High-Performance Passive Plasma Separation on OSTE Pillar Forest
Abstract
:1. Introduction
2. Materials and Methods
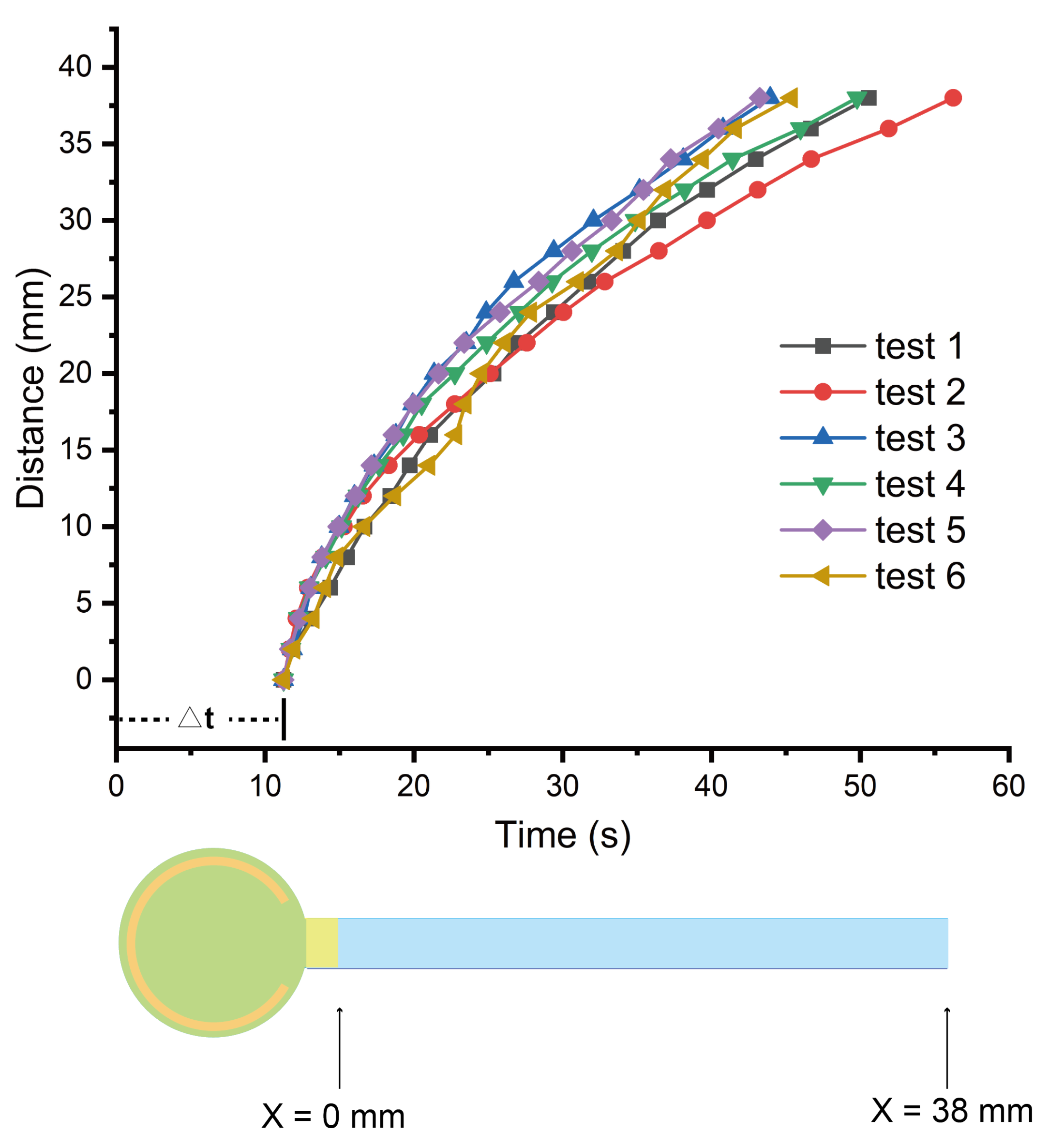
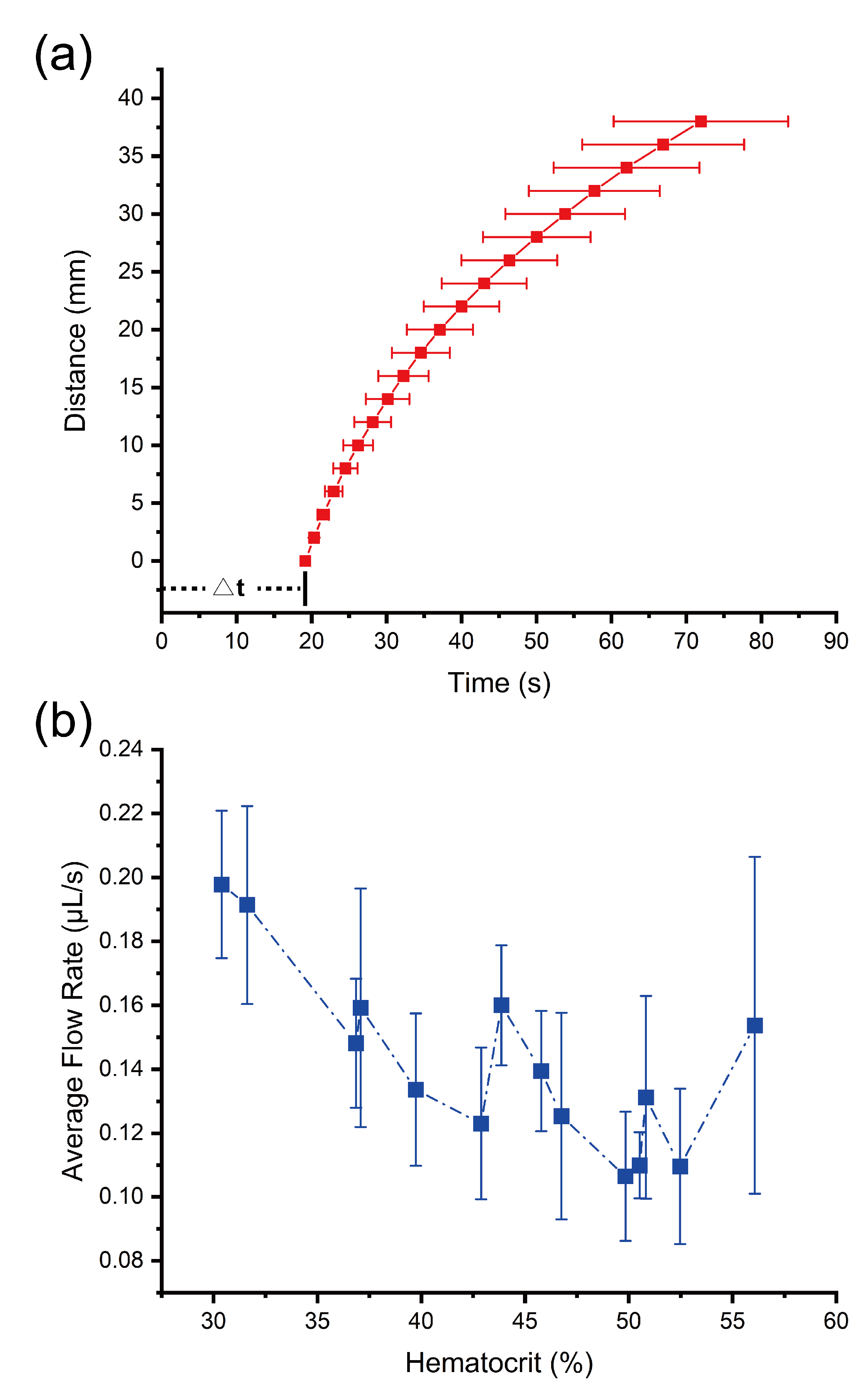
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
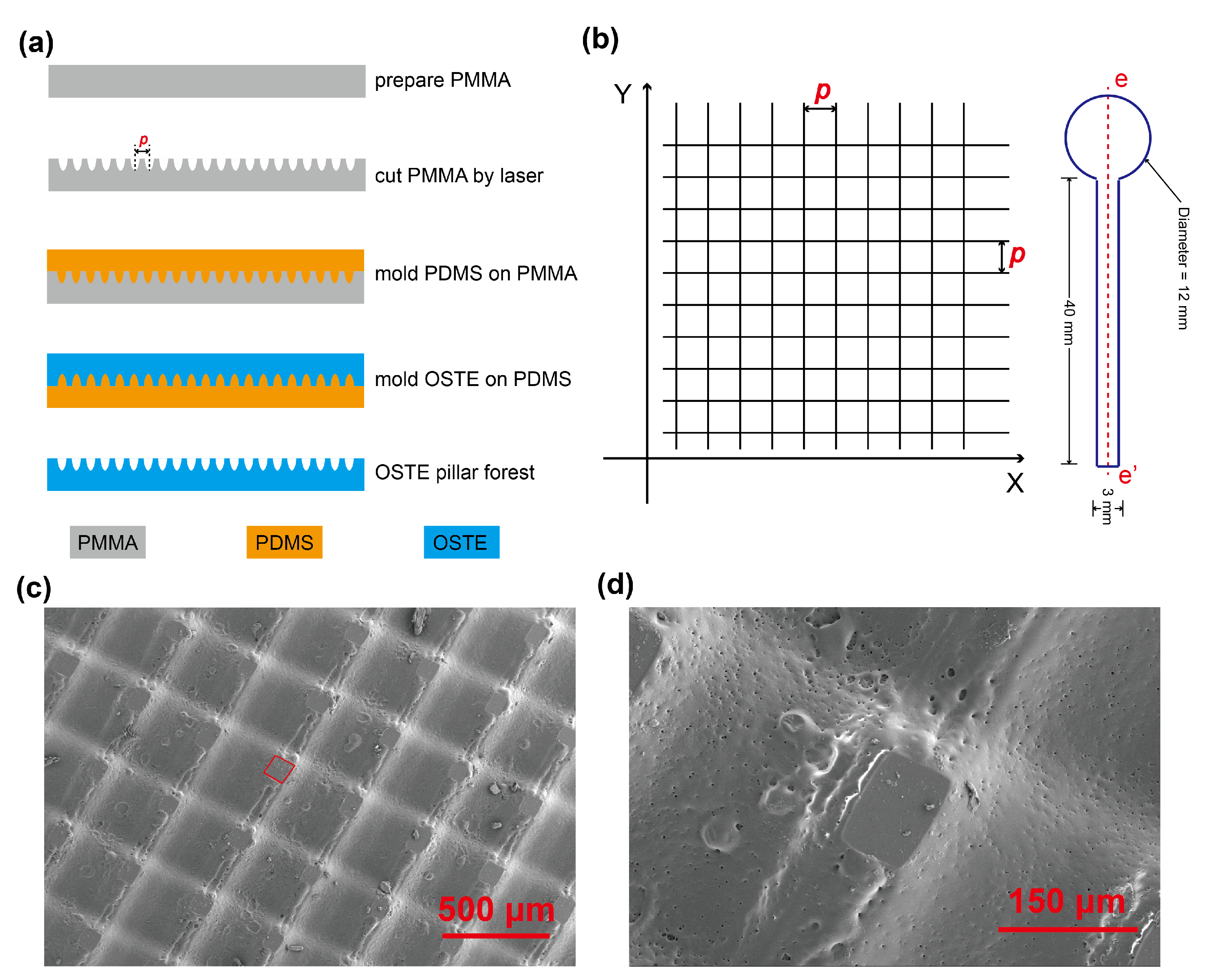
Appendix A.1. Fabrication Setup of Molding OSTE on PDMS
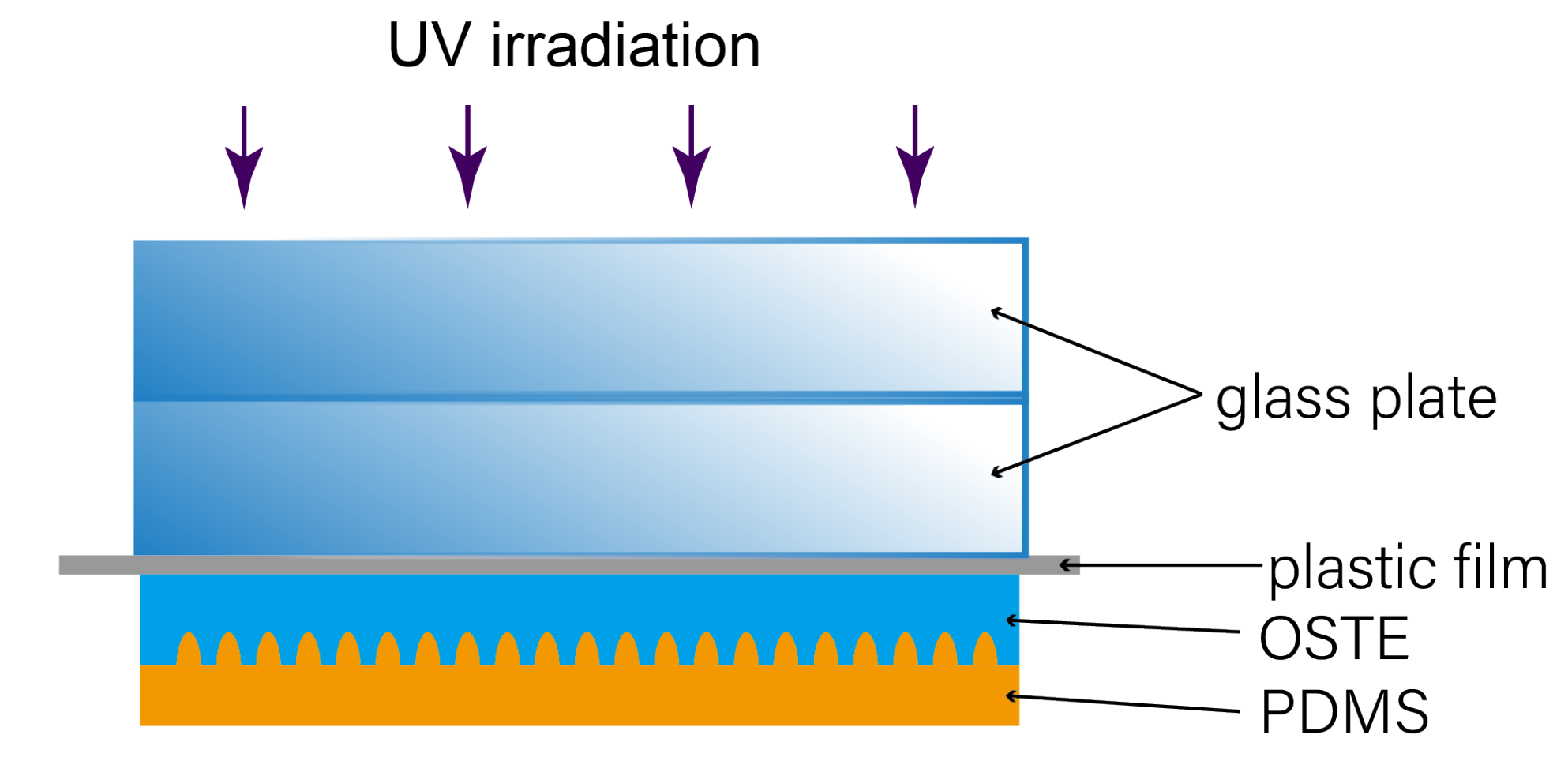
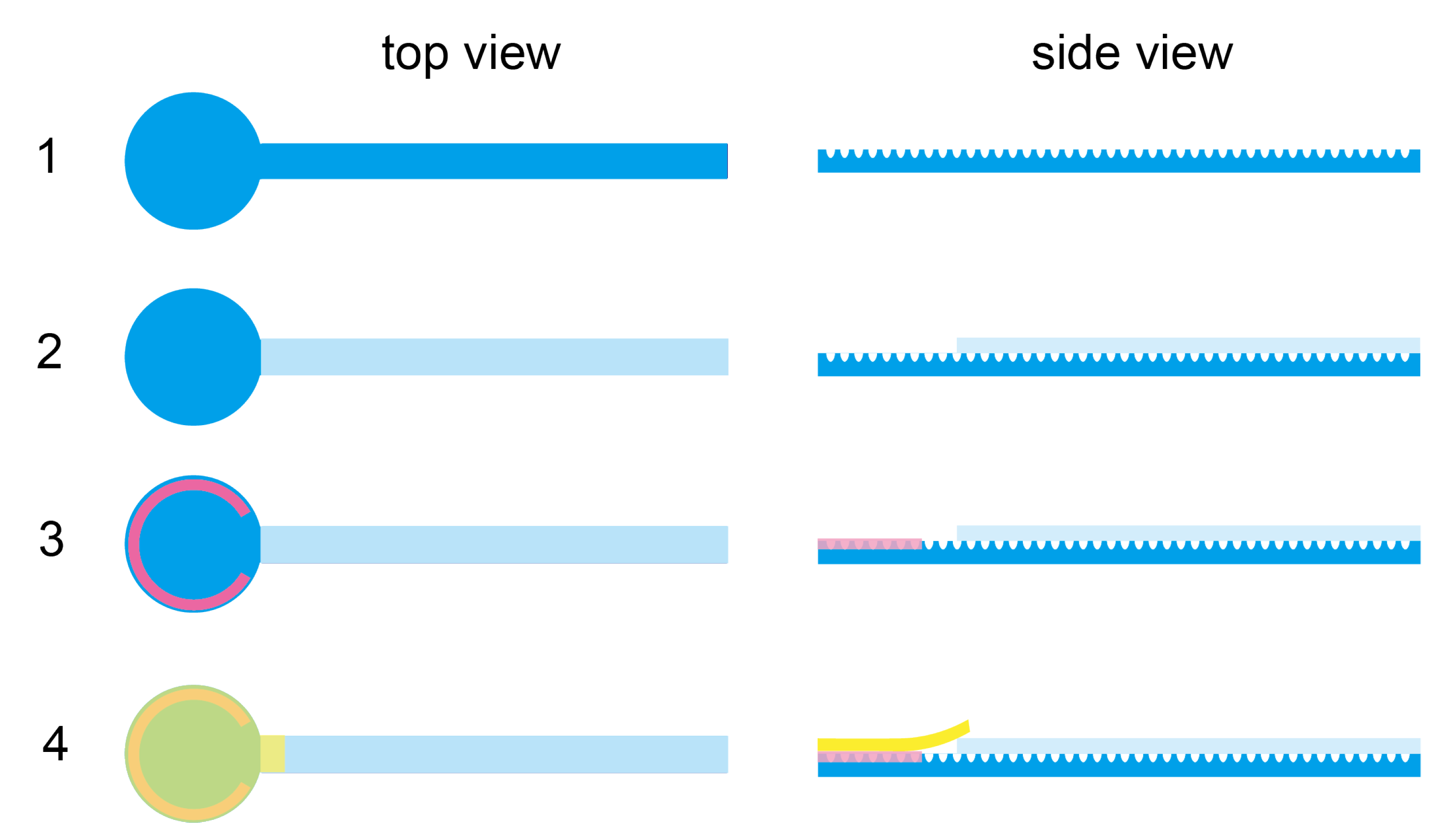
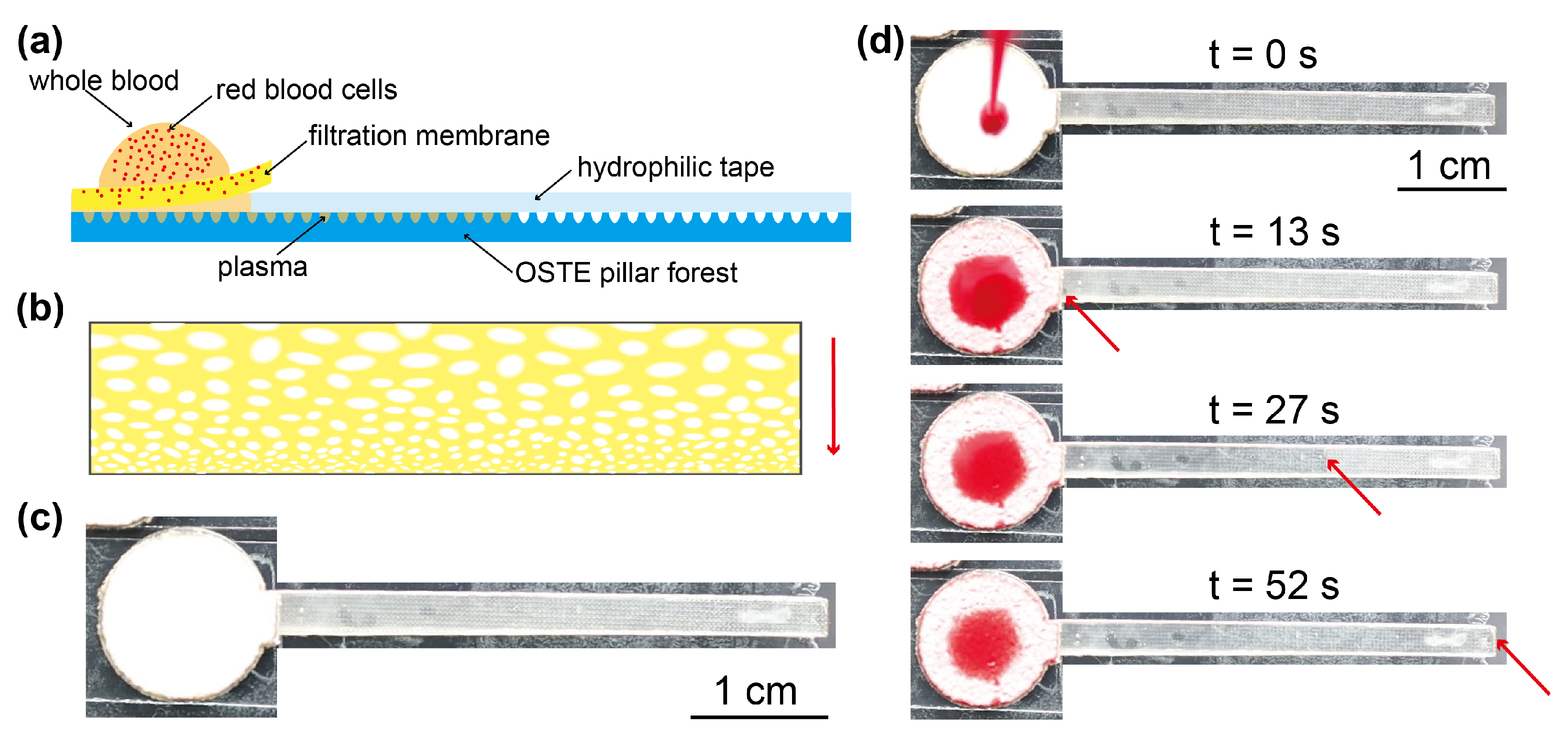
Appendix A.2. Assembly of the Microfluidic Device for Plasma Separation
Appendix A.3. Purity Check and Protein Concentration Measurement by BCA Assay
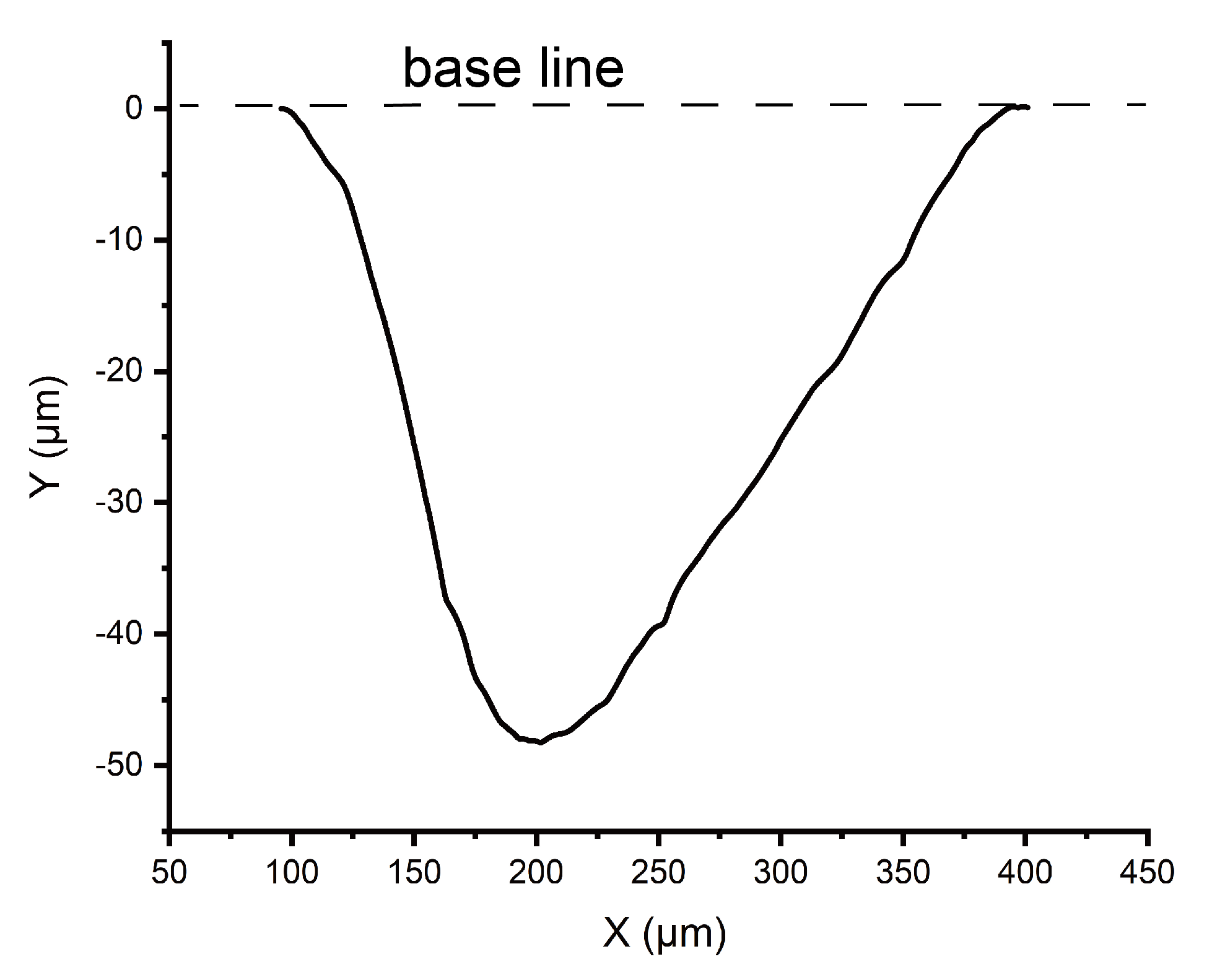
Appendix A.4. Appendix Profile of Micro Grooves on OSTE
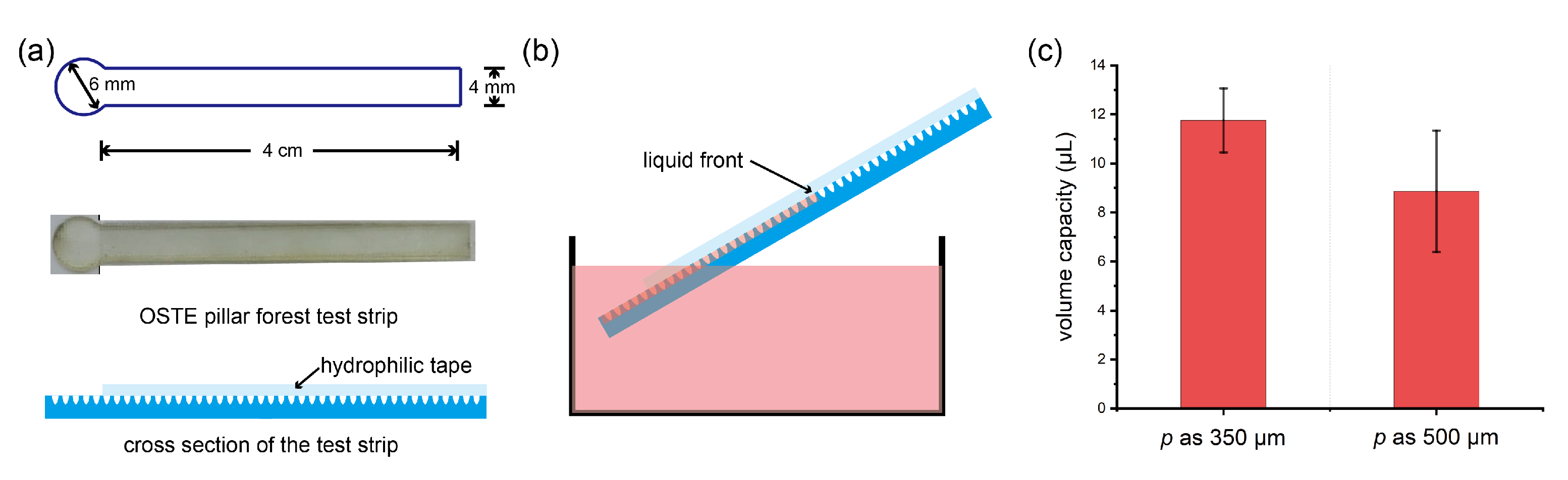
Appendix A.5. Appendix Volume Capacity of Water Absorption on OSTE Pillar Forest
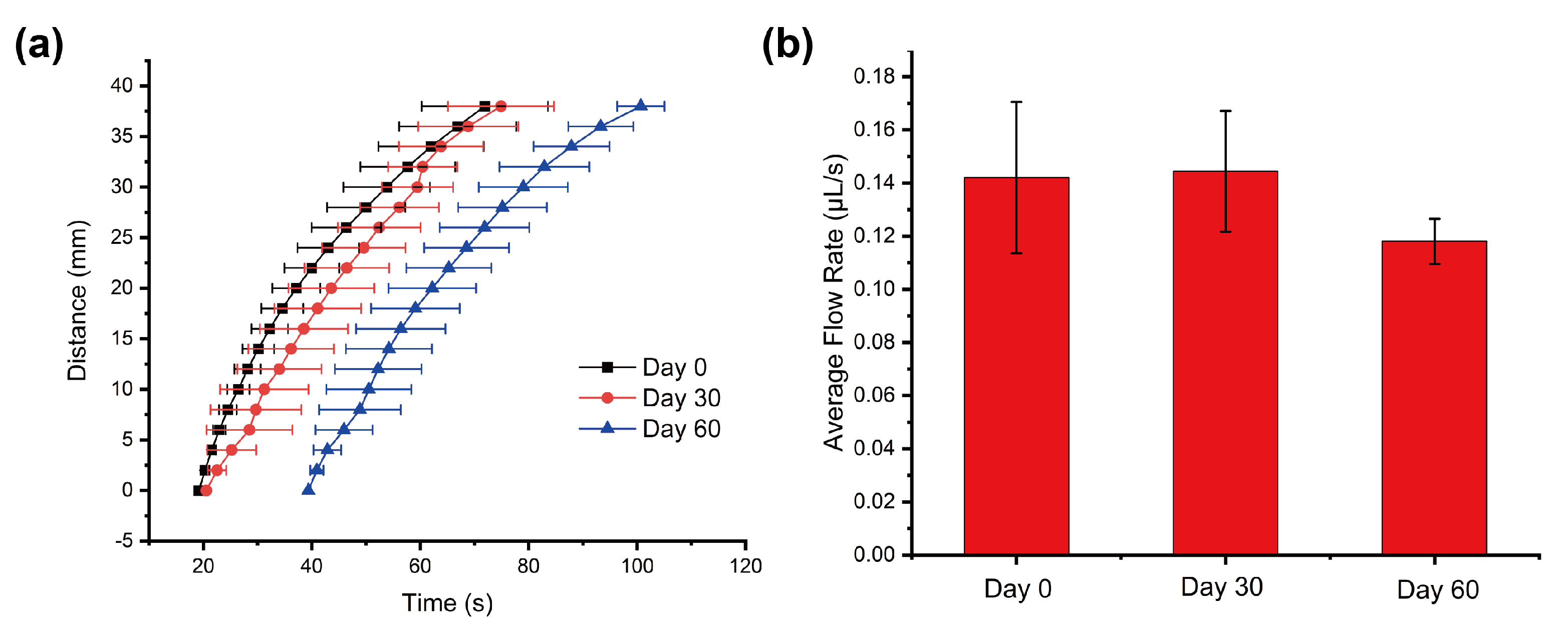
Appendix A.6. Appendix Shelf Life of Our Devices
Appendix A.7. Appendix Estimation of Cost of Our Device
| Material | Unit cost | Material per Device | Cost per Device (USD) |
|---|---|---|---|
| Blood filtration membrane | 0.000233 USD/mm | 165 mm | 0.0384 |
| Hydrophilic tape | 0.0000682 USD/mm | 120 mm | 0.00818 |
| Free nail glue | 0.0302 USD/mL | 0.01 mL | 0.000302 |
| OSTE | 0.127 USD/g | 1.43 g | 0.182 |
| Total: | 0.229 |
References
- Ma, C.B.; Zhang, Y.; Liu, Q.; Du, Y.; Wang, E. Enhanced stability of enzyme immobilized in rationally designed amphiphilic aerogel and its application for sensitive glucose detection. Anal. Chem. 2020, 92, 5319–5328. [Google Scholar] [CrossRef]
- Lu, Z.; O’Dell, D.; Srinivasan, B.; Rey, E.; Wang, R.; Vemulapati, S.; Mehta, S.; Erickson, D. Rapid diagnostic testing platform for iron and vitamin A deficiency. Proc. Natl. Acad. Sci. USA 2017, 114, 13513–13518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dejous, C.; Krishnan, U.M. Sensors for diagnosis of prostate cancer: Looking beyond the prostate specific antigen. Biosens. Bioelectron. 2021, 173, 112790. [Google Scholar] [CrossRef] [PubMed]
- Hauser, J.; Lenk, G.; Hansson, J.; Beck, O.; Stemme, G.; Roxhed, N. High-yield passive plasma filtration from human finger prick blood. Anal. Chem. 2018, 90, 13393–13399. [Google Scholar] [CrossRef]
- Tripathi, S.; Kumar, Y.B.; Agrawal, A.; Prabhakar, A.; Joshi, S.S. Microdevice for plasma separation from whole human blood using bio-physical and geometrical effects. Sci. Rep. 2016, 6, 1–15. [Google Scholar]
- Bilatto, S.E.; Adly, N.Y.; Correa, D.S.; Wolfrum, B.; Offenhäusser, A.; Yakushenko, A. Printed microfluidic filter for heparinized blood. Biomicrofluidics 2017, 11, 034101. [Google Scholar] [CrossRef] [Green Version]
- Rafeie, M.; Zhang, J.; Asadnia, M.; Li, W.; Warkiani, M.E. Multiplexing slanted spiral microchannels for ultra-fast blood plasma separation. Lab Chip 2016, 16, 2791–2802. [Google Scholar] [CrossRef]
- Amasia, M.; Madou, M. Large-volume centrifugal microfluidic device for blood plasma separation. Bioanalysis 2010, 2, 1701–1710. [Google Scholar] [CrossRef] [Green Version]
- Karthick, S.; Sen, A. Improved understanding of acoustophoresis and development of an acoustofluidic device for blood plasma separation. Phys. Rev. Appl. 2018, 10, 034037. [Google Scholar] [CrossRef]
- Nakashima, Y.; Hata, S.; Yasuda, T. Blood plasma separation and extraction from a minute amount of blood using dielectrophoretic and capillary forces. Sens. Actuators B Chem. 2010, 145, 561–569. [Google Scholar] [CrossRef]
- Wong, A.P.; Gupta, M.; Shevkoplyas, S.S.; Whitesides, G.M. Egg beater as centrifuge: Isolating human blood plasma from whole blood in resource-poor settings. Lab Chip 2008, 8, 2032–2037. [Google Scholar] [CrossRef]
- Jeong, S.W.; Park, Y.M.; Jo, S.H.; Lee, S.J.; Kim, Y.T.; Lee, K.G. Smartphone operable centrifugal system (SOCS) for on-site DNA extraction from foodborne bacterial pathogen. Biomicrofluidics 2019, 13, 034111. [Google Scholar] [CrossRef]
- Bhamla, M.S.; Benson, B.; Chai, C.; Katsikis, G.; Johri, A.; Prakash, M. Hand-powered ultralow-cost paper centrifuge. Nat. Biomed. Eng. 2017, 1, 1–7. [Google Scholar] [CrossRef]
- Maria, M.S.; Rakesh, P.; Chandra, T.; Sen, A. Capillary flow-driven microfluidic device with wettability gradient and sedimentation effects for blood plasma separation. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, J.H.; Lee, S.H.; Hong, S.; Park, S.M.; Lee, J.; Dickey, A.M.; Lee, L.P. Hemolysis-free blood plasma separation. Lab Chip 2014, 14, 2287–2292. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, A.; Shamloo, A.; Mohammadaliha, N.; Hajghassem, H.; Mehrabadi, J.F.; Bazzaz, M. High throughput blood plasma separation using a passive PMMA microfluidic device. Microsyst. Technol. 2016, 22, 2447–2454. [Google Scholar] [CrossRef]
- Guo, W.; Hansson, J.; van der Wijngaart, W. Synthetic Paper Separates Plasma from Whole Blood with Low Protein Loss. Anal. Chem. 2020, 92, 6194–6199. [Google Scholar] [CrossRef]
- Baillargeon, K.R.; Murray, L.P.; Deraney, R.N.; Mace, C.R. High-Yielding Separation and Collection of Plasma from Whole Blood Using Passive Filtration. Anal. Chem. 2020, 92, 16245–16252. [Google Scholar] [CrossRef]
- Tripathi, S.; Kumar, Y.B.V.; Prabhakar, A.; Joshi, S.S.; Agrawal, A. Passive blood plasma separation at the microscale: A review of design principles and microdevices. J. Micromech. Microeng. 2015, 25, 083001. [Google Scholar] [CrossRef]
- Lu, Z.; Rey, E.; Vemulapati, S.; Srinivasan, B.; Mehta, S.; Erickson, D. High-yield paper-based quantitative blood separation system. Lab Chip 2018, 18, 3865–3871. [Google Scholar] [CrossRef]
- Hale, R.; Ranjan, R.; Hidrovo, C. Capillary flow through rectangular micropillar arrays. Int. J. Heat Mass Transf. 2014, 75, 710–717. [Google Scholar] [CrossRef]
- Xiao, R.; Enright, R.; Wang, E.N. Prediction and optimization of liquid propagation in micropillar arrays. Langmuir 2010, 26, 15070–15075. [Google Scholar] [CrossRef]
- Kim, J.; Moon, M.W.; Lee, K.R.; Mahadevan, L.; Kim, H.Y. Hydrodynamics of writing with ink. Phys. Rev. Lett. 2011, 107, 264501. [Google Scholar] [CrossRef]
- Holzner, G.; Kriel, F.H.; Priest, C. Pillar cuvettes: Capillary-filled, microliter quartz cuvettes with microscale path lengths for optical spectroscopy. Anal. Chem. 2015, 87, 4757–4764. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, C.; Aronsson, M.; Rundström, G.; Pettersson, C.; Mendel-Hartvig, I.; Bakker, J.; Martinsson, E.; Liedberg, B.; MacCraith, B.; Öhman, O.; et al. Silane–dextran chemistry on lateral flow polymer chips for immunoassays. Lab Chip 2008, 8, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Melin, J.; Rundström, G.; Peterson, C.; Bakker, J.; MacCraith, B.D.; Read, M.; Öhman, O.; Jönsson, C. A multiplexed point-of-care assay for C-reactive protein and N-terminal pro-brain natriuretic peptide. Anal. Biochem. 2011, 409, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.M.; Gandhiraman, R.; Volcke, C.; Cafolla, A.A.; Daniels, S.; Killard, A.J. Plasma surface modification of cyclo-olefin polymers and its application to lateral flow bioassays. Langmuir 2009, 25, 11155–11161. [Google Scholar] [CrossRef] [PubMed]
- Carlborg, C.F.; Haraldsson, T.; Öberg, K.; Malkoch, M.; Van Der Wijngaart, W. Beyond PDMS: Off-stoichiometry thiol–ene (OSTE) based soft lithography for rapid prototyping of microfluidic devices. Lab Chip 2011, 11, 3136–3147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.; Gustafsson, L.; Jansson, R.; Hedhammar, M.; van der Wijngaart, W. Formation of a thin-walled spider silk tube on a micromachined scaffold. In Proceedings of the 2018 IEEE Micro Electro Mechanical Systems (MEMS), Belfast, UK, 21–25 January 2018; pp. 83–85. [Google Scholar]
- Guo, W.; Hansson, J.; Gustafsson, L.; van der Wijngaart, W. “Bend-and-Bond” Polymer Microfluidic Origami. In Proceedings of the 2021 IEEE 34th International Conference on Micro Electro Mechanical Systems (MEMS), Gainesville, FL, USA, 25–29 January 2021; pp. 222–225. [Google Scholar]
- Guo, W.; Vilaplana, L.; Hansson, J.; Marco, M.P.; van Der Wijngaart, W. Immunoassays on thiol-ene synthetic paper generate a superior fluorescence signal. Biosens. Bioelectron. 2020, 163, 112279. [Google Scholar] [CrossRef]
- Guo, W.; Hansson, J.; van der Wijngaart, W. Synthetic microfluidic paper with superior fluorescent signal readout. In Proceedings of the the 23rd International Conference on Miniaturized Systems for Chemistry and Life Sciences (μTAS 2019), Basel, Switzerland, 27–31 October 2019; pp. 1056–1057. [Google Scholar]
- Zandi Shafagh, R.; Vastesson, A.; Guo, W.; Van Der Wijngaart, W.; Haraldsson, T. E-beam nanostructuring and direct click biofunctionalization of thiol–ene resist. ACS Nano 2018, 12, 9940–9946. [Google Scholar] [CrossRef]
- Hansson, J.; Yasuga, H.; Haraldsson, T.; Van der Wijngaart, W. Synthetic microfluidic paper: High surface area and high porosity polymer micropillar arrays. Lab Chip 2016, 16, 298–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alda, J. Laser and Gaussian beam propagation and transformation. Encycl. Opt. Eng. 2003, 999–1013. [Google Scholar]
- Yang, S.; Ündar, A.; Zahn, J.D. A microfluidic device for continuous, real time blood plasma separation. Lab Chip 2006, 6, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Kersaudy-Kerhoas, M.; Kavanagh, D.M.; Dhariwal, R.S.; Campbell, C.J.; Desmulliez, M.P. Validation of a blood plasma separation system by biomarker detection. Lab Chip 2010, 10, 1587–1595. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Liu, C.; Xu, Z.; Li, J. Extraction of plasma from whole blood using a deposited microbead plug (DMBP) in a capillary-driven microfluidic device. Biomed. Microdevices 2012, 14, 565–572. [Google Scholar] [CrossRef]
- Washburn, E.W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273. [Google Scholar] [CrossRef]
- Guo, W.; Hansson, J.; van der Wijngaart, W. Capillary pumping independent of liquid sample viscosity. Langmuir 2016, 32, 12650–12655. [Google Scholar] [CrossRef]
- Xiao, Z.; Yang, Y.; Zhang, X.; Guo, W. Controlling Capillary Flow Rate on Lateral Flow Test Substrates by Tape. Micromachines 2021, 12, 562. [Google Scholar] [CrossRef]
- Frantz, E.; Li, H.; Steckl, A.J. Quantitative hematocrit measurement of whole blood in a point-of-care lateral flow device using a smartphone flow tracking app. Biosens. Bioelectron. 2020, 163, 112300. [Google Scholar] [CrossRef]
- Gao, Q.; Chang, Y.; Deng, Q.; You, H. A simple and rapid method for blood plasma separation driven by capillary force with an application in protein detection. Anal. Methods 2020, 12, 2560–2570. [Google Scholar] [CrossRef]
- Dudek, M.M.; Gandhiraman, R.P.; Volcke, C.; Daniels, S.; Killard, A.J. Evaluation of a range of surface modifications for the enhancement of lateral flow assays on cyclic polyolefin micropillar devices. Plasma Process. Polym. 2009, 6, 620–630. [Google Scholar] [CrossRef]
- Samy, R.; Sen, A. Elastocapillary flow driven lab-on-a-membrane device based on differential wetting and sedimentation effect for blood plasma separation. J. Micromech. Microeng. 2019, 29, 065001. [Google Scholar] [CrossRef]
- Kuo, J.N.; Zhan, Y.H. Microfluidic chip for rapid and automatic extraction of plasma from whole human blood. Microsyst. Technol. 2015, 21, 255–261. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, W.; Li, H.; Wu, W.; Wang, W. A 3D filter for plasma separation from whole blood. In Proceedings of the 2017 19th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Kaohsiung, Taiwan, 18–22 June 2017; pp. 564–567. [Google Scholar]
| Reference | Methods | Hematocrit | Separation Yield | Protein Recovery Rate | Volume/Time (Process Rate) |
|---|---|---|---|---|---|
| Gao et al. [43] | Membrane filter | 45% | 71.70% | 82.3% | 60 L/360 s |
| Samy et al. [45] | Wetting and Sedimentation | - | 22–49% | - | - |
| Shamsi et al. [16] | Zweifach–Fung effect | - | 66.6 % | - | - |
| Kuo et al. [46] | Fishbone filtration | 45% | 15% | - | 10 L/75 s |
| Baillargeon et al. [18] | Membrane filter | 30% | 53.8% | - | - |
| Liu et al. [47] | 3D Parylene filter | - | 42% | - | 2000 L/300 s |
| Son et al. [15] | Membrane filter | 38% | 20% | 89% | - |
| Maria et al. [14] | Wetting and Sedimentation | 45% | - | - | 10 L/900 s |
| Our device | Membrane filter | 30.4–56.1% | 60.0% | 85.5% | 45 L/72 s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Z.; Sun, L.; Yang, Y.; Feng, Z.; Dai, S.; Yang, H.; Zhang, X.; Sheu, C.-L.; Guo, W. High-Performance Passive Plasma Separation on OSTE Pillar Forest. Biosensors 2021, 11, 355. https://doi.org/10.3390/bios11100355
Xiao Z, Sun L, Yang Y, Feng Z, Dai S, Yang H, Zhang X, Sheu C-L, Guo W. High-Performance Passive Plasma Separation on OSTE Pillar Forest. Biosensors. 2021; 11(10):355. https://doi.org/10.3390/bios11100355
Chicago/Turabian StyleXiao, Zhiqing, Lexin Sun, Yuqian Yang, Zitao Feng, Sihan Dai, Hao Yang, Xingwei Zhang, Chia-Lin Sheu, and Weijin Guo. 2021. "High-Performance Passive Plasma Separation on OSTE Pillar Forest" Biosensors 11, no. 10: 355. https://doi.org/10.3390/bios11100355
APA StyleXiao, Z., Sun, L., Yang, Y., Feng, Z., Dai, S., Yang, H., Zhang, X., Sheu, C.-L., & Guo, W. (2021). High-Performance Passive Plasma Separation on OSTE Pillar Forest. Biosensors, 11(10), 355. https://doi.org/10.3390/bios11100355






