Study of Stem Cells Influence on Cardiac Cells Cultured with a Cyanide-P-Trifluoromethoxyphenylhydrazone in Organ-on-a-Chip System
Abstract
1. Introduction
2. Materials and Methods
2.1. PLLA and PU Nanofiber Mats
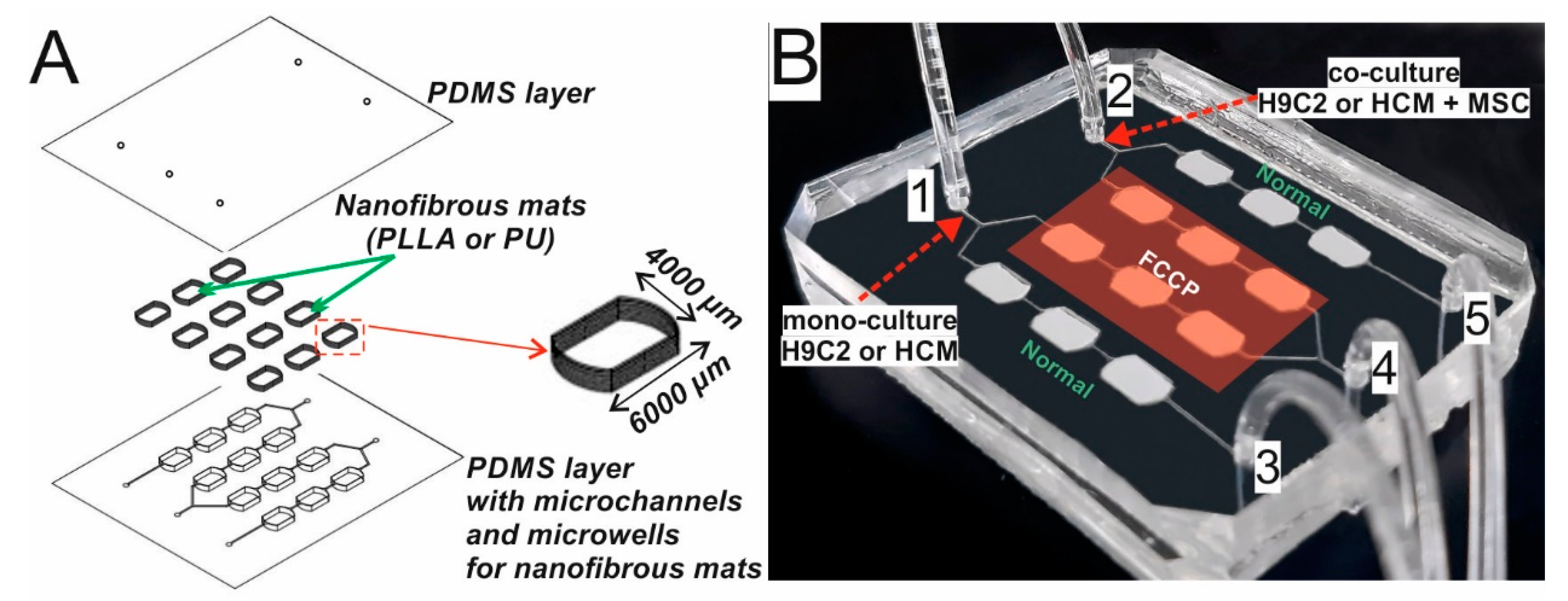
2.2. Development of Organ-on-a-Chip System
2.3. Flow Simulation in the Microsystem
2.4. Cell Lines
2.5. Cell Culture in the Microsystem
2.6. Cell Incubation with FCCP
2.7. Evaluation of Changes in the Potential of the Inner Mitochondrial Membrane
2.8. Evaluation of the Metabolic Activity of Cardiac and Stem Cells
2.9. Statistical Analysis
3. Results
3.1. Initial Studies in Macroscale
3.2. Working of the Microsystem
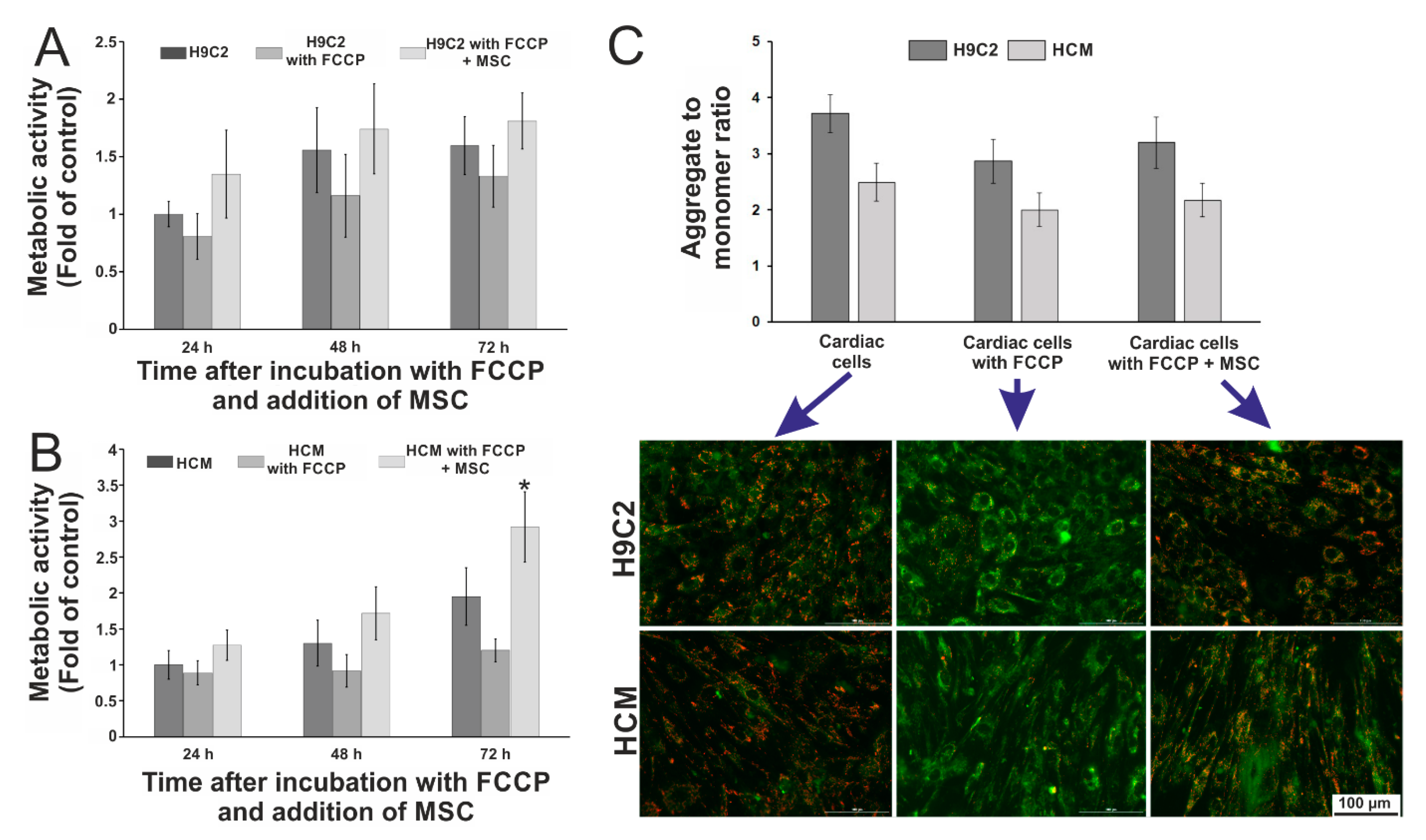
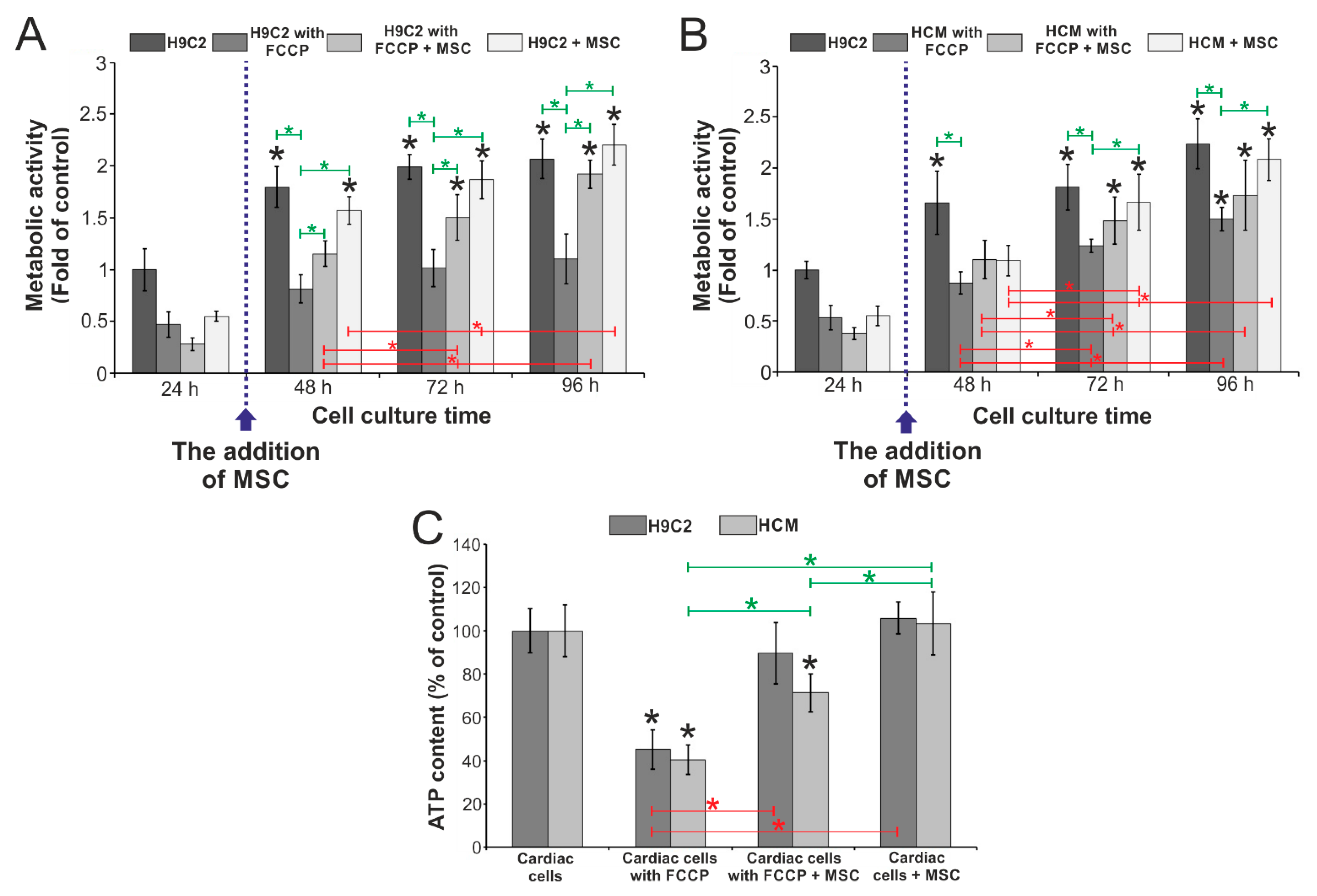
3.3. Evaluation of the Influence of Stem Cells on the Metabolic Activity of Cardiac Cells Incubated with FCCP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Atlas on Cardiovascular Disease Prevention and Control; World Health Organization in Collaboration with the World Heart Federation: Geneva, Switzerland, 2011; ISBN 9789241564373. [Google Scholar]
- Katz, M.; Freimark, D.; Raichlin, E.; Har-Zahav, Y.; Arad, M.; Kassif, Y.; Peled, A.; Asher, E.; Elian, D.; Kogan, A.; et al. Risk of early, intermediate, and late rejection following heart transplantation: Trends over the past 25 years and relation to changes in medical management. Tertiary center experience: The Sheba Heart Transplantation Registry. Clin. Transplant. 2017, 31, e13063. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, H.L.; Thorsteinsdóttir, H.; Perry, G.; Renihan, J.; Singer, P.A.; Daar, A.S. Regenerative medicine: New opportunities for developing countries. Int. J. Biotechnol. 2006, 8, 60–77. [Google Scholar] [CrossRef]
- Matta, R.; Davies, J.E. Bioengineering and Regenerative Medicine in Surgery. In Bioengineering for Surgery: The Critical Engineer Surgeon Interface; Elsevier: London, UK, 2016; pp. 189–203. ISBN 978-0-08-100123-3. [Google Scholar]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [PubMed]
- Kobuszewska, A.; Sokolowska, P.; Jastrzebska, E. Cardiac cell culture microtechnologies based on stem cells. In Cardiac Cell Culture Technologies: Microfluidic and On-Chip Systems; Springer International Publishing: Basel, Switzerland, 2017; ISBN 9783319706856. [Google Scholar]
- Batalov, I.; Feinberg, A.W. Differentiation of cardiomyocytes from human pluripotent stem cells using monolayer culture. Biomark. Insights 2015, 10, 71–76. [Google Scholar] [CrossRef]
- Blau, H.M.; Daley, G.Q. Stem cells in the treatment of disease. N. Engl. J. Med. 2019, 380, 1748–1760. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Ginard, B.; Ellison, G.M.; Torella, D. The cardiac stem cell compartment is indispensable for myocardial cell homeostasis, repair and regeneration in the adult. Stem Cell Res. 2014, 13, 615–630. [Google Scholar] [CrossRef]
- Heng, B.C.; Haider, H.K.; Sim, E.K.W.; Cao, T.; Ng, S.C. Strategies for directing the differentiation of stem cells into the cardiomyogenic lineage in vitro. Cardiovasc. Res. 2004, 62, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, T.M.; Burch, J.B.E.; Lassar, A.B. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997, 11, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sun, B. Transforming growth factor-β2 enhances differentiation of cardiac myocytes from embryonic stem cells. Biochem. Biophys. Res. Commun. 2005, 332, 135–141. [Google Scholar] [CrossRef]
- Rosenblatt-Velin, N.; Lepore, M.G.; Cartoni, C.; Beermann, F.; Pedrazzini, T. FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J. Clin. Investig. 2005, 115, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Ding, Q.; Wang, J.; Deng, L.; Yang, L.; Tao, L.; Lei, H.; Lu, S. 5-Azacytidine delivered by mesoporous silica nanoparticles regulates the differentiation of P19 cells into cardiomyocytes. Nanoscale 2016, 8, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Zandstra, P.W.; Bauwens, C.; Yin, T.; Liu, Q.; Schiller, H.; Zweigerdt, R.; Pasumarthi, K.B.S.; Field, L.J. Scalable production of embryonic stem cell-derived cardiomyocytes. Tissue Eng. 2003, 9, 767–778. [Google Scholar] [CrossRef]
- Henderson, K.; Sligar, A.D.; Le, V.P.; Lee, J.; Baker, A.B. Biomechanical Regulation of Mesenchymal Stem Cells for Cardiovascular Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700556. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jia, X.; Bai, K.; Gong, X.; Fan, Y. Effect of fluid shear stress on cardiomyogenic differentiation of rat bone marrow mesenchymal stem cells. Arch. Med. Res. 2010, 41, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Connell, J.P.; Wadhwa, L.; Ruano, R.; Jacot, J.G. Amniotic Fluid-Derived Stem Cells Demonstrated Cardiogenic Potential in Indirect Co-culture with Human Cardiac Cells. Ann. Biomed. Eng. 2014, 42, 2490–2500. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.J.; Zhao, E.J.; Chiao, M.; Lim, C.J. Co-culture of induced pluripotent stem cells with cardiomyocytes is sufficient to promote their differentiation into cardiomyocytes. PLoS ONE 2020, 15, e0230966. [Google Scholar] [CrossRef] [PubMed]
- Kshitiz; Park, J.; Kim, P.; Helen, W.; Engler, A.J.; Levchenko, A.; Kim, D.H. Control of stem cell fate and function by engineering physical microenvironments. Integr. Biol. 2012, 4, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Meyvantsson, I.; Beebe, D.J. Cell Culture Models in Microfluidic Systems. Annu. Rev. Anal. Chem. 2008, 1, 423–449. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Kitsara, M.; Agbulut, O.; Kontziampasis, D.; Chen, Y.; Menasché, P. Fibers for hearts: A critical review on electrospinning for cardiac tissue engineering. Acta Biomater. 2017, 48, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, J.Y.; Jin, S.; Yoon, S.; Kwak, J.Y.; Jeong, Y.H. A microfluidic chip embracing a nanofiber scaffold for 3D cell culture and real-time monitoring. Nanomaterials 2019, 9, 588. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, W.; Wang, S.; Qin, J. Regulation of Fibrochondrogenesis of Mesenchymal Stem Cells in an Integrated Microfluidic Platform Embedded with Biomimetic Nanofibrous Scaffolds. PLoS ONE 2013, 8, 1–13. [Google Scholar] [CrossRef]
- Hesari, Z.; Soleimani, M.; Atyabi, F.; Sharifdini, M.; Nadri, S.; Warkiani, M.E.; Zare, M.; Dinarvand, R. A hybrid microfluidic system for regulation of neural differentiation in induced pluripotent stem cells. J. Biomed. Mater. Res. Part A 2016, 104, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Toh, Y.C.; Blagović, K.; Voldman, J. Advancing stem cell research with microtechnologies: Opportunities and challenges. Integr. Biol. 2010, 2, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Sokolowska, P.; Zukowski, K.; Lasocka, I.; Szulc-Dabrowska, L.; Jastrzebska, E. Human mesenchymal stem cell (hMSC) differentiation towards cardiac cells using a new microbioanalytical method. Analyst 2020, 145, 3017–3028. [Google Scholar] [CrossRef] [PubMed]
- Mummery, C.; Ward-van Oostwaard, D.; Doevendans, P.; Spijker, R.; Van den Brink, S.; Hassink, R.; Van der Heyden, M.; Opthof, T.; Pera, M.; Brutel de la Riviere, A.; et al. Differentiation of human embryonic stem cells to cardiomyocytes: Role of coculture with visceral endoderm-like cells. Circulation 2003, 107, 2733–2740. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.Y.; Khryapenkova, T.G.; Vasileva, A.K.; Marey, M.V.; Galkina, S.I.; Isaev, N.K.; Sheval, E.V.; Polyakov, V.Y.; Sukhikh, G.T.; Zorov, D.B. Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. J. Cell. Mol. Med. 2008, 12, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Li, J.; Lu, Y.; Zhang, C.; Li, Q.; Mao, J.; Li, L. hong The mechanism between epithelial mesenchymal transition in breast cancer and hypoxia microenvironment. Biomed. Pharmacother. 2016, 80, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Tomecka, E.; Wojasinski, M.; Jastrzebska, E.; Chudy, M.; Ciach, T.; Brzozka, Z. Poly(L-lactic acid) and polyurethane nanofibers fabricated by solution blow spinning as potential substrates for cardiac cell culture. Mater. Sci. Eng. C 2017, 75, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Kobuszewska, A.; Kolodziejek, D.; Wojasinski, M.; Jastrzebska, E.; Ciach, T.; Brzozka, Z. Lab-on-a-chip system integrated with nanofiber mats used as a potential tool to study cardiovascular diseases (CVDs). Sens. Actuators B Chem. 2021, 330, 129291. [Google Scholar] [CrossRef]
- Sgarbi, G.; Barbato, S.; Costanzini, A.; Solaini, G.; Baracca, A. The role of the ATPase inhibitor factor 1 (IF1) in cancer cells adaptation to hypoxia and anoxia. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 99–109. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Chen, Z.; Lin, Y.; Yu, P.; Mao, L. Continuous electrochemical monitoring of extracellular lactate production from neonatal rat cardiomyocytes following myocardial hypoxia. Anal. Chem. 2012, 84, 5285–5291. [Google Scholar] [CrossRef]
- Han, Y.H.; Kim, S.H.; Kim, S.Z.; Park, W.H. Carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP) as an O2{radical dot}- generator induces apoptosis via the depletion of intracellular GSH contents in Calu-6 cells. Lung Cancer 2009, 63, 201–209. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ma, C.; Liu, W.; Wang, J. On-chip monitoring of skeletal myoblast transplantation for the treatment of hypoxia-induced myocardial injury. Analyst 2014, 139, 4482–4490. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef]
- Li, T.; Chen, L.; Yu, Y.; Yang, B.; Li, P.; Tan, X.Q. Resveratrol alleviates hypoxia/reoxygenation injury-induced mitochondrial oxidative stress in cardiomyocytes. Mol. Med. Rep. 2019, 19, 2774–2780. [Google Scholar] [CrossRef]
- Mathur, A.; Hong, Y.; Kemp, B.K.; Barrientos, A.A.; Erusalimsky, J.D. Evaluation of fluorescent dyes for the detection of mitochondrial membrane potential changes in cultured cardiomyocytes. Cardiovasc. Res. 2000, 46, 126–138. [Google Scholar] [CrossRef]
- Kobuszewska, A.; Jastrzębska, E.; Żukowski, K.; Brzózka, Z. Simulation of hypoxia of myocardial cells in microfluidic systems. Sci. Rep. 2020, 10, 15524. [Google Scholar] [CrossRef]
- Portillo-Lara, R.; Spencer, A.R.; Walker, B.W.; Shirzaei Sani, E.; Annabi, N. Biomimetic cardiovascular platforms for in vitro disease modeling and therapeutic validation. Biomaterials 2019, 198, 78–94. [Google Scholar] [CrossRef]
- Harink, B.; Le Gac, S.; Truckenmüller, R.; Van Blitterswijk, C.; Habibovic, P. Regeneration-on-a-chip? the perspectives on use of microfluidics in regenerative medicine. Lab Chip 2013, 13, 3512–3528. [Google Scholar] [CrossRef]
- El-Ali, J.; Sorger, P.K.; Jensen, K.F. Cells on chips. Nature 2006, 442, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Bagno, L.; Hatzistergos, K.E.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol. Ther. 2018, 26, 1610–1623. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Liu, Q.; Liu, H.; Yang, H.; Yun, J.X.; Eisenberg, C.; Borg, T.K.; Xu, M.; Gao, B.Z. Laser-patterned stem-cell bridges in a cardiac muscle model for on-chip electrical conductivity analyses. Lab Chip 2012, 12, 566–573. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobuszewska, A.; Kolodziejek, D.; Wojasinski, M.; Ciach, T.; Brzozka, Z.; Jastrzebska, E. Study of Stem Cells Influence on Cardiac Cells Cultured with a Cyanide-P-Trifluoromethoxyphenylhydrazone in Organ-on-a-Chip System. Biosensors 2021, 11, 131. https://doi.org/10.3390/bios11050131
Kobuszewska A, Kolodziejek D, Wojasinski M, Ciach T, Brzozka Z, Jastrzebska E. Study of Stem Cells Influence on Cardiac Cells Cultured with a Cyanide-P-Trifluoromethoxyphenylhydrazone in Organ-on-a-Chip System. Biosensors. 2021; 11(5):131. https://doi.org/10.3390/bios11050131
Chicago/Turabian StyleKobuszewska, Anna, Dominik Kolodziejek, Michal Wojasinski, Tomasz Ciach, Zbigniew Brzozka, and Elzbieta Jastrzebska. 2021. "Study of Stem Cells Influence on Cardiac Cells Cultured with a Cyanide-P-Trifluoromethoxyphenylhydrazone in Organ-on-a-Chip System" Biosensors 11, no. 5: 131. https://doi.org/10.3390/bios11050131
APA StyleKobuszewska, A., Kolodziejek, D., Wojasinski, M., Ciach, T., Brzozka, Z., & Jastrzebska, E. (2021). Study of Stem Cells Influence on Cardiac Cells Cultured with a Cyanide-P-Trifluoromethoxyphenylhydrazone in Organ-on-a-Chip System. Biosensors, 11(5), 131. https://doi.org/10.3390/bios11050131






