Versatile Cell and Animal Models for Advanced Investigation of Lead Poisoning
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Drug Pretreatments
2.2. Fly Strains and Transgenic Fly Constructs
2.3. Preparation of Fly Samples
2.4. FRET-Based Pb Imaging
2.5. Data Analysis
3. Results
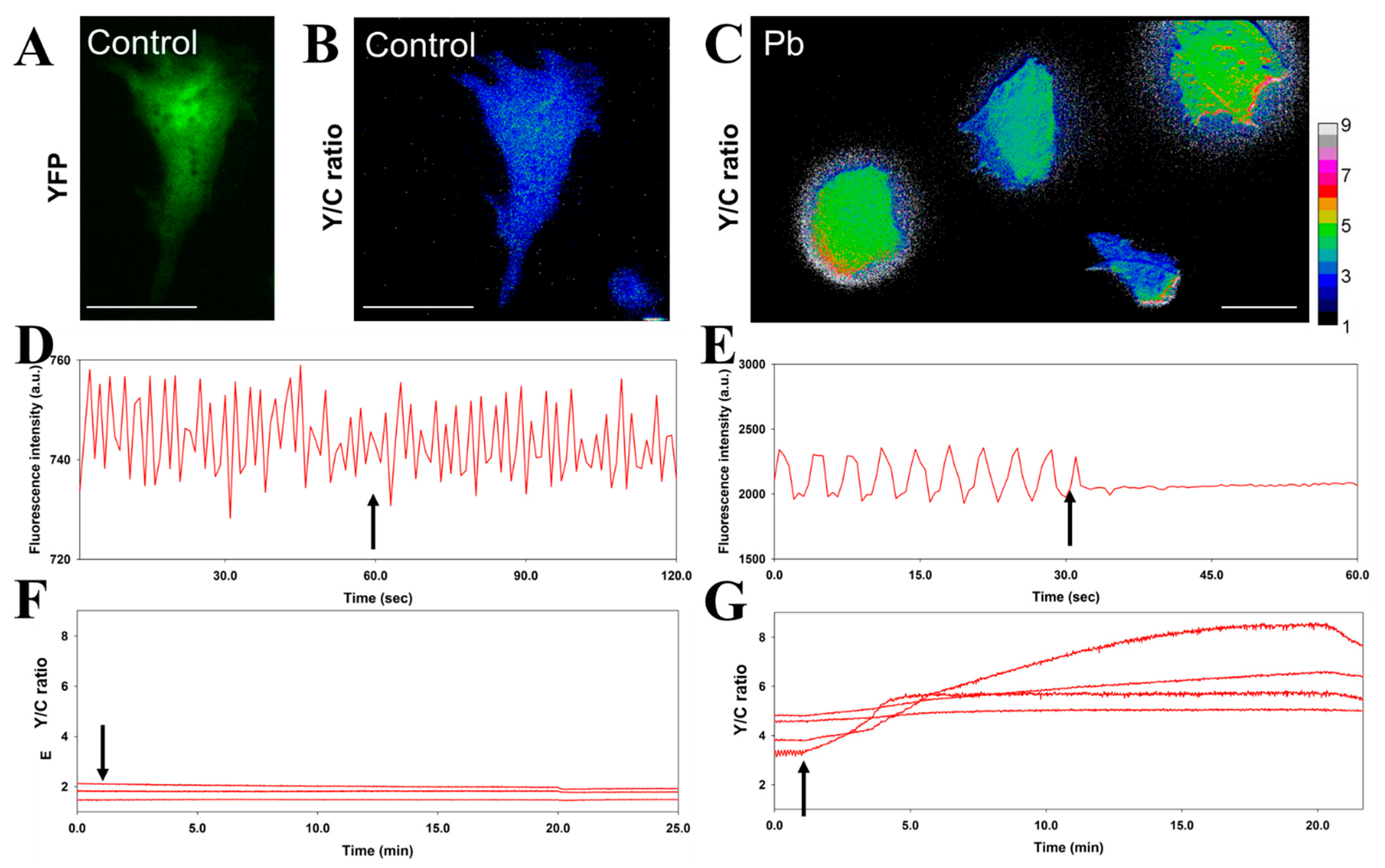
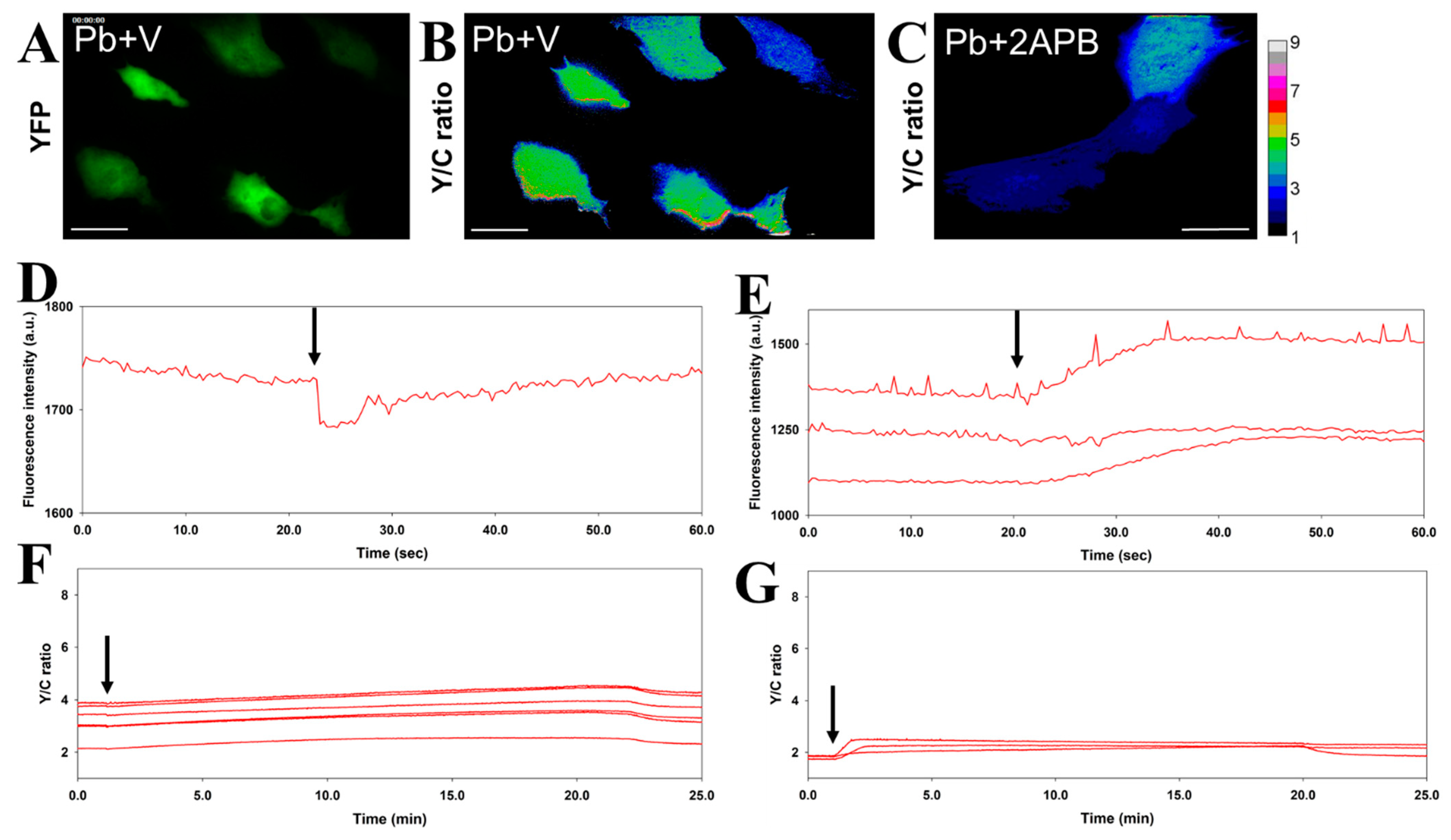
3.1. Simultaneous Monitoring of Physical Cardiomyocyte Activity and Pb Content
3.2. In Vivo Pb Sensing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beattie, O.B.; Geiger, J. Frozen in Time: Unlocking the Secrets of the Franklin Expedition; Western Producer Prairie Books: Saskatoon, SK, Canda, 1987; ISBN 978-0-88833-303-2. [Google Scholar]
- Kowall, W.; Beattie, O.B.; Baadsgaard, H.; Krahn, P.M. Did solder kill Franklins men? Nature 1990, 343, 319–320. [Google Scholar] [CrossRef]
- Kowall, W.A.; Krahnl, P.M.; Beattie, O.B. Lead levels in human tissues from the Franklin Forensic Project. Int. J. Environ. Anal. Chem. 1988, 35, 119–126. [Google Scholar] [CrossRef]
- Swanston, T.; Varney, T.L.; Kozachuk, M.; Choudhury, S.; Bewer, B.; Coulthard, I.; Keenleyside, A.; Nelson, A.; Martin, R.R.; Stenton, D.R.; et al. Franklin expedition lead exposure: New insights from high resolution confocal X-ray fluorescence imaging of skeletal microstructure. PLoS ONE 2018, 13, e0202983. [Google Scholar] [CrossRef]
- Martin, R.R.; Naftel, S.; Macfie, S.; Jones, K.; Nelson, A. Pb distribution in bones from the Franklin expedition: Synchrotron X-ray fluorescence and laser ablation/mass spectroscopy. Appl. Phys. A 2013, 111, 23–29. [Google Scholar] [CrossRef][Green Version]
- Millar, K.; Bowman, A.; Battersby, W. A re-analysis of the supposed role of lead poisoning in Sir John Franklin’s last expedition, 1845–1848—CORRIGENDUM. Polar Rec. 2015, 51, 341–342. [Google Scholar] [CrossRef]
- Smith, K.E.; Weis, E.; Chauvel, C.; Moulin, S. Honey Maps the Pb Fallout from the 2019 Fire at Notre-Dame Cathedral, Paris: A Geochemical Perspective. Environ. Sci. Tech. Lett. 2020, 7, 753–759. [Google Scholar] [CrossRef]
- Vallée, A.; Sorbets, E.; Lelong, H.; Langrand, J.; Blacher, J. The lead story of the fire at the Notre-Dame cathedral of Paris. Environ. Pollut. 2021, 269, 116140. [Google Scholar] [CrossRef]
- Central News Agency of Chinese Medicine Doctors, Dealer Detained in Lead Poisoning Case. Taipei News. 7 August 2020. Available online: https://www.taiwannews.com.tw/en/news/3982844 (accessed on 7 August 2020).
- Rocha, A.; Trujillo, K.A. Neurotoxicity of low-level lead exposure: History, mechanisms of action, and behavioral effects in humans and preclinical models. Neurotoxicology 2019, 73, 58–80. [Google Scholar] [CrossRef]
- Lin, C.Y.; Huang, P.C.; Wu, C.; Sung, F.C.; Su, T.C. Association between urine lead levels and cardiovascular disease risk factors, carotid intima-media thickness and metabolic syndrome in adolescents and young adults. Int. J. Hyg. Eviron. Health 2020, 223, 248–255. [Google Scholar] [CrossRef]
- Mitra, P.; Sharma, S.; Purohit, P.; Sharma, P. Clinical and molecular aspects of lead toxicity: An update. Crit. Rev. Clin. Lab. Sci. 2017, 54, 506–528. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Zhang, Y.; Liu, X.; Liu, S.; Li, B.; Zhang, M.; Qin, L.; Yi, H.; Li, M.; Li, L.; et al. Electrochemical biosensor for amplified detection of Pb2+ based on perfect match of reduced graphene oxide-gold nanoparticles and single-stranded DNAzyme. Anal. Bioanal. Chem. 2019, 411, 7499–7509. [Google Scholar] [CrossRef]
- Miyawaki, A.; Llopis, J.; Heim, R.; McCaffery, J.M.; Adams, J.A.; Ikura, M.; Tsien, R.Y. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 1997, 388, 882–887. [Google Scholar] [CrossRef]
- Hochreiter, B.; Garcia, A.P.; Schmid, J.A. Fluorescent proteins as genetically encoded FRET biosensors in life sciences. Sensors 2015, 15, 26281–26314. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Y.; Yang, X.; Tang, Y.; Han, S.; Kang, A.; Deng, H.; Chi, Y.; Zhu, D.; Lu, Y. FÖrster resonance energy transfer (FRET)-based biosensors for biological applications. Biosens. Bioelectron. 2019, 138, 111314. [Google Scholar] [CrossRef]
- Vinkenborg, J.L.; Nicolson, T.J.; Bellomo, E.A.; Koay, M.S.; Rutter, G.A.; Merkx, M. Genetically encoded FRET sensors to monitor intracellular Zn2+ homeostasis. Nat. Methods 2009, 6, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Wegner, S.V.; Arslan, H.; Sunbul, M.; Yin, J.; He, C. Dynamic copper(I) imaging in mammalian cells with a genetically encoded fluorescent copper(I) sensor. J. Am. Chem. Soc. 2010, 132, 2567–2569. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.Y.; Yang, D.M. Intracellular Pb2+ content monitoring using a protein-based Pb2+ indicator. Toxicol. Sci. 2012, 126, 436–445. [Google Scholar] [CrossRef]
- Chiu, T.Y.; Chen, P.H.; Chang, C.L.; Yang, D.M. Live-cell dynamic sensing of Cd(2+) with a FRET-based indicator. PLoS ONE 2013, 8, e65853. [Google Scholar] [CrossRef]
- Chen, Y.J.; Liu, C.Y.; Tsai, D.Y.; Yeh, Y.C. A portable fluorescence resonance energy transfer biosensor for rapid detection of silver ions. Sens. Actuators B 2018, 259, 784–788. [Google Scholar] [CrossRef]
- Yang, D.M.; Fu, T.F.; Lin, C.S.; Chiu, T.Y.; Huang, C.C.; Huang, H.Y.; Chung, M.W.; Lin, Y.S.; Manurung, R.V.; Nguyen, P.; et al. High-performance FRET biosensors for single-cell and in vivo lead detection. Biosens. Bioelectron. 2020, 168, 112571. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.C.; Zhang, P.; Poon, E.; Kong, C.W.; Boheler, K.R.; Huang, Y.; Li, R.A.; Yao, X. Nitric oxide-cGMP-PKG pathway acts on Orai1 to inhibit the hypertrophy of human embryonic stem cell-derived cardiomyocytes. Stem Cell 2015, 33, 2973–2984. [Google Scholar] [CrossRef] [PubMed]
- Uzun, A.U.; Mannhardt, I.; Breckwoldt, K.; Horváth, A.; Johannsen, S.S.; Hansen, A.; Eschenhagen, T.; Christ, T. Ca2+-currents in human induced pluripotent stem cell-derived cardiomyocytes effects of two different culture conditions. Front. Pharmacol. 2016, 7, 300. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.M.; Manurung, R.V.; Lin, Y.S.; Chiu, T.Y.; Lai, W.Q.; Chang, Y.F.; Fu, T.F. Monitoring the heavy metal lead inside living Drosophila with a FRET-based biosensor. Sensors 2020, 20, 1712. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Mattos, G.; Costa, C.; Savio, F.; Alonso, M.; Nicolson, G.L. Lead poisoning: Acute exposure of the heart to lead ions promotes changes in cardiac function and Cav1.2 ion channels. Biophys. Rev. 2017, 9, 807–825. [Google Scholar] [CrossRef]
- Lanphear, B.P.; Rauch, P.; Allen, R.W.; Hornung, R.W. Low-level lead exposure and mortality in US adults: A population-based cohort study. Lancet Publich Health 2018, 3, e177–e184. [Google Scholar] [CrossRef]
- Chang, Y.F.; Teng, H.C.; Cheng, S.Y.; Wang, C.T.; Chiou, S.H.; Kao, L.S.; Kao, F.J.; Chiou, A.; Yang, D.M. Orai1-STIM1 formed store-operated Ca2+ channels (SOCs) as the molecular components needed for Pb2+ entry in living cells. Toxicol. Appl. Pharmacol. 2008, 227, 430–439. [Google Scholar] [CrossRef]
- Chiu, T.Y.; Teng, H.C.; Huang, P.C.; Kao, F.J.; Yang, D.M. Dominant role of Orai1 with STIM1 on the cytosolic entry and cytotoxicity of lead ions. Toxicol. Sci. 2009, 110, 353–362. [Google Scholar] [CrossRef]
- Obeng-Gyasi, E.; Armijos, R.X.; Weigel, M.M.; Filippelli, G.M.; Sayegh, M.A. Cardiovascular-Related Outcomes in U.S. Adults Exposed to Lead. Int. J. Environ. Res. Public Health. 2018, 15, 759. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lee, H.L.; Hwang, Y.T.; Huang, P.C.; Wang, C.; Sung, F.C.; Wu, C.; Su, T.C. Urinary heavy metals, DNA methylation, and subclinical atherosclerosis. Ecotoxicol. Environ. Safe. 2020, 204, 111039. [Google Scholar] [CrossRef]
- Changela, A.; Chen, K.; Xue, Y.; Holschen, J.; Outten, C.E.; O’Halloran, T.V.; Mondragón, A. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science 2003, 301, 1383–1387. [Google Scholar] [CrossRef]
- Algar, W.R.; Hildebrandt, N.; Vogel, S.S.; Medintz, I.L. FRET as a biomolecular research tool—Understanding its potential while avoiding pitfalls. Nat. Methods 2019, 16, 815–829. [Google Scholar] [CrossRef]
- Chang, T.J.; Lai, W.Q.; Chang, Y.F.; Wang, C.L.; Yang, D.M. Development and optimization of heavy metal lead biosensors in biomedical and environmental applications. J. Chin. Med. Assoc. 2021, 84, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Besunder, J.B.; Super, D.M.; Anderson, R.L. Comparison of dimercaptosuccinic acid and calcium disodium ethylenediaminetetraacetic acid versus dimercaptopropanol and ethylenediaminetetraacetic acid in children with lead poisoning. J. Pediatr. 1997, 130, 966–971. [Google Scholar] [CrossRef]
- Bjørklund, G.; Mutter, J.; Aaseth, J. Metal chelators and neurotoxicity: Lead, mercury, and arsenic. Arch. Toxicol. 2017, 91, 3787–3797. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Piatkevich, K.D.; Mc Larney, B.; Abdelfattah, A.S.; Mehta, S.; Murdock, M.H.; Gottschalk, S.; Molina, R.S.; Zhang, W.; Chen, Y.; et al. A genetically encoded near-infrared fluorescent calcium ion indicator. Nat. Methods 2019, 16, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Cosio, D.; Piatkevich, K.D.; Aufmkolk, S.; Su, W.C.; Celiker, O.T.; Schohl, A.; Murdock, M.H.; Aggarwal, A.; Chang, Y.F.; et al. Improved genetically encoded near-infrared fluorescent calcium ion indicators for in vivo imaging. PLoS Biol. 2020, 18, e3000965. [Google Scholar] [CrossRef]




| iPSC Experimental Sets | Delta Pb (FRET Y/C Ratio) | Beating Frequency |
|---|---|---|
| Control | NA 1 (basal maintained at 1.97 ± 0.15) | 24 bpm 2 → 24 bpm |
| Pb (50 μM) | 2.06 (4.18 ± 0.32 → 6.24 ± 0.17) | 20 bpm → NA |
| Pb (50 μM) + Verapamil | 0.19 (3.06 ± 0.14 → 3.25 ± 0.73) | NA |
| Pb (50 μM) + 2-APB | 0.34 (1.91 ± 0.1 → 2.25 ± 0.47) | 20 bpm → 16 bpm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.-M.; Chang, Y.-F. Versatile Cell and Animal Models for Advanced Investigation of Lead Poisoning. Biosensors 2021, 11, 371. https://doi.org/10.3390/bios11100371
Yang D-M, Chang Y-F. Versatile Cell and Animal Models for Advanced Investigation of Lead Poisoning. Biosensors. 2021; 11(10):371. https://doi.org/10.3390/bios11100371
Chicago/Turabian StyleYang, De-Ming, and Yu-Fen Chang. 2021. "Versatile Cell and Animal Models for Advanced Investigation of Lead Poisoning" Biosensors 11, no. 10: 371. https://doi.org/10.3390/bios11100371
APA StyleYang, D.-M., & Chang, Y.-F. (2021). Versatile Cell and Animal Models for Advanced Investigation of Lead Poisoning. Biosensors, 11(10), 371. https://doi.org/10.3390/bios11100371





