Evaluation of the Diagnostic Accuracy of a New Biosensors-Based Rapid Diagnostic Test for the Point-Of-Care Diagnosis of Previous and Recent Dengue Infections in Malaysia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Index Test
2.4. Reference Standard
2.5. Analysis
2.6. Ethical Statement
3. Results
3.1. Description of the Study Participants
3.2. Diagnostic Accuracy of ViroTrack Dengue Serostate
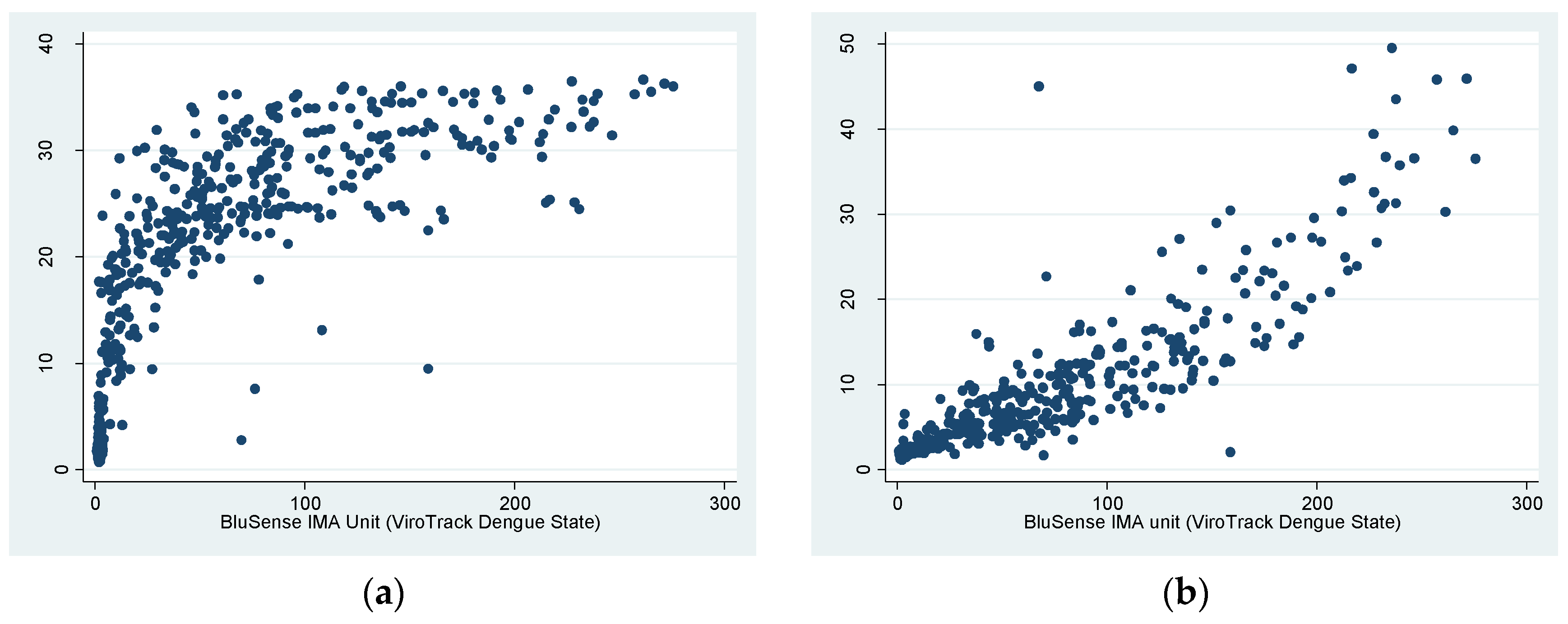
3.3. Correlation of ViroTrack Dengue Serostate and Panbio Dengue IgG ELISA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control—New Edition; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- World Health Organization. Global Strategy for Dengue Prevention and Control 2012–2020; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the Global Spatial Limits of Dengue Virus Transmission by Evidence-Based Consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef] [PubMed]
- Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Stanaway, J.D. The global economic burden of dengue: A systematic analysis. Lancet Infect. Dis. 2016, 16, 935–941. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, Z.; Wen, Z.; Liu, Y.; Zeng, C.; Xiao, D.; Ou, M.; Han, Y.; Huang, S.; Liu, D.; et al. Global Epidemiology of Dengue Outbreaks in 1990-2015: A Systematic Review and Meta-Analysis. Front. Cell Infect. Microbiol. 2017, 7, 317. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Stanaway, J.D.; Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Coffeng, L.E.; Brady, O.J.; Hay, S.I.; Bedi, N.; Bensenor, I.M.; Castañeda-Orjuela, C.A.; et al. The global burden of dengue: An analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2016, 16, 712–723. [Google Scholar] [CrossRef]
- Khetarpal, N.; Khanna, I. Dengue Fever: Causes, Complications, and Vaccine Strategies. J. Immunol. Res. 2016, 2016, 6803098. [Google Scholar] [CrossRef]
- Halstead, S.B.; Dans, L.F. Dengue infection and advances in dengue vaccines for children. Lancet Child. Adolesc. Health 2019, 3, 734–741. [Google Scholar] [CrossRef]
- Imai, N.; Dorigatti, I.; Cauchemez, S.; Ferguson, N.M. Estimating dengue transmission intensity from sero-prevalence surveys in multiple countries. PLoS Negl. Trop. Dis. 2015, 9, e0003719. [Google Scholar] [CrossRef]
- Fritzell, C.; Rousset, D.; Adde, A.; Kazanji, M.; Van Kerkhove, M.D.; Flamand, C. Current challenges and implications for dengue, chikungunya and Zika seroprevalence studies worldwide: A scoping review. PLoS Negl. Trop. Dis. 2018, 12, e0006533. [Google Scholar] [CrossRef]
- Lim, J.K.; Carabali, M.; Lee, J.S.; Lee, K.S.; Namkung, S.; Lim, S.K.; Ridde, V.; Fernandes, J.; Lell, B.; Matendechero, S.H.; et al. Evaluating dengue burden in Africa in passive fever surveillance and seroprevalence studies: Protocol of field studies of the Dengue Vaccine Initiative. BMJ Open 2018, 8, e017673. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Artsob, H.; Pelegrino, J.L.; Buchy, P.; Cardosa, M.J.; Devi, S.; Enria, D.A.; Farrar, J.; Gubler, D.J.; Guzman, M.G.; et al. Evaluation of diagnostic tests: Dengue. Nat. Rev. Microbiol. 2010, 8, S30–S38. [Google Scholar] [CrossRef]
- Miller, E.; Sikes, H.D. Addressing Barriers to the Development and Adoption of Rapid Diagnostic Tests in Global Health. Nanobiomedicine 2015, 2, 6. [Google Scholar] [CrossRef]
- Blacksell, S.D. Commercial Dengue Rapid Diagnostic Tests for Point-of-Care Application: Recent Evaluations and Future Needs? J. Biomed. Biotechnol. 2012, 2012, 151967. [Google Scholar] [CrossRef]
- Pang, J.; Chia, P.Y.; Lye, D.C.; Leo, Y.S. Progress and Challenges towards Point-of-Care Diagnostic Development for Dengue. J. Clin. Microbiol. 2017, 55, 3339–3349. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, D.W.; Nisalak, A.; Kalayanarooj, S.; Solomon, T.; Dung, N.M.; Cuzzubbo, A.; Devine, P.L. Evaluation of a rapid immunochromatographic test for diagnosis of dengue virus infection. J. Clin. Microbiol. 1998, 36, 234–238. [Google Scholar] [CrossRef]
- Luo, S.; Cui, W.; Li, C.; Ling, F.; Fu, T.; Liu, Q.; Ren, J.; Sun, J. Seroprevalence of dengue IgG antibodies in symptomatic and asymptomatic individuals three years after an outbreak in Zhejiang Province, China. BMC Infect. Dis. 2018, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Antunes, P.; Watterson, D.; Parmvi, M.; Burger, R.; Boisen, A.; Young, P.; Cooper, M.A.; Hansen, M.F.; Ranzoni, A.; Donolato, M. Quantification of NS1 dengue biomarker in serum via optomagnetic nanocluster detection. Sci. Rep. 2015, 5, 16145. [Google Scholar] [CrossRef]
- Chong, Z.L.; Sekaran, S.D.; Soe, H.J.; Peramalah, D.; Rampal, S.; Ng, C.W. Diagnostic accuracy and utility of three dengue diagnostic tests for the diagnosis of acute dengue infection in Malaysia. BMC Infect. Dis. 2020, 20, 210. [Google Scholar] [CrossRef] [PubMed]
- Imai, N.; Ferguson, N.M. Targeting vaccinations for the licensed dengue vaccine: Considerations for serosurvey design. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.; et al. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. Clin. Chem. 2015, 61, 1446–1452. [Google Scholar] [CrossRef]
- Idengue. Official Portal: Idengue for Community (Version 3.0). Available online: https://idengue.mysa.gov.my/index.php (accessed on 7 January 2021).
- Chew, C.H.; Woon, Y.L.; Amin, F.; Adnan, T.H.; Abdul Wahab, A.H.; Ahmad, Z.E.; Bujang, M.A.; Abdul Hamid, A.M.; Jamal, R.; Chen, W.S.; et al. Rural-urban comparisons of dengue seroprevalence in Malaysia. BMC Public Health 2016, 16, 824. [Google Scholar] [CrossRef]
- ESRI. Malaysia Average Household Size. Available online: http://www.arcgis.com/home/item.html?id=69f2d666483f4bf79e3e7b9c42a12d39 (accessed on 4 November 2016).
- Diagnostics, B. BluSense Diagnostics: Products. Available online: https://blusense-diagnostics.com/products/ (accessed on 3 April 2021).
- Liao, T.; Wang, X.; Donolato, M.; Harris, E.; Cruz, M.M.; Balmaseda, A.; Wang, R.Y.L. Evaluation of ViroTrack Sero Zika IgG/IgM, a New Rapid and Quantitative Zika Serological Diagnostic Assay. Diagnostics 2020, 10, 372. [Google Scholar] [CrossRef]
- Diagnostics, B. BluSense Diagnostics: Technology. Available online: https://blusense-diagnostics.com/technology/ (accessed on 3 April 2021).
- Donolato, M.; Antunes, P.; de la Torre, T.Z.; Hwu, E.T.; Chen, C.H.; Burger, R.; Rizzi, G.; Bosco, F.G.; Strømme, M.; Boisen, A.; et al. Quantification of rolling circle amplified DNA using magnetic nanobeads and a Blu-ray optical pick-up unit. Biosens. Bioelectron. 2015, 67, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Comin, A.; Toft, N.; Stegeman, A.; Klinkenberg, D.; Marangon, S. Serological diagnosis of avian influenza in poultry: Is the haemagglutination inhibition test really the ‘gold standard’? Influenza Other Respir. Viruses 2012, 7, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Maeda, J. Review of diagnostic plaque reduction neutralization tests for flavivirus infection. Vet. J. 2013, 195, 33–40. [Google Scholar] [CrossRef]
- Lukman, N.; Salim, G.; Kosasih, H.; Susanto, N.H.; Parwati, I.; Fitri, S.; Alisjahbana, B.; Widjaja, S.; Williams, M. Comparison of the Hemagglutination Inhibition Test and IgG ELISA in Categorizing Primary and Secondary Dengue Infections Based on the Plaque Reduction Neutralization Test. Biomed. Res. Int. 2016, 2016, 5253842. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, S.; Hafner, G.; Ruiz, D.; Calzada, N.; Guzman, M.G. Evaluation of immunoglobulin M and G capture enzyme-linked immunosorbent assay Panbio kits for diagnostic dengue infections. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2007, 39, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Blacksell, S.D.; Jarman, R.G.; Gibbons, R.V.; Tanganuchitcharnchai, A.; Mammen, M.P., Jr.; Nisalak, A.; Kalayanarooj, S.; Bailey, M.S.; Premaratna, R.; de Silva, H.J.; et al. Comparison of seven commercial antigen and antibody enzyme-linked immunosorbent assays for detection of acute dengue infection. Clin. Vaccine Immunol. 2012, 19, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Schüttoff, T.; Adam, A.; Reiche, S.; Jassoy, C. Enhancing the concordance of two commercial dengue IgG ELISAs by exchange of the calibrator sample. J. Clin. Virol. 2019, 118, 1–5. [Google Scholar] [CrossRef]
- Clarke, D.H.; Casals, J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am. J. Trop. Med. Hyg. 1958, 7, 561–573. [Google Scholar] [CrossRef]
- Wang, S.M.; Sekaran, S.D. Early diagnosis of Dengue infection using a commercial Dengue Duo rapid test kit for the detection of NS1, IGM, and IGG. Am. J. Trop. Med. Hyg. 2010, 83, 690–695. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Plaque Reduction Neutralization Testing of Human Antibodies to Dengue Viruses; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Ngwe Tun, M.M.; Muta, Y.; Inoue, S.; Morita, K. Persistence of Neutralizing Antibody Against Dengue Virus 2 After 70 Years from Infection in Nagasaki. Biores. Open Access 2016, 5, 188–191. [Google Scholar] [CrossRef]
- Endy, T.P.; Nisalak, A.; Chunsuttitwat, S.; Vaughn, D.W.; Green, S.; Ennis, F.A.; Rothman, A.L.; Libraty, D.H. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J. Infect. Dis. 2004, 189, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Ngwe Tun, M.M.; Thant, K.Z.; Inoue, S.; Kurosawa, Y.; Lwin, Y.Y.; Lin, S.; Aye, K.T.; Thet Khin, P.; Myint, T.; Htwe, K.; et al. Serological characterization of dengue virus infections observed among dengue hemorrhagic fever/dengue shock syndrome cases in upper Myanmar. J. Med. Virol. 2013, 85, 1258–1266. [Google Scholar] [CrossRef]
- Šimundić, A.-M. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC 2009, 19, 203–211. [Google Scholar] [PubMed]
- Chan, Y.H. Biostatistics 104: Correlational analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
- WMA. Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Available online: http://www.wma.net/en/30publications/10policies/b3/ (accessed on 8 November 2016).
- Raafat, N.; Blacksell, S.D.; Maude, R.J. A review of dengue diagnostics and implications for surveillance and control. Trans. R Soc. Trop. Med. Hyg. 2019, 113, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Kohn, M.A.; Carpenter, C.R.; Newman, T.B. Understanding the Direction of Bias in Studies of Diagnostic Test Accuracy. Acad. Emerg. Med. 2013, 20. [Google Scholar] [CrossRef]
- Mohd-Zaki, A.H.; Brett, J.; Ismail, E.; L’Azou, M. Epidemiology of dengue disease in Malaysia (2000-2012): A systematic literature review. PLoS Negl. Trop. Dis. 2014, 8, e3159. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.A.; George, A.; Salas, R.A.; Williams, S.A.; Doon, R.; Chadee, D.D. Seroprevalence of dengue in Trinidad using rapid test kits: A cord blood survey. Acta Trop. 2007, 101, 153–158. [Google Scholar] [CrossRef]
- Imai, N.; Dorigatti, I.; Cauchemez, S.; Ferguson, N.M. Estimating Dengue Transmission Intensity from Case-Notification Data from Multiple Countries. PLoS Negl. Trop. Dis. 2016, 10, e0004833. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.M. Quadas-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]


| Sample Characteristics | n (%) | ||
|---|---|---|---|
| Positive | Equivocal | Negative | |
| 383 (79.1) | - | 101 (20.9) |
| 363 (75.0) | 11 (2.3) | 110 (22.7) | |
| 356 (73.6) | - | 128 (26.4) | |
| 414 (85.5) | - | 70 (14.5) | |
| 298 (61.6) | - | 186 (38.4) | |
| 299 (61.8) | - | 185 (38.2) | |
| 296 (61.2) | - | 188 (38.8) | |
| 265 (54.8) | - | 219 (45.2) | |
| 41 (8.47) | 12 (2.48) | 431 (89.05) |
| (IgG capture ELISA only) | |||
| Estimate, % (95%CI) | Previous Dengue | Recent Dengue |
|---|---|---|
| Sensitivity | 349/383 91.1 (87.8–93.8) | 37/41 90.2 (76.9–97.3) |
| Specificity | 92/101 91.1 (83.8–95.8) | 413/443 93.2 (90.5–95.4) |
| Positive Predictive Value | 349/358 97.5 (95.3–98.8) | 37/67 55.2 (42.6–67.4) |
| Negative Predictive Value | 92/126 73.0 (64.4–80.5) | 413/417 99.0 (97.6–99.7) |
| Estimate, % (95%CI) | IgG Indirect ELISA Only | Hemagglutination Inhibition Only | FRNT95 Only |
|---|---|---|---|
| Sensitivity | 346/363 95.3 (92.6–97.3) | 326/356 91.6 (88.2–94.2) | 313/414 75.6 (71.2–79.7) |
| Specificity | 109/121 90.1 (83.3–94.8) | 96/128 75.0 (66.6–82.2) | 25/70 35.7 (24.6–48.1) |
| Positive Predictive Value | 346/358 96.7 (94.2–98.3) | 326/358 91.1 (87.6–93.8) | 313/358 87.4 (83.5–90.7) |
| Negative Predictive Value | 109/126 86.5 (79.3–91.9) | 96/126 76.2 (67.8–83.3) | 25/126 19.8 (13.3–27.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chong, Z.L.; Soe, H.J.; Ismail, A.A.; Mahboob, T.; Chandramathi, S.; Sekaran, S.D. Evaluation of the Diagnostic Accuracy of a New Biosensors-Based Rapid Diagnostic Test for the Point-Of-Care Diagnosis of Previous and Recent Dengue Infections in Malaysia. Biosensors 2021, 11, 129. https://doi.org/10.3390/bios11050129
Chong ZL, Soe HJ, Ismail AA, Mahboob T, Chandramathi S, Sekaran SD. Evaluation of the Diagnostic Accuracy of a New Biosensors-Based Rapid Diagnostic Test for the Point-Of-Care Diagnosis of Previous and Recent Dengue Infections in Malaysia. Biosensors. 2021; 11(5):129. https://doi.org/10.3390/bios11050129
Chicago/Turabian StyleChong, Zhuo Lin, Hui Jen Soe, Amni Adilah Ismail, Tooba Mahboob, Samudi Chandramathi, and Shamala Devi Sekaran. 2021. "Evaluation of the Diagnostic Accuracy of a New Biosensors-Based Rapid Diagnostic Test for the Point-Of-Care Diagnosis of Previous and Recent Dengue Infections in Malaysia" Biosensors 11, no. 5: 129. https://doi.org/10.3390/bios11050129
APA StyleChong, Z. L., Soe, H. J., Ismail, A. A., Mahboob, T., Chandramathi, S., & Sekaran, S. D. (2021). Evaluation of the Diagnostic Accuracy of a New Biosensors-Based Rapid Diagnostic Test for the Point-Of-Care Diagnosis of Previous and Recent Dengue Infections in Malaysia. Biosensors, 11(5), 129. https://doi.org/10.3390/bios11050129





