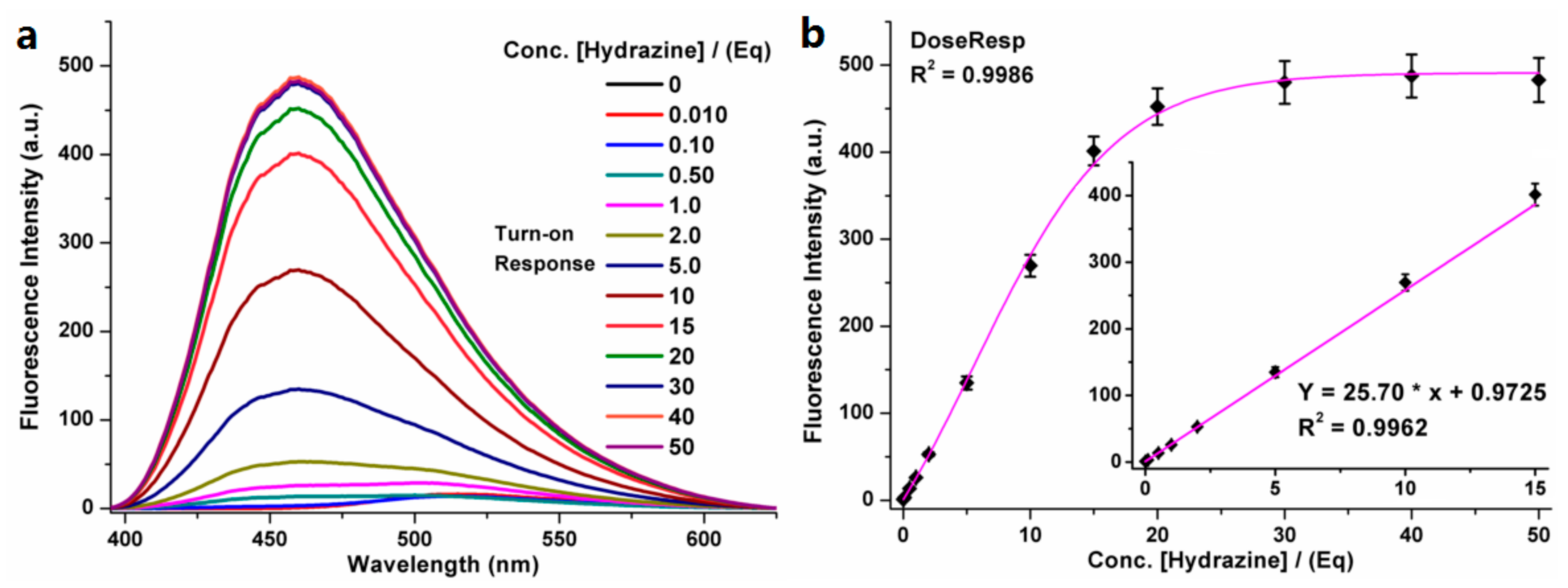
3.2. Fluorescent Properties for Detecting Hydrazine
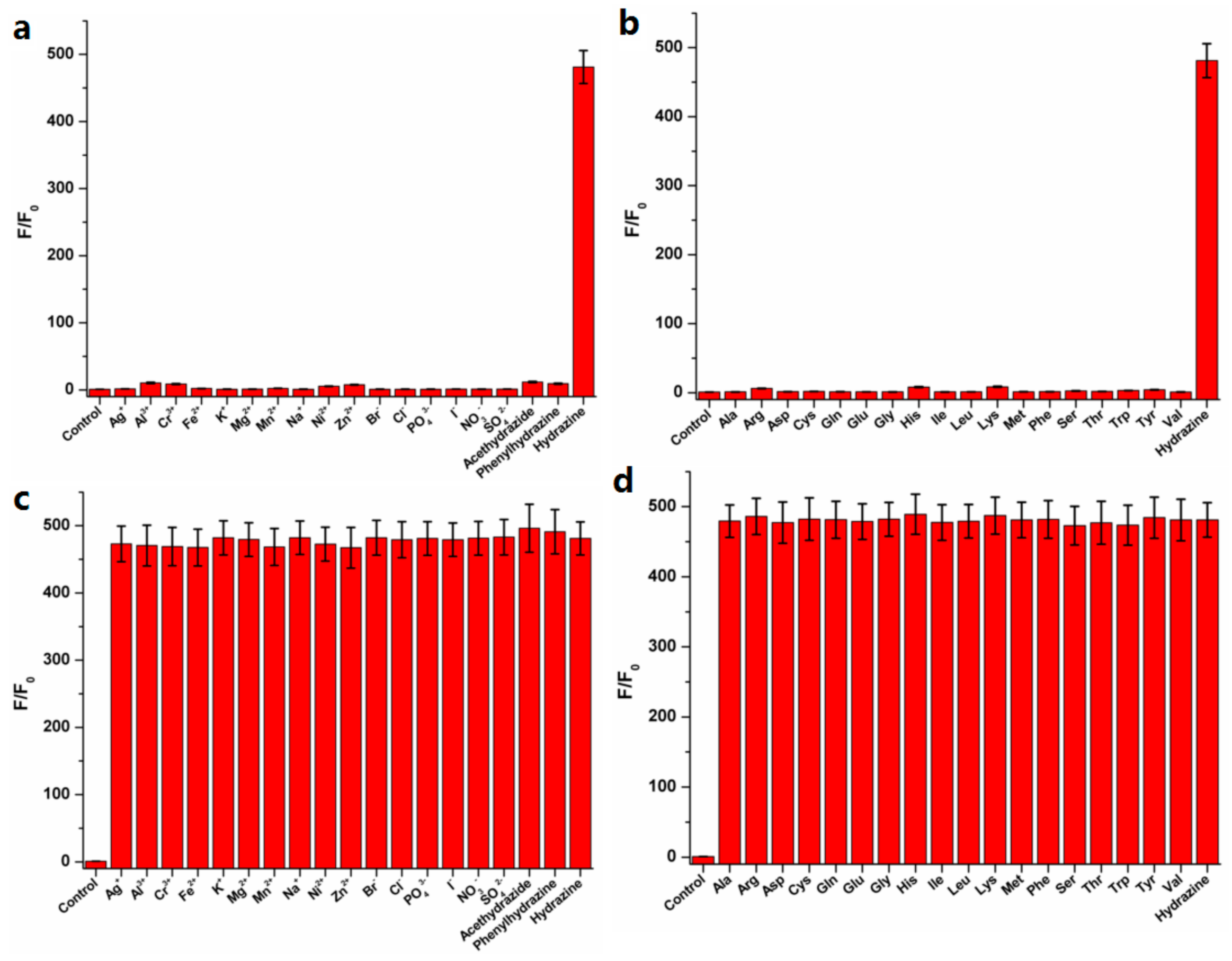
3.3. Selectivity for Hydrazine
3.4. The Reaction Mechanism
3.5. Application in Water Samples
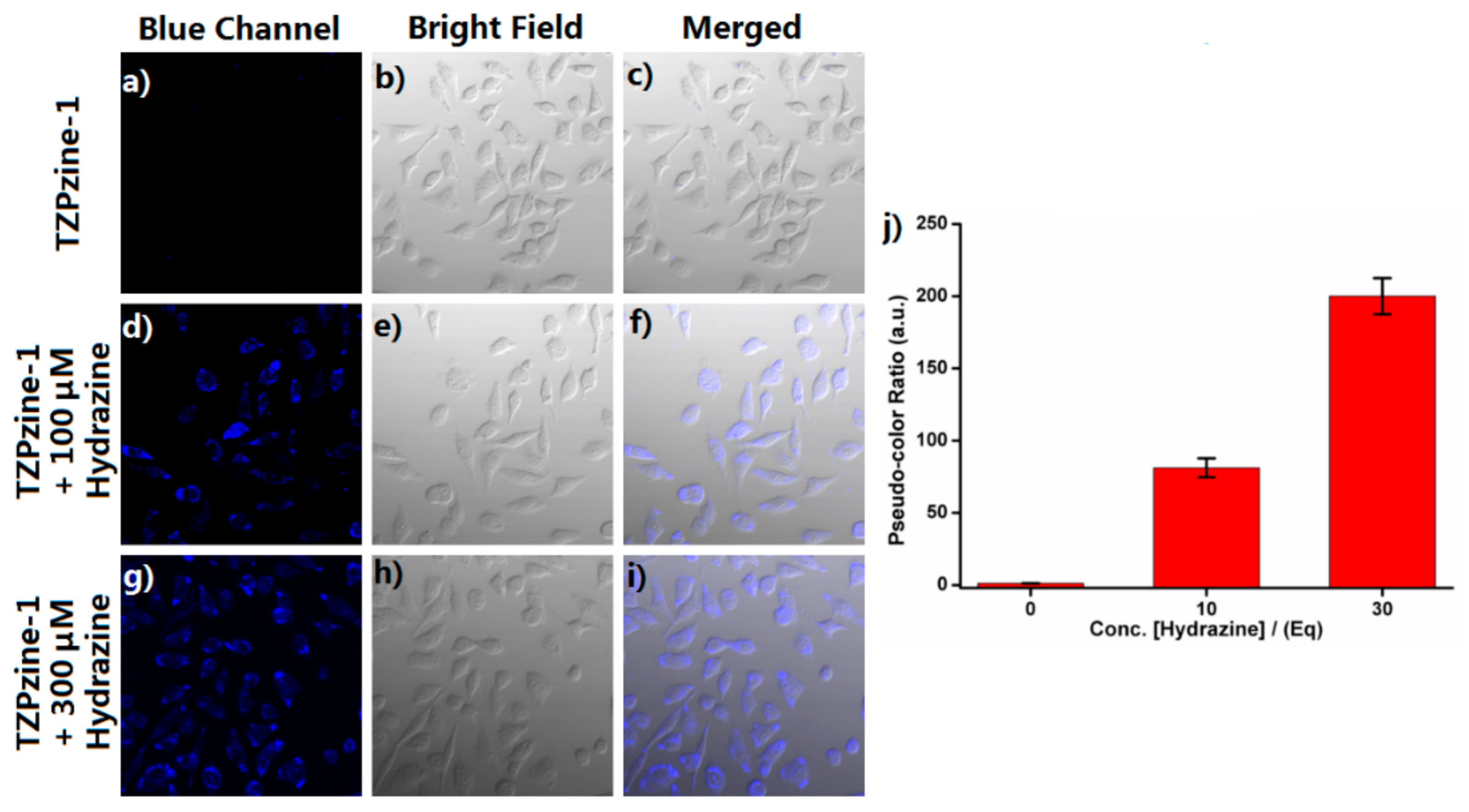
3.6. Cell Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The evolution and future of Earth’s nitrogen cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, U. Nonalternating inorganic heterocycles containing hydrazine as building block. Coord. Chem. Rev. 2002, 235, 53–91. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yang, Y.S.; Wang, W.; Jiao, Q.C.; Zhu, H.L. Fluorescent sensors for the detection of hydrazine in environmental and biological systems: Recent advances and future prospects. Coord. Chem. Rev. 2020, 417, 213367. [Google Scholar] [CrossRef]
- Gan, L.; Li, B.; Chen, Y.; Yu, B.; Chen, Z. Green synthesis of reduced graphene oxide using bagasse and its application in dye removal: A waste-to-resource supply chain. Chemosphere 2019, 219, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Tafazoli, S.; Mashregi, M.; O’Brien, P.J. Role of hydrazine in isoniazid-induced hepatotoxicity in a hepatocyte inflammation model. Toxicol. Appl. Pharm. 2008, 229, 94–101. [Google Scholar] [CrossRef]
- Zelnick, S.; Mattie, D.; Stepaniak, P. Occupational exposure to hydrazines: Treatment of acute central nervous system toxicity. Aviat. Space Environ. Med. 2003, 74, 1285–1291. [Google Scholar]
- Daemi, S.; Ashkarran, A.A.; Bahari, A.; Ghasemi, S. Fabrication of a gold nanocage/graphene nanoscale platform forelectrocatalytic detection of hydrazine. Sens. Actuat. B Chem. 2017, 245, 55–65. [Google Scholar] [CrossRef]
- Gu, X.; Camden, J.P. Surface-enhanced Raman spectroscopy-based approach for ultrasensitive and selective detection of hydrazine. Anal. Chem. 2015, 87, 6460–6464. [Google Scholar] [CrossRef]
- Arulraj, A.D.; Vijayan, M.; Vasantha, V.S. Spectrophotometric determination of pico-molar level of hydrazine by using Alizarin red in water and urine samples. Spectrochim. Acta A 2015, 148, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Baezzat, M. Flow injection chemiluminescence determination of hydrazine. Anal. Chim. Acta 1998, 358, 121–125. [Google Scholar] [CrossRef]
- Collins, G.; Rose-Pehrsson, S. Fluorescent detection of hydrazine, monomethylhydrazine, and 1,1-dimethylhydrazine by derivatization with aromatic dicarbaldehydes. Analyst 1994, 119, 1907–1913. [Google Scholar] [CrossRef]
- Malone, H. Determination of mixtures of hydrazine and 1,1-dimethylhydrazine. Anal. Chem. 1961, 33, 575–577. [Google Scholar] [CrossRef]
- Qian, Y.; Lin, J.; Han, L.J.; Lin, L.; Zhu, H.L. A resorufin-based colorimetric and fluorescent probe for live-cell monitoring of hydrazine. Biosens. Bioelectron. 2014, 58, 282–286. [Google Scholar] [CrossRef]
- Goswami, S.; Das, S.; Aich, K.; Pakhira, B.; Panja, S.; Mukherjee, S.K.; Sarkart, S. A chemodosimeter for the ratiometric detection of hydrazine based on return of ESIPT and its application in live-cell imaging. Org. Lett. 2013, 15, 5412–5415. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Yoon, B.; Kim, J.S.; Sessler, J.L. Naphthalimide trifluoroacetyl acetonate: A hydrazine-selective chemodosimetric sensor. Chem. Sci. 2013, 4, 4121–4126. [Google Scholar] [CrossRef]
- Li, J.; Cui, Y.C.; Bi, C.X.; Feng, S.Q.; Yu, F.Z.; Yuan, E.; Xu, S.Z.; Hu, Z.; Sun, Q.; Wei, D.G.; et al. Oligo (ethylene glycol)-functionalized ratiometric fluorescent probe for the detection of hydrazine in vitro and in vivo. Anal. Chem. 2019, 91, 7360–7365. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Wang, Z.Y.; Du, Z.F.; Miao, J.Y.; Zhao, B.X. A simple but effective near-infrared ratiometric fluorescent probe for hydrazine and its application in bioimaging. Sens. Actuat. B Chem. 2016, 232, 369–374. [Google Scholar] [CrossRef]
- Sun, M.D.; Guo, J.; Yang, Q.B.; Xiao, N.; Li, Y.X. A new fluorescent and colorimetric sensor for hydrazine and its application in biological systems. J. Mater. Chem. B 2014, 2, 1846–1851. [Google Scholar] [CrossRef]
- Wang, W.D.; Hu, Y.; Li, Q.; Hu, S.L. A carbazole-based turn-on fluorescent probe for the detection of hydrazine in aqueous solution. Inorg. Chim. Acta 2018, 477, 206–211. [Google Scholar] [CrossRef]
- Lu, Z.L.; Fan, W.L.; Shi, X.M.; Lu, Y.A.; Fan, C.H. Two distinctly separated emission colorimetric NIR fluorescent probe for fast hydrazine detection in living cells and mice upon independent excitations. Anal. Chem. 2017, 89, 9918–9925. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.R.; Huo, F.J.; Chao, J.B.; Yin, C.X. A ratiometric fluorescent probe for hydrazine based on novel cyclization mechanism and its application in living cells. Sens. Actuat. B Chem. 2018, 260, 609–616. [Google Scholar] [CrossRef]
- Choi, M.G.; Hwang, J.; Moon, J.O.; Sung, J.; Chang, S.K. Hydrazine-selective chromogenic and fluorogenic probe based on levulinated coumarin. Org. Lett. 2011, 13, 5260–5263. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.Q.; Zheng, J.L.; Zhang, W.C.; Wang, J.B.; Liang, W.L.; Stadler, F.J. A new quinoline-derived highly-sensitive fluorescent probe for the detection of hydrazine with excellent large-emission-shift ratiometric response. Talanta 2019, 195, 857–864. [Google Scholar] [CrossRef]
- Ali, F.; Anila, H.A.; Taye, N.; Mogare, D.G.; Chattopadhyay, S.; Das, A. Specific receptor for hydrazine: Mapping the in situ release of hydrazine in live cells and in an in vitro enzymatic assay. Chem. Commun. 2016, 52, 6166–6169. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Ju, I.G.; Choe, Y.H.; Kim, Y.; Park, S.; Hyun, Y.M.; Oh, M.S.; Kim, D. Hydrazine expos: The next-generation fluorescent probe. ACS Sens. 2019, 4, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Pan, J.; Ye, Z.; Zeng, L.T.; Su, D.D. A smart fluorescent probe for discriminative detection of hydrazine and bisulfite from different emission channels. Sens. Actuat. B Chem. 2018, 274, 274–284. [Google Scholar] [CrossRef]
- Qi, Y.L.; Chen, J.; Zhang, B.; Li, H.; Li, D.D.; Wang, B.Z.; Yang, Y.S.; Zhu, H.L. A turn-on fluorescent sensor for selective detection of hydrazine and its application in Arabidopsis thaliana. Spectrochim. Acta A 2020, 227. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Yu, Z.Y.; Li, N.; Jia, H.S.; Wei, H.; Wang, J.; Song, Y.T. Design and synthesis of dual-excitation fluorescent probe, Tb3+-dtpa-bis(fluorescein), and application in detection of hydrazine in environmental water samples and live cells. Dyes Pigment. 2019, 162, 281–294. [Google Scholar] [CrossRef]
- Meher, N.; Panda, S.; Kumar, S.; Lyer, P.K. Aldehyde group driven aggregation-induced enhanced emission in naphthalimides and its application for ultradetection of hydrazine on multiple platforms. Chem. Sci. 2018, 9, 3978–3985. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Zhang, G.; Gao, Y.; Yang, X.Y.; Li, Y.H. A novel detection technique of hydrazine hydrate: Modality change of hydrogen bonding-induced rapid and ultrasensitive colorimetric assay. Chem. Commun. 2011, 47, 12816–12818. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, H.; Tang, H.R.; Yang, C.; Guan, X.P.; Li, Y.X. Amperometric sensing of hydrazine by using single gold nanopore electrodes filled with Prussian Blue and coated with polypyrrole and carbon dots. Microchim. Acta 2019, 186, e350. [Google Scholar] [CrossRef]
- Wang, B.; Wang, L.R.; Liu, L.L.; Wang, W.; Man, R.J.; Zheng, D.J.; Deng, Y.S.; Yang, Y.S.; Xu, C.; Zhu, H.L. A novel series of benzothiazepine derivatives as tubulin polymerization inhibitors with anti-tumor potency. Bioorg. Chem. 2021, 108, 104585. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, R.; Dickson, J.; Brown, T.; Stewart, M.; Pati, H.N.; VanDerveer, D.; Arman, H.; Harris, J.; Pennington, W.; Holt, H.L., Jr.; et al. Synthesis and cytotoxicity of epoxide and pyrazole analogs of the combretastatins. Bioorg. Med. Chem. 2005, 13, 6025–6034. [Google Scholar] [CrossRef]
- Ducki, S. Antimitotic chalcones and related compounds as inhibitors of tubulin assembly. Anticancer Agents Med. Chem. 2009, 9, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Hartle, M.D.; Sommer, S.K.; Dietrich, S.R.; Pluth, M.D. Chemically reversible reactions of hydrogen sulfide with metal phthalocyanines. Inorg. Chem. 2014, 53, 7800–7802. [Google Scholar] [CrossRef]
- Strianese, M.; Lamberti, M.; Pellecchia, C. Chemically reversible binding of H2S to a zinc porphyrin complex: Towards implementation of a reversible sensor via a “coordinative-based approach”. Dalton Trans. 2017, 46, 1872–1877. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Man, R.-J.; Wu, M.-K.; Yang, B.; Yang, Y.-S. A Novel Fluorescent Probe for Selective Detection of Hydrazine and Its Application in Imaging. Biosensors 2021, 11, 130. https://doi.org/10.3390/bios11050130
Man R-J, Wu M-K, Yang B, Yang Y-S. A Novel Fluorescent Probe for Selective Detection of Hydrazine and Its Application in Imaging. Biosensors. 2021; 11(5):130. https://doi.org/10.3390/bios11050130
Chicago/Turabian StyleMan, Ruo-Jun, Meng-Ke Wu, Bing Yang, and Yu-Shun Yang. 2021. "A Novel Fluorescent Probe for Selective Detection of Hydrazine and Its Application in Imaging" Biosensors 11, no. 5: 130. https://doi.org/10.3390/bios11050130
APA StyleMan, R.-J., Wu, M.-K., Yang, B., & Yang, Y.-S. (2021). A Novel Fluorescent Probe for Selective Detection of Hydrazine and Its Application in Imaging. Biosensors, 11(5), 130. https://doi.org/10.3390/bios11050130



