Sub-Part-Per-Billion Level Sensing of Fentanyl Residues from Wastewater Using Portable Surface-Enhanced Raman Scattering Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the SERS Substrate
2.2. Fentanyl SERS Measurement
2.3. Multivariate Analysis
3. Results and Discussion
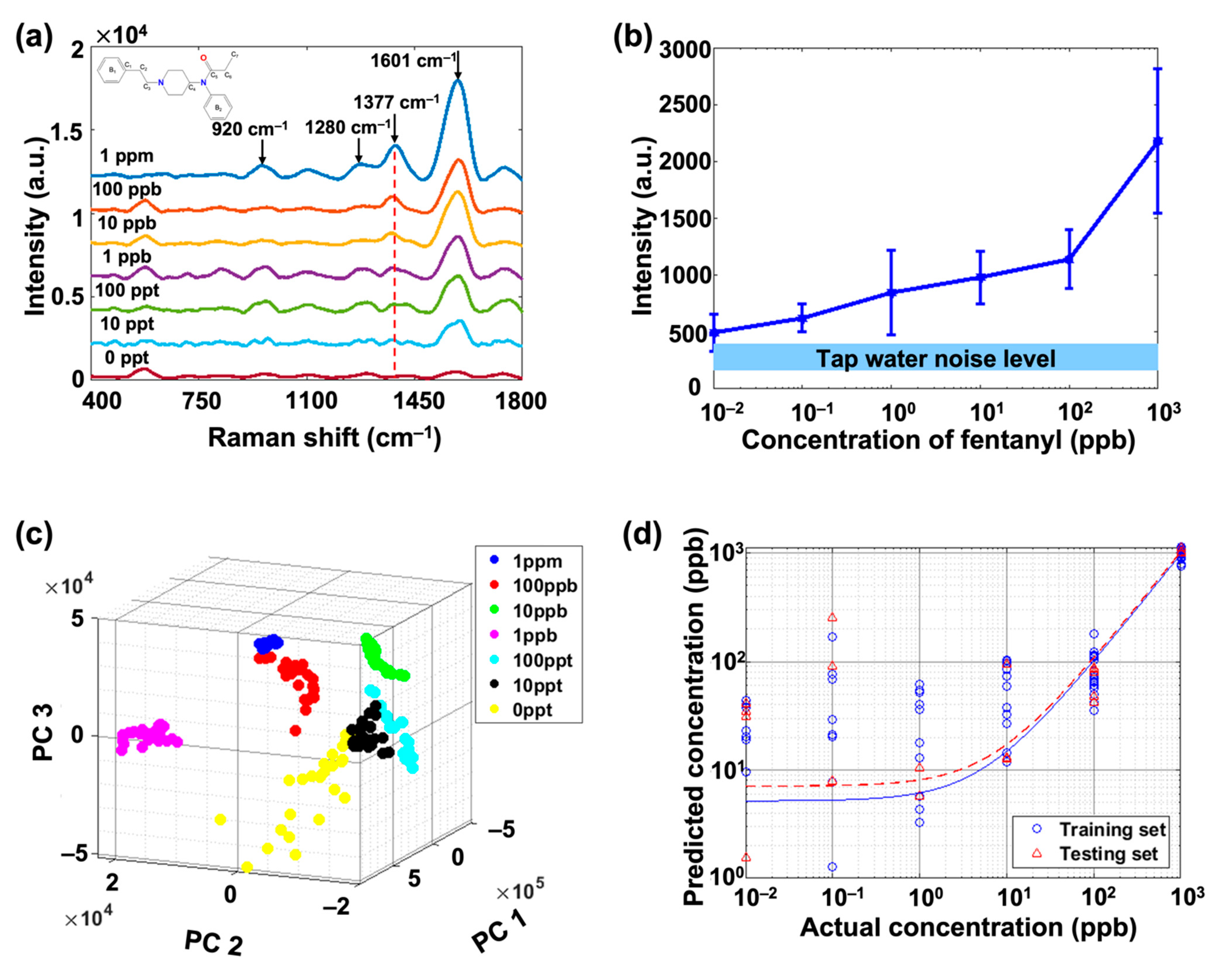
3.1. Fentanyl SERS Detection in Artificially Contaminated Tap Water
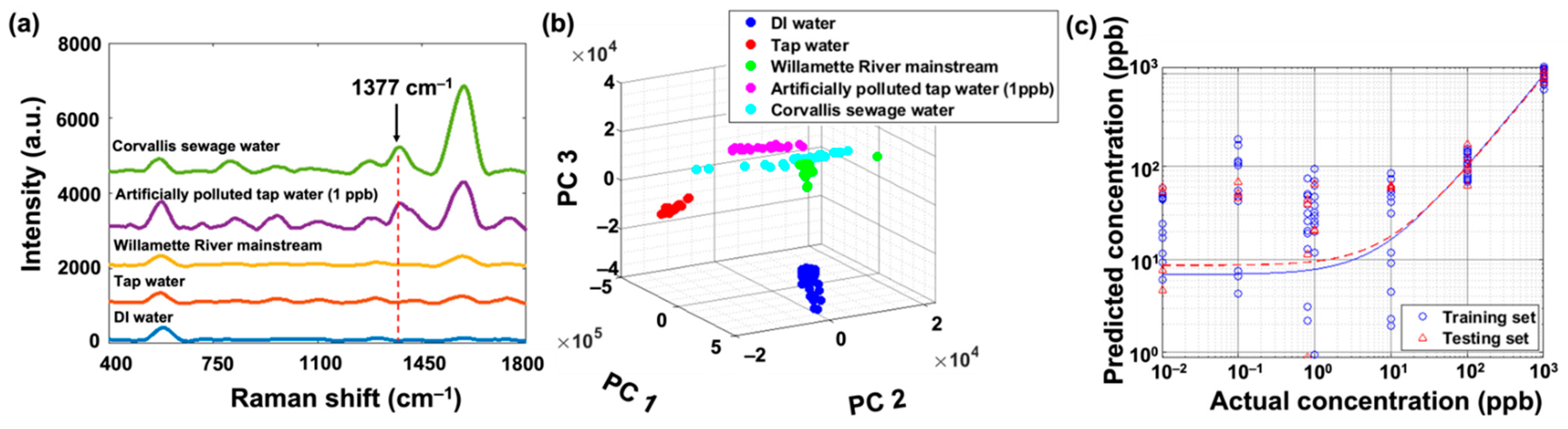
3.2. Fentanyl SERS Detection in Sewage Water from the City of Corvallis
3.3. Fentanyl SERS Detection from Different Sewage Treatment Plants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Nuijs, A.L.; Castiglioni, S.; Tarcomnicu, I.; Postigo, C.; de Alda, M.L.; Neels, H.; Zuccato, E.; Barcelo, D.; Covaci, A. Illicit Drug Consumption Estimations Derived from Wastewater Analysis: A Critical Review. Sci. Total Environ. 2011, 409, 3564–3577. [Google Scholar] [CrossRef]
- Zuccato, E.; Chiabrando, C.; Castiglioni, S.; Bagnati, R.; Fanelli, R. Estimating Community Drug Abuse by Wastewater Analysis. Environ. Health Perspect. 2008, 116, 1027–1032. [Google Scholar] [CrossRef] [Green Version]
- Postigo, C.; de Alda, M.L.; Barceló, D. Evaluation of Drugs of Abuse Use and Trends in a Prison through Wastewater Analysis. Environ. Int. 2011, 37, 49–55. [Google Scholar] [CrossRef]
- Ort, C.; Van Nuijs, A.L.; Berset, J.D.; Bijlsma, L.; Castiglioni, S.; Covaci, A.; de Voogt, P.; Emke, E.; Fatta-Kassinos, D.; Griffiths, P.; et al. Spatial Differences and Temporal Changes in Illicit Drug Use in Europe Quantified by Wastewater Analysis. Addiction 2014, 109, 1338–1352. [Google Scholar] [CrossRef]
- Baker, D.R.; Barron, L.; Kasprzyk-Hordern, B. Illicit and Pharmaceutical Drug Consumption Estimated via Wastewater Analysis. Part A: Chemical Analysis and Drug Use Estimates. Sci. Total Environ. 2014, 487, 629–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postigo, C.; de Alda, M.J.L.; Barceló, D. Drugs of Abuse and Their Metabolites in the Ebro River Basin: Occurrence in Sewage and Surface Water, Sewage Treatment Plants Removal Efficiency, and Collective Drug Usage Estimation. Environ. Int. 2010, 36, 75–84. [Google Scholar] [CrossRef]
- Postigo, C.; Lopez de Alda, M.J.; Barceló, D. Fully Automated Determination in the Low Nanogram per Liter Level of Different Classes of Drugs of Abuse in Sewage Water by On-Line Solid-Phase Extraction-Liquid Chromatography−Electrospray-Tandem Mass Spectrometry. Anal. Chem. 2008, 80, 3123–3134. [Google Scholar] [CrossRef]
- Krizman-Matasic, I.; Kostanjevecki, P.; Ahel, M.; Terzic, S. Simultaneous Analysis of Opioid Analgesics and Their Metabolites in Municipal Wastewaters and River Water by Liquid Chromatography–tandem Mass Spectrometry. J. Chromatogr. A 2018, 1533, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Ultraperformance Liquid Chromatography—Tandem Mass Spectrometry Analysis of Stimulatory Drugs of Abuse in Wastewater and Surface Waters. Anal. Chem. 2007, 79, 3821–3829. [Google Scholar] [CrossRef]
- Berset, J.D.; Brenneisen, R.; Mathieu, C. Analysis of llicit and Illicit Drugs in Waste, Surface and Lake Water Samples using Large Volume Direct Injection High Performance Liquid Chromatography Electrospray Tandem Mass Spectrometry (HPLC–MS/MS). Chemosphere 2010, 81, 859–866. [Google Scholar] [CrossRef]
- Castiglioni, S.; Zuccato, E.; Crisci, E.; Chiabrando, C.; Fanelli, R.; Bagnati, R. Identification and Measurement of Illicit Drugs and Their Metabolites in Urban Wastewater by Liquid Chromatography tandem Mass Spectrometry. Anal. Chem. 2006, 78, 8421–8429. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.; De Jong, M.; De Wael, K. Electrochemical strategies for the Detection of Forensic Drugs. Curr. Opin. Electrochem. 2018, 11, 34–40. [Google Scholar] [CrossRef]
- Yang, Z.; Castrignanò, E.; Estrela, P.; Frost, C.G.; Kasprzyk-Hordern, B. Community Sewage Sensors towards Evaluation of Drug Use Trends: Detection of Cocaine in Wastewater with DNA-directed Immobilization Aptamer sensors. Sci. Rep. 2016, 6, 21024–21034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrido, J.M.P.J.; Delerue-Matos, C.; Borges, F.; Macedo, T.R.; Oliveira-Brett, A.M. Voltammetric Oxidation of Drugs of Abuse I. Morphine and Metabolites. Electroanalysis 2004, 16, 1419–1426. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.P.; Metters, J.P.; Irving, C.; Sutcliffe, O.B.; Banks, C.E. Forensic Electrochemistry: The Electroanalytical Sensing of Synthetic Cathinone-Derivatives and their Accompanying Adulterants in “Legal High” Products. Analyst 2014, 139, 389–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagheri, H.; Khoshsafar, H.; Afkhami, A.; Amidi, S. Sensitive and Simple Simultaneous Determination of Morphine and Codeine Using a Zn2SnO4 Nanoparticle/Graphene Composite Modified Electrochemical Sensor. New J. Chem. 2016, 40, 7102–7112. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously Intense Raman Spectra of Pyridine at a Silver Electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2019, 14, 28–117. [Google Scholar] [CrossRef] [Green Version]
- Dasary, S.S.; Singh, A.K.; Senapati, D.; Yu, H.; Ray, P.C. Gold Nanoparticle Based Label-free SERS Probe for Ultrasensitive and Selective Detection of Trinitrotoluene. J. Am. Chem. Soc. 2009, 131, 13806–13812. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Ge, M.; Li, P.; Xie, Q.; Yang, L. Development of Surface-enhanced Raman Spectroscopy Application for Determination of Illicit Drugs: Towards a Practical Sensor. Talanta 2019, 191, 1–10. [Google Scholar] [CrossRef]
- Inscore, F.; Shende, C.; Sengupta, A.; Huang, H.; Farquharson, S. Detection of Drugs of Abuse in Saliva by Surface-enhanced Raman Spectroscopy (SERS). Appl. Spectrosc. 2011, 65, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, N.; Ji, D.; Song, H.; Liu, Y.; Zhou, L.; Sun, Z.; Jornet, J.M.; Thompson, A.C.; Collins, R.L.; et al. Superabsorbing Metasurfaces with Hybrid Ag–Au Nanostructures for Surface-Enhanced Raman Spectroscopy Sensing of Drugs and Chemicals. Small Methods 2018, 2, 1800045. [Google Scholar] [CrossRef]
- Rana, V.; Cañamares, M.V.; Kubic, T.; Leona, M.; Lombardi, J.R. Surface-Enhanced Raman Spectroscopy for Trace Identification of Controlled Substances: Morphine, Codeine, and Hydrocodone. J. Forensic Sci. 2011, 56, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Cunningham, B.T. Point-of-Care Detection and Real-Time Monitoring of Intravenously Delivered Drugs via Tubing with an Integrated SERS Sensor. Nanoscale 2014, 6, 5162–5171. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Chong, X.; Squire, K.; Wang, A.X. Microfluidic Diatomite Analytical Devices for Illicit Drug Sensing with ppb-Level Sensitivity. Sens. Actuators B 2018, 259, 587–595. [Google Scholar] [CrossRef]
- Tan, A.; Zhao, Y.; Sivashanmugan, K.; Squire, K.; Wang, A.X. Quantitative TLC-SERS Detection of Histamine in Seafood with Support Vector Machine Analysis. Food Control 2019, 103, 111–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single Molecule Detection Using Surface-enhanced Raman Scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667. [Google Scholar] [CrossRef] [Green Version]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Arnob, M.M.P.; Shih, W.C. 3-Dimensional Plasmonic Substrates Based on Chicken Eggshell Bio-Templates for SERS-Based Bio-sensing. Micromachines 2017, 8, 196. [Google Scholar] [CrossRef] [Green Version]
- Sivashanmugan, K.; Squire, K.; Tan, A.; Zhao, Y.; Kraai, J.A.; Rorrer, G.L.; Wang, A.X. Trace Detection of Tetrahydrocannabinol in Body Fluid via Surface-Enhanced Raman Scattering and Principal Component Analysis. ACS Sens. 2019, 4, 1109–1117. [Google Scholar] [CrossRef]
- Alharbi, O.; Xu, Y.; Goodacre, R. Simultaneous Multiplexed Quantification of Caffeine and its Major Metabolites Theobromine and Paraxanthine Using Surface-Enhanced Raman Scattering. Anal. Bioanal. Chem. 2015, 407, 8253–8261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, X.; Li, E.; Squire, K.; Liu, Y.; Bo, W.; Cheng, L.; Wang, A.X. Plasmonic nanoparticles-decorated diatomite biosilica: Extending the horizon of on-chip chromatography and label-free biosensing. J. Biophotonics. 2017, 10, 1473–1484. [Google Scholar] [CrossRef]
- Kong, X.; Yu, Q.; Li, E.; Wang, Q.; Liu, R.; Wang, A.X. Diatomite photonics crystals for facile on-ship chromatography and sensing for harmful ingredients from food. Materials 2018, 11, 539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bista, R.S.; Lobb, M.; Haywood, A.; Hardy, J.; Tapuni, A.; Norris, R. Development, Validation, and Application of an HPLC-MS/MS Method of the Determination of Fentanyl and Nor-Fentanyl in Human Plasma and Saliva. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 960, 27–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohouli, E.; Keihan, A.H.; Shahdost-Fard, F.; Naghian, E.; Plonska-Brzezinska, M.E.; Rahimi-Nasrabadi, M.; Ahmadi, F. A Glassy Carbon Electrode Modified with Carbon Nanoonions for Electrochemical Determination of Fentanyl. Mater. Sci. Eng. C 2020, 110, 110684. [Google Scholar] [CrossRef] [PubMed]
- Shende, C.; Brouillette, C.; Farquharson, S. Detection of Codeine and Fentanyl in Saliva, Blood Plasma and Whole Blood in 5-minutes Using a SERS Flow-Separation Strip. Analyst 2019, 144, 5449–5454. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Squire, K.; Li, E.; LeDuff, P.; Rorrer, G.L.; Tang, S.; Chen, B.; McKay, C.; Navarro-Gonzalez, R.; Wang, A.X. Chemical and Biological Sensing Using Diatom Photonic Crystal Biosilica with In-Situ Growth Plasmonic Nanoparticles. IEEE Trans. Nanobiosci. 2016, 15, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Selesnick, I.W.; Duval, L. Chromatogram baseline estimation and denoising using sparsity. Chemom. Intell. Lab. Syst. 2014, 139, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Leonard, J.; Haddad, A.; Green, O.; Birke, R.L.; Kubic, T.; Kocak, A.; Lombardi, J.R. SERS, Raman, and DFT Analysis of Fentanyl and Carfentanil: Toward Detection of Trace Sample. J. Raman Spectrosc. 2017, 48, 1323–1329. [Google Scholar] [CrossRef]
- Barbillon, G.; Ivanov, A.; Sarychev, A.K. SERS Amplification in Au/Si Asymmetric Dimer Array Coupled to Efficient Adsorption of Thiophenol Molecules. Nanomaterials 2021, 11, 1521. [Google Scholar] [CrossRef]
- Srichan, C.; Ekpanyapong, M.; Horprathum, M.; Eiamchai, P.; Nuntawong, N.; Phokharatkul, D.; Danvirutai, P.; Bohez, E.; Wisitsoraat, A.; Tuantranont, A. Highly-Sensitive Surface-Enhanced Raman Spectroscopy (SERS)-based Chemical Sensor using 3D Graphene Foam Decorated with Silver Nanoparticles as SERS substrate. Sci. Rep. 2016, 6, 23733. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Singh, P. A review of the structures of oxide glasses by Raman spectroscopy. RSC Adv. 2015, 5, 67583–67609. [Google Scholar] [CrossRef]
- Prescribing and Overdose Data for Oregon. Available online: https://www.oregon.gov/oha/ph/preventionwellness/substanceuse/opioids/pages/data.aspx (accessed on 26 September 2021).
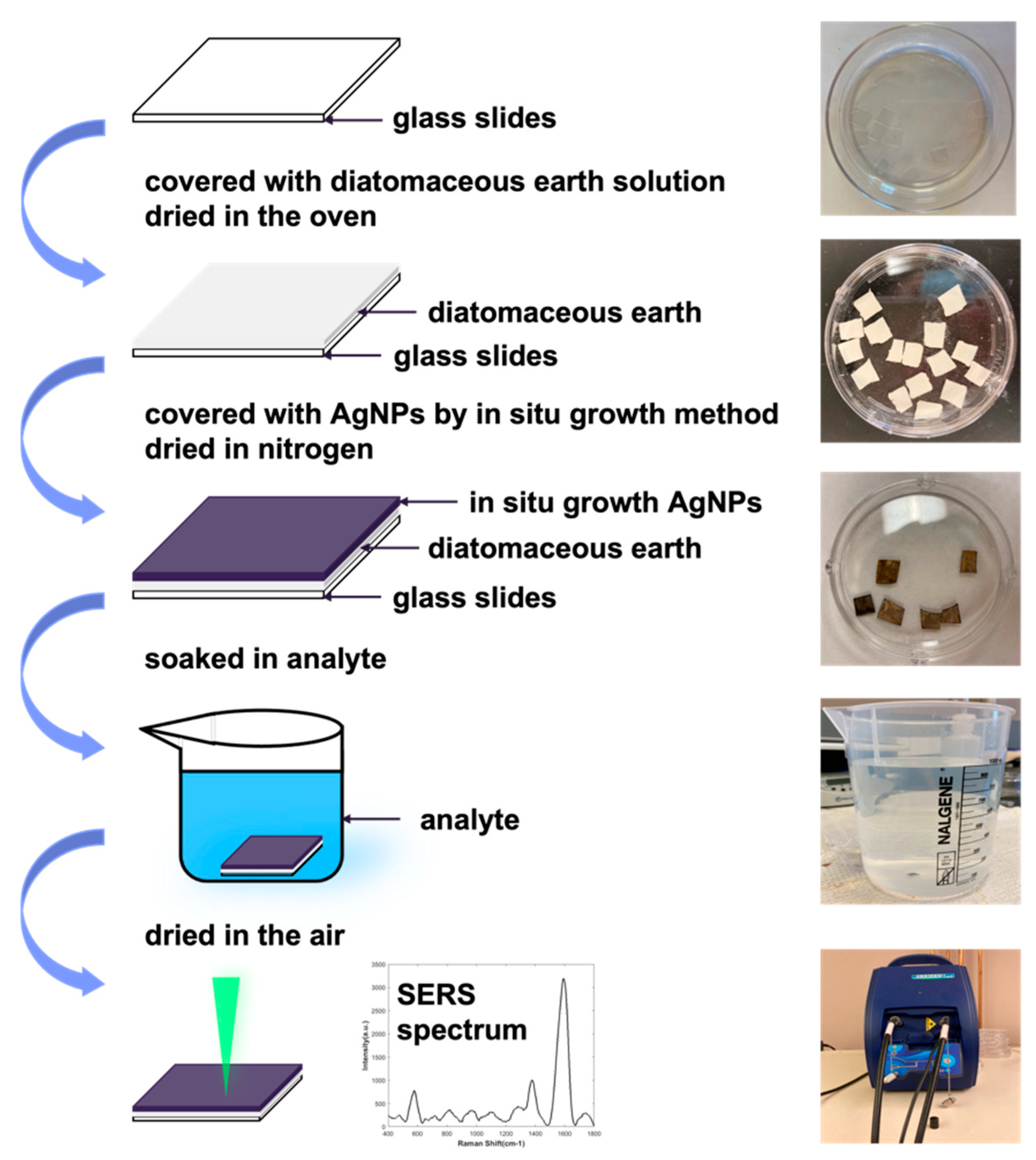

| Methods | Sensitivity | Equipment Requirement | Estimated Sensing Time | Sensor Cost | Reference |
|---|---|---|---|---|---|
| HPLC-MS | 30 ppb | HPLC-MS | 8 min | High | [34] |
| Electrochemical | 5 ppm | Potentiostat | A few minutes | Medium | [35] |
| Previous SERS | 5 ppb | 5lb field-usable Raman spectrometer | 5 min | Low | [36] |
| Our diatomaceous earth SERS | 800 ppt | Portable Raman spectrometer | 2 min | Low | This work |
| Raman Shift/ cm−1 | SERS Peak Assignment |
|---|---|
| 920 | C–H asymmetric out-of-plane trigonal bend of B2 |
| 1280 | C3 C–H twisting |
| 1377 | C7 C–H bonds |
| 1601 | C–C symmetric stretch of B1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Hou, X.; Zhen, C.; Wang, A.X. Sub-Part-Per-Billion Level Sensing of Fentanyl Residues from Wastewater Using Portable Surface-Enhanced Raman Scattering Sensing. Biosensors 2021, 11, 370. https://doi.org/10.3390/bios11100370
Zhang B, Hou X, Zhen C, Wang AX. Sub-Part-Per-Billion Level Sensing of Fentanyl Residues from Wastewater Using Portable Surface-Enhanced Raman Scattering Sensing. Biosensors. 2021; 11(10):370. https://doi.org/10.3390/bios11100370
Chicago/Turabian StyleZhang, Boxin, Xingwei Hou, Cheng Zhen, and Alan X. Wang. 2021. "Sub-Part-Per-Billion Level Sensing of Fentanyl Residues from Wastewater Using Portable Surface-Enhanced Raman Scattering Sensing" Biosensors 11, no. 10: 370. https://doi.org/10.3390/bios11100370
APA StyleZhang, B., Hou, X., Zhen, C., & Wang, A. X. (2021). Sub-Part-Per-Billion Level Sensing of Fentanyl Residues from Wastewater Using Portable Surface-Enhanced Raman Scattering Sensing. Biosensors, 11(10), 370. https://doi.org/10.3390/bios11100370





