Voltammetric Immunosensor to Track a Major Peanut Allergen (Ara h 1) in Food Products Employing Quantum Dot Labels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus and Electrodes
2.2. Reagents and Solutions
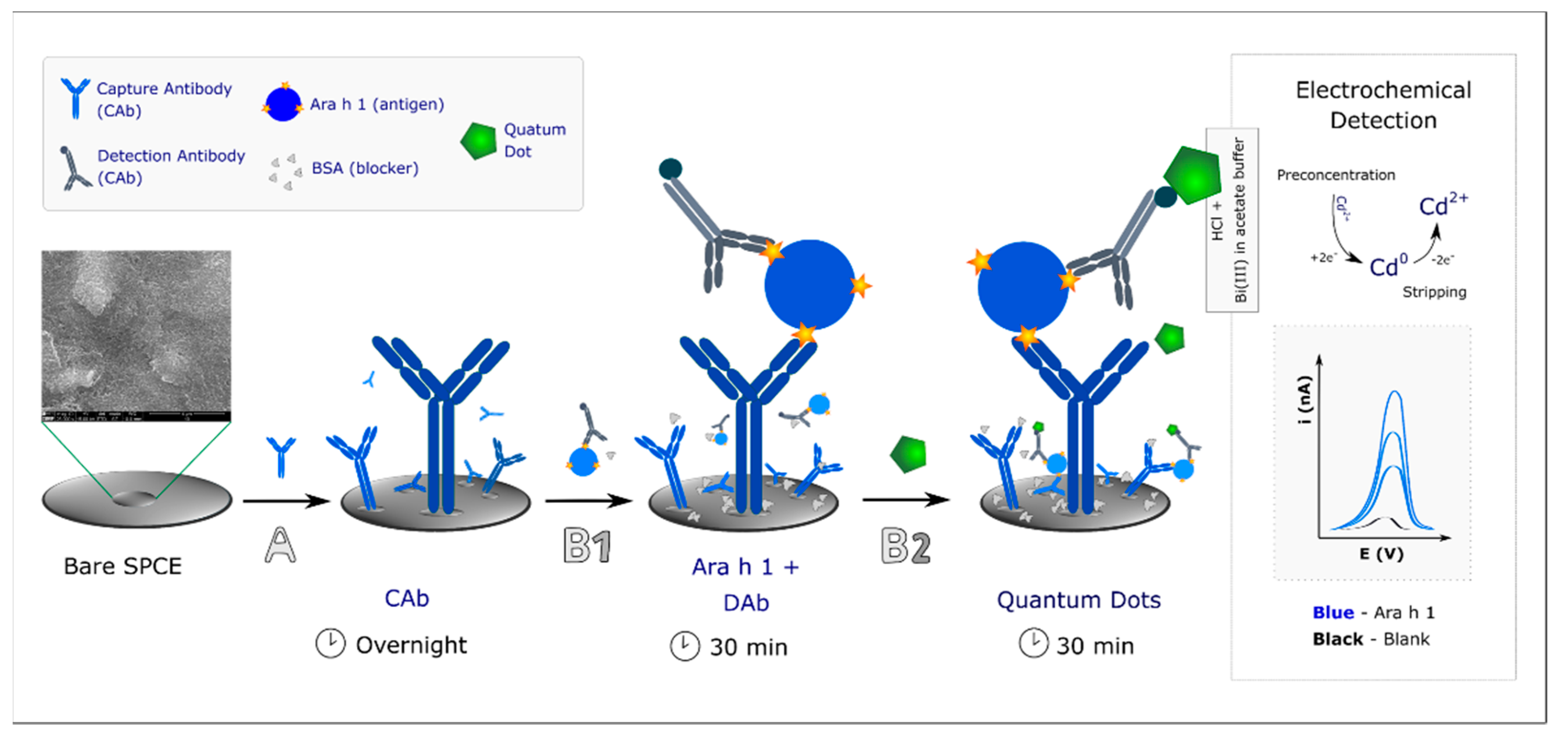
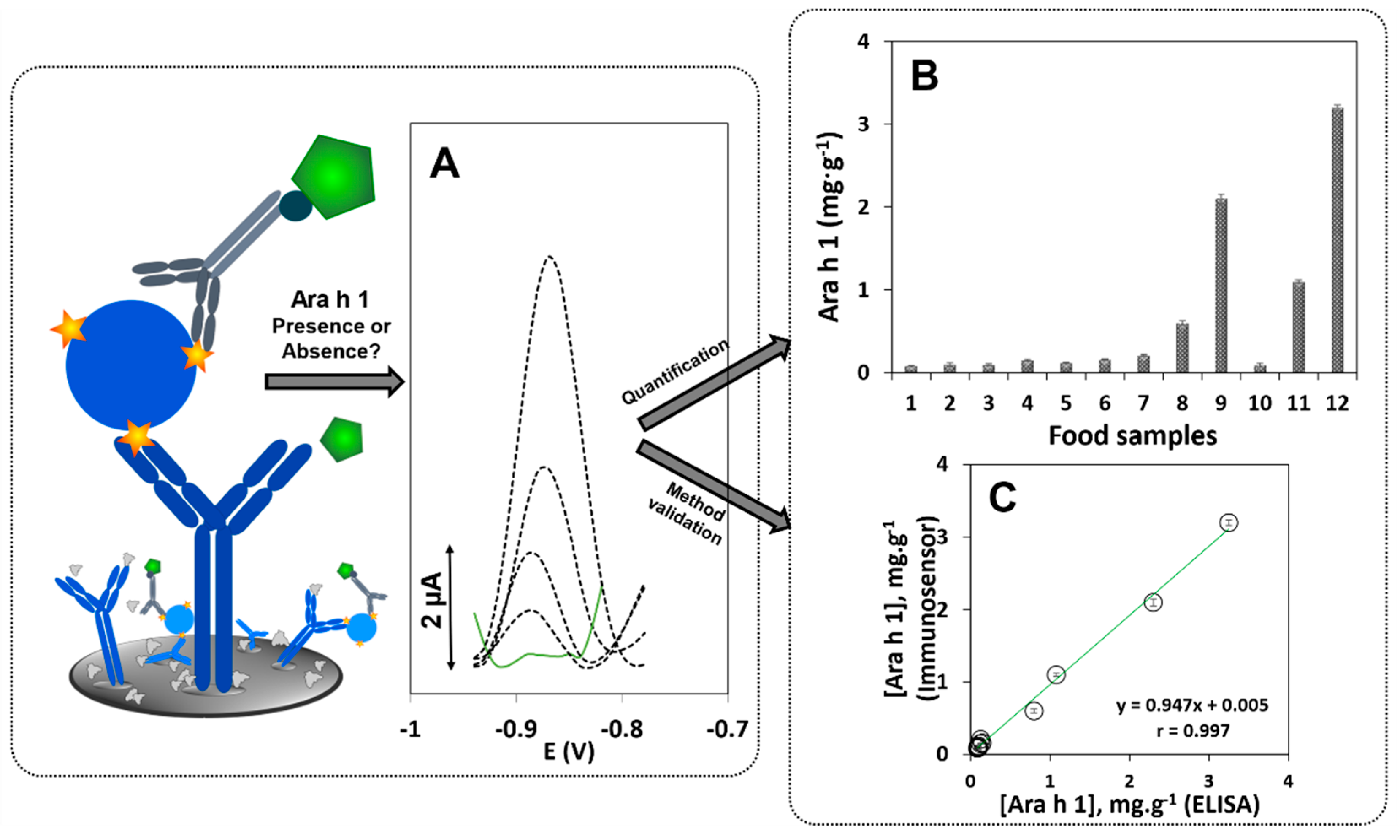
2.3. Immunosensor/Assay Development
2.4. Electrochemical Detection
2.5. Food Sample Preparation
3. Results and Discussion
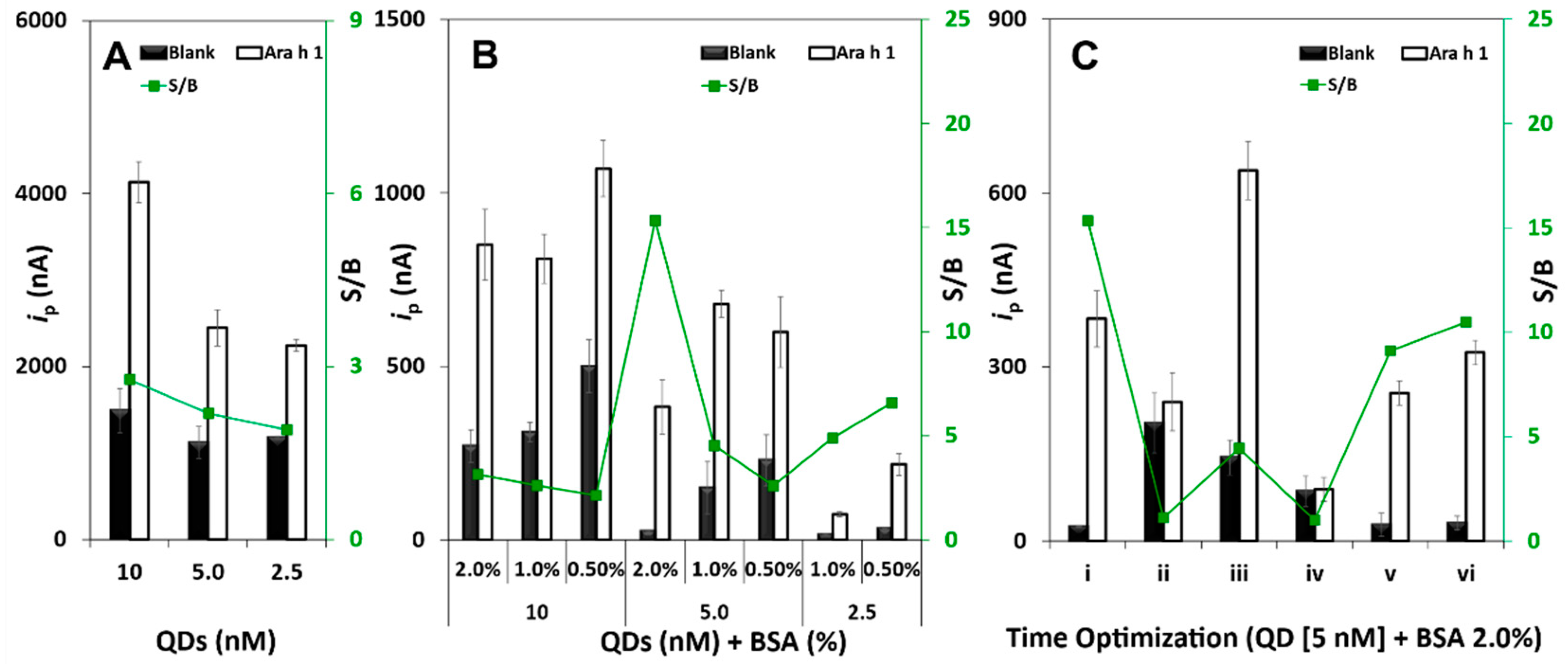
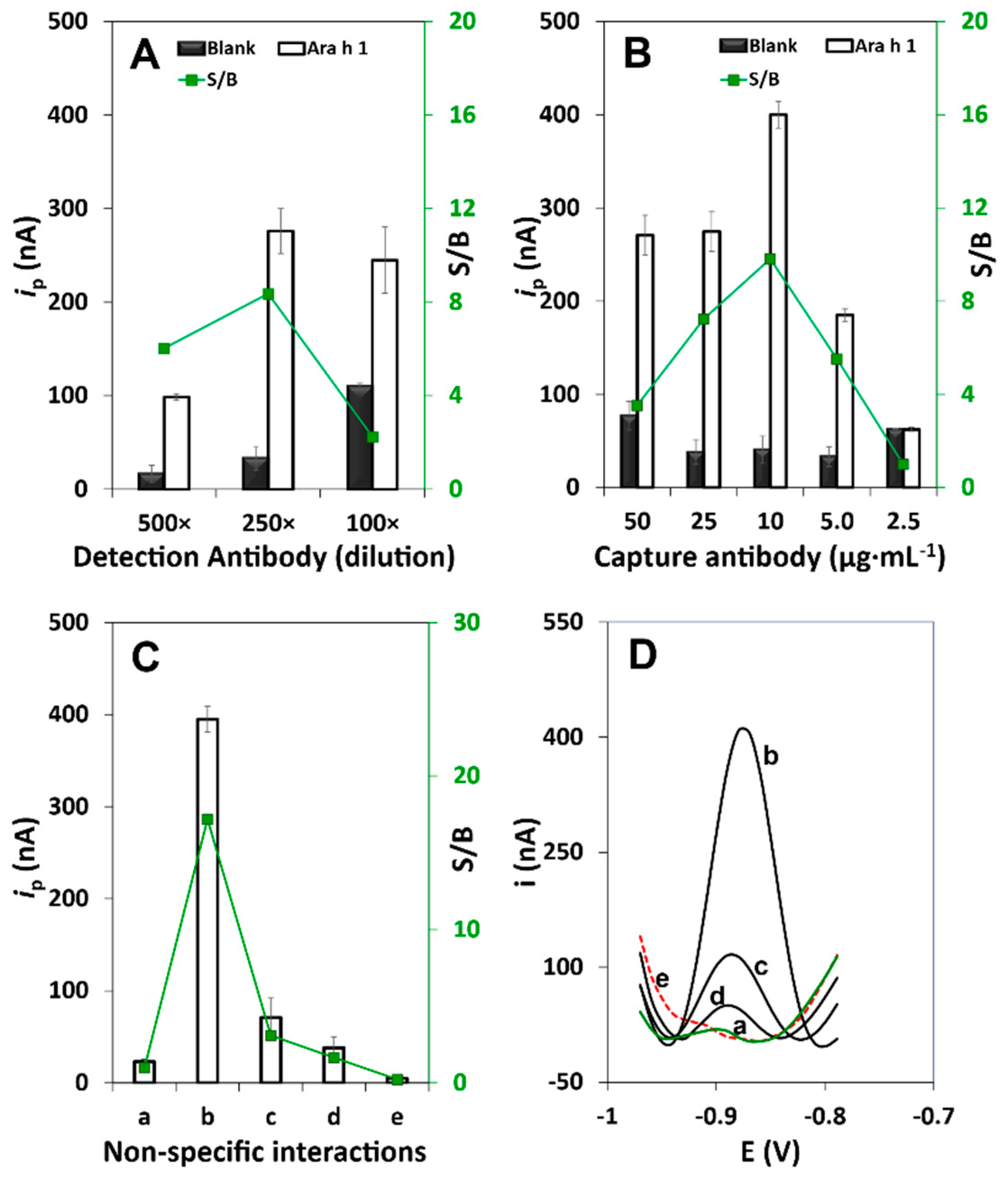
3.1. Method Development and Optimization
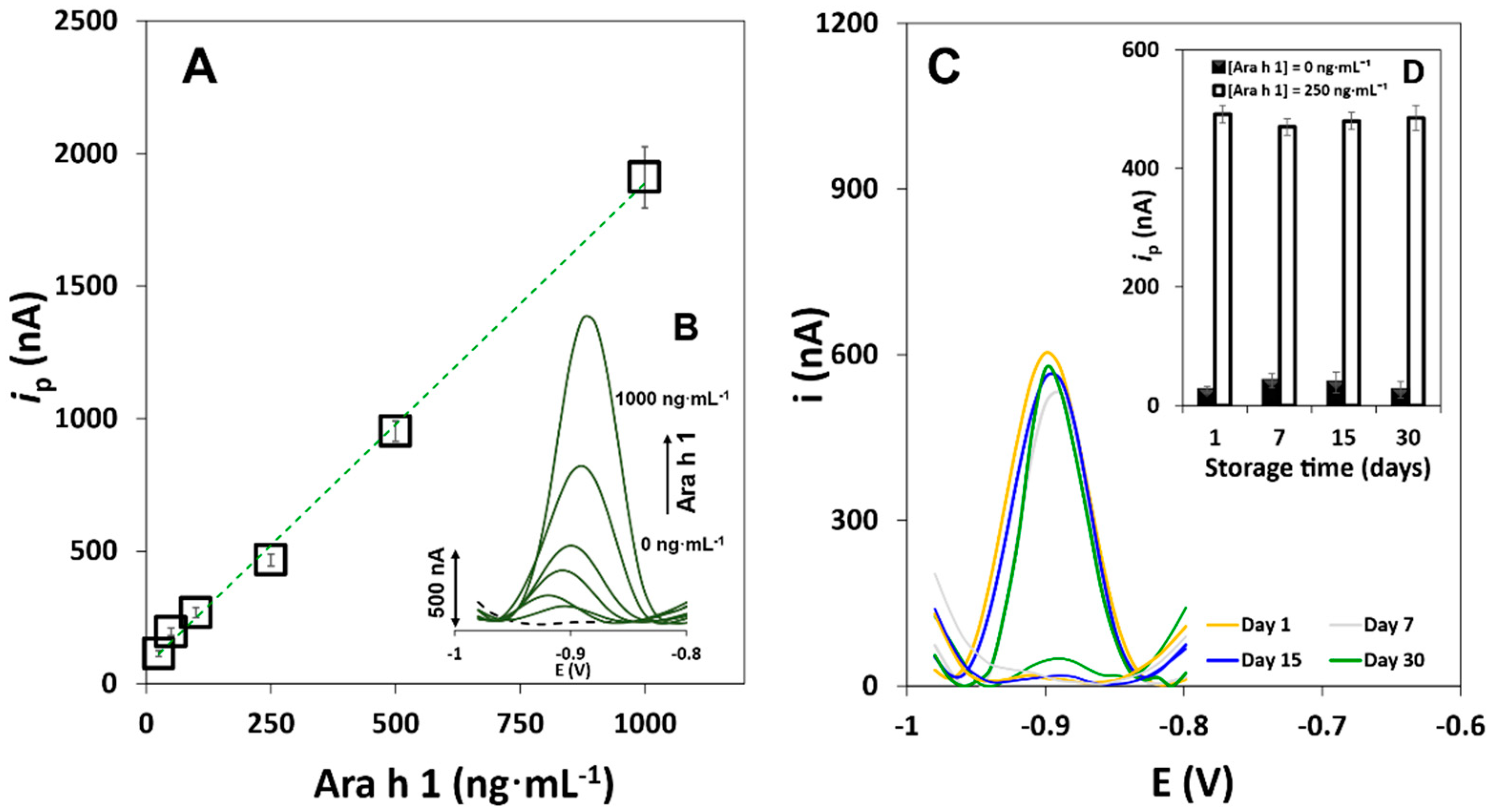
3.2. Method Performance
3.3. Ara h 1 Determination in Food Samples
3.4. Comparison with Other Electrochemical Immunoassays for the Analysis of Ara h 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Ginaldi, L. New Perspectives in Food Allergy. Int. J. Mol. Sci. 2020, 21, 1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, D.; Ciccolini, A.; Avilla, E.; Waserman, S. Food allergy and anaphylaxis. J. Asthma Allergy 2018, 11, 111–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenta, R.; Hochwallner, H.; Linhart, B.; Pahr, S. Food allergies: The basics. Gastroenterology 2015, 148, 1120–1131.e4. [Google Scholar] [CrossRef] [Green Version]
- Sheehan, W.J.; Taylor, S.L.; Phipatanakul, W.; Brough, H.A. Environmental Food Exposure: What Is the Risk of Clinical Reactivity From Cross-Contact and What Is the Risk of Sensitization. J. Allergy Clin. Immunol. Pract. 2018, 6, 1825–1832. [Google Scholar] [CrossRef]
- Lieberman, J.A.; Gupta, R.S.; Knibb, R.C.; Haselkorn, T.; Tilles, S.; Mack, D.P.; Pouessel, G. The global burden of illness of peanut allergy: A comprehensive literature review. Allergy 2021, 76, 1367–1384. [Google Scholar] [CrossRef]
- Magnusdottir, H.; Vidarsdóttir, A.G.; Ludviksson, B.R.; Clausen, M.; Lund, S.H.; Jensen, A.B.; Sigurdardottir, S.T. Ara h 1 and Ara h 6 Sensitization Causes Clinical Peanut Allergy in Ara h 2-Negative Individuals. Int. Arch. Allergy Immunol. 2019, 178, 66–75. [Google Scholar] [CrossRef]
- Agyemang, A.; Sicherer, S. The importance of early peanut ingestion in the prevention of peanut allergy. Expert Rev. Clin. Immunol. 2019, 15, 487–495. [Google Scholar] [CrossRef]
- Soon, J.M.; Manning, L. “May Contain” Allergen Statements: Facilitating or Frustrating Consumers? J. Consum. Policy 2017, 40, 447–472. [Google Scholar] [CrossRef] [Green Version]
- EC. Regulation (EU) No 1169/2011 on the Provision of Food Information to Consumers. 2011. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R1169 (accessed on 7 August 2021).
- FDA (Food and Drug Administration). Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA); FDA: Silver Spring, MD, USA, 2004. [Google Scholar]
- Hosu, O.; Selvolini, G.; Marrazza, G. Recent advances of immunosensors for detecting food allergens. Curr. Opin. Electrochem. 2018, 10, 149–156. [Google Scholar] [CrossRef]
- Khedri, M.; Ramezani, M.; Rafatpanah, H.; Abnous, K. Detection of food-born allergens with aptamer-based biosensors. TrAC Trends Anal. Chem. 2018, 103, 126–136. [Google Scholar] [CrossRef]
- Freitas, M.; Neves, M.M.P.S.; Nouws, H.P.A.; Delerue-Matos, C. Electrochemical Immunosensor for the Simultaneous Determination of Two Main Peanut Allergenic Proteins (Ara h 1 and Ara h 6) in Food Matrices. Foods 2021, 10, 1718. [Google Scholar] [CrossRef]
- Alves, R.C.; Barroso, M.F.; González-García, M.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. New Trends in Food Allergens Detection: Toward Biosensing Strategies. Crit. Rev. Food Sci. Nutr. 2016, 56, 2304–2319. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical Affinity Biosensors Based on Selected Nanostructures for Food and Environmental Monitoring. Sensors 2020, 20, 5125. [Google Scholar] [CrossRef] [PubMed]
- Neethirajan, S.; Weng, X.; Tah, A.; Cordero, J.O.; Ragavan, K.V. Nano-biosensor platforms for detecting food allergens—New trends. Sens. Bio-Sens. Res. 2018, 18, 13–30. [Google Scholar] [CrossRef]
- Gómez-Arribas, L.N.; Benito-Peña, E.; Hurtado-Sánchez, M.D.C.; Moreno-Bondi, M.C. Biosensing Based on Nanoparticles for Food Allergens Detection. Sensors 2018, 18, 1087. [Google Scholar] [CrossRef] [Green Version]
- Campuzano, S.; Ruiz-Valdepeñas Montiel, V.; Serafín, V.; Yáñez-Sedeño, P.; Pingarrón, J.M. Cutting-Edge Advances in Electrochemical Affinity Biosensing at Different Molecular Level of Emerging Food Allergens and Adulterants. Biosensors 2020, 10, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sena-Torralba, A.; Pallás-Tamarit, Y.; Morais, S.; Maquieira, Á. Recent advances and challenges in food-borne allergen detection. TrAC Trends Anal. Chem. 2020, 132, 116050. [Google Scholar] [CrossRef]
- Ross, G.M.S.; Bremer, M.G.E.G.; Nielen, M.W.F. Consumer-friendly food allergen detection: Moving towards smartphone-based immunoassays. Anal. Bioanal. Chem. 2018, 410, 5353–5371. [Google Scholar] [CrossRef] [Green Version]
- Vasilescu, A.; Nunes, G.; Hayat, A.; Latif, U.; Marty, J.-L. Electrochemical Affinity Biosensors Based on Disposable Screen-Printed Electrodes for Detection of Food Allergens. Sensors 2016, 16, 1863. [Google Scholar] [CrossRef] [Green Version]
- Freitas, M.; Nouws, H.P.A.; Delerue-Matos, C. Electrochemical Biosensing in Cancer Diagnostics and Follow-up. Electroanalysis 2018, 30, 1584–1603. [Google Scholar] [CrossRef] [Green Version]
- Borrill, A.J.; Reily, N.E.; Macpherson, J.V. Addressing the practicalities of anodic stripping voltammetry for heavy metal detection: A tutorial review. Analyst 2019, 144, 6834–6849. [Google Scholar] [CrossRef]
- Merkoçi, A.; Marcolino-Junior, L.H.; Marín, S.; Fatibello-Filho, O.; Alegret, S. Detection of cadmium sulphide nanoparticles by using screen-printed electrodes and a handheld device. Nanotechnology 2007, 18, 035502. [Google Scholar] [CrossRef] [PubMed]
- Martín-Yerga, D.; González-García, M.B.; Costa-García, A. Biosensor array based on the in situ detection of quantum dots as electrochemical label. Sens. Actuators B Chem. 2013, 182, 184–189. [Google Scholar] [CrossRef]
- Freitas, M.; Neves, M.M.P.S.; Nouws, H.P.A.; Delerue-Matos, C. Quantum dots as nanolabels for breast cancer biomarker HER2-ECD analysis in human serum. Talanta 2020, 208, 120430. [Google Scholar] [CrossRef]
- Kokkinos, C.; Prodromidis, M.; Economou, A.; Petrou, P.; Kakabakos, S. Disposable integrated bismuth citrate-modified screen-printed immunosensor for ultrasensitive quantum dot-based electrochemical assay of C-reactive protein in human serum. Anal. Chim. Acta 2015, 886, 29–36. [Google Scholar] [CrossRef]
- Livas, D.; Trachioti, M.; Banou, S.; Angelopoulou, M.; Economou, A.; Prodromidis, M.; Petrou, P.; Kakabakos, S.; Kokkinos, C. 3D printed microcell featuring a disposable nanocomposite Sb/Sn immunosensor for quantum dot-based electrochemical determination of adulteration of ewe/goat’s cheese with cow’s milk. Sens. Actuators B Chem. 2021, 334, 129614. [Google Scholar] [CrossRef]
- Kokkinos, C.; Angelopoulou, M.; Economou, A.; Prodromidis, M.; Florou, A.; Haasnoot, W.; Petrou, P.; Kakabakos, S. Lab-on-a-Membrane Foldable Devices for Duplex Drop-Volume Electrochemical Biosensing Using Quantum Dot Tags. Anal. Chem. 2016, 88, 6897–6904. [Google Scholar] [CrossRef]
- Murasova, P.; Kovarova, A.; Kasparova, J.; Brozkova, I.; Hamiot, A.; Pekarkova, J.; Dupuy, B.; Drbohlavova, J.; Bilkova, Z.; Korecka, L. Direct culture-free electrochemical detection of Salmonella cells in milk based on quantum dots-modified nanostructured dendrons. J. Electroanal. Chem. 2020, 863, 114051. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Singh, C.; Kotnala, R.K.; Sumana, G. Graphene quantum dots-based nano-biointerface platform for food toxin detection. Anal. Bioanal. Chem. 2018, 410, 7313–7323. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Singh, C.; Kumar Pandey, M.; Sumana, G. Star shaped zinc sulphide quantum dots self-assembled monolayers: Preparation and applications in food toxin detection. Sens. Actuators B Chem. 2016, 231, 624–633. [Google Scholar] [CrossRef]
- Masikini, M.; Mailu, S.N.; Tsegaye, A.; Njomo, N.; Molapo, K.M.; Ikpo, C.O.; Sunday, C.E.; Rassie, C.; Wilson, L.; Baker, P.G.L.; et al. A Fumonisins Immunosensor Based on Polyanilino-Carbon Nanotubes Doped with Palladium Telluride Quantum Dots. Sensors 2015, 15, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Delgado, N.; Herrera, M.; Tavabi, A.H.; Luysberg, M.; Dunin-Borkowski, R.E.; Rodriguez-Cantó, P.J.; Abargues, R.; Martínez-Pastor, J.P.; Molina, S.I. Structural and chemical characterization of CdSe-ZnS core-shell quantum dots. Appl. Surf. Sci. 2018, 457, 93–97. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Guo, Q.; Hou, H.; You, T. Enzyme-free ethanol sensor based on electrospun nickel nanoparticle-loaded carbon fiber paste electrode. Anal. Chim. Acta 2010, 663, 153–157. [Google Scholar] [CrossRef]
- de la Cruz, S.; López-Calleja, I.; Martín, R.; González, I.; Alcocer, M.; García, T. Recent Advances in the Detection of Allergens in Foods. In Food Allergens: Methods and Protocols; Lin, J., Alcocer, M., Eds.; Springer: New York, NY, USA, 2017; pp. 263–295. ISBN 978-1-4939-6925-8. [Google Scholar]
- Walczyk, N.E.; Smith, P.M.C.; Tovey, E.R.; Roberts, T.H. Peanut protein extraction conditions strongly influence yield of allergens Ara h 1 and 2 and sensitivity of immunoassays. Food Chem. 2017, 221, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.; Pimentel, F.B.; Nouws, H.P.A.; Silva, T.H.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. Improving the extraction of Ara h 6 (a peanut allergen) from a chocolate-based matrix for immunosensing detection: Influence of time, temperature and additives. Food Chem. 2017, 218, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Freitas, M.; Nouws, H.P.A.; Keating, E.; Fernandes, V.C.; Delerue-Matos, C. Immunomagnetic bead-based bioassay for the voltammetric analysis of the breast cancer biomarker HER2-ECD and tumour cells using quantum dots as detection labels. Microchim. Acta 2020, 187, 184. [Google Scholar] [CrossRef]
- Freitas, M.; Correr, W.; Cancino-Bernardi, J.; Barroso, M.F.; Delerue-Matos, C.; Zucolotto, V. Impedimetric immunosensors for the detection of Cry1Ab protein from genetically modified maize seeds. Sens. Actuators B Chem. 2016, 237, 702–709. [Google Scholar] [CrossRef]
- Alves, R.C.; Pimentel, F.B.; Nouws, H.P.A.; Marques, R.C.B.; González-García, M.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. Detection of Ara h 1 (a major peanut allergen) in food using an electrochemical gold nanoparticle-coated screen-printed immunosensor. Biosens. Bioelectron. 2015, 64, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Valdepeñas Montiel, V.; Pellicanò, A.; Campuzano, S.; Torrente-Rodríguez, R.M.; Reviejo, Á.J.; Cosio, M.S.; Pingarrón, J.M. Electrochemical detection of peanuts at trace levels in foods using a magnetoimmunosensor for the allergenic protein Ara h 2. Sens. Actuators B Chem. 2016, 236, 825–833. [Google Scholar] [CrossRef]
- Montiel, V.R.-V.; Campuzano, S.; Pellicanò, A.; Torrente-Rodríguez, R.M.; Reviejo, A.J.; Cosio, M.S.; Pingarrón, J.M. Sensitive and selective magnetoimmunosensing platform for determination of the food allergen Ara h 1. Anal. Chim. Acta 2015, 880, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Sobhan, A.; Oh, J.-H.; Park, M.-K.; Kim, S.W.; Park, C.; Lee, J. Single walled carbon nanotube based biosensor for detection of peanut allergy-inducing protein ara h1. Korean J. Chem. Eng. 2018, 35, 172–178. [Google Scholar] [CrossRef]
- Huang, Y.; Bell, M.C.; Suni, I.I. Impedance Biosensor for Peanut Protein Ara h 1. Anal. Chem. 2008, 80, 9157–9161. [Google Scholar] [CrossRef] [PubMed]

| Sensing Surface | Food Sample | Assay Strategy | LOD | Ref | ||
|---|---|---|---|---|---|---|
| Construction * | Long-Term Stability | Detection Scheme | Assay Time | |||
| Sandwich-type assay. Capture antibody immobilized on bare SPCE by adsorption (~12 h) | 30 days | Cookies, cereal and protein bars | QDs used as label. Detection by DPASV | 1 h | 3.5 ng·mL−1 | This work |
| Sandwich-type assay. Capture antibody immobilized by adsorption on SPCE modified with AuNP (~12 h) | n.d. | Cookies, chocolate | AP used as label. Detection by LSV | 3 h 50 min | 3.8 ng·mL−1 | [41] |
| Sandwich-type assay. Capture antibody immobilized on MBs and using SPCE as transducer (~2 h) | 25 days | Hazelnuts, peanut based food; saliva | HRP used as label. H2O2/HQ system with HQ monitored by amperometry | 2 h | 6.3 ng·mL−1 | [43] |
| Label-free assay. Capture antibody covalently immobilized on a silicon wafer functionalized with SWCNT and (>15 h) | n.d. | n.d. | (Label-free) Detection by LSV | 30 min | 1 ng·mL−1 | [44] |
| Label-free assay. Capture antibody immobilized on 11-MUA/AuE (~19 h) | n.d. | n.d. | Fe(CN)63−/4− used as redox probe. Detection by EIS | 5 min | 0.3 nM | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, M.; Nouws, H.P.A.; Delerue-Matos, C. Voltammetric Immunosensor to Track a Major Peanut Allergen (Ara h 1) in Food Products Employing Quantum Dot Labels. Biosensors 2021, 11, 426. https://doi.org/10.3390/bios11110426
Freitas M, Nouws HPA, Delerue-Matos C. Voltammetric Immunosensor to Track a Major Peanut Allergen (Ara h 1) in Food Products Employing Quantum Dot Labels. Biosensors. 2021; 11(11):426. https://doi.org/10.3390/bios11110426
Chicago/Turabian StyleFreitas, Maria, Henri P. A. Nouws, and Cristina Delerue-Matos. 2021. "Voltammetric Immunosensor to Track a Major Peanut Allergen (Ara h 1) in Food Products Employing Quantum Dot Labels" Biosensors 11, no. 11: 426. https://doi.org/10.3390/bios11110426
APA StyleFreitas, M., Nouws, H. P. A., & Delerue-Matos, C. (2021). Voltammetric Immunosensor to Track a Major Peanut Allergen (Ara h 1) in Food Products Employing Quantum Dot Labels. Biosensors, 11(11), 426. https://doi.org/10.3390/bios11110426







