Detection Technologies for Reactive Oxygen Species: Fluorescence and Electrochemical Methods and Their Applications
Abstract
1. Introduction
2. Superoxide Radical ( Detection
2.1. Introduction to Superoxide Radical () Detection
2.2. Fluorescence Method for Superoxide Radical () Detection
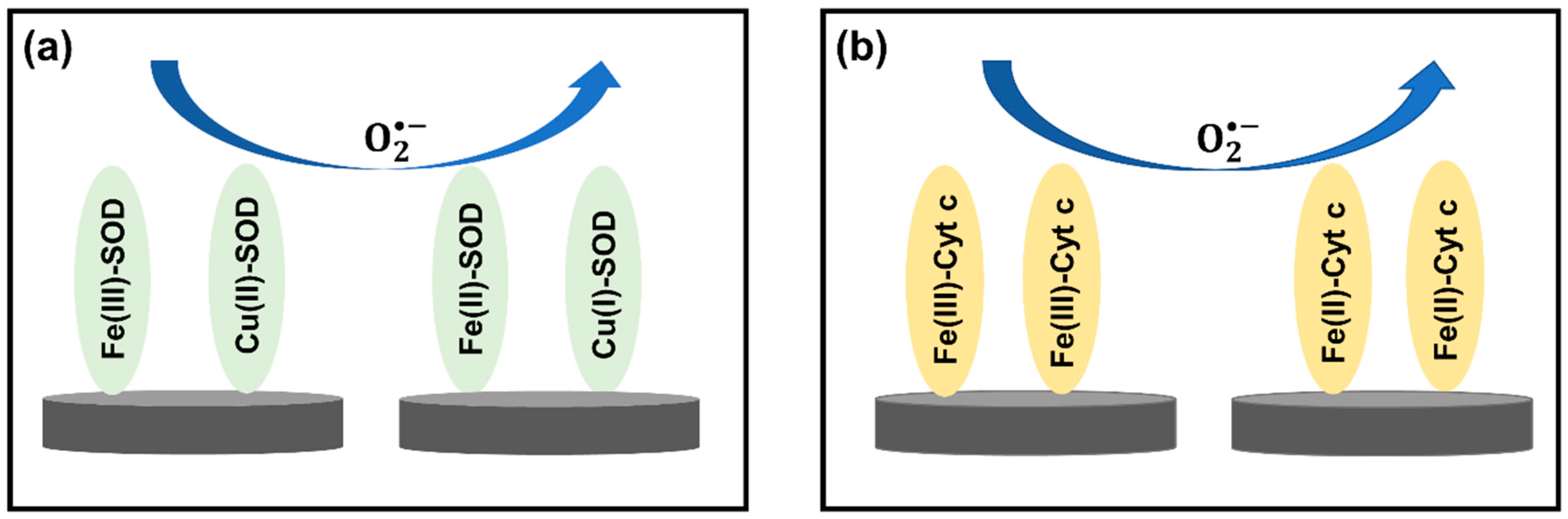
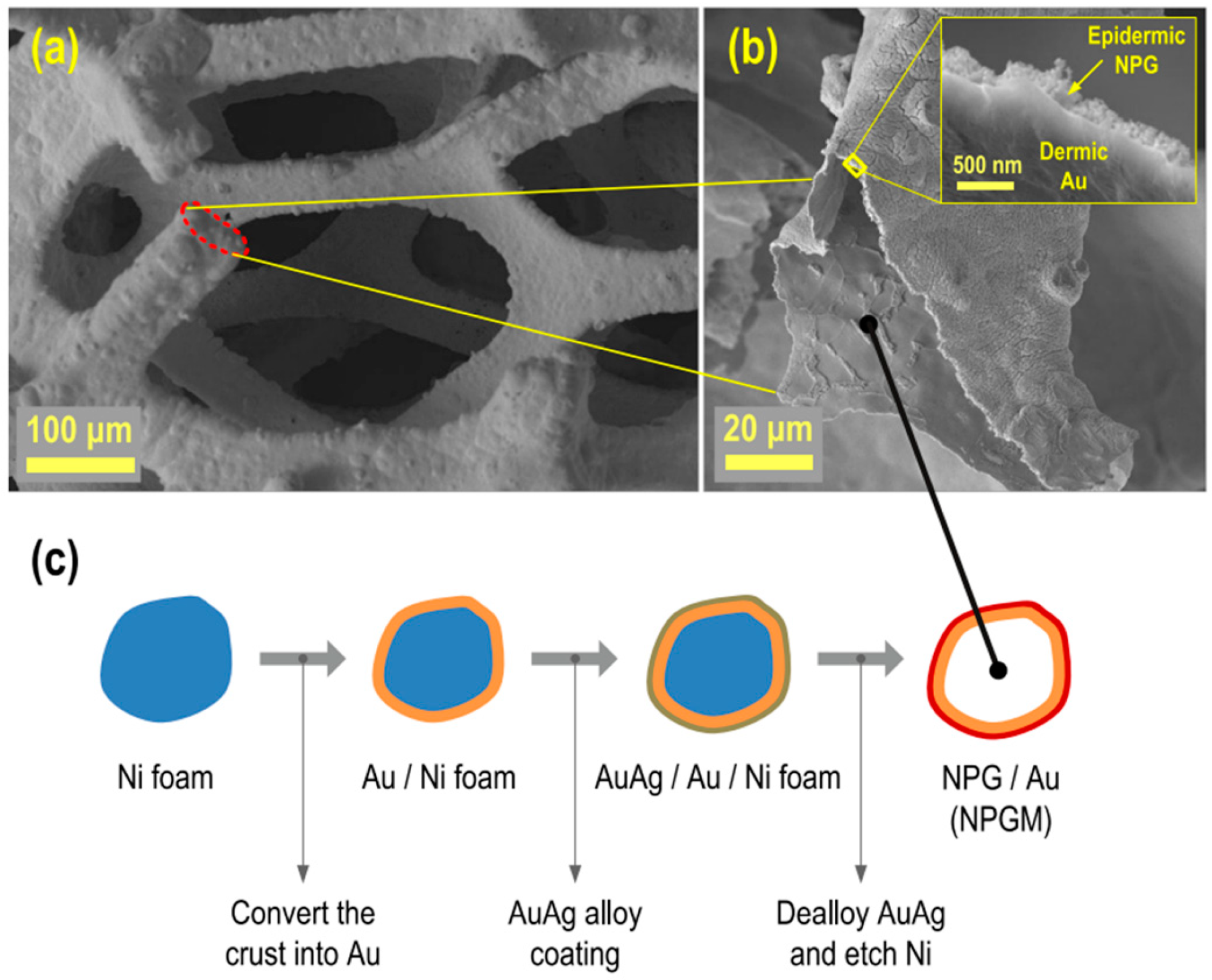
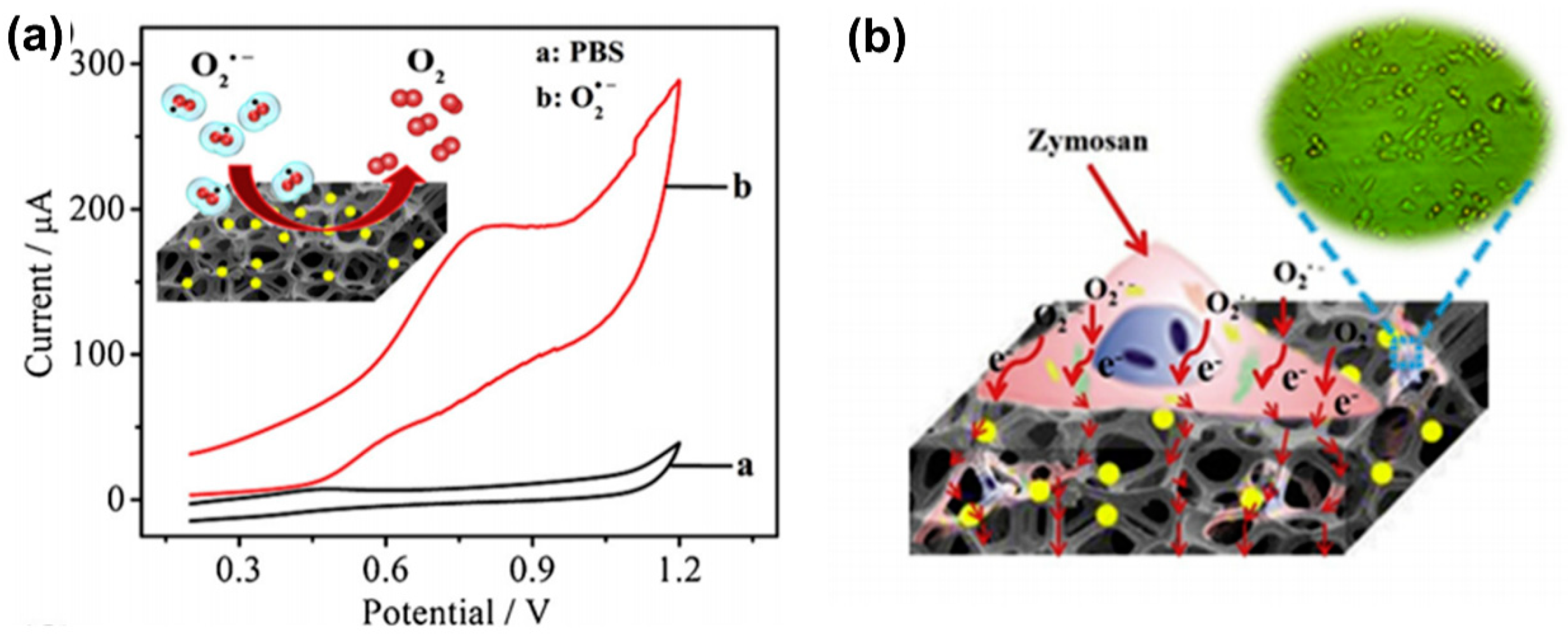
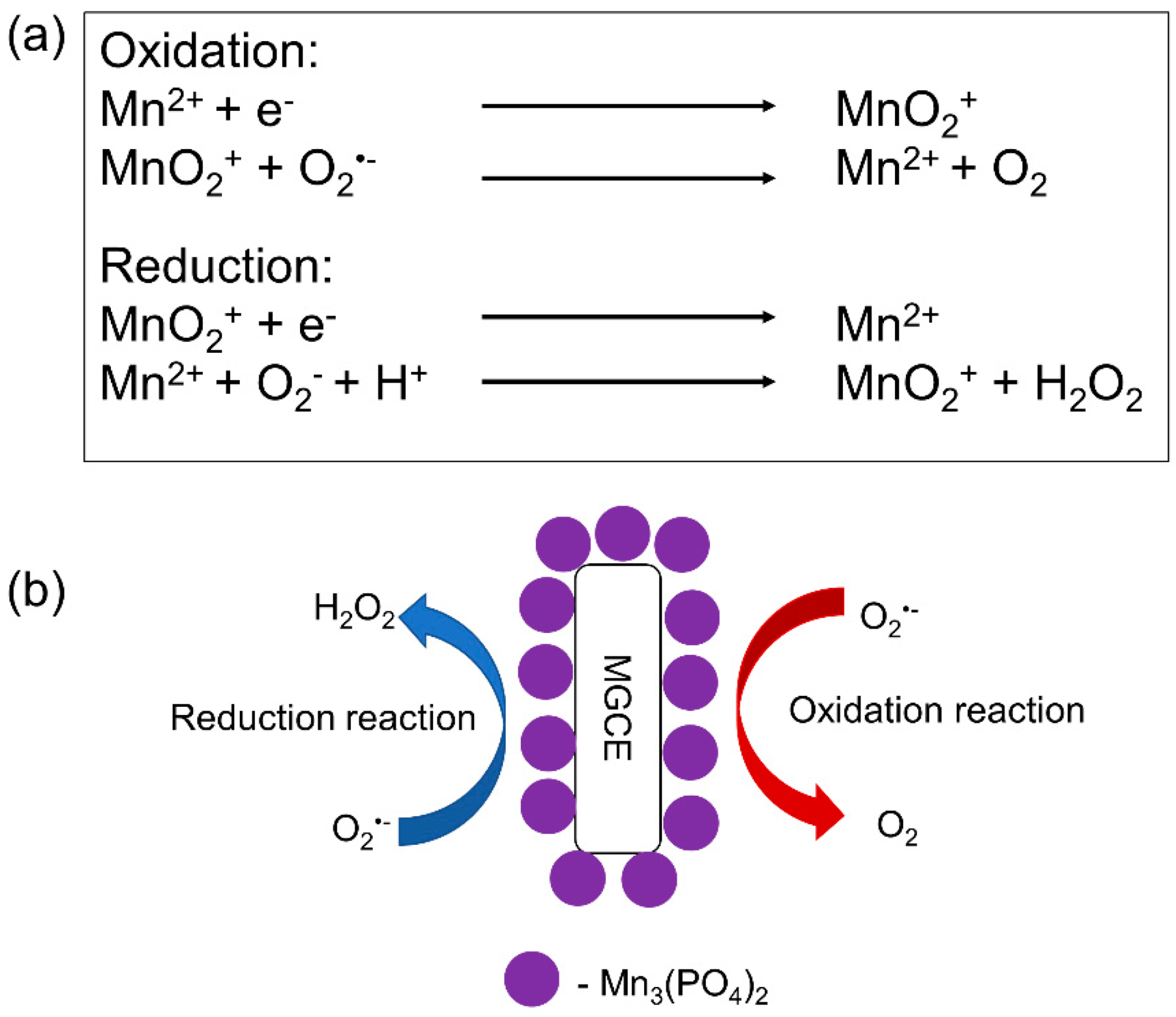
2.3. Electrochemical Method for Superoxide Radical Detection
3. Hydrogen Peroxide (H2O2) Detection
3.1. Introduction to Hydrogen Peroxide (H2O2) Detection
3.2. Electrochemical Techniques for Hydrogen Peroxide (H2O2) Detection
4. Hydroxyl Radical (•OH) Detection
4.1. Introduction to Hydroxyl Radical (•OH) Detection
4.2. Fluorescence Method for Hydroxyl Radical (•OH) Detection
4.3. Electrochemical Methods for Hydroxyl Radical (•OH) Detection
5. Conclusions and Future Perspectives
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Koopman, W.J.H.; Nijtmans, L.G.J.; Dieteren, C.E.J.; Roestenberg, P.; Valsecchi, F.; Smeitink, J.A.M.; Willems, P.H.G.M. Mammalian mitochondrial complex I: Biogenesis, regulation, and reactive oxygen species generation. Antioxid. Redox Signal. 2010, 12, 1431–1470. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2008, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Anglada, J.M.; Martins-Costa, M.; Francisco, J.S.; Ruiz-López, M.F. Interconnection of reactive oxygen species chemistry across the interfaces of atmospheric, environmental, and biological processes. Acc. Chem. Res. 2015, 48, 575–583. [Google Scholar] [CrossRef]
- Blough, N.V.; Zepp, R.G. Reactive Oxygen Species in Natural Waters. In Active Oxygen in Chemistry; Foote, C.S., Valentine, J.S., Greenberg, A., Liebman, J.F., Eds.; Springer: Glasgow, UK, 1995; pp. 280–333. [Google Scholar]
- Church, D.F.; Pryor, W.A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect. 1985, 64, 111–126. [Google Scholar] [CrossRef]
- Weschler, C.J.; Shields, H.C. Production of the Hydroxyl Radical in Indoor Air. Environ. Sci. Technol. 1996, 30, 3250–3258. [Google Scholar] [CrossRef]
- Burns, J.M.; Cooper, W.J.; Ferry, J.L.; King, D.W.; DiMento, B.P.; McNeill, K.; Miller, C.J.; Miller, W.L.; Peake, B.M.; Rusak, S.A.; et al. Methods for reactive oxygen species (ROS) detection in aqueous environments. Aquat. Sci. 2012, 74, 683–734. [Google Scholar] [CrossRef]
- Boursiac, Y.; Prak, S.; Boudet, J.; Postaire, O.; Luu, D.-T.; Tournaire-Roux, C.; Santoni, V.; Maurel, C. The response of Arabidopsis root water transport to a challenging environment implicates reactive oxygen species- and phosphorylation-dependent internalization of aquaporins. Plant Signal Behav. 2008, 3, 1096–1098. [Google Scholar] [CrossRef]
- Miller, G.A.D.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R.O.N. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Cadenas, E.; Davies, K.J.A. Mitochondrial free radical generation, oxidative stress, and aging11This article is dedicated to the memory of our dear friend, colleague, and mentor Lars Ernster (1920–1998), in gratitude for all he gave to us. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Glasauer, A.; Chandel, N.S. Targeting antioxidants for cancer therapy. Biochem. Pharmacol. 2014, 92, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Oxidative burst without phagocytes: The role of respiratory proteins. Nat. Immunol. 2007, 8, 1029–1031. [Google Scholar] [CrossRef] [PubMed]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Majzunova, M.; Dovinova, I.; Barancik, M.; Chan, J.Y.H. Redox signaling in pathophysiology of hypertension. J. Biomed. Sci. 2013, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Ammendola, R.; Faraonio, R.; Russo, T.; Cimino, F. Redox Control of Signal Transduction, Gene Expression and Cellular Senescence. Neurochem. Res. 2004, 29, 617–628. [Google Scholar] [CrossRef]
- Soh, N. Recent advances in fluorescent probes for the detection of reactive oxygen species. Anal. Bioanal. Chem. 2006, 386, 532–543. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Subedi, L.; Lee, T.H.; Wahedi, H.M.; Baek, S.-H.; Kim, S.Y. Resveratrol-Enriched Rice Attenuates UVB-ROS-Induced Skin Aging via Downregulation of Inflammatory Cascades. Oxid. Med. Cell. Longev. 2017, 2017, 8379539. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Haber, E.P.; Hirabara, S.M.; Rebelato, E.L.O.; Procopio, J.; Morgan, D.; Oliveira-Emilio, H.C.; Carpinelli, A.R.; Curi, R. Diabetes associated cell stress and dysfunction: Role of mitochondrial and non-mitochondrial ROS production and activity. J. Physiol. 2007, 583, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Bechtel, W.; Bauer, G. Catalase protects tumor cells from apoptosis induction by intercellular ROS signaling. Anticancer Res. 2009, 29, 4541–4557. [Google Scholar] [PubMed]
- Emerit, J.; Edeas, M.; Bricaire, F. Neurodegenerative diseases and oxidative stress. Biomed. Pharmacotherap. 2004, 58, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Tabner, B.J.; Turnbull, S.; El-Aganf, O.; Allsop, D. Production of reactive oxygen species from aggregating proteins implicated in Alzheimer’s disease, Parkinson’s disease and other neurodegenerative diseases. Curr. Top. Med. Chem. 2001, 1, 507–517. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Singh, S.P.; Häder, D.-P.; Sinha, R.P. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem. Biophys. Res. Commun. 2010, 397, 603–607. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- Prabhakaran, V.; Arges, C.G.; Ramani, V. In situ fluorescence spectroscopy correlates ionomer degradation to reactive oxygen species generation in an operating fuel cell. Phys. Chem. Chem. Phys. 2013, 15, 18965–18972. [Google Scholar] [CrossRef]
- He, W.; Liu, Y.; Wamer, W.G.; Yin, J.-J. Electron spin resonance spectroscopy for the study of nanomaterial-mediated generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 49–63. [Google Scholar] [CrossRef]
- Utsumi, H.; Yasukawa, K.; Soeda, T.; Yamada, K.-I.; Shigemi, R.; Yao, T.; Tsuneyoshi, M. Noninvasive Mapping of Reactive Oxygen Species by In Vivo Electron Spin Resonance Spectroscopy in Indomethacin-Induced Gastric Ulcers in Rats. J. Pharmacol. Exp. Ther. 2006, 317, 228. [Google Scholar] [CrossRef]
- Barbin, L.E.; Saquy, P.C.; Guedes, D.F.C.; Sousa-Neto, M.D.; Estrela, C.; Pécora, J.D. Determination of para-Chloroaniline and Reactive Oxygen Species in Chlorhexidine and Chlorhexidine Associated with Calcium Hydroxide. J. Endod. 2008, 34, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Blaškovičová, J.; Sochr, J.; Koutsogiannis, A.; Diamantidou, D.; Kopel, P.; Adam, V.; Labuda, J. Detection of ROS Generated by UV-C Irradiation of CdS Quantum Dots and their Effect on Damage to Chromosomal and Plasmid DNA. Electroanalysis 2018, 30, 698–704. [Google Scholar] [CrossRef]
- Martínez, S.R.; Miana, G.E.; Albesa, I.; Mazzieri, M.R.; Becerra, M.C. Evaluation of Antibacterial Activity and Reactive Species Generation of N-Benzenesulfonyl Derivatives of Heterocycles. Chem. Pharm. Bull. 2016, 64, 135–141. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Cheng, G.; Hardy, M.; Ouari, O.; Bennett, B.; Zielonka, J. Teaching the basics of reactive oxygen species and their relevance to cancer biology: Mitochondrial reactive oxygen species detection, redox signaling, and targeted therapies. Redox Biol. 2018, 15, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wu, Q.; Li, Q.; Shao, S.; Guo, Y. Fluorescence and HPLC Detection of Hydroxyl Radical by a Rhodamine-Nitroxide Probe and its Application in Cell Imaging. J. Fluoresc. 2014, 24, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Parrondo, J.; Sankarasubramanian, S.; Ramani, V. Detection of Reactive Oxygen Species in Anion Exchange Membrane Fuel Cells using In Situ Fluorescence Spectroscopy. ChemSusChem 2017, 10, 3056–3062. [Google Scholar] [CrossRef]
- Wojtala, A.; Bonora, M.; Malinska, D.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Chapter Thirteen—Methods to Monitor ROS Production by Fluorescence Microscopy and Fluorometry. In Methods in Enzymology; Galluzzi, L., Kroemer, G., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 542, pp. 243–262. [Google Scholar]
- Yang, Y.; Zhang, H.; Wang, Z.; Li, X.; Abdelsamie, A.A.A.; Yuan, X.; Fan, X.; Zhang, R.; Chang, H. Highly Sensitive Electrochemical Detection of Reactive Oxygen Species in Living Cancer Cells Using Monolithic Metallic Foam Electrodes. ChemElectroChem 2020, 7, 2485–2492. [Google Scholar] [CrossRef]
- Kumar, V.; Sachdev, A.; Matai, I. Self-assembled reduced graphene oxide–cerium oxide nanocomposite@cytochrome c hydrogel as a solid electrochemical reactive oxygen species detection platform. New J. Chem. 2020, 44, 11248–11255. [Google Scholar] [CrossRef]
- Fisher, A.B. Redox Signaling Across Cell Membranes. Antioxid. Redox Signal. 2009, 11, 1349–1356. [Google Scholar] [CrossRef]
- Sen, C.K.; Packer, L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996, 10, 709–720. [Google Scholar] [CrossRef]
- Zhou, D.; Shao, L.; Spitz, D.R. Chapter One—Reactive Oxygen Species in Normal and Tumor Stem Cells. In Advances in Cancer Research; Townsend, D.M., Tew, K.D., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 122, pp. 1–67. [Google Scholar]
- Chen, Y.; Azad, M.B.; Gibson, S.B. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009, 16, 1040. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: A mini review. Oxid. Med. Cell. Longev. 2016, 2016, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-Q.; Ye, C.; Bao, S.-J.; Xu, M.-W.; Zhang, Y.; Wang, L.; Ma, X.-Q.; Guo, J.; Li, C.-M. Nanostructured cobalt phosphates as excellent biomimetic enzymes to sensitively detect superoxide anions released from living cells. Biosens. Bioelectron. 2017, 87, 998–1004. [Google Scholar] [CrossRef]
- Deshmukh, P.P.; Navalkar, A.; Maji, S.K.; Manjare, S.T. Phenylselenyl containing turn-on dibodipy probe for selective detection of superoxide in mammalian breast cancer cell line. Sens. Actuators B Chem. 2019, 281, 8–13. [Google Scholar] [CrossRef]
- Zhang, N.; He, Y.; Tang, Q.; Wang, Y.; Zheng, Q.; Hu, P. A mitochondrial targeting two-channel responsive fluorescence probe for imaging the superoxide radical anion in vitro and in vivo. Talanta 2019, 194, 79–85. [Google Scholar] [CrossRef]
- Malferrari, M.; Ghelli, A.; Roggiani, F.; Valenti, G.; Paolucci, F.; Rugolo, M.; Rapino, S. Reactive Oxygen Species Produced by Mutated Mitochondrial Respiratory Chains of Entire Cells Monitored Using Modified Microelectrodes. ChemElectroChem 2019, 6, 627–633. [Google Scholar] [CrossRef]
- Amatore, C.; Arbault, S.; Bouton, C.; Drapier, J.-C.; Ghandour, H.; Koh, A.C.W. Real-Time Amperometric Analysis of Reactive Oxygen and Nitrogen Species Released by Single Immunostimulated Macrophages. ChemBioChem 2008, 9, 1472–1480. [Google Scholar] [CrossRef]
- Ueno, T.; Nagano, T. Fluorescent probes for sensing and imaging. Nat. Methods 2011, 8, 642–645. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, J.; Li, P.; Wang, X.; Bi, S.; Zhang, J.; Zhang, W.; Wang, H.; Tang, B. In situ and real-time imaging of superoxide anion and peroxynitrite elucidating arginase 1 nitration aggravating hepatic ischemia-reperfusion injury. Biomaterials 2019, 225, 119499. [Google Scholar] [CrossRef]
- Sedgwick, A.C.; Han, H.-H.; Gardiner, J.E.; Bull, S.D.; He, X.-P.; James, T.D. Long-wavelength fluorescent boronate probes for the detection and intracellular imaging of peroxynitrite. Chem. Commun. 2017, 53, 12822–12825. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, F.; Hyun, J.Y.; Wei, T.; Qiang, J.; Ren, X.; Shin, I.; Yoon, J. Recent progress in the development of fluorescent, luminescent and colorimetric probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 2016, 45, 2976–3016. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Chen, G.; Liu, Y.; Cao, Z.; Zhao, X.; Li, G.; Yu, F.; Chen, L.; Wang, H.; You, J. In situ quantification and evaluation of ClO−/H2S homeostasis in inflammatory gastric tissue by applying a rationally designed dual-response fluorescence probe featuring a novel H+-activated mechanism. Analyst 2017, 142, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochim. Biophys. Acta 2014, 1840, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Magidson, V.; Khodjakov, A. Chapter 23—Circumventing Photodamage in Live-Cell Microscopy. In Methods in Cell Biology; Sluder, G., Wolf, D.E., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 114, pp. 545–560. [Google Scholar]
- Ettinger, A.; Wittmann, T. Chapter 5—Fluorescence live cell imaging. In Methods in Cell Biology; Waters, J.C., Wittman, T., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 123, pp. 77–94. [Google Scholar]
- Wang, X.; Fang, H.; Huang, Z.; Shang, W.; Hou, T.; Cheng, A.; Cheng, H. Imaging ROS signaling in cells and animals. J. Mol. Med. 2013, 91, 917–927. [Google Scholar] [CrossRef]
- Li, R.-Q.; Mao, Z.-Q.; Rong, L.; Wu, N.; Lei, Q.; Zhu, J.-Y.; Zhuang, L.; Zhang, X.-Z.; Liu, Z.-H. A two-photon fluorescent probe for exogenous and endogenous superoxide anion imaging in vitro and in vivo. Biosens. Bioelectron. 2017, 87, 73–80. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, F.; Wang, J.; Chen, L. Near-Infrared Fluorescence Probe for In Situ Detection of Superoxide Anion and Hydrogen Polysulfides in Mitochondrial Oxidative Stress. Anal. Chem. 2016, 88, 4122–4129. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, X.; Wu, C.; Wu, Y.; Li, P.; Guo, X.; Tang, B. A new endoplasmic reticulum-targeted two-photon fluorescent probe for imaging of superoxide anion in diabetic mice. Biosens. Bioelectron. 2017, 91, 449–455. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Wang, H.; Tan, H.; Xie, X.; Zhang, X.; Liu, C.; Qu, X.; Hua, J. A turn-on near-infrared fluorescence probe with aggregation-induced emission based on dibenzo[a,c]phenazine for detection of superoxide anions and its application in cell imaging. Analyst 2018, 143, 1242–1249. [Google Scholar] [CrossRef]
- Zhang, W.; Li, P.; Yang, F.; Hu, X.; Sun, C.; Zhang, W.; Chen, D.; Tang, B. Dynamic and Reversible Fluorescence Imaging of Superoxide Anion Fluctuations in Live Cells and In Vivo. J. Am. Chem. Soc. 2013, 135, 14956–14959. [Google Scholar] [CrossRef]
- Si, F.; Liu, Y.; Yan, K.; Zhong, W. A mitochondrion targeting fluorescent probe for imaging of intracellular superoxide radicals. Chem. Commun. 2015, 51, 7931–7934. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Zhang, L.; Huang, Y.; Bi, L.; Lv, C.; Chen, L. A ratiometric fluorescent probe for detecting the endogenous biological signaling molecule superoxide anion and bioimaging during tumor treatment. J. Mater. Chem. B 2020, 8, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhu, Z.; Zheng, Y.; Cheng, J.; Zhang, N.; Long, Y.-T.; Zheng, J.; Qian, X.; Yang, Y. A Three-Channel Fluorescent Probe That Distinguishes Peroxynitrite from Hypochlorite. J. Am. Chem. Soc. 2012, 134, 18479–18482. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Li, J.; Zheng, J.; Xu, X.; Yang, R. Simultaneous Intracellular β-d-Glucosidase and Phosphodiesterase I Activities Measurements Based on A Triple-Signaling Fluorescent Probe. Anal. Chem. 2011, 83, 1268–1274. [Google Scholar] [CrossRef]
- Peng, M.; Wang, Y.; Fu, Q.; Sun, F.; Na, N.; Ouyang, J. Melanosome-Targeting Near-Infrared Fluorescent Probe with Large Stokes Shift for In Situ Quantification of Tyrosinase Activity and Assessing Drug Effects on Differently Invasive Melanoma Cells. Anal. Chem. 2018, 90, 6206–6213. [Google Scholar] [CrossRef]
- Yu, F.; Gao, M.; Li, M.; Chen, L. A dual response near-infrared fluorescent probe for hydrogen polysulfides and superoxide anion detection in cells and in vivo. Biomaterials 2015, 63, 93–101. [Google Scholar] [CrossRef]
- Li, P.; Zhang, W.; Li, K.; Liu, X.; Xiao, H.; Zhang, W.; Tang, B. Mitochondria-Targeted Reaction-Based Two-Photon Fluorescent Probe for Imaging of Superoxide Anion in Live Cells and In Vivo. Anal. Chem. 2013, 85, 9877–9881. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Li, P.; Huang, F.; Wang, H.; Zhang, W.; Tang, B. Elucidating the relationship between superoxide anion levels and lifespan using an enhanced two-photon fluorescence imaging probe. Chem. Commun. 2015, 51, 9710–9713. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, X.; Wang, Y.; Liu, Q.; Yu, F.; Huang, Y.; Ding, C.; Chen, L. Sequential Detection of Superoxide Anion and Hydrogen Polysulfides under Hypoxic Stress via a Spectral-Response-Separated Fluorescent Probe Functioned with a Nitrobenzene Derivative. Anal. Chem. 2019, 91, 7774–7781. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Xu, W.; Yang, Z.; Yan, Y.; Xie, X.; Wang, Y.; Yi, T.; Wang, C.; Hua, J. Mitochondria-Targeted Ratiometric Fluorescent Probe Based on Diketopyrrolopyrrole for Detecting and Imaging of Endogenous Superoxide Anion in Vitro and In Vivo. Anal. Chem. 2019, 91, 5786–5793. [Google Scholar] [CrossRef] [PubMed]
- Piperi, C.; Adamopoulos, C.; Dalagiorgou, G.; Diamanti-Kandarakis, E.; Papavassiliou, A.G. Crosstalk between Advanced Glycation and Endoplasmic Reticulum Stress: Emerging Therapeutic Targeting for Metabolic Diseases. J. Clin. Endocrinol. Metab. 2012, 97, 2231–2242. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, A.; Mendez, R.; Zhang, K. Cross Talk Between ER Stress, Oxidative Stress, and Inflammation in Health and Disease. In Stress Responses: Methods and Protocols; Oslowski, C.M., Ed.; Springer: New York, NY, USA, 2015; pp. 205–214. [Google Scholar]
- Bronsart, L.L.; Stokes, C.; Contag, C.H. Chemiluminescence Imaging of Superoxide Anion Detects Beta-Cell Function and Mass. PLoS ONE 2016, 11, e0146601. [Google Scholar] [CrossRef]
- Foufelle, F.; Fromenty, B. Role of endoplasmic reticulum stress in drug-induced toxicity. Pharmacol. Res. Perspect. 2016, 4, e00211. [Google Scholar] [CrossRef]
- Iorga, A.; Dara, L.; Kaplowitz, N. Drug-Induced Liver Injury: Cascade of Events Leading to Cell Death, Apoptosis or Necrosis. Int. J. Mol. Sci. 2017, 18, 1018. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.; Wu, Q.; Lv, Y.; Gong, X.; Cheng, D. Imaging of endoplasmic reticulum superoxide anion fluctuation in a liver injury model by a selective two-photon fluorescent probe. New J. Chem. 2020, 44, 5457–5462. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, M.; Chen, Q.; Yu, F.; Jiang, G.; Chen, L. Associated Detection of Superoxide Anion and Mercury(II) under Chronic Mercury Exposure in Cells and Mice Models via a Three-Channel Fluorescent Probe. Anal. Chem. 2018, 90, 9769–9778. [Google Scholar] [CrossRef]
- Nazarewicz, R.R.; Bikineyeva, A.; Dikalov, S.I. Rapid and Specific Measurements of Superoxide Using Fluorescence Spectroscopy. J. Biomol. Screen. 2013, 18, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Madhurantakam, S.; Selvaraj, S.; Nesakumar, N.; Sethuraman, S.; Rayappan, J.B.B.; Krishnan, U.M. Electrochemical enzymeless detection of superoxide employing naringin–copper decorated electrodes. Biosens. Bioelectron. 2014, 59, 134–139. [Google Scholar] [CrossRef]
- Liu, N.; Hao, J.; Cai, K.; Zeng, M.; Huang, Z.; Chen, L.; Peng, B.; Li, P.; Wang, L.; Song, Y. Ratiometric fluorescence detection of superoxide anion based on AuNPs-BSA@Tb/GMP nanoscale coordination polymers. Luminescence 2018, 33, 119–124. [Google Scholar] [CrossRef]
- Shen, X.; Wang, Q.; Liu, Y.; Xue, W.; Ma, L.; Feng, S.; Wan, M.; Wang, F.; Mao, C. Manganese Phosphate Self-assembled Nanoparticle Surface and Its application for Superoxide Anion Detection. Sci. Rep. 2016, 6, 28989. [Google Scholar] [CrossRef] [PubMed]
- Ganesana, M.; Erlichman, J.S.; Andreescu, S. Real-time monitoring of superoxide accumulation and antioxidant activity in a brain slice model using an electrochemical cytochrome c biosensor. Free Radic. Biol. Med. 2012, 53, 2240–2249. [Google Scholar] [CrossRef] [PubMed]
- Crulhas, B.P.; Recco, L.C.; Delella, F.K.; Pedrosa, V.A. A Novel Superoxide Anion Biosensor for Monitoring Reactive Species of Oxygen Released by Cancer Cells. Electroanalysis 2017, 29, 1252–1257. [Google Scholar] [CrossRef]
- Ding, A.; Wang, B.; Ma, X.; Diao, J.; Zheng, J.; Chen, J.; Li, C. DNA-induced synthesis of biomimetic enzyme for sensitive detection of superoxide anions released from live cell. RSC Adv. 2018, 8, 12354–12359. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Liu, Y.; Liu, G.; Ding, L.; Lu, X. Construction of a highly sensitive non-enzymatic sensor for superoxide anion radical detection from living cells. Biosens. Bioelectron. 2017, 90, 39–45. [Google Scholar] [CrossRef]
- Liu, X.; Ran, M.; Liu, G.; Liu, X.; Xue, Z.; Lu, X. A sensitively non-enzymatic amperometric sensor and its application in living cell superoxide anion radical detection. Talanta 2018, 186, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Essafi, M.; Lee, C.; Haoues, M.; Diouani, M.F.; Kim, H.; Kim, Y. Ultrasensitive detection of hazardous reactive oxygen species using flexible organic transistors with polyphenol-embedded conjugated polymer sensing layers. J. Hazard Mater. 2018, 355, 17–24. [Google Scholar] [CrossRef]
- Madhurantakam, S.; Selvaraj, S.; Rayappan, J.B.B.; Krishnan, U.M. Exploring hesperidin-copper complex as an enzyme mimic for monitoring macrophage activity. J. Solid State Electrochem. 2018, 22, 1893–1899. [Google Scholar] [CrossRef]
- Peshavariya, H.M.; Dusting, G.J.; Selemidis, S. Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free Radic. Res. 2007, 41, 699–712. [Google Scholar] [CrossRef]
- Feron, K.; Lim, R.; Sherwood, C.; Keynes, A.; Brichta, A.; Dastoor, P.C. Organic Bioelectronics: Materials and Biocompatibility. Int. J. Mol. Sci. 2018, 19, 2382. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Devel. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef]
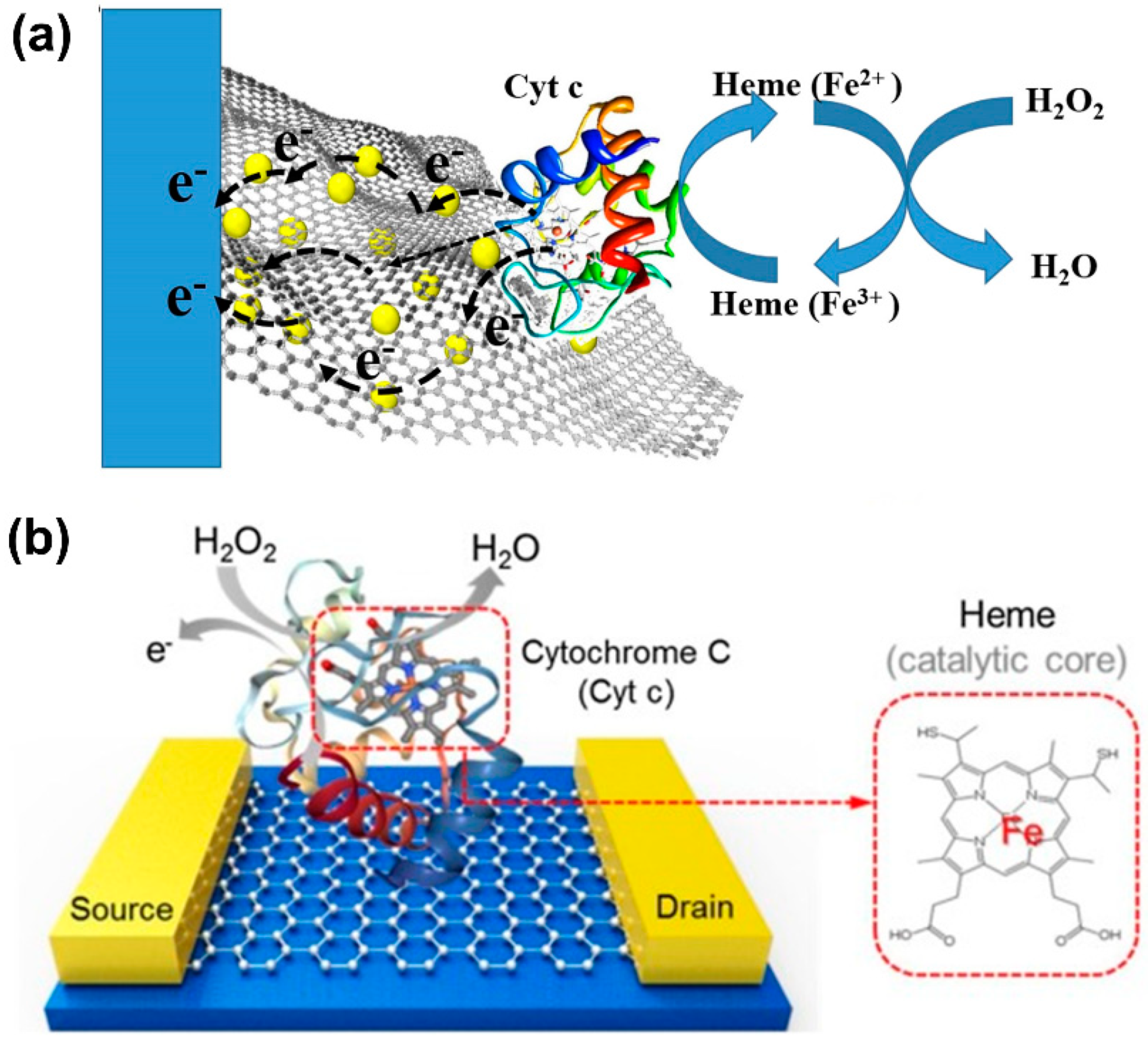
- Chen, X.J.; West, A.C.; Cropek, D.M.; Banta, S. Detection of the Superoxide Radical Anion Using Various Alkanethiol Monolayers and Immobilized Cytochrome c. Anal. Chem. 2008, 80, 9622–9629. [Google Scholar] [CrossRef] [PubMed]
- Lisdat, F.; Ge, B.; Ehrentreich-Förster, E.; Reszka, R.; Scheller, F.W. Superoxide Dismutase Activity Measurement Using Cytochrome c-Modified Electrode. Anal. Chem. 1999, 71, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wen, W.; Xiong, H.; Zhang, X.; Gu, H.; Wang, S. A novel amperometric biosensor for superoxide anion based on superoxide dismutase immobilized on gold nanoparticle-chitosan-ionic liquid biocomposite film. Anal. Chim. Acta 2013, 758, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Manickam, P.; Vashist, A.; Madhu, S.; Sadasivam, M.; Sakthivel, A.; Kaushik, A.; Nair, M. Gold nanocubes embedded biocompatible hybrid hydrogels for electrochemical detection of H2O2. Bioelectrochemistry 2020, 131, 107373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, D.; Sun, L.-H.; Xue, P.; Wang, M.-Q.; Lu, Z.; Wang, F.; Xia, Q.; Xu, M.-W.; Bao, S.-J. Constructing high effective nano-Mn3(PO4)2-chitosan in situ electrochemical detection interface for superoxide anions released from living cell. Biosens. Bioelectron. 2019, 133, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexanfria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Chen, W.; Cai, S.; Ren, Q.-Q.; Wen, W.; Zhao, Y.-D. Recent advances in electrochemical sensing for hydrogen peroxide: A review. Analyst 2012, 137, 49–58. [Google Scholar] [CrossRef]
- Endo, K.; Miyasaka, T.; Mochizuki, S.; Aoyagi, S.; Himi, N.; Asahara, H.; Tsujioka, K.; Sakai, K. Development of a superoxide sensor by immobilization of superoxide dismutase. Sens. Actuators B Chem. 2002, 83, 30–34. [Google Scholar] [CrossRef]
- Scheller, W.; Jin, W.; Ehrentreich-Förster, E.; Ge, B.; Lisdat, F.; Büttemeier, R.; Wollenberger, U.; Scheller, F.W. Cytochrome C Based Superoxide Sensor for In Vivo Application. Electroanalysis 1999, 11, 703–706. [Google Scholar] [CrossRef]
- Sadeghian, R.B.; Han, J.; Ostrovidov, S.; Salehi, S.; Bahraminejad, B.; Ahadian, S.; Chen, M.; Khademhosseini, A. Macroporous mesh of nanoporous gold in electrochemical monitoring of superoxide release from skeletal muscle cells. Biosens. Bioelectron. 2017, 88, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Rong, G.; Corrie, S.R.; Clark, H.A. In vivo biosensing: Progress and perspectives. ACS Sens. 2017, 2, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Bimbo, L.M.; Sarparanta, M.; Santos, H.A.; Airaksinen, A.J.; Mäkilä, E.; Laaksonen, T.; Peltonen, L.; Lehto, V.-P.; Hirvonen, J.; Salonen, J. Biocompatibility of Thermally Hydrocarbonized Porous Silicon Nanoparticles and their Biodistribution in Rats. ACS Nano 2010, 4, 3023–3032. [Google Scholar] [CrossRef]
- Peng, F.; Xu, T.; Wu, F.; Ma, C.; Liu, Y.; Li, J.; Zhao, B.; Mao, C. Novel biomimetic enzyme for sensitive detection of superoxide anions. Talanta 2017, 174, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, G.; Cai, Z.; Oyama, M.; Chen, X. Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim. Acta 2014, 181, 689–705. [Google Scholar] [CrossRef]
- Liu, T.; Niu, X.; Shi, L.; Zhu, X.; Zhao, H.; Lana, M. Electrocatalytic analysis of superoxide anion radical using nitrogen-doped graphene supported Prussian Blue as a biomimetic superoxide dismutase. Electrochim. Acta 2015, 176, 1280–1287. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, H.; Shi, L.; Lan, M.; Zhang, H.; Yu, C. Enzyme- and metal-free electrochemical sensor for highly sensitive superoxide anion detection based on nitrogen doped hollow mesoporous carbon spheres. Electrochim. Acta 2017, 227, 69–76. [Google Scholar] [CrossRef]
- Hu, F.X.; Guo, C.; Yang, H.B.; Shi, Z.; Wang, M.; Xue, Y.H.; Zhu, L.; Chen, T.; Dai, L.; Li, C.M. 3D Pt/Graphene foam bioplatform for highly sensitive and selective in-situ adsorption and detection of superoxide anions released from living cells. Sens. Actuators B Chem. 2019, 287, 209–217. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, M.; Liu, Y.; Wang, C.; Guan, L.; Li, K.; Lin, Y. Fabrication of Cobalt Nanocomposites as Enzyme Mimetic with Excellent Electrocatalytic Activity for Superoxide Oxidation and Cellular Release Detection. ACS Sustain. Chem. Eng. 2019, 7, 10227–10233. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef]
- Zaidi, S.A.; Shahzad, F.; Batool, S. Progress in cancer biomarkers monitoring strategies using graphene modified support materials. Talanta 2020, 210, 120669. [Google Scholar] [CrossRef]
- Booth, M.A.; Gowers, S.A.N.; Leong, C.L.; Rogers, M.L.; Samper, I.C.; Wickham, A.P.; Boutelle, M.G. Chemical Monitoring in Clinical Settings: Recent Developments toward Real-Time Chemical Monitoring of Patients. Anal. Chem. 2018, 90, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.K. Antimycin-insensitive oxidation of succinate and reduced nicotinamide-adenine dinucleotide in electron-transport particles I. pH dependency and hydrogen peroxide formation. Biochim. Biophys. Acta 1966, 122, 157–166. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Clifford, D.P.; Repine, J.E. Hydrogen peroxide mediated killing of bacteria. Mol. Cell. Biochem. 1982, 49, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. H2O2, a Necessary Evil for Cell Signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef]
- Netto, L.E.S.; Antunes, F. The Roles of Peroxiredoxin and Thioredoxin in Hydrogen Peroxide Sensing and in Signal Transduction. Mol. Cells 2016, 39, 65–71. [Google Scholar] [CrossRef]
- Chihara, R.; Kitajima, H.; Ogawa, Y.; Nakamura, H.; Tsutsui, S.; Mizutani, M.; Kino-oka, M.; Ezoe, S. Effects of residual H2O2 on the growth of MSCs after decontamination. Regan. Ther. 2018, 9, 111–115. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Chang, C.J. A targetable fluorescent probe for imaging hydrogen peroxide in the mitochondria of living cells. J. Am. Chem. Soc. 2008, 130, 9638–9639. [Google Scholar] [CrossRef]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef]
- Halliwell, B.; Clement, M.V.; Long, L.H. Hydrogen peroxide in the human body. FEBS Lett. 2000, 486, 10–13. [Google Scholar] [CrossRef]
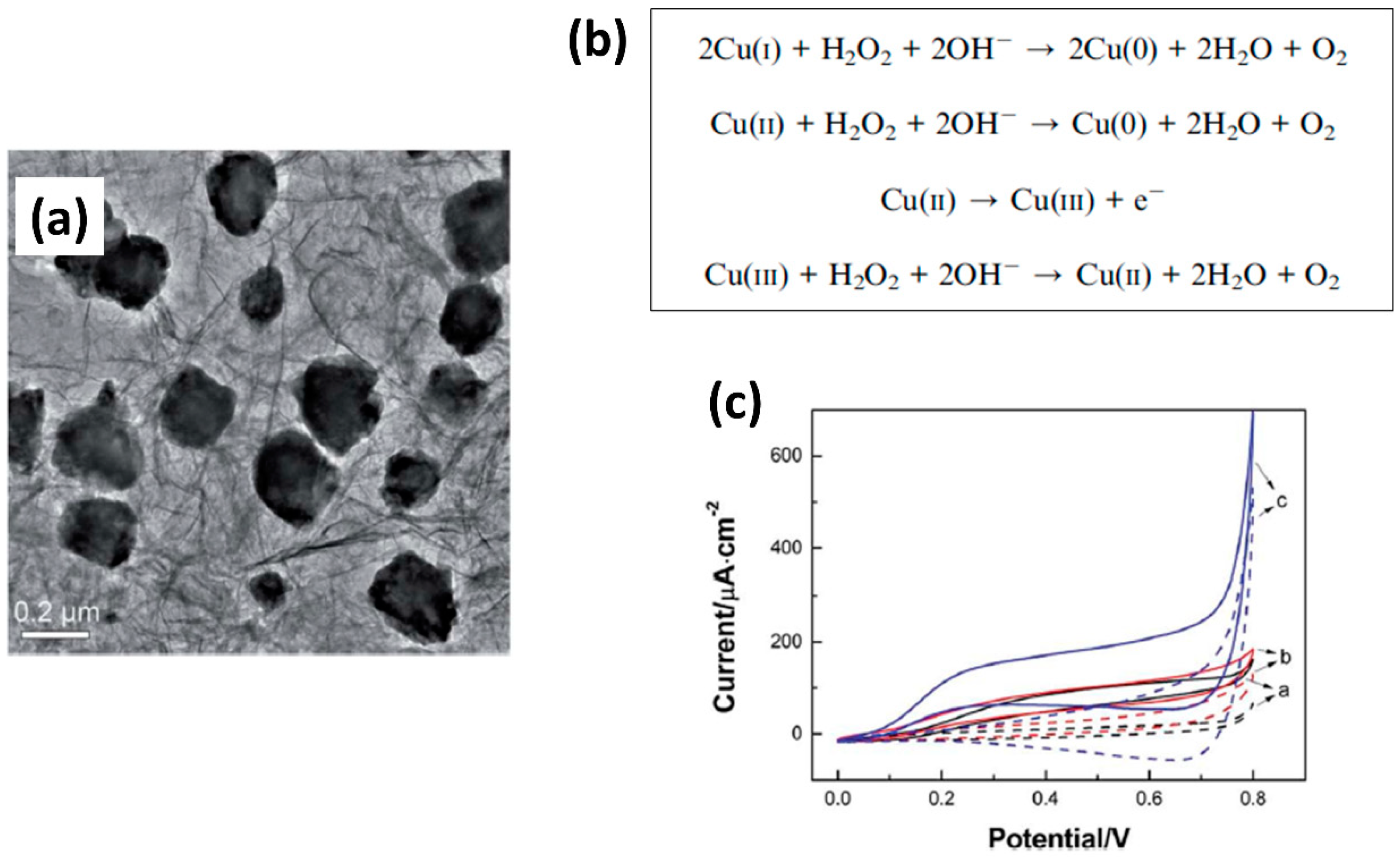
- Song, M.-J.; Hwang, S.W.; Whang, D. Non-enzymatic electrochemical CuO nanoflowers sensor for hydrogen peroxide detection. Talanta 2010, 80, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Deac, A.R.; Morar, C.; Turdean, G.L.; Darabantu, M.; Gál, E.; Bende, A.; Muresan, L.M. Glassy carbon electrode modified with hemin and new melamine compounds for H2O2 amperometric detection. J. Solid State Electrochem. 2016, 20, 3071–3081. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, K.; Guo, K.; Kong, J.; Marty, J.-L.; Yu, C.; Liu, B. Electrochemistry and biosensing activity of cytochrome c immobilized in macroporous materials. Microchim. Acta 2011, 175, 87. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, C.-Y. A novel nitrite biosensor based on direct electron transfer of hemoglobin immobilized on a graphene oxide/Au nanoparticles/multiwalled carbon nanotubes nanocomposite film. RSC Adv. 2014, 4, 31573–31580. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Kim, S.J. An enzymatic biosensor for hydrogen peroxide based on one-pot preparation of CeO2-reduced graphene oxide nanocomposite. RSC Adv. 2015, 5, 12937–12943. [Google Scholar] [CrossRef]
- Okawa, Y.; Yokoyama, N.; Sakai, Y.; Shiba, F. Direct electron transfer biosensor for hydrogen peroxide carrying nanocomplex composed of horseradish peroxidase and Au-nanoparticle–Characterization and application to bienzyme systems. Anal. Chem. Res. 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, J.; Wang, J.; Xu, J.; Hayat, T.; Alharbi, N.S. Direct electrochemistry of cytochrome c immobilized on one dimensional Au nanoparticles functionalized magnetic N-doped carbon nanotubes and its application for the detection of H2O2. Sens. Actuators B Chem. 2019, 282, 85–95. [Google Scholar] [CrossRef]
- Aghamiri, Z.S.; Mohsennia, M.; Rafiee-Pour, H.-A. Immobilization of cytochrome c on polyaniline/polypyrrole/carboxylated multi-walled carbon nanotube/glassy carbon electrode: Biosensor fabrication. J. Solid State Electrochem. 2019, 23, 2233–2242. [Google Scholar] [CrossRef]
- Thirumalai, D.; Kathiresan, V.; Lee, J.; Jin, S.-H.; Chang, S.-C. Electrochemical reactive oxygen species detection by cytochrome c immobilized with vertically aligned and electrochemically reduced graphene oxide on a glassy carbon electrode. Analyst 2017, 142, 4544–4552. [Google Scholar] [CrossRef]
- Dinesh, B.; Mani, V.; Saraswathi, R.; Chen, S.-M. Direct electrochemistry of cytochrome c immobilized on a graphene oxide–carbon nanotube composite for picomolar detection of hydrogen peroxide. RSC Adv. 2014, 4, 28229–28237. [Google Scholar] [CrossRef]
- Kong, B.; Liu, W.; Li, Q.; Chen, L.; Liu, X.; Zhou, J. A Hydrogen Peroxide Biosensor Based on Cytochrome c Immobilized Graphene-L-Cysteine Modified Glassy Carbon Electrode. Sens. Lett. 2015, 13, 267–272. [Google Scholar] [CrossRef]
- Eguílaz, M.; Gutiérrez, A.; Rivas, G. Non-covalent functionalization of multi-walled carbon nanotubes with cytochrome c: Enhanced direct electron transfer and analytical applications. Sens. Actuators B Chem. 2016, 225, 74–80. [Google Scholar] [CrossRef]
- Aghamiri, Z.S.; Mohsennia, M.; Rafiee-Pour, H.-A. Fabrication and characterization of cytochrome c-immobilized polyaniline/multi-walled carbon nanotube composite thin film layers for biosensor applications. Thin Solid Films 2018, 660, 484–492. [Google Scholar] [CrossRef]
- Mohseni, G.; Negahdary, M.; Malekzadeh, R.; Manoochehri, J.; Hadaegh, A.; Sayad, A.; Akbari-dastjerdi, H.; Fazilati, M.; Rezaei-Zarchi, S. Direct electron transfer of cytochrome c on ZrO2 nanoparticles modified glassy carbon electrode. Int. J. Electrochem. Sci. 2012, 7, 7033–7044. [Google Scholar]
- Akhtar, N.; El-Safty, S.A.; Khairy, M.; El-Said, W.A. Fabrication of a highly selective nonenzymatic amperometric sensor for hydrogen peroxide based on nickel foam/cytochrome c modified electrode. Sens. Actuators B Chem. 2015, 207, 158–166. [Google Scholar] [CrossRef]
- Rui, Q.; Komori, K.; Tian, Y.; Liu, H.; Luo, Y.; Sakai, Y. Electrochemical biosensor for the detection of H2O2 from living cancer cells based on ZnO nanosheets. Anal. Chim. Acta 2010, 670, 57–62. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, H.; Rui, Q.; Tian, Y. Detection of Extracellular H2O2 Released from Human Liver Cancer Cells Based on TiO2 Nanoneedles with Enhanced Electron Transfer of Cytochrome c. Anal. Chem. 2009, 81, 3035–3041. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, Y.; Hou, J.; Jia, Z.; Zhong, D.; Zhou, S.; Huo, D.; Yang, M.; Hou, C. Electrochemical biointerface based on electrodeposition AuNPs on 3D graphene aerogel: Direct electron transfer of Cytochrome c and hydrogen peroxide sensing. J. Electroanal. Chem. 2019, 842, 16–23. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, K.H.; Seo, S.E.; Kim, M.I.; Park, S.J.; Kwon, O.S. Cytochrome C-decorated graphene field-effect transistor for highly sensitive hydrogen peroxide detection. J. Ind. Eng. Chem. 2020, 83, 29–34. [Google Scholar] [CrossRef]
- Turdean, G.L.; Popescu, I.C.; Curulli, A.; Palleschi, G. Iron(III) protoporphyrin IX—single-wall carbon nanotubes modified electrodes for hydrogen peroxide and nitrite detection. Electrochim. Acta 2006, 51, 6435–6441. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Hu, J.-M. Hydrogen peroxide biosensor based on the direct electrochemistry of myoglobin immobilized on silver nanoparticles doped carbon nanotubes film. Biosens. Bioelectron. 2009, 24, 2149–2154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-L.; Zou, X.-Z.; Lai, G.-S.; Han, D.-Y.; Wang, F. Direct Electrochemistry of Hemoglobin Immobilized on Carbon-Coated Iron Nanoparticles for Amperometric Detection of Hydrogen Peroxide. Electroanalysis 2007, 19, 1869–1874. [Google Scholar] [CrossRef]
- Xu, X.; Song, L.; Zheng, Q.; Cao, X.; Yao, C. General Preparation of Heme Protein Functional Fe3O4@ Au-Nps Magnetic Nanocomposite for Sensitive Detection of Hydrogen Peroxide. Electroanalysis 2017, 29, 765–772. [Google Scholar] [CrossRef]
- Kong, F.-Y.; Li, W.-W.; Wang, J.-Y.; Fang, H.-L.; Fan, D.-H.; Wang, W. Direct electrolytic exfoliation of graphite with hemin and single-walled carbon nanotube: Creating functional hybrid nanomaterial for hydrogen peroxide detection. Anal. Chim. Acta 2015, 884, 37–43. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, Z.; Liu, H.; Yang, M.; Lin, L.; Li, Q. Hemin-graphene oxide-pristine carbon nanotubes complexes with intrinsic peroxidase-like activity for the detection of H2O2 and simultaneous determination for Trp, AA, DA, and UA. Sens. Actuators B Chem. 2013, 188, 496–501. [Google Scholar] [CrossRef]
- Le, H.T.N.; Jeong, H.K. Electrochemical supramolecular recognition of hemin-carbon composites. Chem. Phys. Lett. 2018, 698, 102–109. [Google Scholar] [CrossRef]
- Wu, H.; Wei, T.; Li, X.; Yang, J.; Zhang, J.; Fan, S.; Zhang, H. Synergistic-effect-controlled tetraoctylammonium bromide/multi-walled carbon nanotube@ hemin hybrid material for construction of electrochemical sensor. J. Electrochem. Soc. 2017, 164, B147–B151. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, Z.; Zhou, G.; Zhu, L.; Feng, Y.; Lin, T.; Hou, H.; Guo, Q. A sensitive hydrogen peroxide sensor based on a three-dimensional N-doped carbon nanotube-hemin modified electrode. Anal. Methods 2015, 7, 8439–8444. [Google Scholar] [CrossRef]
- Zhao, Y.-D.; Bi, Y.-H.; Zhang, W.-D.; Luo, Q.-M. The interface behavior of hemoglobin at carbon nanotube and the detection for H2O2. Talanta 2005, 65, 489–494. [Google Scholar] [CrossRef]
- Valentini, F.; Cristofanelli, L.; Carbone, M.; Palleschi, G. Glassy carbon electrodes modified with hemin-carbon nanomaterial films for amperometric H2O2 and NO2− detection. Electrochim. Acta 2012, 63, 37–46. [Google Scholar] [CrossRef]
- Deac, A.R.; Muresan, L.M.; Cotet, L.C.; Baia, L.; Turdean, G.L. Hybrid composite material based on graphene and polyhemin for electrochemical detection of hydrogen peroxide. J. Electroanal. Chem. 2017, 802, 40–47. [Google Scholar] [CrossRef]
- Qin, F.-X.; Jia, S.-Y.; Wang, F.-F.; Wu, S.-H.; Song, J.; Liu, Y. Hemin@ metal–organic framework with peroxidase-like activity and its application to glucose detection. Catal. Sci. Technol. 2013, 3, 2761–2768. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Sun, J.; Jiao, S.; Chen, H.; Gao, F.; Wang, L. Fluorescent blood glucose monitor by hemin-functionalized graphene quantum dots based sensing system. Anal. Chim. Acta 2014, 810, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, H.; He, J.; Zhang, Y.; Yu, J.; Song, Y. Cu-hemin metal-organic-frameworks/chitosan-reduced graphene oxide nanocomposites with peroxidase-like bioactivity for electrochemical sensing. Electrochim. Acta 2016, 213, 691–697. [Google Scholar] [CrossRef]
- Kumar, S.; Bhushan, P.; Bhattacharya, S. Facile synthesis of Au@ Ag–hemin decorated reduced graphene oxide sheets: A novel peroxidase mimetic for ultrasensitive colorimetric detection of hydrogen peroxide and glucose. RSC Adv. 2017, 7, 37568–37577. [Google Scholar] [CrossRef]
- Kong, J.; Yu, X.; Hu, W.; Hu, Q.; Shui, S.; Li, L.; Han, X.; Xie, H.; Zhang, X.; Wang, T. A biomimetic enzyme modified electrode for H2O2 highly sensitive detection. Analyst 2015, 140, 7792–7798. [Google Scholar] [CrossRef]
- Zhang, L.; Han, G.; Liu, Y.; Tang, J.; Tang, W. Immobilizing haemoglobin on gold/graphene–chitosan nanocomposite as efficient hydrogen peroxide biosensor. Sens. Actuators B Chem. 2014, 197, 164–171. [Google Scholar] [CrossRef]
- Zhao, P.; Chen, S.; Zhou, J.; Zhang, S.; Huo, D.; Hou, C. A novel Fe-hemin-metal organic frameworks supported on chitosan-reduced graphene oxide for real-time monitoring of H2O2 released from living cells. Anal. Chim. Acta 2020, 1128, 90–98. [Google Scholar] [CrossRef]
- Wang, W.; Tang, H.; Wu, Y.; Zhang, Y.; Li, Z. Highly electrocatalytic biosensor based on Hemin@AuNPs/reduced graphene oxide/chitosan nanohybrids for non-enzymatic ultrasensitive detection of hydrogen peroxide in living cells. Biosens. Bioelectron. 2019, 132, 217–223. [Google Scholar] [CrossRef]
- Yang, Z.; Qi, C.; Zheng, X.; Zheng, J. Sensing hydrogen peroxide with a glassy carbon electrode modified with silver nanoparticles, AlOOH and reduced graphene oxide. Microchim. Acta 2016, 183, 1131–1136. [Google Scholar] [CrossRef]
- Kumar, V.; Gupta, R.K.; Gundampati, R.K.; Singh, D.K.; Mohan, S.; Hasan, S.H.; Malviya, M. Enhanced electron transfer mediated detection of hydrogen peroxide using a silver nanoparticle–reduced graphene oxide–polyaniline fabricated electrochemical sensor. RSC Adv. 2018, 8, 619–631. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Huang, N.; Lu, Q.; Liu, M.; Li, H.; Zhang, Y.; Yao, S. Sensitive detection of hydrogen peroxide and nitrite based on silver/carbon nanocomposite synthesized by carbon dots as reductant via one step method. Electrochim. Acta 2016, 211, 36–43. [Google Scholar] [CrossRef]
- Yusoff, N.; Rameshkumar, P.; Mehmood, M.S.; Pandikumar, A.; Lee, H.W.; Huang, N.M. Ternary nanohybrid of reduced graphene oxide-nafion@silver nanoparticles for boosting the sensor performance in non-enzymatic amperometric detection of hydrogen peroxide. Biosens. Bioelectron. 2017, 87, 1020–1028. [Google Scholar] [CrossRef]
- Li, C.; Chen, D.; Wang, Y.; Lai, X.; Peng, J.; Wang, X.; Zhang, K.; Cao, Y. Simultaneous Electrochemical Detection of Nitrite and Hydrogen Peroxide Based on 3D Au-rGO/FTO Obtained Through a One-Step Synthesis. Sensors 2019, 19, 1304. [Google Scholar] [CrossRef]
- Maji, S.K.; Sreejith, S.; Mandal, A.K.; Ma, X.; Zhao, Y. Immobilizing gold nanoparticles in mesoporous silica covered reduced graphene oxide: A hybrid material for cancer cell detection through hydrogen peroxide sensing. ACS Appl. Mater. Interfaces 2014, 6, 13648–13656. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Yu, H.; Li, X. In situ growth of noble metal nanoparticles on graphene oxide sheets and direct construction of functionalized porous-layered structure on gravimetric microsensors for chemical detection. Chem. Commun. 2012, 48, 10784–10786. [Google Scholar] [CrossRef]
- Nellaiappan, S.; Kumar, A.S.; Nisha, S.; Pillai, K.C. In-situ preparation of Au(111) oriented nanoparticles trapped carbon nanofiber-chitosan modified electrode for enhanced bifunctional electrocatalysis and sensing of formaldehyde and hydrogen peroxide in neutral pH solution. Electrochim. Acta 2017, 249, 227–240. [Google Scholar] [CrossRef]
- Lin, X.-Q.; Deng, H.-H.; Wu, G.-W.; Peng, H.-P.; Liu, A.-L.; Lin, X.-H.; Xia, X.-H.; Chen, W. Platinum nanoparticles/graphene-oxide hybrid with excellent peroxidase-like activity and its application for cysteine detection. Analyst 2015, 140, 5251–5256. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Zhang, Y.; Liu, Y.; Li, M.; Lu, X. Green and rapid synthesis of a water-dispersible Pt–reduced graphene oxide hybrid material for hydrogen peroxide detection. Anal. Methods 2016, 8, 816–823. [Google Scholar] [CrossRef]
- Li, Y.; Huan, K.; Deng, D.; Tang, L.; Wang, J.; Luo, L. Facile Synthesis of ZnMn2O4@rGO Microspheres for Ultrasensitive Electrochemical Detection of Hydrogen Peroxide from Human Breast Cancer Cells. ACS Appl. Mater. Interfaces 2020, 12, 3430–3437. [Google Scholar] [CrossRef] [PubMed]
- Salih, E.; Mekawy, M.; Hassan, R.Y.A.; El-Sherbiny, I.M. Synthesis, characterization and electrochemical-sensor applications of zinc oxide/graphene oxide nanocomposite. J. Nanostruct. Chem. 2016, 6, 137–144. [Google Scholar] [CrossRef]
- Hussain, M.; Sun, H.; Karim, S.; Nisar, A.; Khan, M.; Ul Haq, A.; Iqbal, M.; Ahmad, M. Noble metal nanoparticle-functionalized ZnO nanoflowers for photocatalytic degradation of RhB dye and electrochemical sensing of hydrogen peroxide. J. Nanopart. Res. 2016, 18, 95. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, D.; Li, Q. Manganese oxide ultrathin nanosheets sensors for non-enzymatic detection of H2O2. Mater. Lett. 2014, 125, 202–205. [Google Scholar] [CrossRef]
- Zeng, F.; Pan, Y.; Yang, Y.; Li, Q.; Li, G.; Hou, Z.; Gu, G. Facile construction of Mn3O4-MnO2 hetero-nanorods/graphene nanocomposite for highly sensitive electrochemical detection of hydrogen peroxide. Electrochim. Acta 2016, 196, 587–596. [Google Scholar] [CrossRef]
- Hou, C.; Xu, Q.; Yin, L.; Hu, X. Metal–organic framework templated synthesis of Co3O4 nanoparticles for direct glucose and H2O2 detection. Analyst 2012, 137, 5803–5808. [Google Scholar] [CrossRef]
- Liu, M.; He, S.; Chen, W. Co3O4 nanowires supported on 3D N-doped carbon foam as an electrochemical sensing platform for efficient H2O2 detection. Nanoscale 2014, 6, 11769–11776. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, X.; Huang, D.; Li, S.; Yuan, H.; Wang, M.; Shen, Y. Co9S8 hollow spheres for enhanced electrochemical detection of hydrogen peroxide. Talanta 2015, 141, 73–79. [Google Scholar] [CrossRef]
- Li, Z.; Xin, Y.; Wu, W.; Fu, B.; Zhang, Z. Topotactic Conversion of Copper(I) Phosphide Nanowires for Sensitive Electrochemical Detection of H2O2 Release from Living Cells. Anal. Chem. 2016, 88, 7724–7729. [Google Scholar] [CrossRef]
- Zeng, H.-H.; Qiu, W.-B.; Zhang, L.; Liang, R.-P.; Qiu, J.-D. Lanthanide coordination polymer nanoparticles as an excellent artificial peroxidase for hydrogen peroxide detection. Anal. Chem. 2016, 88, 6342–6348. [Google Scholar] [CrossRef]
- Goud, K.Y.; Kumar, V.S.; Hayat, A.; Catanante, G.; Gobi, K.V.; Marty, J.L. Polymer scaffold layers of screen-printed electrodes for homogeneous deposition of silver nanoparticles: Application to the amperometric detection of hydrogen peroxide. Microchim. Acta 2019, 186, 810. [Google Scholar] [CrossRef] [PubMed]
- Preethi, S.; Sangaranarayanan, M.V. Shape-controlled electrodeposition of silver using chitosan as structure-directing agent on disposable pencil graphite electrodes: Low-cost electrocatalysts for the detection of hydrogen peroxide and hydrazine hydrate. J. Solid State Electrochem. 2020, 24, 2773–2788. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.Z.; Bian, C.; Tong, J.H.; Dong, H.P.; Zhang, H.; Xia, S.H. Copper nano-clusters prepared by one-step electrodeposition and its application on nitrate sensing. AIP Advances 2015, 5, 041312. [Google Scholar] [CrossRef]
- Liu, Y.; Han, Y.; Chen, R.; Zhang, H.; Liu, S.; Liang, F. In situ Immobilization of Copper Nanoparticles on Polydopamine Coated Graphene Oxide for H2O2 Determination. PLoS ONE 2016, 11, e0157926. [Google Scholar] [CrossRef]
- Ma, G.; Yang, M.; Zhao, G.; Zhang, Y.; Xu, F.; Wang, L. Facile preparation of a three-dimensional macroporous graphene wrapped cuprous oxide composite by one-step hydrothermal assembly for stable and sensitive hydrogen peroxide detection. Anal. Methods 2016, 8, 7405–7412. [Google Scholar] [CrossRef]
- Park, B.K.; Kim, D.; Jeong, S.; Moon, J.; Kim, J.S. Direct writing of copper conductive patterns by ink-jet printing. Thin Solid Films 2007, 515, 7706–7711. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Y.; Hu, Z.-W.; Yu, S.-H. Comparison Study on the Stability of Copper Nanowires and Their Oxidation Kinetics in Gas and Liquid. ACS Nano 2016, 10, 3823–3834. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xu, Z.; Zhang, T.; Shao, Y.; Xi, P.; Li, H.; Xu, C. Cu2O/CuO@rGO heterostructure derived from metal–organic-frameworks as an advanced electrocatalyst for non-enzymatic electrochemical H2O2 sensor. RSC Adv. 2016, 6, 103116–103123. [Google Scholar] [CrossRef]
- Golsheikh, A.M.; Yeap, G.-Y.; Yam, F.K.; Lim, H.S. Facile fabrication and enhanced properties of copper-based metal organic framework incorporated with graphene for non-enzymatic detection of hydrogen peroxide. Synth. Met. 2020, 260, 116272. [Google Scholar] [CrossRef]
- Bui, Q.-T.; Yu, I.-K.; Gopalan, A.I.; Saianand, G.; Kim, W.; Choi, S.-H. Facile Fabrication of Metal Oxide Based Catalytic Electrodes by AC Plasma Deposition and Electrochemical Detection of Hydrogen Peroxide. Catalysts 2019, 9, 888. [Google Scholar] [CrossRef]
- Amala, G.; Saravanan, J.; Yoo, D.J.; Kim, A.R. An environmentally benign one pot green synthesis of reduced graphene oxide based composites for the enzyme free electrochemical detection of hydrogen peroxide. New J. Chem. 2017, 41, 4022–4030. [Google Scholar] [CrossRef]
- Fu, Y.; Huang, D.; Li, C.; Zou, L.; Ye, B. Graphene blended with SnO2 and Pd-Pt nanocages for sensitive non-enzymatic electrochemical detection of H2O2 released from living cells. Anal. Chim. Acta 2018, 1014, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, Y.; Bao, J.; Huo, D.; Fa, H.; Shen, X.; Hou, C. One-step electrodeposition of Au-Pt bimetallic nanoparticles on MoS2 nanoflowers for hydrogen peroxide enzyme-free electrochemical sensor. Electrochim. Acta 2017, 250, 152–158. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, Y.; Lin, S.; Wang, L.; Wang, C. Synthesis of PtAu bimetallic nanoparticles on graphene–carbon nanotube hybrid nanomaterials for nonenzymatic hydrogen peroxide sensor. Talanta 2013, 112, 111–116. [Google Scholar] [CrossRef]
- Liu, W.; Hiekel, K.; Hübner, R.; Sun, H.; Ferancova, A.; Sillanpää, M. Pt and Au bimetallic and monometallic nanostructured amperometric sensors for direct detection of hydrogen peroxide: Influences of bimetallic effect and silica support. Sens. Actuators B Chem. 2018, 255, 1325–1334. [Google Scholar] [CrossRef]
- Janyasupab, M.; Zhang, Y.; Lin, P.-Y.; Bartling, B.; Xu, J.; Liu, C.-C. Bimetallic Pt-Ru Nanoparticle Catalyst for Hydrogen Peroxide Detection. J. Nanotechnol. 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Heydaryan, K.; Almasi-Kashi, M.; Sharifi, N.; Ranjbar-Azad, M. Efficiency improvement in non-enzymatic H2O2 detection induced by the simultaneous synthesis of Au and Ag nanoparticles in an RGO/Au/Fe3O4/Ag nanocomposite. New J. Chem. 2020, 44, 9037–9045. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Zhao, X.; Deng, Y.; Li, Q.; Xia, Y. Green Preparation of Ag-Au Bimetallic Nanoparticles Supported on Graphene with Alginate for Non-Enzymatic Hydrogen Peroxide Detection. Nanomaterials 2018, 8, 507. [Google Scholar] [CrossRef]
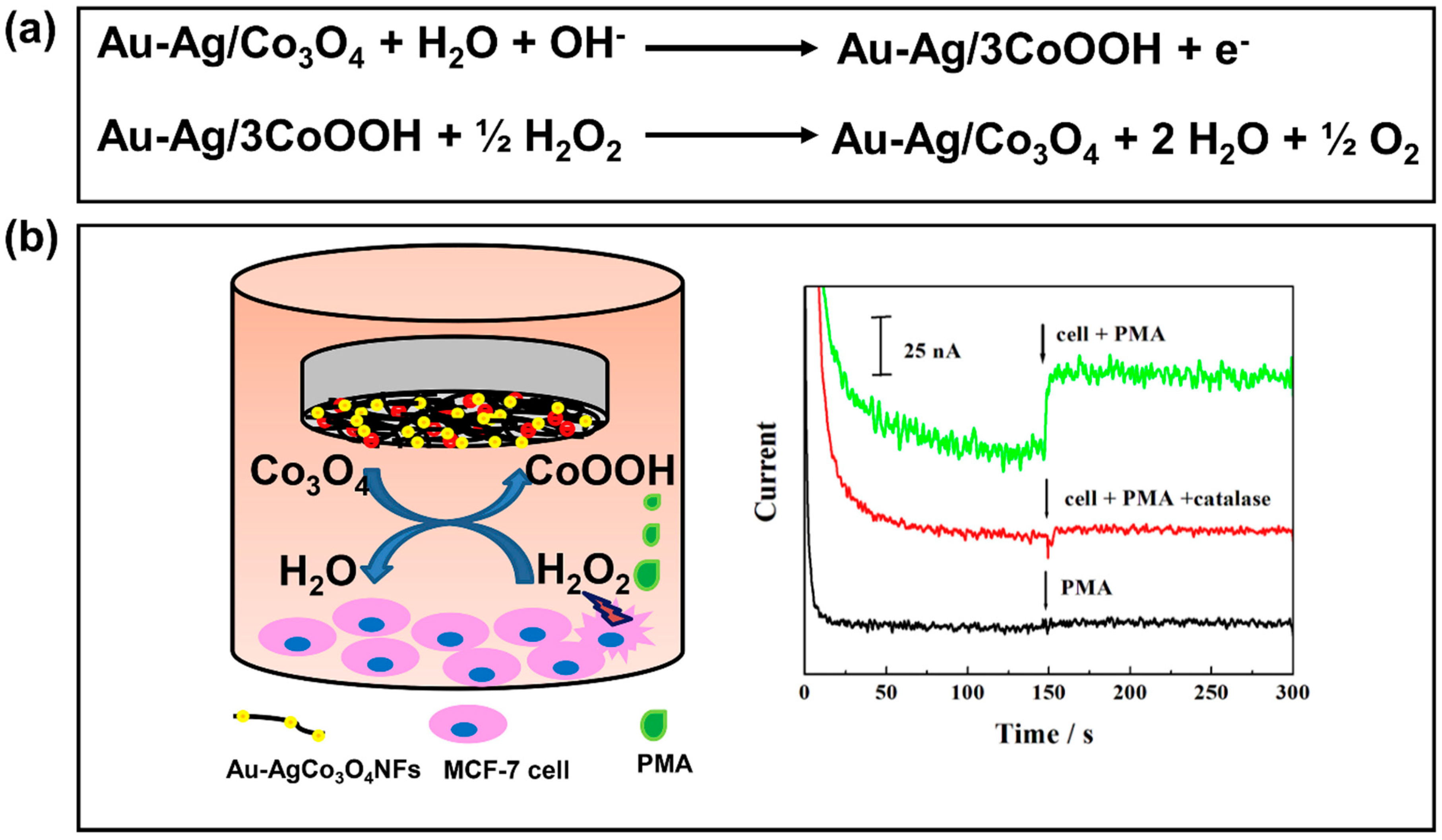
- Zhang, Y.; Deng, D.; Zhu, X.; Liu, S.; Zhu, Y.; Han, L.; Luo, L. Electrospun bimetallic Au-Ag/Co3O4 nanofibers for sensitive detection of hydrogen peroxide released from human cancer cells. Anal. Chim. Acta 2018, 1042, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Bach, L.G.; Thi, M.L.N.; Son, N.T.; Bui, Q.B.; Nhac-Vu, H.T.; Ai-Le, P.H. Mesoporous gold nanoparticles supported cobalt nanorods as a free-standing electrochemical sensor for sensitive hydrogen peroxide detection. J. Electroanal. Chem. 2019, 848, 113359. [Google Scholar] [CrossRef]
- Zhang, T.; Xing, Y.; Song, Y.; Gu, Y.; Yan, X.; Lu, N.; Liu, H.; Xu, Z.; Xu, H.; Zhang, Z.; et al. AuPt/MOF–Graphene: A Synergistic Catalyst with Surprisingly High Peroxidase-Like Activity and Its Application for H2O2 Detection. Anal. Chem. 2019, 91, 10589–10595. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.; Tran, V.-K.; Son, S.E.; Hur, W.; Choi, H.; Seong, G.H. Characterization of Au@PtNP/GO nanozyme and its application to electrochemical microfluidic devices for quantification of hydrogen peroxide. Sens. Actuators B Chem. 2019, 294, 166–176. [Google Scholar] [CrossRef]
- Shang, L.; Zeng, B.; Zhao, F. Fabrication of Novel Nitrogen-Doped Graphene–Hollow AuPd Nanoparticle Hybrid Films for the Highly Efficient Electrocatalytic Reduction of H2O2. ACS Appl. Mater. Interfaces 2015, 7, 122–128. [Google Scholar] [CrossRef]
- Liang, X.; Li, W.; Han, H.; Ma, Z. Non-enzymatic electrochemical sensor based on Cu2O/AuCu/Cu composite for hydrogen peroxide detection. J. Electroanal. Chem. 2019, 834, 43–48. [Google Scholar] [CrossRef]
- Dang, W.; Sun, Y.; Jiao, H.; Xu, L.; Lin, M. AuNPs-NH2/Cu-MOF modified glassy carbon electrode as enzyme-free electrochemical sensor detecting H2O2. J. Electroanal. Chem. 2020, 856, 113592. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Q.; Cheng, X.; Wang, Y.; Zhang, Y.; Fan, G. Sustainable and scalable in-situ fabrication of Au nanoparticles and Fe3O4 hybrids as highly efficient electrocatalysts for the enzyme-free sensing of H2O2 in neutral and basic solutions. Sens. Actuators B Chem. 2020, 314, 128067. [Google Scholar] [CrossRef]
- Huang, S.; Jiang, G.; Wu, X.; Zhang, H.; Chen, Z.; Chen, A.; Zhang, J. Scalable synthesis of heterostructure of Fe2O3–Au nanomaterials for application in biological detection. Mater. Res. Express 2020, 6, 1250b5. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Gong, S.; Chen, Y.; Xie, R.; Wu, Q.; Tao, J.; Meng, F.; Zhao, P. A novel non-enzymatic electrochemical biosensor based on the nanohybrid of bimetallic PdCu nanoparticles/carbon black for highly sensitive detection of H2O2 released from living cells. Sens. Actuators B Chem. 2019, 290, 249–257. [Google Scholar] [CrossRef]
- Ramaraj, S.; Sakthivel, M.; Chen, S.-M.; Lou, B.-S.; Ho, K.-C. Defect and Additional Active Sites on the Basal Plane of Manganese-Doped Molybdenum Diselenide for Effective Enzyme Immobilization: In Vitro and In Vivo Real-Time Analyses of Hydrogen Peroxide Sensing. ACS Appl. Mater. Interfaces 2019, 11, 7862–7871. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Shanthi, S.; Peng, T.-K.; Lin, H.-Y.; Ikeda, H.; Hayakawa, Y.; Ponnusamy, S.; Muthamizhchelvan, C.; Huang, S.-T. Real-time quantification of hydrogen peroxide production in living cells using NiCo2S4@CoS2 heterostructure. Sens. Actuators B Chem. 2019, 287, 124–130. [Google Scholar] [CrossRef]
- Kung, C.-C.; Lin, P.-Y.; Buse, F.J.; Xue, Y.; Yu, X.; Dai, L.; Liu, C.-C. Preparation and characterization of three dimensional graphene foam supported platinum–ruthenium bimetallic nanocatalysts for hydrogen peroxide based electrochemical biosensors. Biosens. Bioelectron. 2014, 52, 1–7. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Du, X.; Chen, Y.; Dong, W.; Han, B.; Chen, Q. Facile fabrication of Pt-Ag bimetallic nanoparticles decorated reduced graphene oxide for highly sensitive non-enzymatic hydrogen peroxide sensing. Talanta 2016, 159, 280–286. [Google Scholar] [CrossRef]
- Sakthivel, K.; Mani, G.; Chen, S.-M.; Lin, S.-H.; Muthumariappan, A.; Mani, V. A novel synthesis of non-aggregated spinel nickel ferrite nanosheets for developing non-enzymatic reactive oxygen species sensor in biological samples. J. Electroanal. Chem. 2018, 820, 161–167. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Chapter 31—Cytochrome P450 and Oxidative Stress in the Liver. In Liver Pathophysiology; Muriel, P., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 401–419. [Google Scholar]
- Lyngsie, G.; Krumina, L.; Tunlid, A.; Persson, P. Generation of hydroxyl radicals from reactions between a dimethoxyhydroquinone and iron oxide nanoparticles. Sci. Rep. 2018, 8, 10834. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Ramachandran, A.; Jaeschke, H. Oxidative stress and acute hepatic injury. Curr. Opin. Toxicol. 2018, 7, 17–21. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, Y.-J.; Xu, D.-P.; Wang, F.; Zhou, Y.; Zheng, J.; Li, Y.; Zhang, J.-J.; Li, H.-B. Protective Effects of Lemon Juice on Alcohol-Induced Liver Injury in Mice. Biomed. Res. Int. 2017, 2017, 7463571. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Morón, E.; Abad-Jiménez, Z.; de Marañón, A.M.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef]
- Umeno, A.; Biju, V.; Yoshida, Y. In vivo ROS production and use of oxidative stress-derived biomarkers to detect the onset of diseases such as Alzheimer’s disease, Parkinson’s disease, and diabetes. Free Radic. Res. 2017, 51, 413–427. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Cao, Y.; Tian, X.; Zeng, R.; Liao, D.-F.; Cao, D. Oxidative Stress and Carbonyl Lesions in Ulcerative Colitis and Associated Colorectal Cancer. Oxid. Med. Cell. Longev. 2016, 2016, 9875298. [Google Scholar] [CrossRef]
- Saed, G.M.; Diamond, M.P.; Fletcher, N.M. Updates of the role of oxidative stress in the pathogenesis of ovarian cancer. Gynecol. Oncol. 2017, 145, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Ball, N.; Teo, W.-P.; Chandra, S.; Chapman, J. Parkinson’s Disease and the Environment. Front. Neurol. 2019, 10, 218. [Google Scholar] [CrossRef]
- Han, S.K.; Hwang, T.-M.; Yoon, Y.; Kang, J.-W. Evidence of singlet oxygen and hydroxyl radical formation in aqueous goethite suspension using spin-trapping electron paramagnetic resonance (EPR). Chemosphere 2011, 84, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Burkitt, M.J.; Mason, R.P. Direct evidence for in vivo hydroxyl-radical generation in experimental iron overload: An ESR spin-trapping investigation. Proc. Natl. Acad. Sci. USA 1991, 88, 8440. [Google Scholar] [CrossRef]
- Newton, G.L.; Milligan, J.R. Fluorescence detection of hydroxyl radicals. Radiat. Phys. Chem. 2006, 75, 473–478. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Song, J. Ratiometric fluorescent detection of intracellular hydroxyl radicals based on a hybrid coumarin–cyanine platform. Chem. Commun. 2010, 46, 7930–7932. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Deng, D.; Zhang, K.; Lu, T.; Su, Y.; Lv, Y. Silicon carbon nanoparticles-based chemiluminescence probe for hydroxyl radical in PM2.5. Chem. Commun. 2016, 52, 11259–11262. [Google Scholar] [CrossRef]
- Cao, Y.; Sui, D.; Zhou, W.; Lu, C. Highly selective chemiluminescence detection of hydroxyl radical via increased π-electron densities of rhodamine B on montmorillonite matrix. Sens. Actuators B Chem. 2016, 225, 600–606. [Google Scholar] [CrossRef]
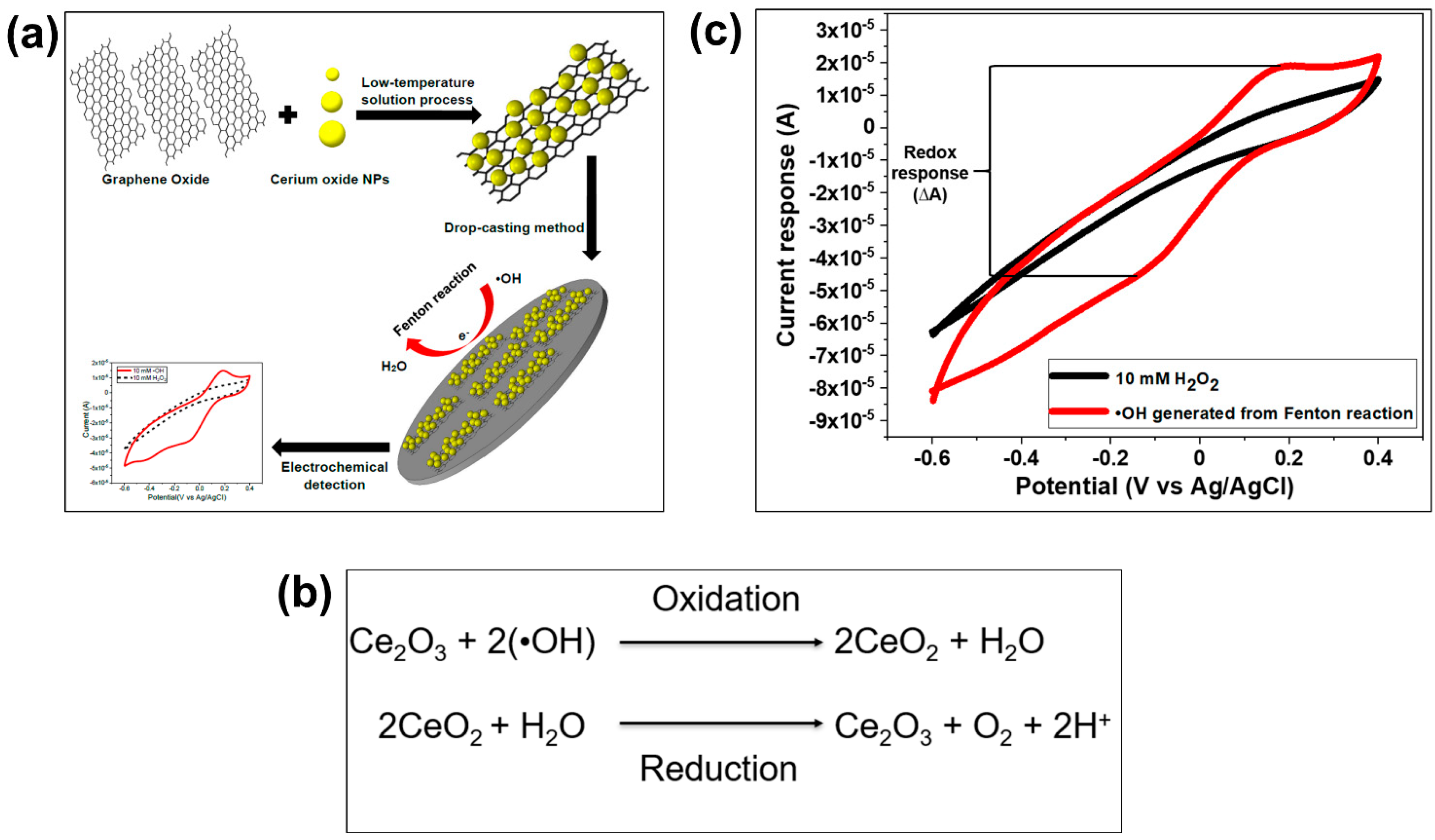
- Duanghathaipornsuk, S.; Kanel, S.; Haushalter, E.F.; Ruetz, J.E.; Kim, D.-S. Detection of Hydroxyl Radicals Using Cerium Oxide/Graphene Oxide Composite on Prussian Blue. Nanomaterials 2020, 10, 1136. [Google Scholar] [CrossRef]
- Cong, M.; Siraj, N.; Bhattarai, N.; Kolic, P.E.; McCarter, K.S.; Chhotaray, P.K.; Warner, I.M. Ratiometric fluorescence detection of hydroxyl radical using cyanine-based binary nanoGUMBOS. Sens. Actuators B Chem. 2018, 257, 993–1000. [Google Scholar] [CrossRef]
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 1–22. [Google Scholar] [CrossRef]
- Wan, H.; Yue, J.; Zhu, S.; Uno, T.; Zhang, X.; Yang, Q.; Yu, K.; Hong, G.; Wang, J.; Li, L.; et al. A bright organic NIR-II nanofluorophore for three-dimensional imaging into biological tissues. Nat. Commun. 2018, 9, 1171. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhong, D.; Zhou, S. NIR-I-to-NIR-II fluorescent nanomaterials for biomedical imaging and cancer therapy. J. Mater. Chem. B 2018, 6, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zhang, Y.; Li, Z.; Zhai, S.; Lv, W.; Liu, Z. Lighting Up NIR-II Fluorescence In Vivo: An Activable Probe for Noninvasive Hydroxyl Radical Imaging. Anal. Chem. 2019, 91, 15757–15762. [Google Scholar] [CrossRef]
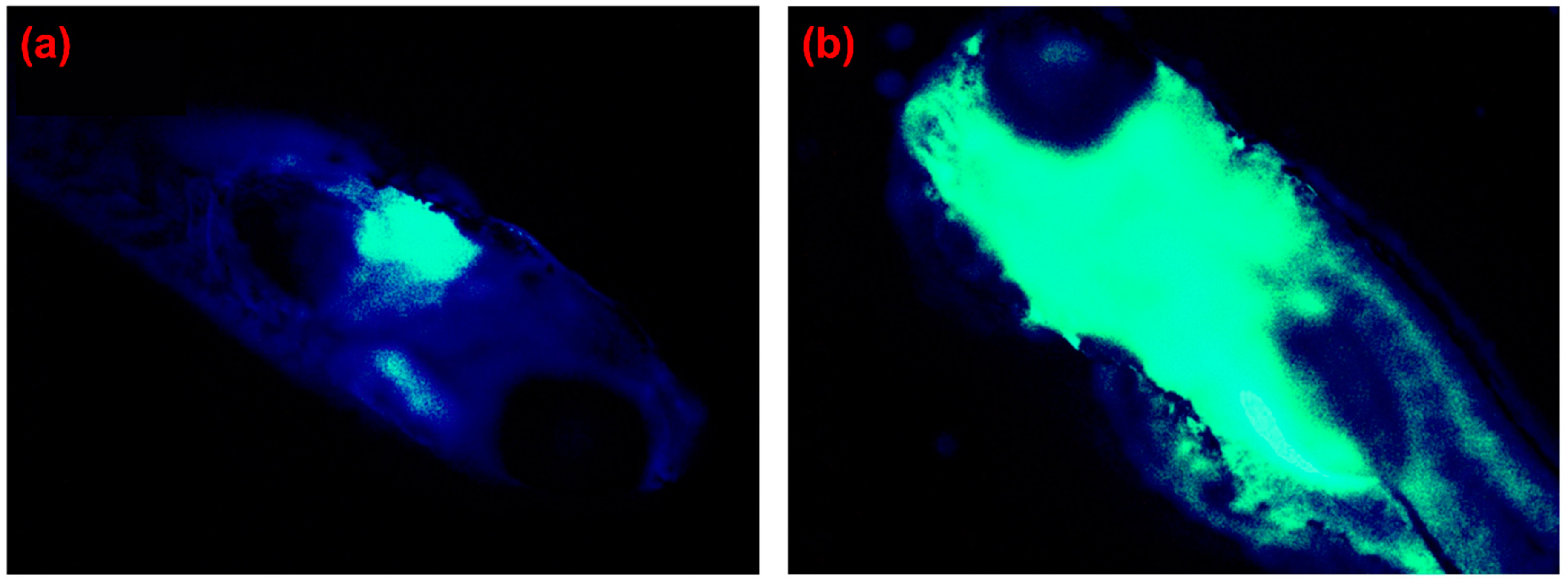
- Yuan, G.; Ding, H.; Sun, H.; Zhou, L.; Lin, Q. A mitochondrion-targeting turn-on fluorescent probe detection of endogenous hydroxyl radicals in living cells and zebrafish. Sens. Actuators B Chem. 2019, 296, 126706. [Google Scholar] [CrossRef]
- Wu, L.; Yang, Y.; Zhang, H.; Zhu, G.; Zhang, X.; Chen, J. Sensitive electrochemical detection of hydroxyl radical with biobarcode amplification. Anal. Chim. Acta 2012, 756, 1–6. [Google Scholar] [CrossRef]
- Huang, Y.; Sinha, A.; Zhao, H.; Dang, X.; Zhang, Y.; Quan, X. Real Time Detection of Hazardous Hydroxyl Radical Using an Electrochemical Approach. ChemistrySelect 2019, 4, 12507–12511. [Google Scholar] [CrossRef]
- Gualandi, I.; Guadagnini, L.; Zappoli, S.; Tonelli, D. A Polypyrrole Based Sensor for the Electrochemical Detection of OH Radicals. Electroanalysis 2014, 26, 1544–1550. [Google Scholar] [CrossRef]
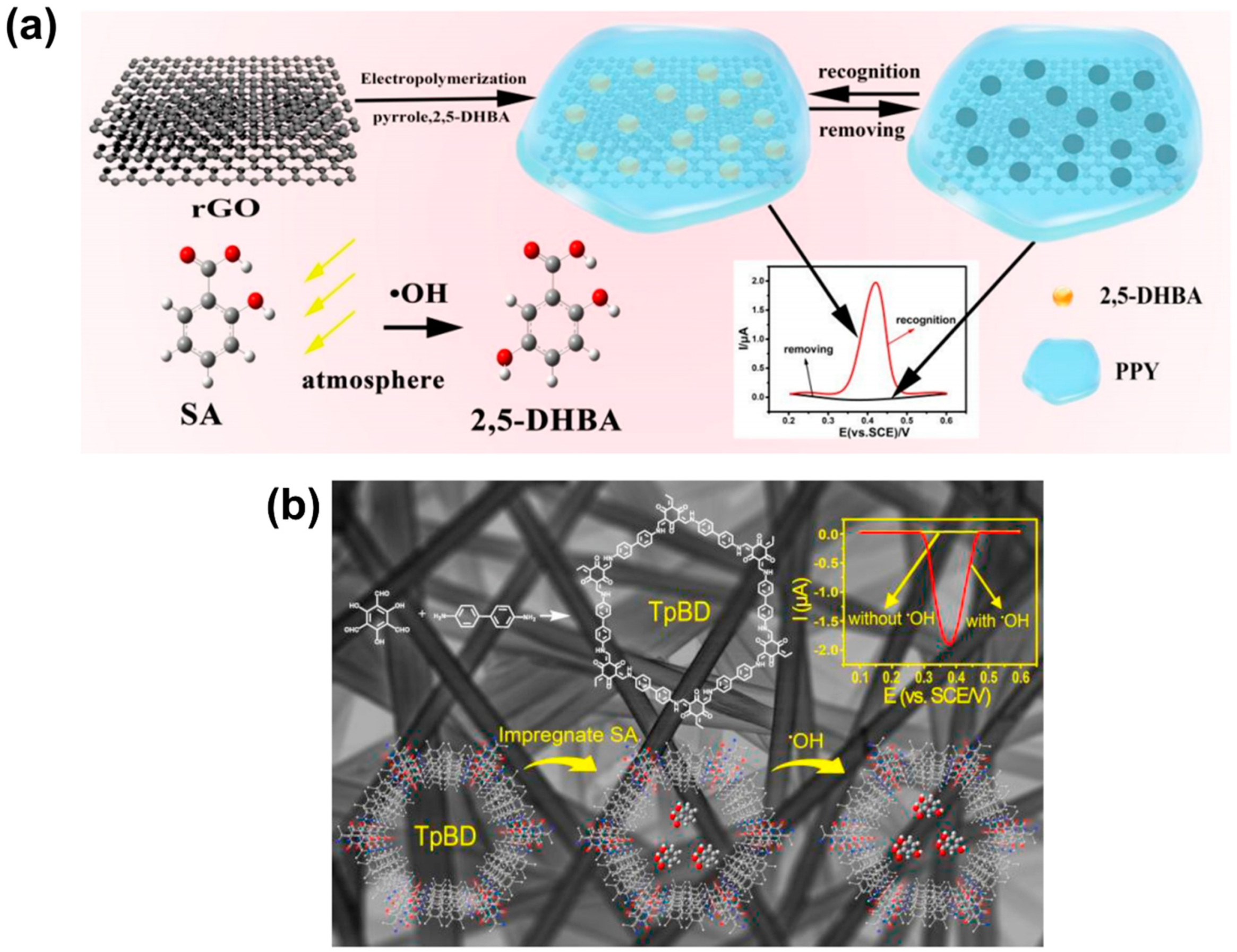
- Huang, Z.; Ying, P.; Huang, L.; Xu, Q.; Hu, X.-Y. Molecularly imprinted polymer functionalized reduced graphene oxide: A new platform for the detection of hydroxyl radicals in the atmosphere. Anal. Methods 2019, 11, 5126–5133. [Google Scholar] [CrossRef]
- Gualandi, I.; Tonelli, D. A new electrochemical sensor for OH radicals detection. Talanta 2013, 115, 779–786. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Chu, C.-H.; Sarang, I.; Fang, K.-C.; Hsu, C.-P.; Huang, Y.-F.; Hsu, C.-H.; Chen, C.-C.; Li, S.-S.; Andrew Yeh, J.; et al. Electronic hydroxyl radical microsensors based on the conductivity change of polyaniline. Sens. Actuators B Chem. 2015, 208, 99–105. [Google Scholar] [CrossRef]
- Jabeen, E.; Janjua, N.K.; Ahmed, S.; Domínguez-Álvarez, E.; Jacob, C. A selective and sensitive monitoring of the OH radical using flavonoid-modified electrodes. Electrochim. Acta 2017, 258, 228–235. [Google Scholar] [CrossRef]
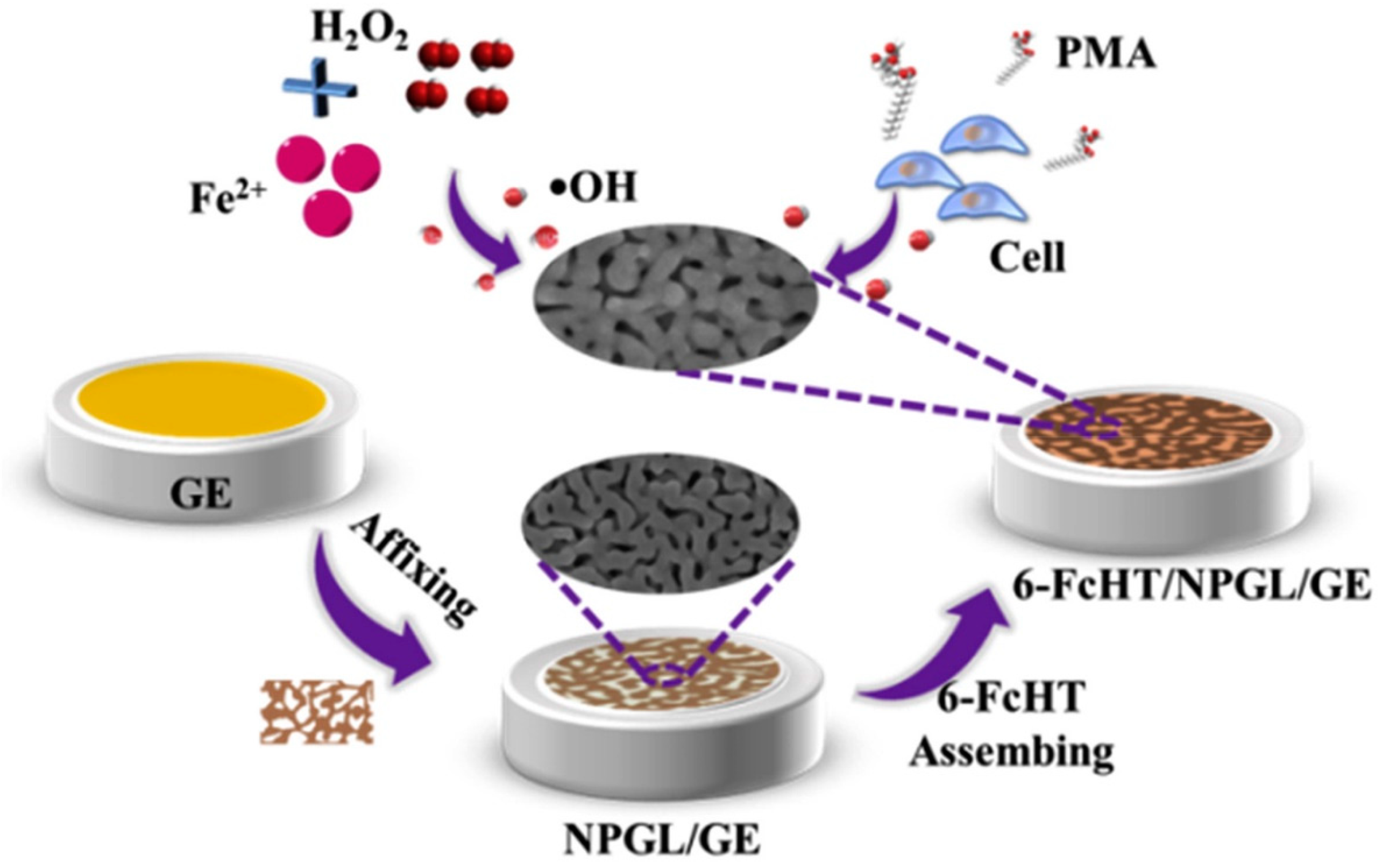
- Xu, Y.; Wang, D.; Zhang, Y.; Zhang, J.; Jiang, S.; Liang, W.; Zhu, T.; Ye, B.-C. A novel electrochemical sensor for determination of hydroxyl radicals in living cells by coupling nanoporous gold layer with self-assembled 6-(Ferrocenyl) hexanethiol. Anal. Chim. Acta 2020, 1096, 69–75. [Google Scholar] [CrossRef]
- Fu, L.; Wang, A.; Lyu, F.; Lai, G.; Yu, J.; Lin, C.-T.; Liu, Z.; Yu, A.; Su, W. A solid-state electrochemical sensing platform based on a supramolecular hydrogel. Sens. Actuators B Chem. 2018, 262, 326–333. [Google Scholar] [CrossRef]
- Duanghathaipornsuk, S.; Alateeq, F.A.O.; Kim, S.S.; Kim, D.-S.; Alba-Rubio, A.C. The effects of size and content of cerium oxide nanoparticles on a composite sensor for hydroxyl radicals detection. Sens. Actuators B Chem. 2020, 321, 128467. [Google Scholar] [CrossRef]
- Fu, L.; Wang, A.; Lyv, F.; Lai, G.; Zhang, H.; Yu, J.; Lin, C.-T.; Yu, A.; Su, W. Electrochemical antioxidant screening based on a chitosan hydrogel. Bioelectrochemistry 2018, 121, 7–10. [Google Scholar] [CrossRef]
- Ouyang, J.; Li, Z.-Q.; Zhang, J.; Wang, C.; Wang, J.; Xia, X.-H.; Zhou, G.-J. A rapid and sensitive method for hydroxyl radical detection on a microfluidic chip using an N-doped porous carbon nanofiber modified pencil graphite electrode. Analyst 2014, 139, 3416–3422. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhang, Y.; Song, Y.; Jiang, Y. A rapid and sensitive electrochemical sensor for hydroxyl free radicals based on self-assembled monolayers of carboxyl functionalized graphene. J. Solid State Electrochem. 2019, 23, 187–194. [Google Scholar] [CrossRef]
- Tang, L.; Reiter, R.J.; Li, Z.-R.; Ortiz, G.G.; Yu, B.P.; Garcia, J.J. Melatonin reduces the increase in 8-hydroxy-deoxyguanosine levels in the brain and liver of kainic acid-treated rats. Mol. Cell. Biochem. 1998, 178, 299–303. [Google Scholar] [CrossRef]
- Daniels, W.M.U.; Van Rensburg, S.J.; Van Zyl, J.M.; Taljaard, J.J.F. Melatonin prevents β-amyloid-induced lipid peroxidation. J. Pineal Res. 1998, 24, 78–82. [Google Scholar] [CrossRef]
- Abdel-Hamid, R.; Newair, E.F. Electrochemical behavior of antioxidants: Part 3. Electrochemical studies of caffeic Acid–DNA interaction and DNA/carbon nanotube biosensor for DNA damage and protection. Arab. J. Chem. 2016, 9, 365–370. [Google Scholar] [CrossRef]
- Barroso, M.F.; de-los-Santos-Álvarez, N.; Delerue-Matos, C.; Oliveira, M.B.P.P. Towards a reliable technology for antioxidant capacity and oxidative damage evaluation: Electrochemical (bio)sensors. Biosens. Bioelectron. 2011, 30, 1–12. [Google Scholar] [CrossRef]
- Wang, T.-T.; Huang, Z.-L.; Xu, Q.; Hu, X.-Y. Simple and sensitive determination of hydroxyl radical in atmosphere based on an electrochemically activated glassy carbon electrode. Int. J. Environ. Anal. Chem. 2018, 98, 477–491. [Google Scholar] [CrossRef]
- Huang, Z.; Ji, Z.; Yin, P.; Shu, Y.; Xu, Q.; Hu, X.-Y. Salicylic acid impregnated activated carbon fiber paper: An effective platform for the simple and sensitive detection of hydroxyl radicals in the atmosphere. Electrochem. Commun. 2019, 100, 113–116. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, Q.; Hu, X. Covalent organic frameworks functionalized carbon fiber paper for the capture and detection of hydroxyl radical in the atmosphere. Chin. Chem. Lett. 2020, 31, 2495–2498. [Google Scholar] [CrossRef]
- Lee, K.-P.; Gopalan, A.I.; Komathi, S. Direct electrochemistry of cytochrome c and biosensing for hydrogen peroxide on polyaniline grafted multi-walled carbon nanotube electrode. Sens. Actuators B Chem. 2009, 141, 518–525. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Biomolecule-Functionalized Carbon Nanotubes: Applications in Nanobioelectronics. ChemPhysChem 2004, 5, 1084–1104. [Google Scholar] [CrossRef]
- Yagati, A.K.; Lee, T.; Min, J.; Choi, J.-W. Electrochemical performance of gold nanoparticle–cytochrome c hybrid interface for H2O2 detection. Colloids Surf. B Biointerfaces 2012, 92, 161–167. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Ganjali, M.R.; Dinarvand, R.; Razavi, T.; Saboury, A.A.; Moosavi-Movahedi, A.A.; Norouzi, P. Direct electrochemistry of cytochrome c on electrodeposited nickel oxide nanoparticles. J. Electroanal. Chem. 2008, 614, 83–92. [Google Scholar] [CrossRef]
- Aghamiri, Z.S.; Mohsennia, M.; Rafiee-Pour, H.-A. Immobilization of cytochrome c and its application as electrochemical biosensors. Talanta 2018, 176, 195–207. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, P.; Lü, Y.; Du, P.; Shi, Y.; Cai, C. Immobilization and direct electrochemistry of cytochrome c at a single-walled carbon nanotube-modified electrode. J. Solid State Electrochem. 2007, 11, 390–397. [Google Scholar] [CrossRef]
- Wang, Z.; Yi, K.; Lin, Q.; Yang, L.; Chen, X.; Chen, H.; Liu, Y.; Wei, D. Free radical sensors based on inner-cutting graphene field-effect transistors. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef]
- Ding, S.; Li, M.; Gong, H.; Zhu, Q.; Shi, G.; Zhu, A. Sensitive and Selective Measurement of Hydroxyl Radicals at Subcellular Level with Tungsten Nanoelectrodes. Anal. Chem. 2020, 92, 2543–2549. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Smith, D.G.; Cappai, R.; Barnham, K.J. The redox chemistry of the Alzheimer’s disease amyloid β peptide. Biochim. Biophys. Acta 2007, 1768, 1976–1990. [Google Scholar] [CrossRef]
- Bauer, I.; Raupach, A. The Role of Heme Oxygenase-1 in Remote Ischemic and Anesthetic Organ Conditioning. Antioxidants 2019, 8, 403. [Google Scholar] [CrossRef]
- del Rosal, B.; Benayas, A. Strategies to Overcome Autofluorescence in Nanoprobe-Driven In Vivo Fluorescence Imaging. Small Methods 2018, 2, 1800075. [Google Scholar] [CrossRef]
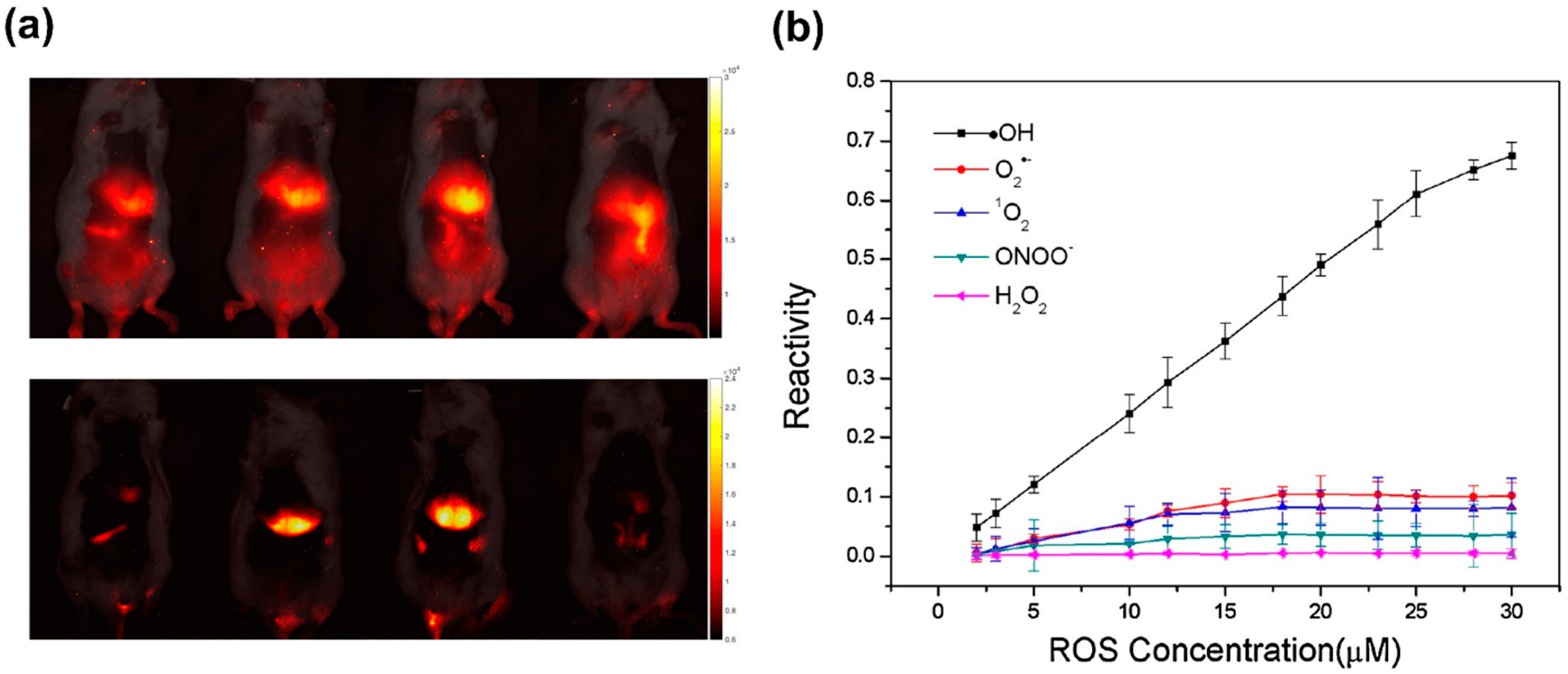
- Zhang, R.; Zhao, J.; Han, G.; Liu, Z.; Liu, C.; Zhang, C.; Liu, B.; Jiang, C.; Liu, R.; Zhao, T.; et al. Real-Time Discrimination and Versatile Profiling of Spontaneous Reactive Oxygen Species in Living Organisms with a Single Fluorescent Probe. J. Am. Chem. Soc. 2016, 138, 3769–3778. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Liu, C.; Yu, P.; Sun, M.; Yan, X.; Guo, J.-P.; Guo, W. Imaging lysosomal highly reactive oxygen species and lighting up cancer cells and tumors enabled by a Si-rhodamine-based near-infrared fluorescent probe. Biomaterials 2017, 133, 60–69. [Google Scholar] [CrossRef]
- Niu, J.; Fan, J.; Wang, X.; Xiao, Y.; Xie, X.; Jiao, X.; Sun, C.; Tang, B. Simultaneous Fluorescence and Chemiluminescence Turned on by Aggregation-Induced Emission for Real-Time Monitoring of Endogenous Superoxide Anion in Live Cells. Anal. Chem. 2017, 89, 7210–7215. [Google Scholar] [CrossRef]
- Lu, D.; Zhou, L.; Wang, R.; Zhang, X.-B.; He, L.; Zhang, J.; Hu, X.; Tan, W. A two-photon fluorescent probe for endogenous superoxide anion radical detection and imaging in living cells and tissues. Sens. Actuators B Chem. 2017, 250, 259–266. [Google Scholar] [CrossRef]
- Chen, L.; Cho, M.K.; Wu, D.; Kim, H.M.; Yoon, J. Two-Photon Fluorescence Probe for Selective Monitoring of Superoxide in Live Cells and Tissues. Anal. Chem. 2019, 91, 14691–14696. [Google Scholar] [CrossRef]
- Han, X.; Wang, R.; Song, X.; Yu, F.; Lv, C.; Chen, L. A mitochondrial-targeting near-infrared fluorescent probe for bioimaging and evaluating endogenous superoxide anion changes during ischemia/reperfusion injury. Biomaterials 2018, 156, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Dan, C.; Dongdong, S.; Chen, M.; Yin, B.-C.; Yuan, L.; Zhang, X.-B. Visualization of oxidative injury in the mouse kidney using selective superoxide anion fluorescent probes. Chem. Sci. 2018, 9, 7606–7613. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Shang, T.; Wu, T.; Liu, G.; Ding, L.; Liu, X. Construction of an ultrasensitive non-enzymatic sensor to investigate the dynamic process of superoxide anion release from living cells. Biosens. Bioelectron. 2018, 100, 8–15. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, X.; Zhao, H.; Sun, W.; Wang, Z.; Lan, M. Enzyme-free electrochemical sensor based on ZIF-67 for the detection of superoxide anion radical released from SK-BR-3 cells. J. Electroanal. Chem. 2019, 855, 113653. [Google Scholar] [CrossRef]
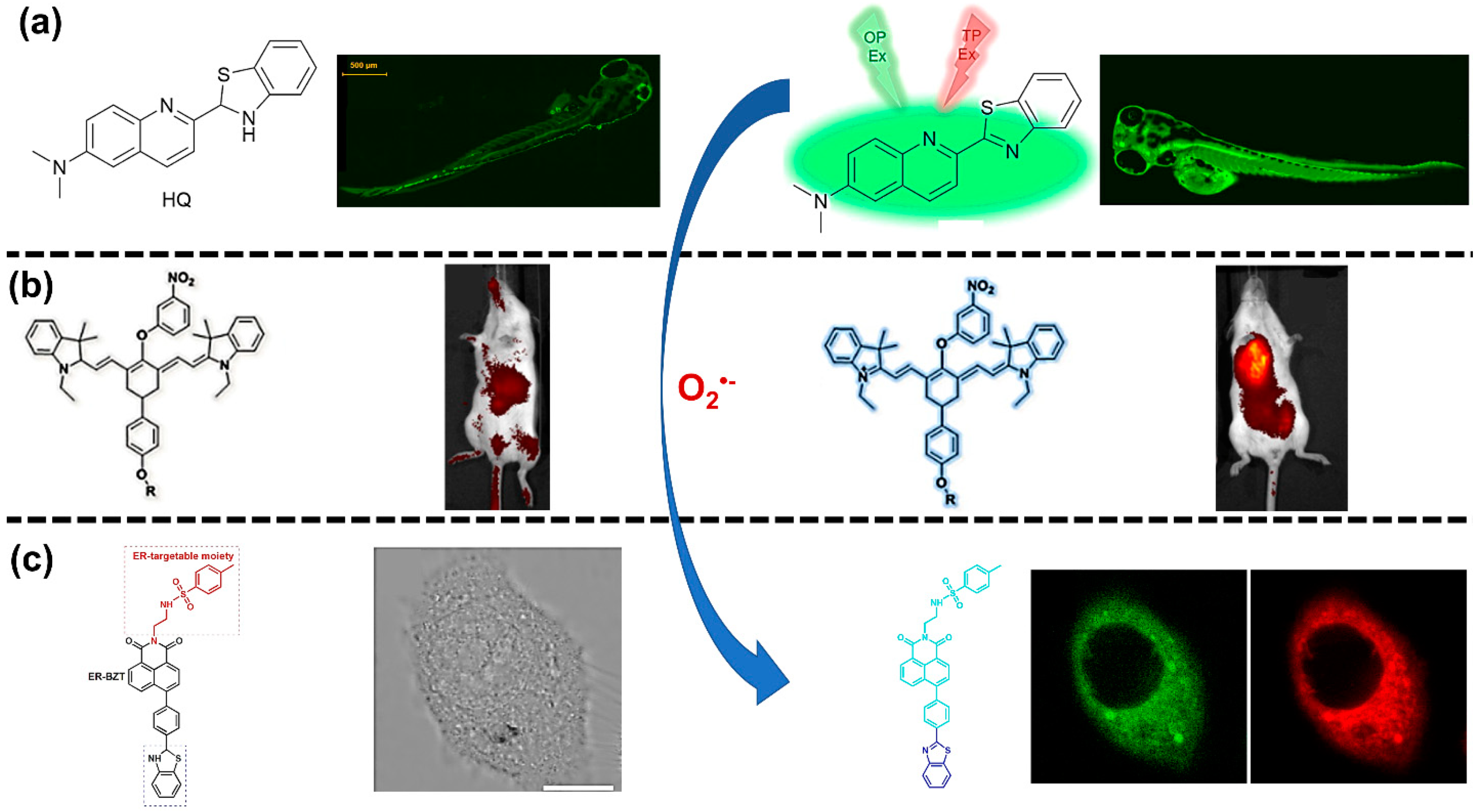
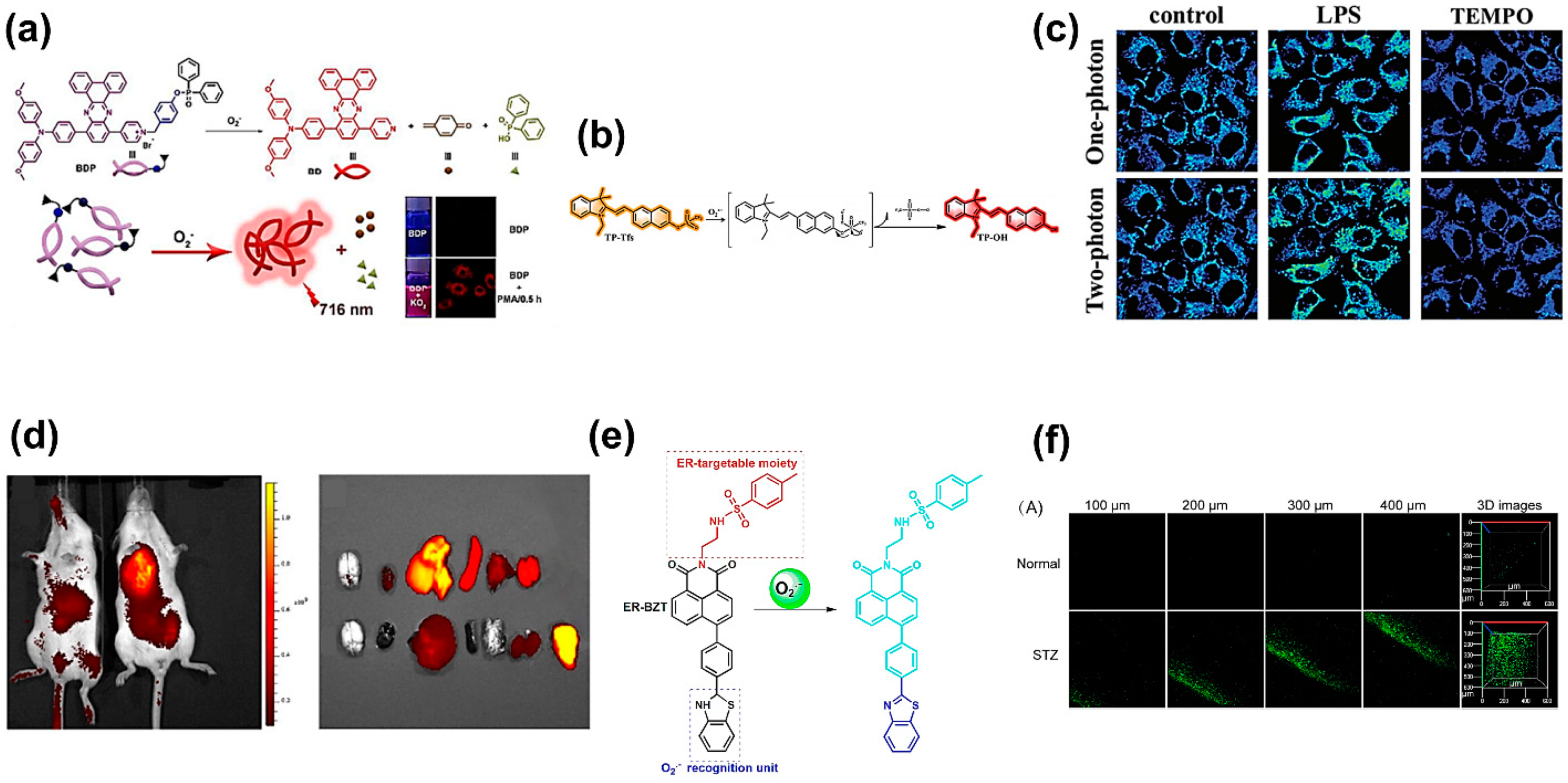
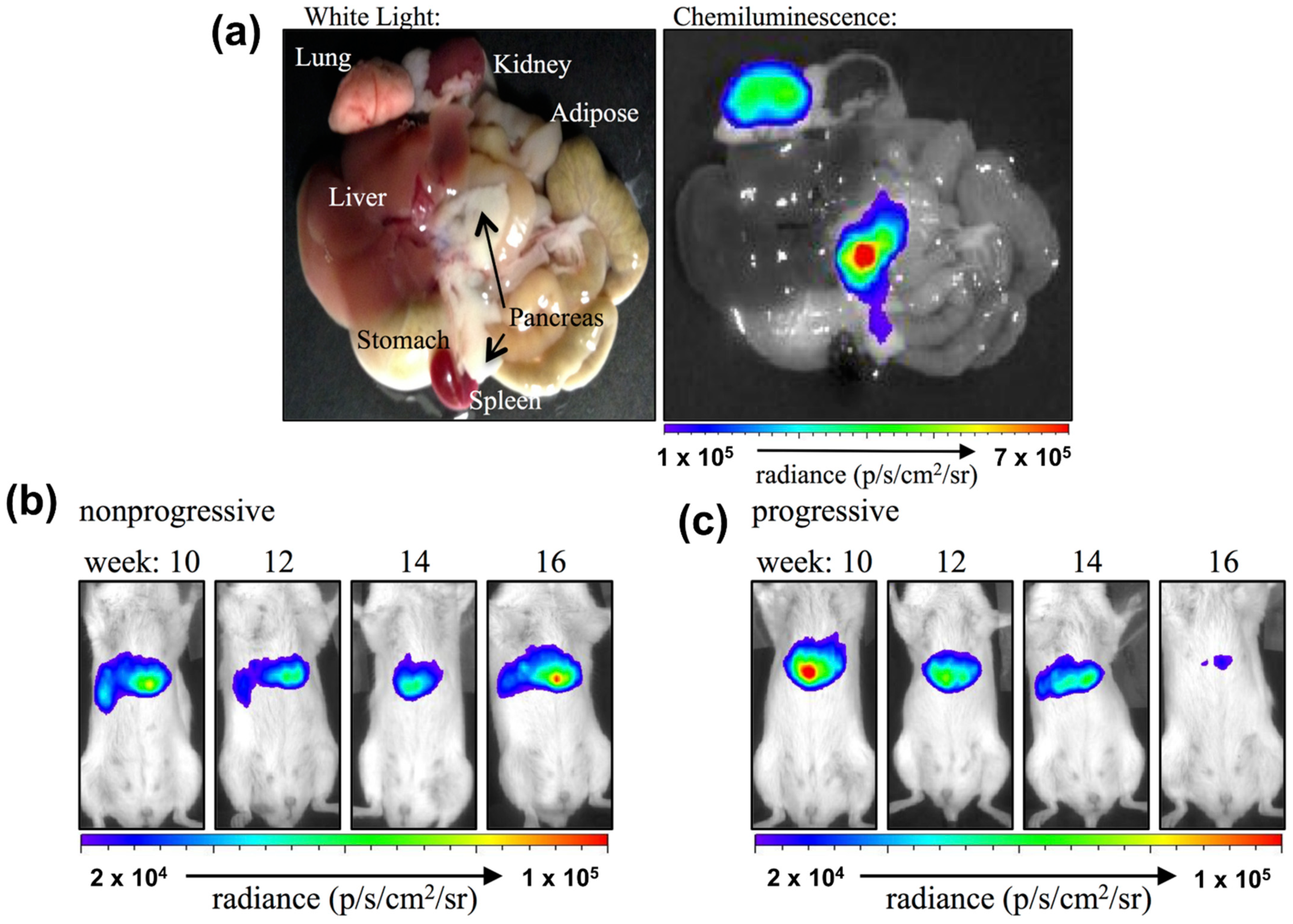
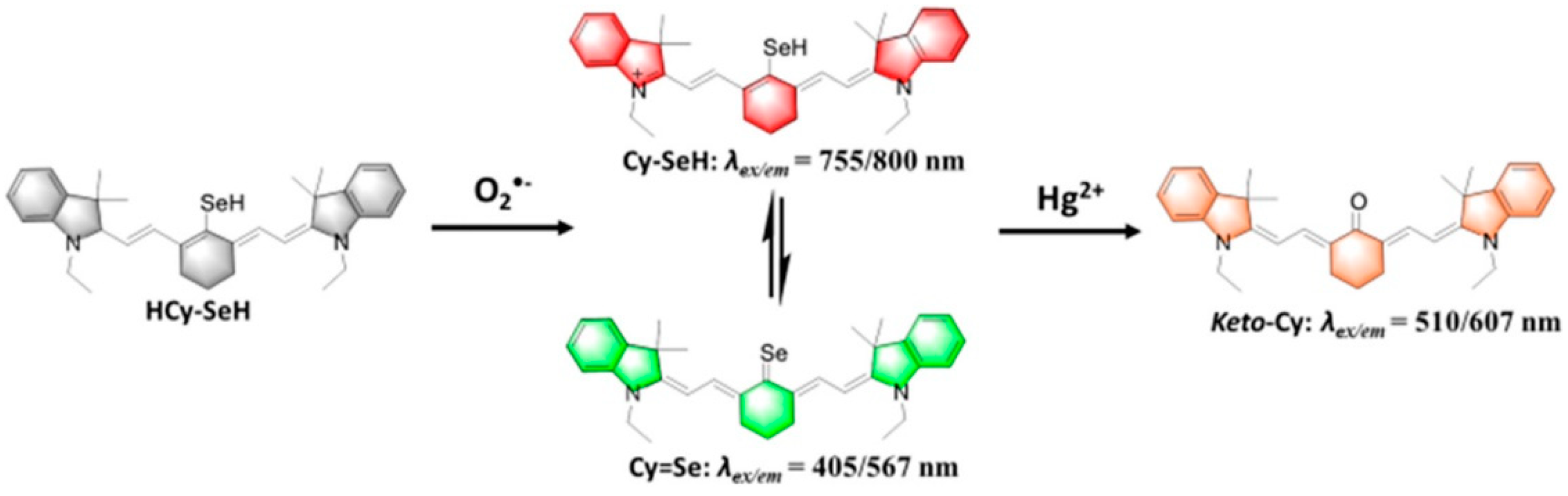
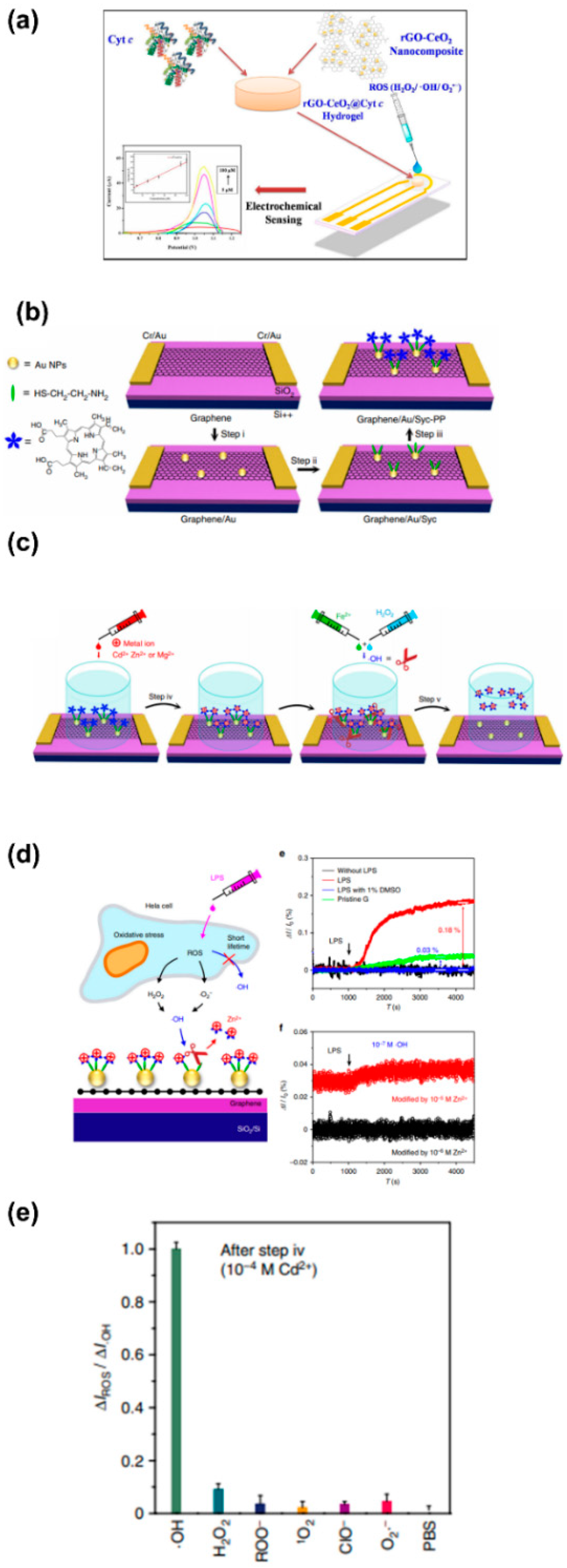
| Reactive Oxygen Species (ROS) Detection | ||||
| Fluorescence Method (make ROS visible in live cells) | Fluorescence Method for ROS Detection | |||
| Advantages | Disadvantages | References | Further Studies and Improvements | |
| A specific generation source of ROS in live cells can be mapped and located. | Biological samples could be damaged due to high-energy light emission, such as photobleaching. | [61,62,71,83] | Novel organic molecules for fluorescent probes are needed to reduce the auto-oxidizing phenomenon. | |
| Fluorescent probes can be easily modified for different types of ROS. | The concentration of ROS can be over-interpreted via false emission light from surrounding tissues. | [275,276,277] | A better design of fluorescent probes is needed to selectively react with specific ROS. | |
| It is an easy sensing procedure. | Limited fluorescent molecules can absorb and emit within the desired wavelength. | [65,68] | The water solubility of fluorescent probes needs to be improved. | |
| It has excellent biocompatibility. | The dynamic concentration of ROS in live cells is hard to evaluate over extended periods of time. | [79,84,278,279,280] | The detection mechanism needs to be studied in detail. | |
| It is a non-invasive method. | [281,282] | |||
| Electrochemical Method for ROS Detection | ||||
| Electrochemical Method (detects ROS by electron exchange) | Organic Electrochemical Method for ROS Detection | |||
| Advantages | Disadvantages | References | Further Studies and Improvements | |
| It is easy to perform without using complicated procedures and toxic chemicals. | Degradation of the sensor can occur in harsh environments. | [47,243,244] | The biological and chemical compatibility of the electrode surface should be improved to be used in live cells. | |
| It shows high selectivity toward the ROS of interest using a specific enzyme. | The sensor performance depends on the surrounding environment and can be inconsistent under unsuitable conditions. | [89,110,134,243,244] | The design for the detection of ROS at their generation sources in live cells should be enhanced. | |
| Real-time detection and fast response are possible. | The signals from other coexisting electroactive species can interfere during a high-potential operation. | [47,101,107,134,135] | The protection of biological/organic molecules on the electrode surface against degradation under harsh conditions should be improved. | |
| Sensor sensitivity can be improved by integrating highly conductive materials. | Biological molecules are costly sensing elements. | A combination of conductive materials with biological/organic molecules should be used to improve the sensor sensitivity and selectivity. | ||
| Inorganic Electrochemical Method for ROS Detection | ||||
| Advantages | Disadvantages | References | Further Studies and Improvements | |
| Detection can be performed in severe environments. | The aggregation of inorganic materials can impair the detection performance. | [143,179,198,214] | Additional development of the methods to prevent aggregation of inorganic materials is required. | |
| Sensor sensitivity and selectivity for individual ROS can be easily adjusted by using different inorganic materials. | The inorganic materials used as sensing elements need to be wisely chosen to selectively detect individual ROS of interest. | [114,252] | Types of inorganic materials as sensing elements should be further investigated to improve the sensor selectivity to specific ROS. | |
| Real-time detection and fast response are possible. | [116,117,118] | To improve overall sensing elements, methods to control the morphology and distribution of inorganic materials as sensing elements could be further developed. | ||
| It shows low-interference signals from other existing electroactive species. | [115,283,284] | |||
| It provides long-term stability with excellent reusability. | [47,112] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duanghathaipornsuk, S.; Farrell, E.J.; Alba-Rubio, A.C.; Zelenay, P.; Kim, D.-S. Detection Technologies for Reactive Oxygen Species: Fluorescence and Electrochemical Methods and Their Applications. Biosensors 2021, 11, 30. https://doi.org/10.3390/bios11020030
Duanghathaipornsuk S, Farrell EJ, Alba-Rubio AC, Zelenay P, Kim D-S. Detection Technologies for Reactive Oxygen Species: Fluorescence and Electrochemical Methods and Their Applications. Biosensors. 2021; 11(2):30. https://doi.org/10.3390/bios11020030
Chicago/Turabian StyleDuanghathaipornsuk, Surachet, Eveline J. Farrell, Ana C. Alba-Rubio, Piotr Zelenay, and Dong-Shik Kim. 2021. "Detection Technologies for Reactive Oxygen Species: Fluorescence and Electrochemical Methods and Their Applications" Biosensors 11, no. 2: 30. https://doi.org/10.3390/bios11020030
APA StyleDuanghathaipornsuk, S., Farrell, E. J., Alba-Rubio, A. C., Zelenay, P., & Kim, D.-S. (2021). Detection Technologies for Reactive Oxygen Species: Fluorescence and Electrochemical Methods and Their Applications. Biosensors, 11(2), 30. https://doi.org/10.3390/bios11020030







