NQS-Doped PDMS Solid Sensor: From Water Matrix to Urine Enzymatic Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Apparatus
2.3. Preparation of PDMS/TEOS-NQS-SiO2 NPs Sensing Membranes
2.4. Analytical Response Measurements
2.4.1. Ammonium Measurement in Aqueous Matrix
2.4.2. Urea Measurement in Urine Matrix
2.4.3. Direct Urea Hydrolysis
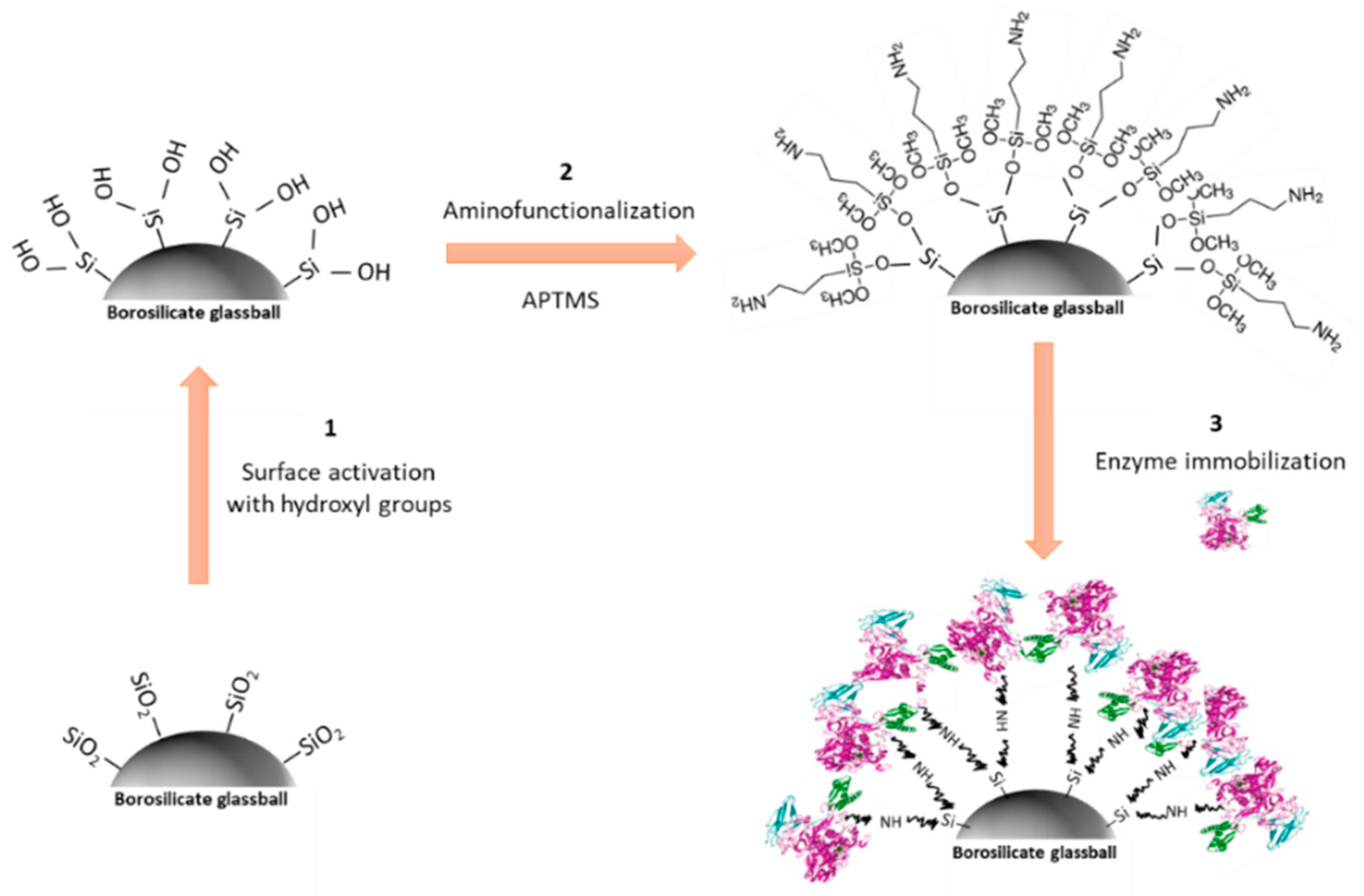
2.4.4. Solid Supported Urea Hydrolysis
3. Results
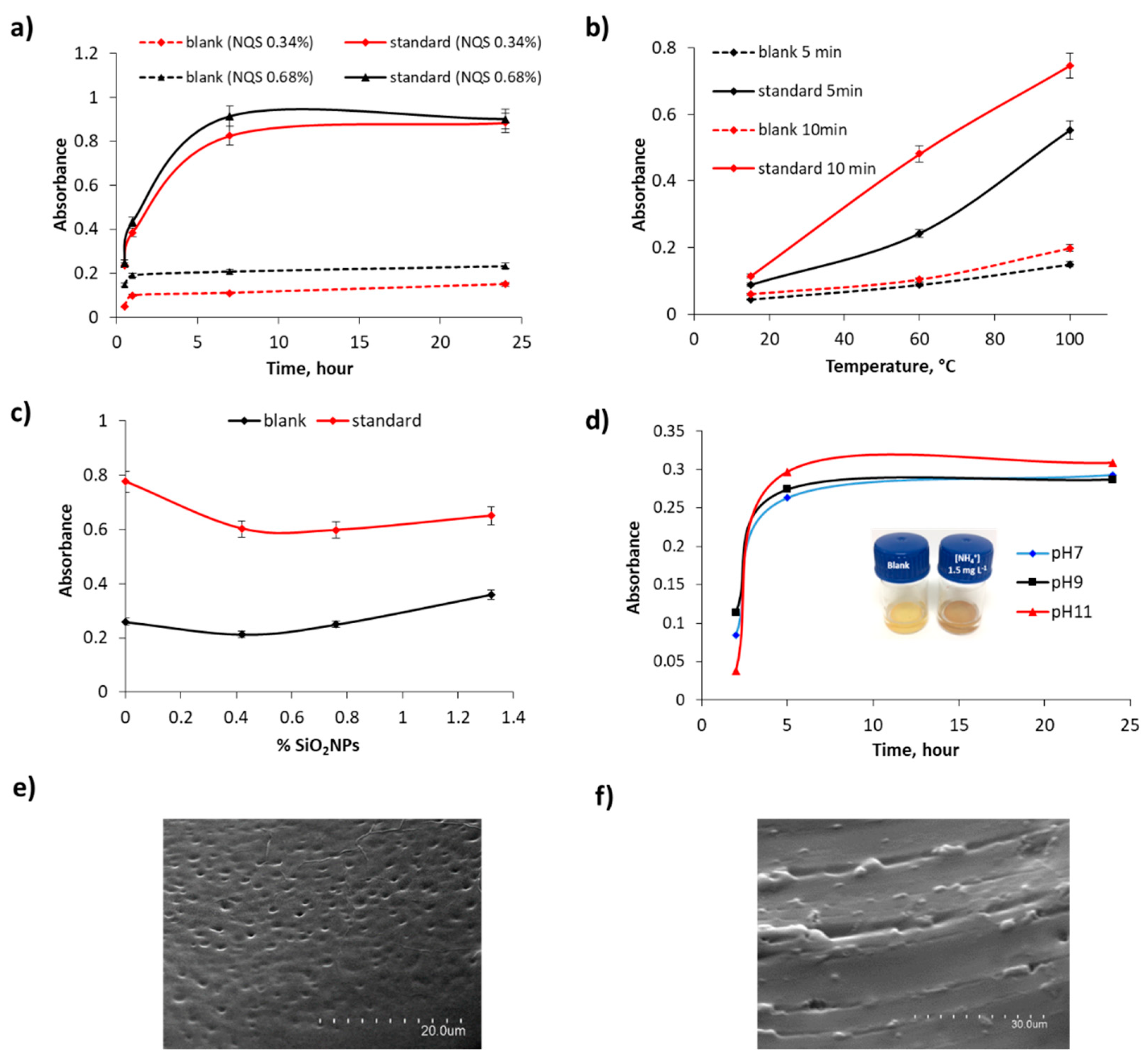
3.1. Performance of Solid Supported NQS-PDMS/TEOS-SiO2 NPs for Ammonium and Urea
3.2. Sensitivity for Ammonium and Urea Standard Solutions
3.3. Sensor Device Performance for Hydrolyzed Urea: Urease in Solution vs. Immobilized on Borosilicate Glass Balls
4. Proof of Concepts
4.1. Water Matrix
4.2. Urine Matrix: Direct Urea and Urease-Catalyzed Hydrolysis Sensing
5. Validation of NQS-Based Sensing Device: Thymol Sensor
6. In Situ Analysis
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ni, J.-Q.; Diehl, C.A.; Chai, L.; Chen, Y.; Heber, A.J.; Lim, T.-T.; Bogan, B.W. Factors and characteristics of ammonia, hydrogen sulfide, carbon dioxide, and particulate matter emissions from two manure-belt layer hen houses. Atmos. Environ. 2017, 156, 113–124. [Google Scholar] [CrossRef]
- Williams Ischer, S.; Farnell, M.B.; Tabler, G.T.; Moreira, M.; O’Shaughnessy, P.T.; Nonnenmann, M.W. Evaluation of a sprinkler cooling system on inhalable dust and ammonia concentrations in broiler chicken production. J. Occup. Environ. Hyg. 2017, 14, 40–48. [Google Scholar] [CrossRef]
- Zhao, L.; Hadlocon, L.J.S.; Manuzon, R.B.; Darr, M.J.; Keener, H.M.; Heber, A.J.; Ni, J. Ammonia concentrations and emission rates at a commercial poultry manure composting facility. Biosyst. Eng. 2016, 150, 69–78. [Google Scholar] [CrossRef]
- Zimmerman, J.B.; Mihelcic, J.R.; Smith, A.J. Global Stressors on Water Quality and Quantity. Environ. Sci. Technol. 2008, 42, 4247–4254. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Watson, C.J.; Yan, M.J.; Lalor, S.; Rafique, R.; Hyde, B.; Lanigan, G.; Richards, K.G.; Holden, N.M.; Humphreys, J. A review of nitrous oxide mitigation by farm nitrogen management in temperate grassland-based agriculture. J. Environ. Manag. 2013, 128, 893–903. [Google Scholar] [CrossRef]
- DIRECTIVE 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy, L-327/1 (2000), Luxembourg. Available online: https://eur-lex.europa.eu/eli/dir/2000/60/oj (accessed on 10 May 2021).
- Di Blasi, J.I.P.; Martínez Torres, J.; García Nieto, P.J.; Alonso Fernández, J.R.; Díaz Muñiz, C.; Taboada, J. Analysis and detection of outliers in water quality parameters from different automated monitoring stations in the Miño river basin (NW Spain). Ecol. Eng. 2013, 60, 60–66. [Google Scholar] [CrossRef]
- Jornet-Martínez, N.; Moliner-Martínez, Y.; Molins-Legua, C.; Campíns-Falcó, P. Trends for the Development of In Situ Analysis Devices. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 1–23. [Google Scholar]
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical sensors: Definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Prieto-Blanco, M.C.; Jornet-Martínez, N.; Moliner-Martínez, Y.; Molins-Legua, C.; Herráez-Hernández, R.; Verdú Andrés, J.; Campins-Falcó, P. Development of a polydimethylsiloxane–thymol/nitroprusside composite based sensor involving thymol derivatization for ammonium monitoring in water samples. Sci. Total Environ. 2015, 503–504, 105–112. [Google Scholar] [CrossRef]
- Prieto-Blanco, M.C.; Ballester-Caudet, A.; Souto-Varela, F.J.; López-Mahía, P.; Campíns-Falcó, P. Rapid evaluation of ammonium in different rain events minimizing needed volume by a cost-effective and sustainable PDMS supported solid sensor. Environ. Pollut. 2020, 265, 114911. [Google Scholar] [CrossRef]
- Xu, M.; Bunes, B.R.; Zang, L. Paper-Based Vapor Detection of Hydrogen Peroxide: Colorimetric Sensing with Tunable Interface. ACS Appl. Mater. Interfaces 2011, 3, 642–647. [Google Scholar] [CrossRef]
- Jornet-Martínez, N.; Hakobyan, L.; Argente-García, A.I.; Molins-Legua, C.; Campíns-Falcó, P. Nylon-Supported Plasmonic Assay Based on the Aggregation of Silver Nanoparticles: In Situ Determination of Hydrogen Sulfide-like Compounds in Breath Samples as a Proof of Concept. ACS Sens. 2019, 4, 2164–2172. [Google Scholar] [CrossRef]
- Muñoz-Ortuño, M.; Argente-García, A.; Moliner-Martínez, Y.; Molins-Legua, C.; Campíns-Falcó, P. Polydimethylsiloxane composites containing 1,2-naphtoquinone 4-sulphonate as unique dispositive for estimation of casein in effluents from dairy industries. Anal. Chim. Acta 2015, 873, 31–37. [Google Scholar] [CrossRef]
- Argente-García, A.; Jornet-Martínez, N.; Herráez-Hernández, R.; Campíns-Falcó, P. A solid colorimetric sensor for the analysis of amphetamine-like street samples. Anal. Chim. Acta 2016, 943, 123–130. [Google Scholar] [CrossRef]
- Jornet-Martínez, N.; Moliner-Martínez, Y.; Herráez-Hernández, R.; Molins-Legua, C.; Verdú-Andrés, J.; Campíns-Falcó, P. Designing solid optical sensors for in situ passive discrimination of volatile amines based on a new one-step hydrophilic PDMS preparation. Sens. Actuators B Chem. 2016, 223, 333–342. [Google Scholar] [CrossRef]
- Campíns-Falcó, P.; Moliner-Martínez, Y.; Herráez-Hernández, R.; Molins-Legua, C.; Verdú-Andrés, J. Passive Sensor for In Situ Amines Detection in Atmospheres. Spain Patent P201300436, 10 February 2018. [Google Scholar]
- Argente-García, A.; Muñoz-Ortuño, M.; Molins-Legua, C.; Moliner-Martínez, Y.; Campíns-Falcó, P. A solid device based on doped hybrid composites for controlling the dosage of the biocide N-(3-aminopropyl)-N-dodecyl-1,3-propanediamine in industrial formulations. Talanta 2016, 147, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Singh, R.K.; Chandra, S. Ionic liquids confined in porous matrices: Physicochemical properties and applications. Prog. Mater. Sci. 2014, 64, 73–120. [Google Scholar] [CrossRef]
- Muginova, S.V.; Myasnikova, D.; Kazarian, S.G.; Shekhovtsova, T.N. Applications of ionic liquids for the development of optical chemical sensors and biosensors. Anal. Sci. 2017, 33, 261. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.I.; Panzer, M.J. Poly(dimethylsiloxane)-Supported Ionogels with a High Ionic Liquid Loading. Angew. Chem. Int. Ed. 2014, 53, 9780–9783. [Google Scholar] [CrossRef]
- Le Bideau, J.; Viau, L.; Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef]
- Clark, K.D.; Zhang, C.; Anderson, J.L. Sample Preparation for Bioanalytical and Pharmaceutical Analysis. Anal. Chem. 2016, 88, 11262–11270. [Google Scholar] [CrossRef]
- Ballester-Caudet, A.; Hakobyan, L.; Moliner-Martinez, Y.; Molins-Legua, C.; Campíns-Falcó, P. Ionic-liquid doped polymeric composite as passive colorimetric sensor for meat freshness as a use case. Talanta 2021, 223, 121778. [Google Scholar] [CrossRef]
- Lambert, D.F.; Sherwood, J.E.; Francis, P.S. The determination of urea in soil extracts and related samples—A review. Soil Res. 2004, 42, 709. [Google Scholar] [CrossRef]
- Safitri, E.; Heng, L.Y.; Ahmad, M.; Ling, T.L. Fluorescence bioanalytical method for urea determination based on water soluble ZnS quantum dots. Sens. Actuators B Chem. 2017, 240, 763–769. [Google Scholar] [CrossRef]
- Alizadeh, T.; Ganjali, M.R.; Rafiei, F. Trace level and highly selective determination of urea in various real samples based upon voltammetric analysis of diacetylmonoxime-urea reaction product on the carbon nanotube/carbon paste electrode. Anal. Chim. Acta 2017, 974, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Tůma, P.; Samcová, E.; Duška, F. Determination of ammonia, creatinine and inorganic cations in urine using CE with contactless conductivity detection. J. Sep. Sci. 2008, 31, 2260–2264. [Google Scholar] [CrossRef]
- Mizobuchi, M.; Tamase, K.; Kitada, Y.; Sasaki, M.; Tanigawa, K. High-performance liquid chromatographic analysis of ammonium in human urine. Anal. Biochem. 1984, 137, 88–92. [Google Scholar] [CrossRef]
- Raphael, K.L.; Carroll, D.J.; Murray, J.; Greene, T.; Beddhu, S. Urine Ammonium Predicts Clinical Outcomes in Hypertensive Kidney Disease. J. Am. Soc. Nephrol. 2017, 28, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.U.; Ibupoto, Z.H.; Salman, S.; Nur, O.; Willander, M.; Danielsson, B. Selective determination of urea using urease immobilized on ZnO nanowires. Sens. Actuators B Chem. 2011, 160, 637–643. [Google Scholar] [CrossRef]
- Prats-Alfonso, E.; Abad, L.; Casañ-Pastor, N.; Gonzalo-Ruiz, J.; Baldrich, E. Iridium oxide pH sensor for biomedical applications. Case urea–urease in real urine samples. Biosens. Bioelectron. 2013, 39, 163–169. [Google Scholar] [CrossRef]
- Velychko, T.P.; Soldatkin, O.O.; Melnyk, V.G.; Marchenko, S.V.; Kirdeciler, S.K.; Akata, B.; Soldatkin, A.P.; El’skaya, A.V.; Dzyadevych, S.V. A Novel Conductometric Urea Biosensor with Improved Analytical Characteristic Based on Recombinant Urease Adsorbed on Nanoparticle of Silicalite. Nanoscale Res. Lett. 2016, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, C. Single-enzyme nanoparticles based urea biosensor. Sens. Actuators B Chem. 2013, 188, 313–317. [Google Scholar] [CrossRef]
- Kuralay, F.; Özyörük, H.; Yıldız, A. Amperometric enzyme electrode for urea determination using immobilized urease in poly(vinylferrocenium) film. Sens. Actuators B Chem. 2006, 114, 500–506. [Google Scholar] [CrossRef]
- Danial, E.N.; Hamza, A.H.; Mahmoud, R.H. Characteristics of Immobilized Urease on Grafted Alginate Bead Systems. Brazilian Arch. Biol. Technol. 2015, 58, 147–153. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef]
- Zucca, P.; Sanjust, E. Inorganic Materials as Supports for Covalent Enzyme Immobilization: Methods and Mechanisms. Molecules 2014, 19, 14139–14194. [Google Scholar] [CrossRef] [PubMed]
- Bocanegra-Rodríguez, S.; Jornet-Martínez, N.; Molins-Legua, C.; Campíns-Falcó, P. New Reusable Solid Biosensor with Covalent Immobilization of the Horseradish Peroxidase Enzyme: In Situ Liberation Studies of Hydrogen Peroxide by Portable Chemiluminescent Determination. ACS Omega 2020, 5, 2419–2427. [Google Scholar] [CrossRef]
- Tanii, T.; Hosaka, T.; Miyake, T.; Zhang, G.-J.; Zako, T.; Funatsu, T.; Ohdomari, I. Preferential immobilization of biomolecules on silicon microstructure array by means of electron beam lithography on organosilane self-assembled monolayer resist. Appl. Surf. Sci. 2004, 234, 102–106. [Google Scholar] [CrossRef]
- Kunduru, K.R.; Basu, A.; Abtew, E.; Tsach, T.; Domb, A.J. Polymeric sensors containing P-dimethylaminocinnamaldehyde: Colorimetric detection of urea nitrate. Sens. Actuators B Chem. 2017, 238, 387–391. [Google Scholar] [CrossRef]
- Suarez, W.T.; de Alvarenga Junior, B.R.; de Oliveira Krambeck Franco, M.; Gabriel, W.L.; de Oliveira, D.M.; dos Santos, V.B. In Situ Determination of Urea in Milk Employing a Portable and Low-Cost LED Photometer. Food Anal. Methods 2018, 11, 1149–1154. [Google Scholar] [CrossRef]
- Hassan, R.Y.A.; Kamel, A.M.; Hashem, M.S.; Hassan, H.N.A.; Abd El-Ghaffar, M.A. A new disposable biosensor platform: Carbon nanotube/poly(o-toluidine) nanocomposite for direct biosensing of urea. J. Solid State Electrochem. 2018, 22, 1817–1823. [Google Scholar] [CrossRef]
- Ibarlucea, B.; Fernández-Sánchez, C.; Demming, S.; Büttgenbach, S.; Llobera, A. Selective functionalisation of PDMS-based photonic lab on a chip for biosensing. Analyst 2011, 136, 3496. [Google Scholar] [CrossRef]
- Kreider, A.; Richter, K.; Sell, S.; Fenske, M.; Tornow, C.; Stenzel, V.; Grunwald, I. Functionalization of PDMS modified and plasma activated two-component polyurethane coatings by surface attachment of enzymes. Appl. Surf. Sci. 2013, 273, 562–569. [Google Scholar] [CrossRef]
- Vianello, F.; Zennaro, L.; Rigo, A. A coulometric biosensor to determine hydrogen peroxide using a monomolecular layer of horseradish peroxidase immobilized on a glass surface. Biosens. Bioelectron. 2007, 22, 2694–2699. [Google Scholar] [CrossRef] [PubMed]
- Tricoli, A.; Righettoni, M.; Teleki, A. Semiconductor Gas Sensors: Dry Synthesis and Application. Angew. Chem. Int. Ed. 2010, 49, 7632–7659. [Google Scholar] [CrossRef]
- Cha, C.; Antoniadou, E.; Lee, M.; Jeong, J.H.; Ahmed, W.W.; Saif, T.A.; Boppart, S.A.; Kong, H. Tailoring Hydrogel Adhesion to Polydimethylsiloxane Substrates Using Polysaccharide Glue. Angew. Chem. Int. Ed. 2013, 52, 6949–6952. [Google Scholar] [CrossRef]
- Ding, H.; Chen, C.; Qi, S.; Han, C.; Yue, C. Smartphone-based spectrometer with high spectral accuracy for mHealth application. Sens. Actuators A Phys. 2018, 274, 94–100. [Google Scholar] [CrossRef]
- Saraji, M.; Bagheri, N. Paper-based headspace extraction combined with digital image analysis for trace determination of cyanide in water samples. Sens. Actuators B Chem. 2018, 270, 28–34. [Google Scholar] [CrossRef]
- Meseguer-Lloret, S.; Molins-Legua, C.; Campins-Falco, P. Ammonium Determination in Water Samples by Using Opa-Nac Reagent: A Comparative Study with Nessler and Ammonium Selective Electrode Methods. Int. J. Environ. Anal. Chem. 2002, 82, 475–489. [Google Scholar] [CrossRef]
- Liang, Y.; Yan, C.; Guo, Q.; Xu, J.; Hu, H. Spectrophotometric determination of ammonia nitrogen in water by flow injection analysis based on NH3-o-phthalaldehyde-Na2SO3 reaction. Anal. Chem. Res. 2016, 10, 1–8. [Google Scholar] [CrossRef]
- Peters, J.J.; Almeida, M.I.G.S.; O’Connor Šraj, L.; McKelvie, I.D.; Kolev, S.D. Development of a micro-distillation microfluidic paper-based analytical device as a screening tool for total ammonia monitoring in freshwaters. Anal. Chim. Acta 2019, 1079, 120–128. [Google Scholar] [CrossRef]
- Kan, Y. An All-Solid-State Ammonium Ion-Selective Electrode Based on Polyaniline as Transducer and Poly (o-phenylenediamine) as Sensitive Membrane. Int. J. Electrochem. Sci. 2016, 11, 9928–9940. [Google Scholar] [CrossRef]
- Saeedfar, K.; Heng, L.; Ling, T.; Rezayi, M. Potentiometric Urea Biosensor Based on an Immobilised Fullerene-Urease Bio-Conjugate. Sensors 2013, 13, 16851–16866. [Google Scholar] [CrossRef]
- Rajesh; Bisht, V.; Takashima, W.; Kaneto, K. An amperometric urea biosensor based on covalent immobilization of urease onto an electrochemically prepared copolymer poly (N-3-aminopropyl pyrrole-co-pyrrole) film. Biomaterials 2005, 26, 3683–3690. [Google Scholar]
- Tsai, H.; Doong, R. Simultaneous determination of pH, urea, acetylcholine and heavy metals using array-based enzymatic optical biosensor. Biosens. Bioelectron. 2005, 20, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- De Melo, J.V.; Cosnier, S.; Mousty, C.; Martelet, C.; Jaffrezic-Renault, N. Urea Biosensors Based on Immobilization of Urease into Two Oppositely Charged Clays (Laponite and Zn-Al Layered Double Hydroxides). Anal. Chem. 2002, 74, 4037–4043. [Google Scholar] [CrossRef] [PubMed]



| Analyte | Instrumentation | Linearity (y = b0 + b1x) | ||
|---|---|---|---|---|
| b0 ± sbo | b1 ± sb1 (L mg−1) | R2 | ||
| Ammonia | Conventional | 0.007 ± 0.010 | 0.035 ± 0.002 | 0.990 |
| Portable | 0.006 ± 0.010 | 0.0316 ± 0.0019 | 0.999 | |
| Smartphone | 193 ± 4 | −5.9 ± 0.7 (no units) | 0.957 | |
| Urea | Conventional | 0.003 ± 0.010 | 0.153 ± 0.008 | 0.995 |
| −0.004 ± 0.009 | 0.149 ± 0.005 | 0.998 | ||
| Ammonia 1 | Conventional | 0.014 ± 0.016 | 0.032 ± 0.003 | 0.990 |
| Ammonia 2 | Conventional | 0.002 ± 0.005 | 0.033 ± 0.001 | 0.998 |
| Vurease (µL) | Abs 590 nm | [NH3] (mg L−1) | Recovery (%) |
|---|---|---|---|
| 0 | 0.2050 | - | - |
| 10 | 0.3820 | 5 | 95 |
| 20 | 0.4806, 0.4782 | 6.2, 6.1 | 117, 116 |
| 40 | 0.4984, 0.4911 | 6.7, 6.5 | 127, 123 |
| PLANT 1 | After Dilution Concentration ± s | Real Concentration ± s | |
 | Entrance | 6.15 ± 0.16 | 123.0 ± 3.2 |
 | Decantation | 6.99 ± 0.11 | 139.8 ± 2.2 |
 | Exit | 2.42 ± 0.09 | 4.82 ± 0.18 |
| PLANT 2 | Found Concentration (mg L−1) (n = 3) | Recovery (%) (n = 3) | |
| Anoxic reactor | 18.0 ± 0.7 1 | 95 ± 5 | |
| 4.8 ± 0.2 2 | 90 ± 7 | ||
| Oxic reactor | 2.5 ± 0.2 1 | 87 ± 7 | |
| <LOD 2 | 100 ± 4 |
| Urine | Absorbance 590 nm | Urea Concentration (g L−1) | |
|---|---|---|---|
| Untreated | Deproteinized | ||
| Sample 1 | 0.6615 | 0.3869 | 27.7 |
| Sample 2 | 0.5654 | 0.2895 | 20.7 |
| Urine | Urea Concentration (g L−1) | |
|---|---|---|
| Hydrolysis in Solution | Glass Supported Hydrolysis | |
| Sample 1 | 12.3 | 11.8 |
| Sample 2 | 12.7 | 11.8 |
| Average | 12.5 ± 0.3 | 12 ± 0 |
| Ammonia | b1 ± sb1 (L mg−1) | R2 |
|---|---|---|
| Hydrolyzed urea | 0.204 ± 0.005 | 0.983 |
| Hydrolyzed urine | 0.194 ± 0.006 | 0.999 |
| 0.193 ± 0.007 | 0.999 |
| AMMONIUM | |||||
|---|---|---|---|---|---|
| Option/Ref | Technique | Reagent(s) | Time of Analysis (s) | LOD (mg·L−1) | Sample |
| Colorimetric sensor by reagent delivering/[10,11] | UV-vis spectroscopy | PDMS, thymol or salicylate, nitroprusside | 600/300 | 0.4/0.03 | Environmental water |
| Ammonia selective electrode/[51] | Potentiometry | Derivatizing OPA-NAC reagents | 300 | 0.07 | Environmental water |
| Chemical reaction/[52] | FIA/UV-vis spectroscopy | OPA-Na2SO3 mixed reagent solution | 600 | 0.13 | Natural water |
| 3D microfluidic paper-based device/[53] | Digital image processing/Reflectance | Nitrazine yellow (NY)/ bromothymol blue (BTB) as indicators | 300 | 0.41/0.6 | Freshwater |
| Ion-selective electrode (ISE)/[54] | Potentiometry | Ag/nano-Ag/polyaniline/poly (o-phenylenediamine) doped electrode | 300 | 0.22 | Tap water |
| Solid colorimetric sensor/this work | Diffuse reflectance spectrophotometry /digital image processing | 1,2-naphthoquinone-4-sulfonate (NQS), PDMS | 600 | 0.4 | Environmental water |
| UREA | |||||
| Urea biosensor/[55] | Potentiometry | Urease, surface-modified fullerene nanomaterial | 55 | 2.4 | Urine |
| Urea biosensor/[56] | Amperometry | Urease, poly(3-aminopropyl-pyrrole-co-pyrrole) support, electrochemical deposition on indium-tin- oxide-coated glass | 42 | 1.2 | Human serum |
| Urea pH sensor/[32] | Potentiometry | Iridium oxide films, silicon-based thin-film platinum microelectrode | 180 | 4.7 | Urine |
| Enzymatic optical biosensor/[57] | Optical | Urease, FITC-dextran sensing probe entrapped in TMOS | 600 | 0.15 | River water, serum |
| Enzyme-based field effect transistors/[58] | Potentiometry | Urease, layered double hydroxide (LDH) clay matrix, glutaraldehyde cross-linker | <12 | 0.21 | Urine, blood |
| Solid colorimetric sensor (this work) | Diffuse reflectance spectrophotometry/digital image processing | 1,2-naphthoquinone-4-sulfonate (NQS), PDMS | 600 | 0.4 | Human urine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Aviñó, A.; Hakobyan, L.; Ballester-Caudet, A.; Moliner-Martínez, Y.; Molins-Legua, C.; Campíns-Falcó, P. NQS-Doped PDMS Solid Sensor: From Water Matrix to Urine Enzymatic Application. Biosensors 2021, 11, 186. https://doi.org/10.3390/bios11060186
Martínez-Aviñó A, Hakobyan L, Ballester-Caudet A, Moliner-Martínez Y, Molins-Legua C, Campíns-Falcó P. NQS-Doped PDMS Solid Sensor: From Water Matrix to Urine Enzymatic Application. Biosensors. 2021; 11(6):186. https://doi.org/10.3390/bios11060186
Chicago/Turabian StyleMartínez-Aviñó, Adrià, Lusine Hakobyan, Ana Ballester-Caudet, Yolanda Moliner-Martínez, Carmen Molins-Legua, and Pilar Campíns-Falcó. 2021. "NQS-Doped PDMS Solid Sensor: From Water Matrix to Urine Enzymatic Application" Biosensors 11, no. 6: 186. https://doi.org/10.3390/bios11060186
APA StyleMartínez-Aviñó, A., Hakobyan, L., Ballester-Caudet, A., Moliner-Martínez, Y., Molins-Legua, C., & Campíns-Falcó, P. (2021). NQS-Doped PDMS Solid Sensor: From Water Matrix to Urine Enzymatic Application. Biosensors, 11(6), 186. https://doi.org/10.3390/bios11060186






