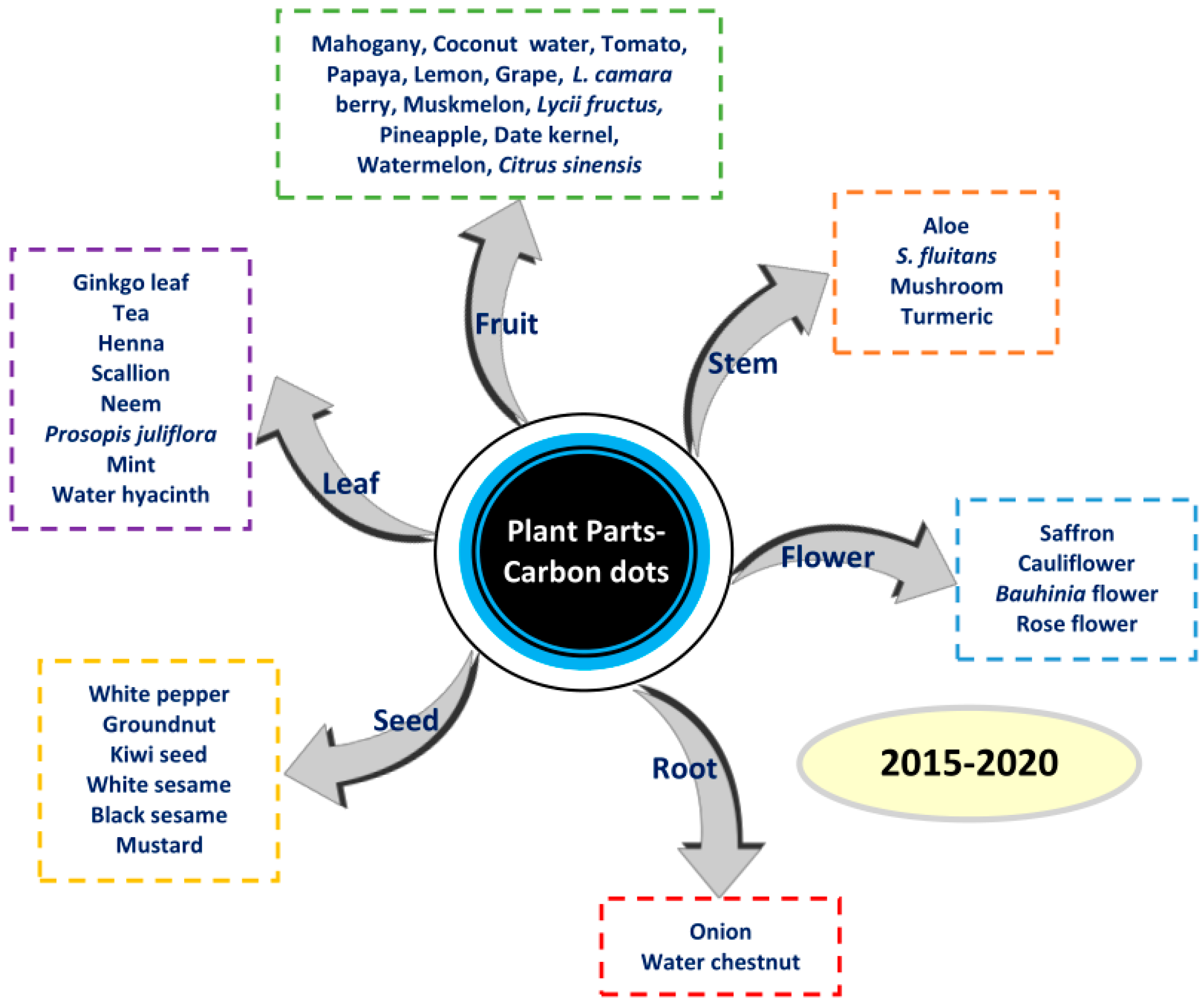
Plant Part-Derived Carbon Dots for Biosensing
Abstract
1. Introduction
2. Synthesis Methods for CDs Using Plant Parts
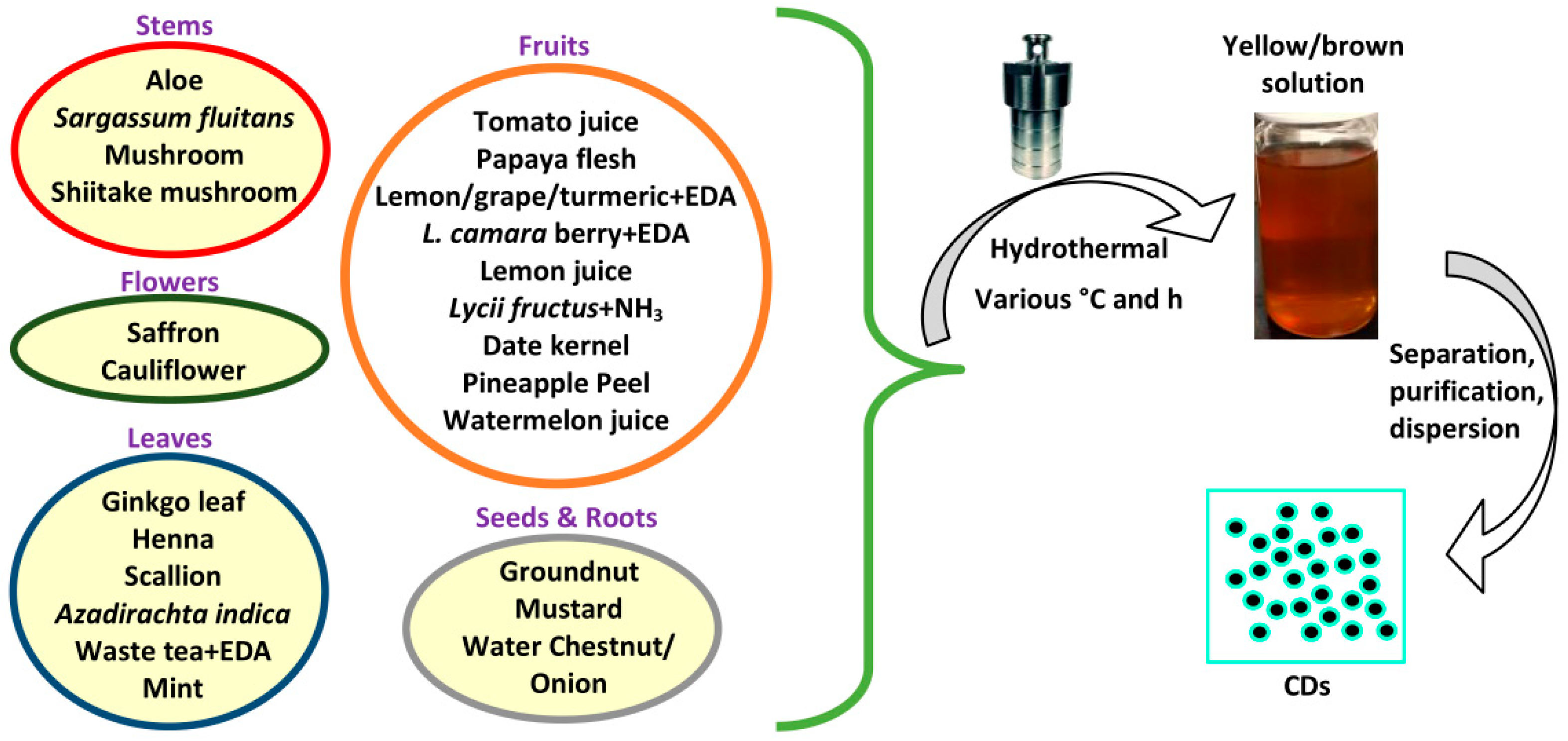
2.1. Hydrothermal Method
2.2. Solvothermal Method
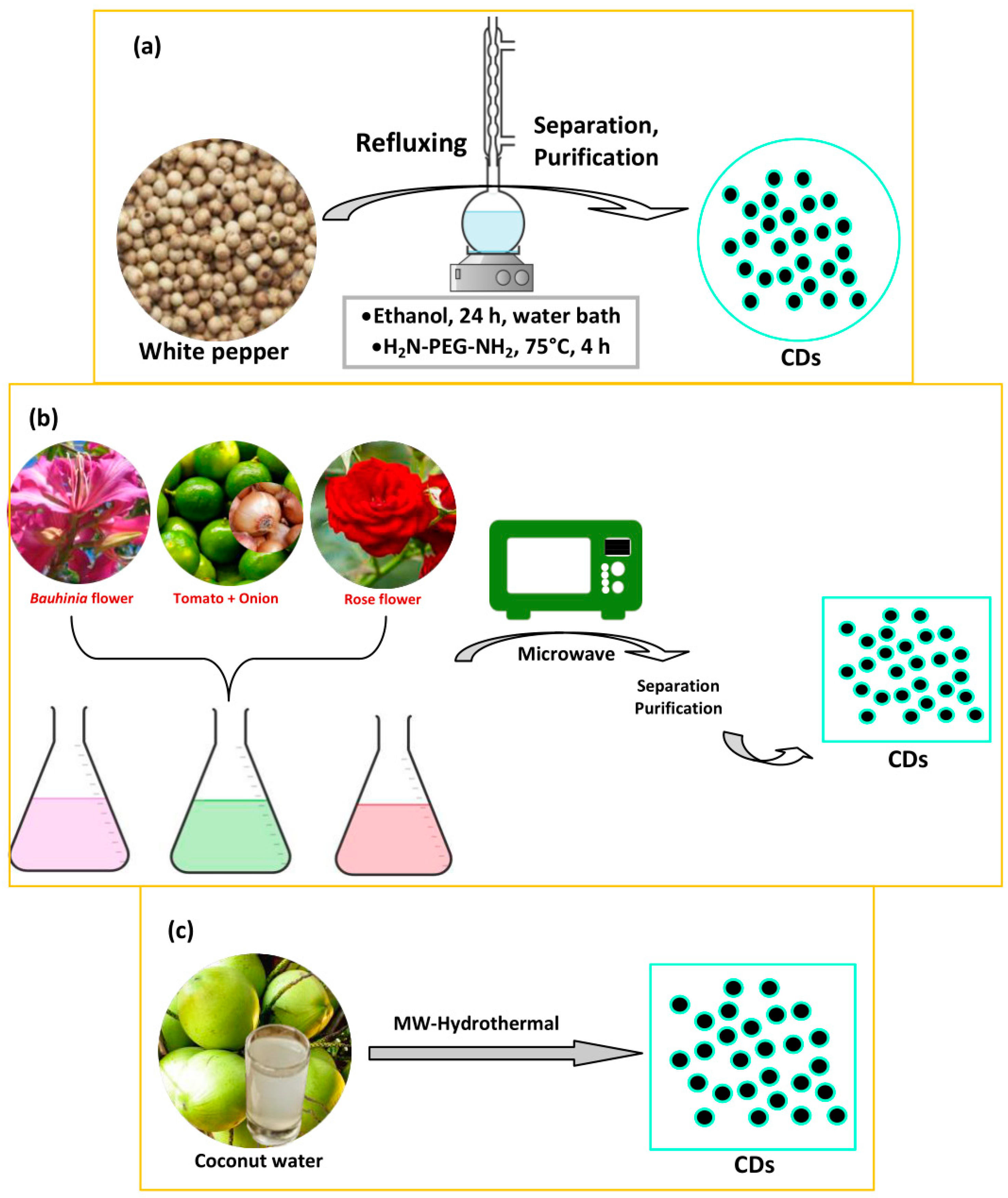
2.3. Microwave Treatment
2.4. Microwave-Assisted Hydrothermal Method
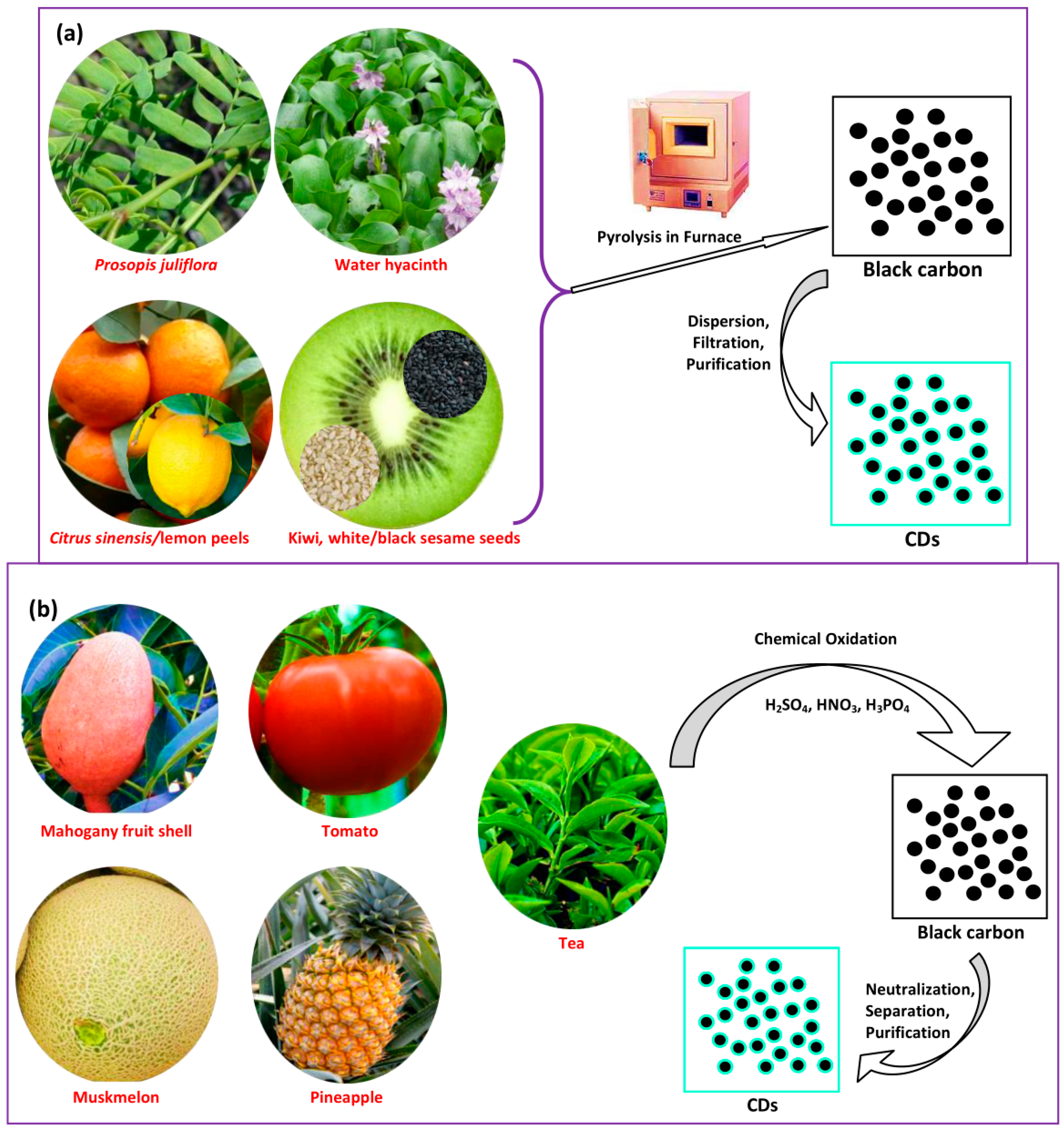
2.5. Pyrolysis Treatment
2.6. Chemical Oxidation
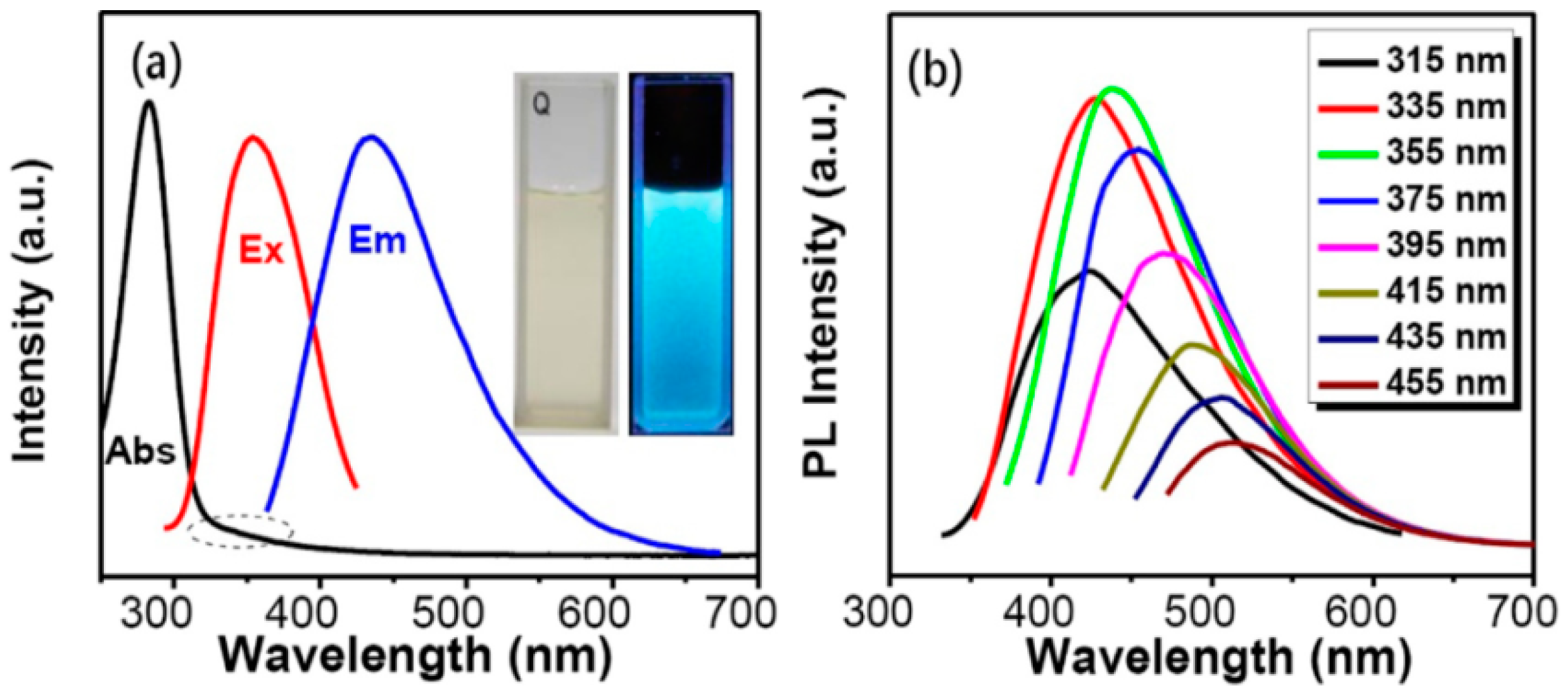
3. Optical Properties of Plant Part-Derived CDs
3.1. UV-vis Absorbance and Emission Color
3.2. Fluorescence and Quantum Yield
4. Biosensing Applications of Plant Part-Derived CDs
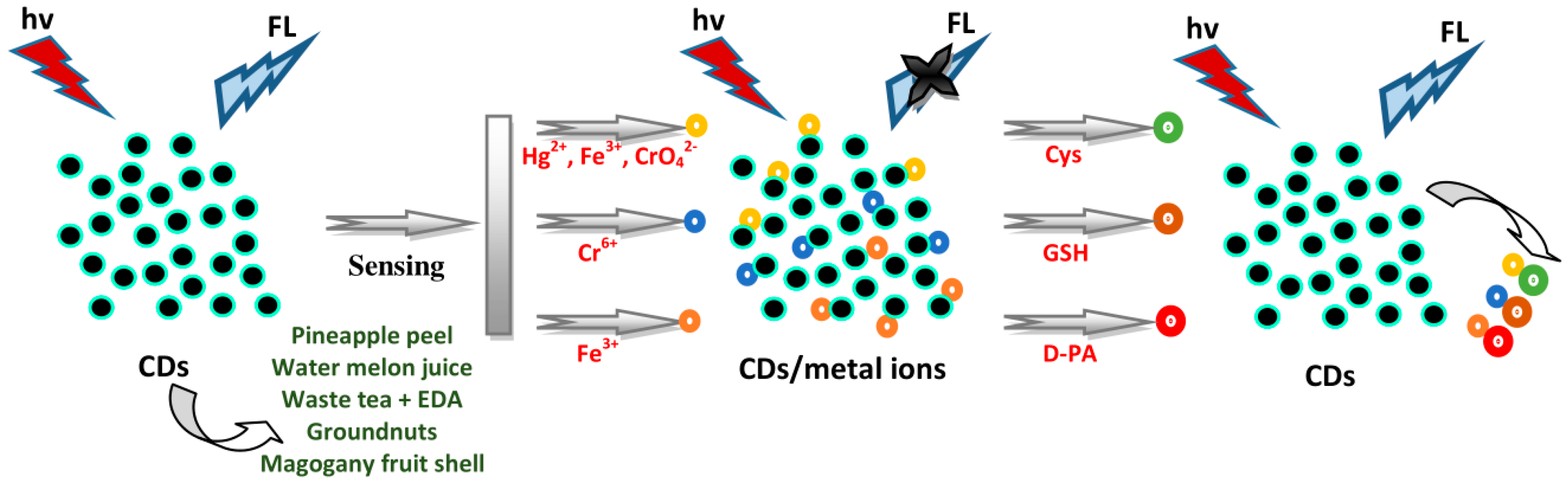
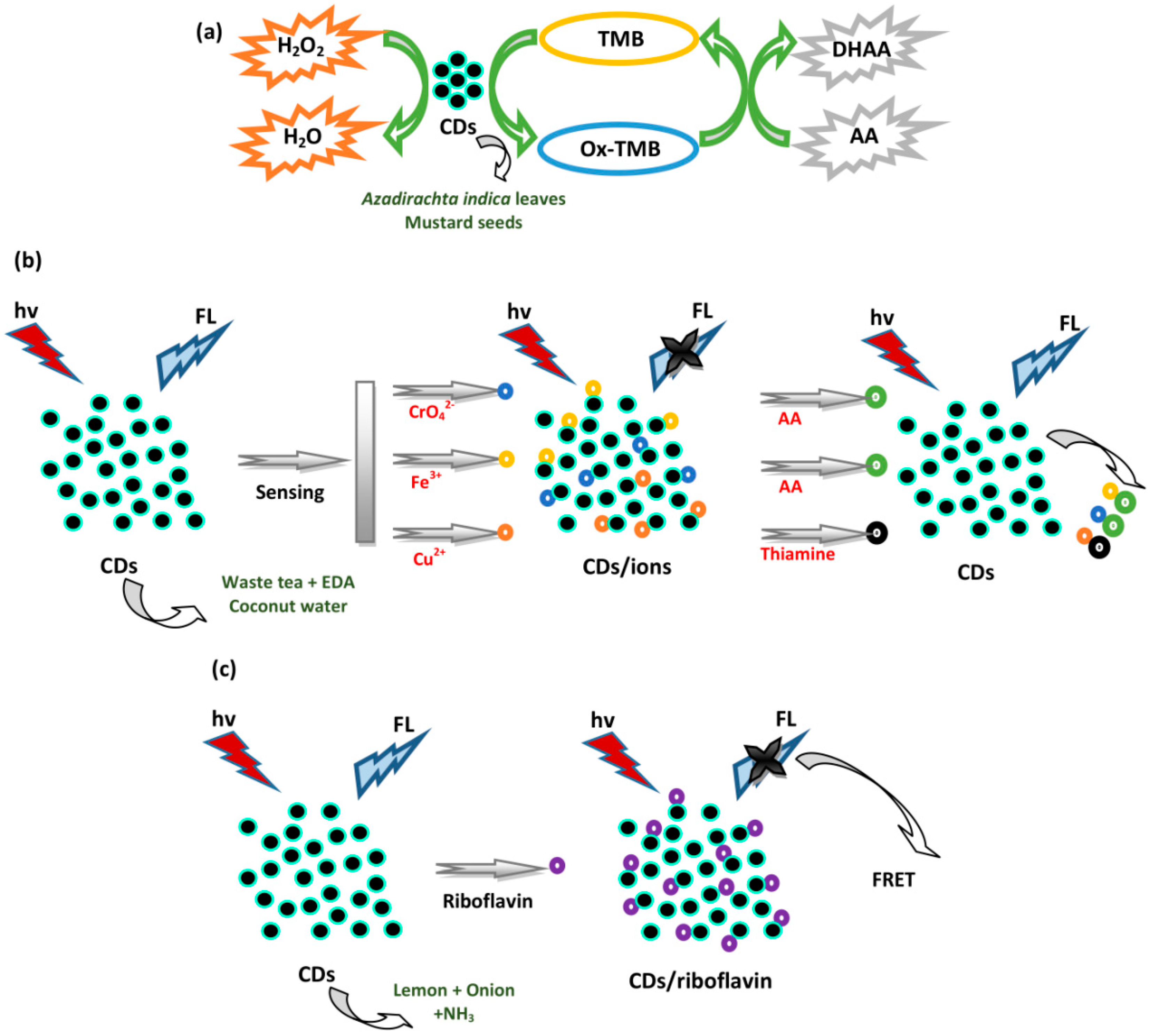
4.1. Sensing of Amino Acids and Thiols
4.2. Sensing of Vitamins
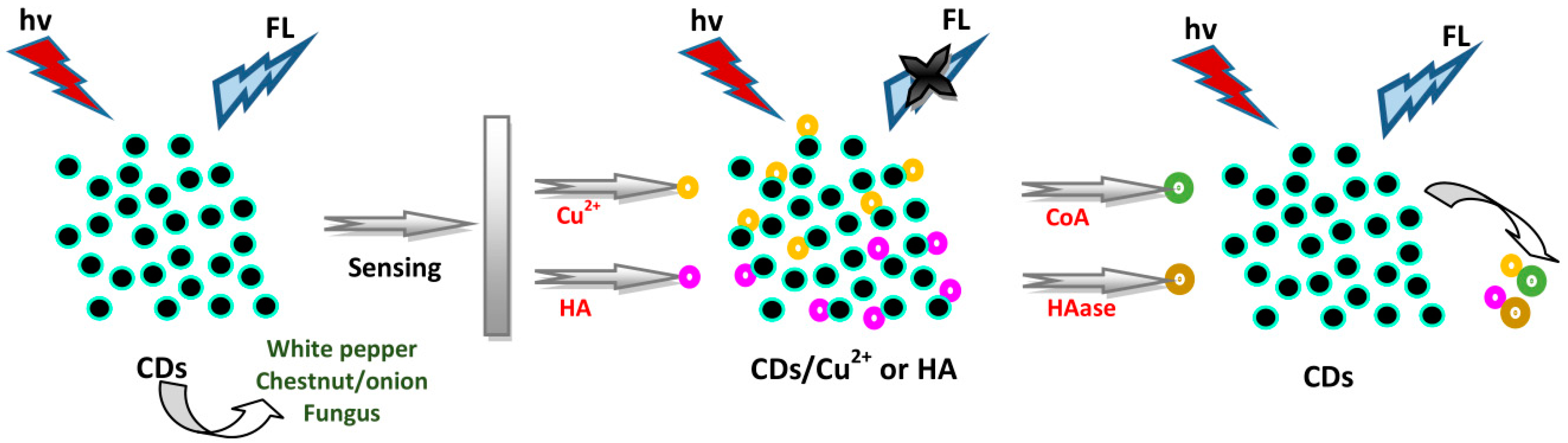
4.3. Sensing of Enzymes
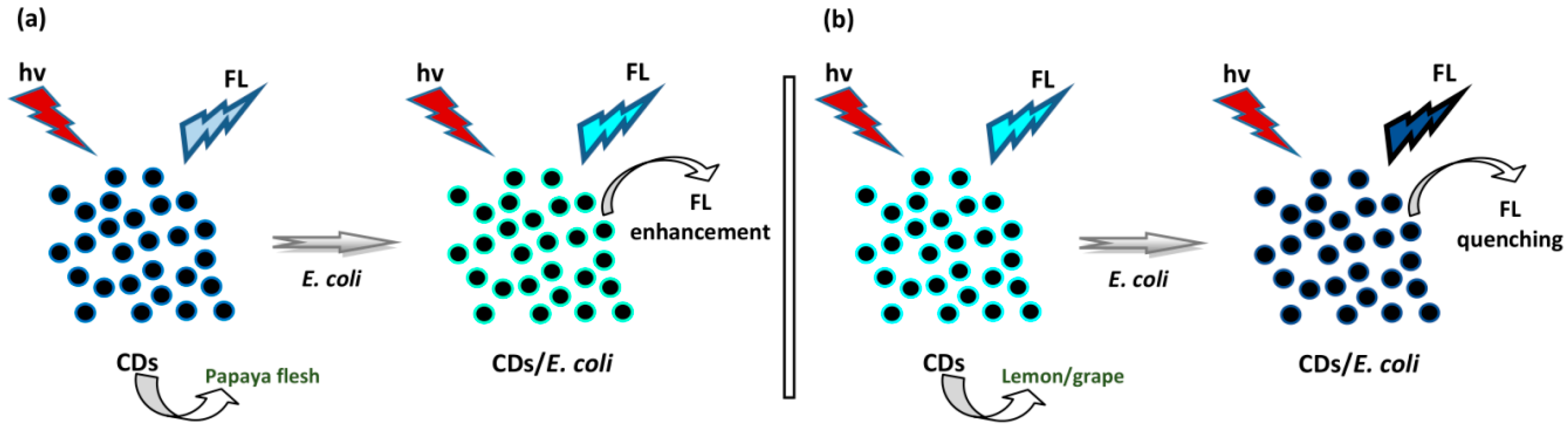
4.4. Sensing of Bacteria
4.5. Sensing of Bio-Pigments
4.6. Sensing of Nucleic Acids and Proteins
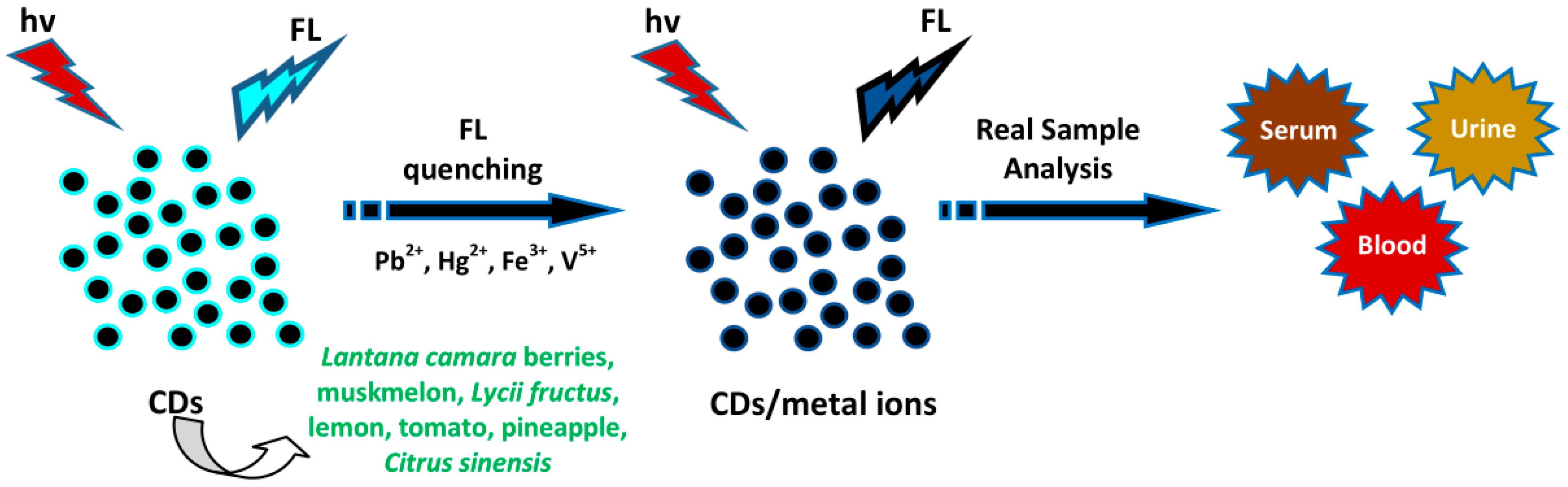
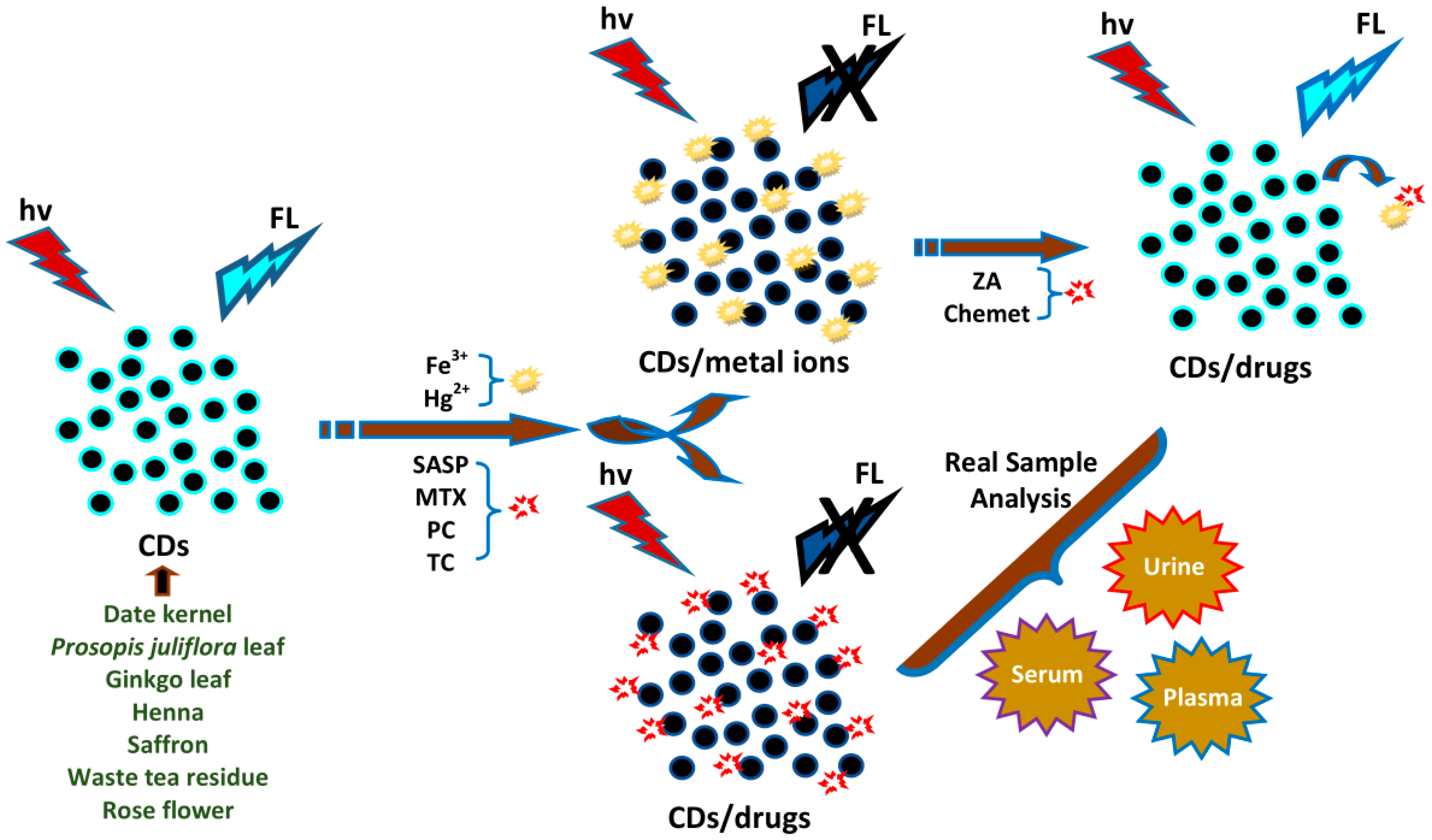
4.7. Sensing of Metal Ions
4.8. Sensing of Drugs
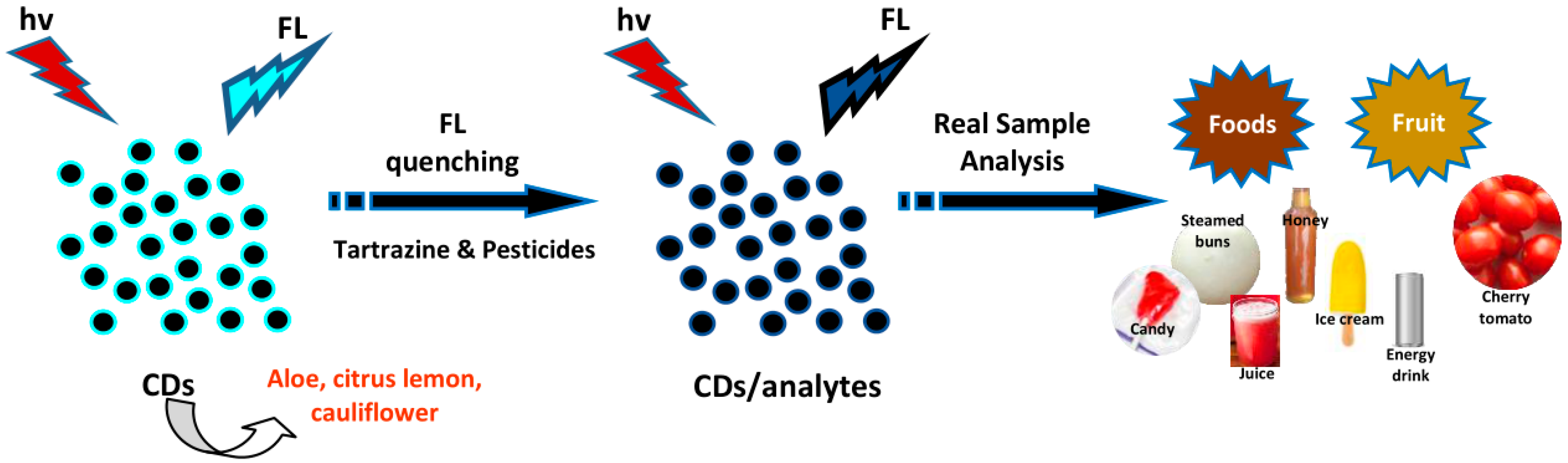
4.9. Sensing of Dyes and Pesticides
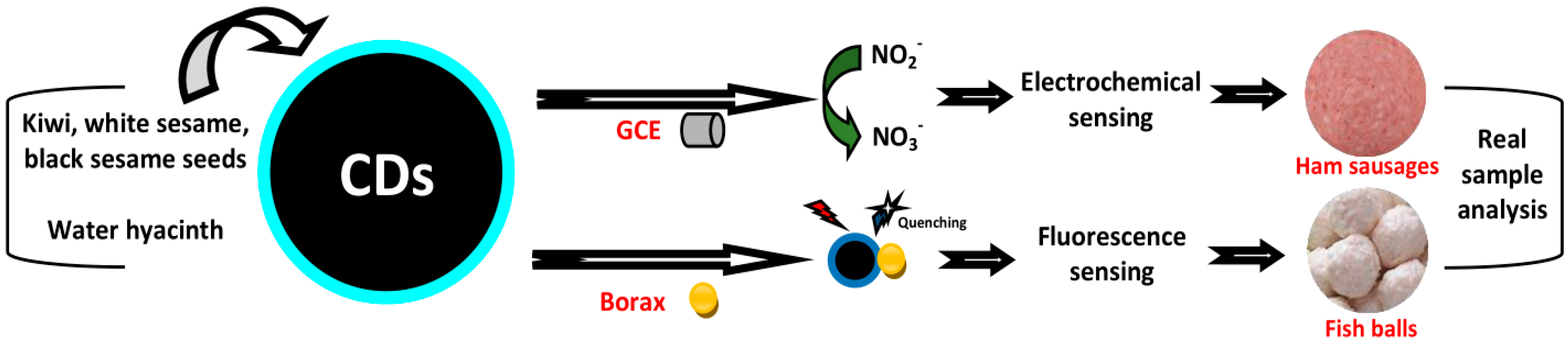
4.10. Sensing of Nitrite and Borax
5. Summary and Perspectives
- CDs should be synthesized from other low-cost waste plant parts or other natural sources.
- In addition to the hydrothermal method, other synthesis methods could be useful for synthesizing plant part-derived CDs.
- The utilization of solid waste residues after separating CDs is needed to explore more beneficial materials.
- New natural resources containing N, S, P, or other heteroatom elements should be explored more in the future.
- An effective synthesis method with a high QY should be developed to fabricate CDs.
- The exploration of plant part-derived CDs for other biomolecules should be investigated.
- The large precursor compositions might lead to heterogeneity in plant part-derived CDs and a focused investigation of separation and purification is required.
- There is no literature on the in vivo application of plant part-derived CDs to detect biologically important small molecules in exposed living things. Therefore, increased attention is required in this field.
Author Contributions
Funding
Conflicts of Interest
References
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Ju, B.; Wang, Y.; Zhang, Y.-M.; Zhang, T.; Liu, Z.; Li, M.; Zhang, S.X.-A. Photostable and low-toxic yellow-green carbon dots for highly selective detection of explosive 2,4,6-Trinitrophenol based on the dual electron transfer mechanism. ACS Appl. Mater. Interfaces 2018, 10, 13040–13047. [Google Scholar] [CrossRef]
- Zhao, P.; Zhu, L. Dispersibility of carbon dots in aqueous and/or organic solvents. Chem. Commun. 2018, 54, 5401–5406. [Google Scholar] [CrossRef]
- Liu, W.; Li, C.; Ren, Y.; Sun, X.; Pan, W.; Li, Y.; Wang, W.; Wang, W. Carbon dots: Surface engineering and applications. J. Mater. Chem. B 2016, 4, 5772–5788. [Google Scholar] [CrossRef]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835–8837. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Yu, S.; Jiang, C. Fluorescent carbon dots: Rational synthesis, tunable optical properties and analytical applications. RSC Adv. 2017, 7, 40973–40989. [Google Scholar] [CrossRef]
- Sun, X.; Lei, Y. Fluorescent carbon dots and their sensing applications. TrAC Trends Anal. Chem. 2017, 89, 163–180. [Google Scholar] [CrossRef]
- Abdelhamidab, H.N.; Chen, Z.-Y.; Wu, H.-F. Surface tuning laser desorption/ionization mass spectrometry (STLDI-MS) for the analysis of small molecules using quantum dots. Anal. Bioanal. Chem. 2017, 409, 4943–4950. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Abdelhamidab, H.N.; Wu, H.-F. Effect of surface capping of quantum dots (CdTe) on proteomics. Rapid Commun. Mass Spectrom. 2016, 30, 1403–1412. [Google Scholar] [CrossRef]
- Abdelhamidab, H.N.; Wu, H.-F. Synthesis and characterization of quantum dots for application in laser soft desorption/ionization mass spectrometry to detect labile metal–drug interactions and their antibacterial activity. RSC Adv. 2015, 5, 76107–76115. [Google Scholar] [CrossRef]
- Abdelhamidab, H.N.; Wu, H.-F. Synthesis and multifunctional applications of quantum nanobeads for label-free and selective metal chemosensing. RSC Adv. 2015, 5, 50494–50504. [Google Scholar] [CrossRef]
- Abdelhamidab, H.N.; Wu, H.-F. Monitoring metallofulfenamic–bovine serum albumin interactions: A novel method for metallodrug analysis. RSC Adv. 2014, 4, 53768–53776. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Park, S.-J.; Zhang, Y.; Kim, T.; Chae, S.; Park, M.; Kim, H.Y. One-step synthesis of robust nitrogen-doped carbon dots: Acid-evoked fluorescence enhancement and their application in Fe3+ detection. J. Mater. Chem. A 2015, 3, 17747–17754. [Google Scholar] [CrossRef]
- Perikala, M.; Bhardwaj, A. Highly stable white-light-emitting carbon dot synthesis using a non-coordinating solvent. ACS Omega 2019, 4, 21223–21229. [Google Scholar] [CrossRef]
- Fiuza, T.; Gomide, G.; Campos, A.F.C.; Messina, F.; Depeyrot, J. On the colloidal stability of nitrogen-rich carbon nanodots aqueous dispersions. J. Carbon Res. 2019, 5, 74. [Google Scholar] [CrossRef]
- Wang, W.; Damm, C.; Walter, J.; Nacken, T.J.; Peukert, W. Photobleaching and stabilization of carbon nanodots produced by solvothermal synthesis. Phys. Chem. Chem. Phys. 2016, 18, 466–475. [Google Scholar] [CrossRef]
- Dias, C.; Vasimalai, N.; Passos, M.; Pinheiro, I.; Vilas-Boas, V.; Peixoto, J.; Espiña, B. Biocompatibility and bioimaging potential of fruit-based carbon dots. Nanomaterials 2019, 9, 199. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Talib, A.; Wu, H.-F. One pot synthesis of gold—Carbon dots nanocomposite and its application for cytosensing of metals for cancer cells. Talanta 2017, 166, 357–363. [Google Scholar] [CrossRef]
- Li, H.; Yan, X.; Kong, D.; Jin, R.; Sun, C.; Dub, D.; Linb, Y.; Lu, G.; Xu, Y. Recent advances in carbon dots for bioimaging applications. Nanoscale Horiz. 2020, 5, 218–234. [Google Scholar] [CrossRef]
- Pardo, J.; Peng, Z.; Zhou, Y. Cancer targeting and drug delivery using carbon-based quantum dots and nanotubes. Molecules 2018, 23, 378. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.; Beach, E. Green chemistry: The emergence of a transformative framework. Green Chem. Lett. Rev. 2007, 1, 9–24. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Zhang, Y.; Qin, X.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Hydrothermal treatment of grass: A low-cost, green route to nitrogen-doped, carbon-rich, photoluminescent polymer nanodots as an effective fluorescent sensing platform for label-free detection of Cu(II) ions. Adv. Mater. 2012, 24, 2037–2041. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Bai, X.; Wang, B.; Liu, Z.; Lu, S.; Yang, B. Biomass-derived carbon dots and their applications. Energy Environ. Mater. 2019, 2, 172–192. [Google Scholar] [CrossRef]
- Zhao, S.; Lan, M.; Zhu, X.; Xue, H.; Ng, T.-W.; Meng, X.; Lee, C.; Wang, P.; Zhang, W. Green synthesis of bifunctional fluorescent carbon dots from garlic for cellular imaging and free radical scavenging. ACS Appl. Mater. Interfaces 2015, 7, 17054–17060. [Google Scholar] [CrossRef]
- Sharma, V.; Tiwari, P.; Mobin, S.M. Sustainable carbon-dots: Recent advances in green carbon dots for sensing and bioimaging. J. Mater. Chem. B 2017, 5, 8904–8924. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Kharisov, B.I.; González, C.M.O.; Méndez, Y.P.; López, I. Greener synthesis of chemical compounds and materials. R. Soc. Open Sci. 2019, 6. [Google Scholar] [CrossRef]
- Wang, R.; Lu, K.-Q.; Tang, Z.-R.; Xu, Y.-J. Recent progress in carbon quantum dots: Synthesis, properties and applications in photocatalysis. J. Mater. Chem. A 2017, 5, 3717–3734. [Google Scholar] [CrossRef]
- Zulfajri, M.; Gedda, G.; Chang, C.-J.; Chang, Y.-P.; Huang, G.G. Cranberry beans derived carbon dots as a potential fluorescence sensor for selective detection of Fe3+ ions in aqueous solution. ACS Omega 2019, 4, 15382–15392. [Google Scholar] [CrossRef]
- Zulfajri, M.; Dayalan, S.; Li, W.-Y.; Huang, G.G.; Chang, C.-J.; Chang, Y.-P. Nitrogen-doped carbon dots from averrhoa carambola fruit extract as a fluorescent probe for methyl orange. Sensors 2019, 19, 5008. [Google Scholar] [CrossRef]
- Namdari, P.; Negahdari, B.; Eatemadi, A. Synthesis, properties and biomedical applications of carbon-based quantum dots: An updated review. Biomed. Pharmacother. 2017, 87, 209–222. [Google Scholar] [CrossRef]
- Atabaev, T.S. Doped carbon dots for sensing and bioimaging applications: A minireview. Nanomaterials 2018, 8, 342. [Google Scholar] [CrossRef]
- Qu, J.-H.; Wei, Q.; Sun, D. Carbon dots: Principles and their applications in food quality and safety detection. Crit. Rev. Food Sci. Nutr. 2018, 58, 2466–2475. [Google Scholar] [CrossRef]
- Feng, H.; Qian, Z. Functional carbon quantum dots: A versatile platform for chemosensing and biosensing. Chem. Rec. 2017, 18, 491–505. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, J. A review of carbon dots in biological applications. J. Mater. Sci. 2016, 51, 4728–4738. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, S.-H. Carbon dots: Large-scale synthesis, sensing and bioimaging. Mater. Today 2016, 19, 382–393. [Google Scholar] [CrossRef]
- Shi, H.; Wei, J.; Qiang, L.; Chen, X.; Meng, X. Fluorescent carbon dots for bioimaging and biosensing applications. J. Biomed. Nanotechnol. 2014, 10, 2677–2699. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon Dots and Graphene Quantum Dots in Electrochemical Biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef]
- Tuerhong, M.; Xu, Y.; Yin, X.-B. Review on carbon dots and their applications. Chin. J. Anal. Chem. 2017, 45, 139–150. [Google Scholar] [CrossRef]
- Molaei, M.J. Carbon quantum dots and their biomedical and therapeutic applications: A review. RSC Adv. 2019, 9, 6460–6481. [Google Scholar] [CrossRef]
- Kang, Z.; Lee, S.-T. Carbon dots: Advances in nanocarbon applications. Nanoscale 2019, 11, 19214–19224. [Google Scholar] [CrossRef] [PubMed]
- Kasouni, A.; Chatzimitakos, T.; Stalikas, C. Bioimaging applications of carbon nanodots: A review. J. Carbon Res. 2019, 5, 19. [Google Scholar] [CrossRef]
- Boakye-Yiadom, K.O.; Kesse, S.; Opoku-Damoah, Y.; Filli, M.S.; Aquib, M.; Joelle, M.M.B.; Farooq, M.A.; Mavlyanova, R.; Raza, F.; Bavi, R.; et al. Carbon dots: Applications in bioimaging and theranostics. Int. J. Pharm. 2019, 564, 308–317. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A mini review on carbon quantum dots: Preparation, properties, and electrocatalytic application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef]
- Semeniuk, M.; Yi, Z.; Poursorkhabi, V.; Tjong, J.; Jaffer, S.; Lu, Z.-H.; Sain, M. Future perspectives and review on organic carbon dots in electronic applications. ACS Nano 2019, 13, 6224–6255. [Google Scholar] [CrossRef]
- Xu, H.; Yang, X.; Li, G.; Zhao, C.; Liao, X. Green synthesis of fluorescent carbon dots for selective detection of tartrazine in food samples. J. Agric. Food Chem. 2015, 63, 6707–6714. [Google Scholar] [CrossRef]
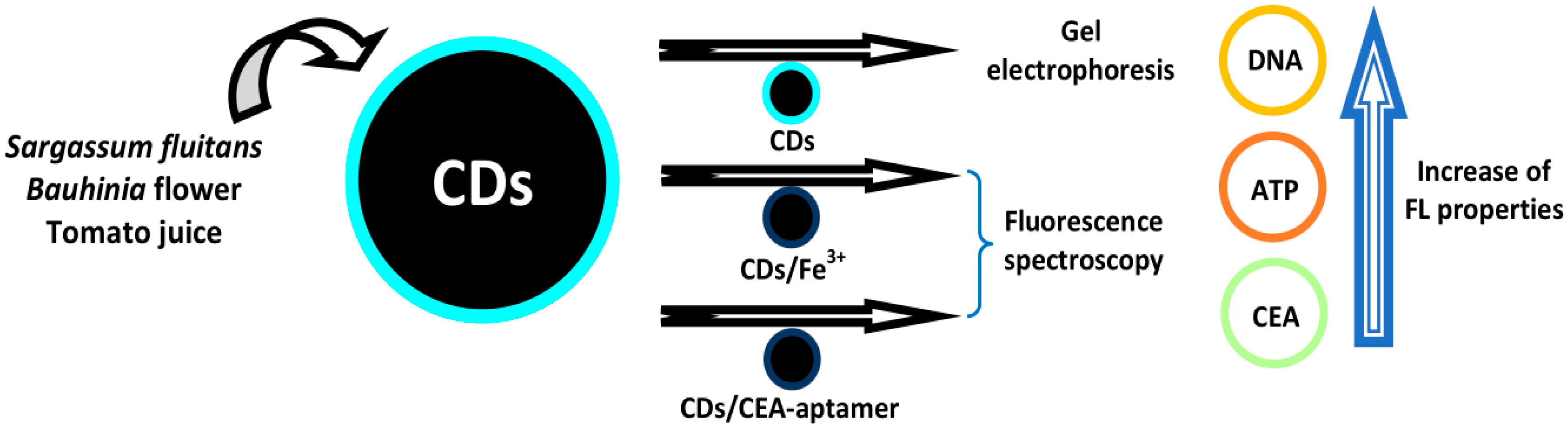
- Godavarthi, S.; Kesarla, M.K.; Vazquez-Velez, E.; Hernández-Eligio, A.; Mahendhiran, M.; Hernandez-Como, N.; Aleman, M.; Gomez, L.M. Nitrogen doped carbon dots derived from Sargassum fluitans as fluorophore for DNA detection. J. Photochem. Photobiol. B Boil. 2017, 172, 36–41. [Google Scholar] [CrossRef]
- Yang, K.; Liu, M.; Wang, Y.; Wang, S.; Miao, H.; Yang, L.; Yang, X. Carbon dots derived from fungus for sensing hyaluronic acid and hyaluronidase. Sens. Actuators B Chem. 2017, 251, 503–508. [Google Scholar] [CrossRef]
- Wang, W.-J.; Xia, J.-M.; Feng, J.; He, M.-Q.; Chen, M.-L.; Wang, J.-H. Green preparation of carbon dots for intracellular pH sensing and multicolor live cell imaging. J. Mater. Chem. B 2016, 4, 7130–7137. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Sefat, S.H.; Kazemifard, N.; Rezaei, B.; Moradi, F. A novel one-step and green synthesis of highly fluorescent carbon dots from saffron for cell imaging and sensing of prilocaine. Sens. Actuators B Chem. 2017, 253, 451–460. [Google Scholar] [CrossRef]
- Tafreshi, F.A.; Fatahi, Z.; Ghasemi, S.F.; Taherian, A.; Esfandiari, N. Ultrasensitive fluorescent detection of pesticides in real sample by using green carbon dots. PLoS ONE 2020, 15, e0230646. [Google Scholar] [CrossRef] [PubMed]
- Roshni, V.; Misra, S.; Santra, M.K.; Ottoor, D. One pot green synthesis of C-dots from groundnuts and its application as Cr(VI) sensor and in vitro bioimaging agent. J. Photochem. Photobiol. A Chem. 2019, 373, 28–36. [Google Scholar]
- Chandra, S.; Singh, V.K.; Yadav, P.K.; Bano, D.; Kumar, V.; Pandey, V.K.; Talat, M.; Hasan, S.H. Mustard seeds derived fluorescent carbon quantum dots and their peroxidase-like activity for colorimetric detection of H2O2 and ascorbic acid in a real sample. Anal. Chim. Acta 2018, 1054, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, L.; Li, X.; Liu, R.; Lin, L.; Zhao, S. Green preparation of S and N Co-doped carbon dots from water chestnut and onion as well as their use as an off–on fluorescent probe for the quantification and imaging of coenzyme A. ACS Sustain. Chem. Eng. 2017, 5, 4992–5000. [Google Scholar] [CrossRef]
- Miao, H.; Wang, L.; Zhuo, Y.; Zhou, Z.; Yang, X. Label-free fluorimetric detection of CEA using carbon dots derived from tomato juice. Biosens. Bioelectron. 2016, 86, 83–89. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Y.; Guo, T.; Yang, T.; Chen, M.-L.; Wang, J.-H. Green preparation of carbon dots with papaya as carbon source for effective fluorescent sensing of Iron (III) and Escherichia Coli. Biosens. Bioelectron. 2016, 85, 68–75. [Google Scholar] [CrossRef]
- Hoan, B.T.; Thanh, T.T.; Tam, P.D.; Trung, N.N.; Cho, S.; Pham, H. A green luminescence of lemon derived carbon quantum dots and their applications for sensing of V5+ ions. Mater. Sci. Eng. B 2019, 251, 114455. [Google Scholar] [CrossRef]
- Ahmadian-Fard-Fini, S.; Salavati-Niasari, M.; Ghanbari, D. Hydrothermal green synthesis of magnetic Fe3O4-carbon dots by lemon and grape fruit extracts and as a photoluminescence sensor for detecting of E. coli bacteria. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 203, 481–493. [Google Scholar] [CrossRef]
- Bandi, R.; Dadigala, R.; Reddy, B. Green synthesis of highly fluorescent nitrogen—Doped carbon dots from Lantana camara berries for effective detection of lead (II) and bioimaging. J. Photochem. Photobiol. B Biol. 2018, 178, 330–338. [Google Scholar] [CrossRef]
- Sun, X.; He, J.; Yang, S.; Zheng, M.; Wang, Y.; Ma, S.; Zheng, H. Green synthesis of carbon dots originated from Lycii Fructus for effective fluorescent sensing of ferric ion and multicolor cell imaging. J. Photochem. Photobiol. B Boil. 2017, 175, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.; Afkhami, A.; Hosseinzadeh, L.; Madrakian, T. Green and cost-effective synthesis of carbon dots from date kernel and their application as a novel switchable fluorescence probe for sensitive assay of Zoledronic acid drug in human serum and cellular imaging. Anal. Chim. Acta 2018, 1030, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Vandarkuzhali, S.A.A.; Natarajan, S.; Jeyabalan, S.; Sivaraman, G.; Singaravadivel, S.; Muthusubramanian, S.; Viswanathan, B. Pineapple peel-derived carbon dots: Applications as sensor, molecular keypad lock, and memory device. ACS Omega 2018, 3, 12584–12592. [Google Scholar] [CrossRef]
- Lu, M.; Duan, Y.; Song, Y.; Tan, J.; Zhou, L. Green preparation of versatile nitrogen-doped carbon quantum dots from watermelon juice for cell imaging, detection of Fe3+ ions and cysteine, and optical thermometry. J. Mol. Liq. 2018, 269, 766–774. [Google Scholar] [CrossRef]
- Jiang, X.; Qin, D.; Mo, G.; Feng, J.; Yu, C.; Mo, W.; Deng, B. Ginkgo leaf-based synthesis of nitrogen-doped carbon quantum dots for highly sensitive detection of salazosulfapyridine in mouse plasma. J. Pharm. Biomed. Anal. 2019, 164, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Shahshahanipour, M.; Rezaei, B.; Ensafi, A.A.; Etemadifar, Z. An ancient plant for the synthesis of a novel carbon dot and its applications as an antibacterial agent and probe for sensing of an anti-cancer drug. Mater. Sci. Eng. C 2019, 98, 826–833. [Google Scholar] [CrossRef] [PubMed]
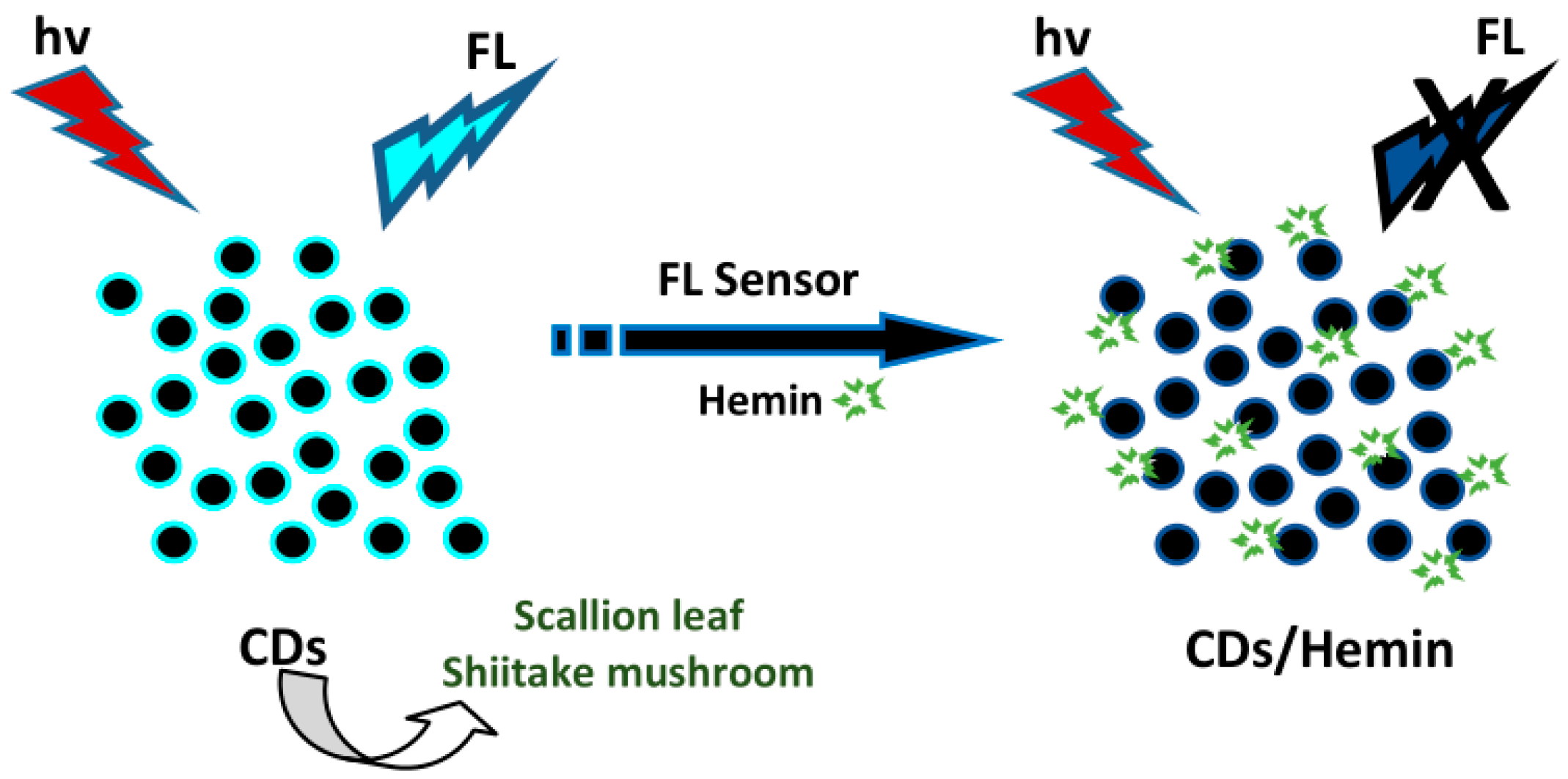
- Zhang, Z.; Hu, B.; Zhuang, Q.; Wang, Y.; Luo, X.; Xie, Y.; Zhou, D. Green synthesis of fluorescent nitrogen–sulfur Co-doped carbon dots from scallion leaves for hemin sensing. Anal. Lett. 2020, 53, 1704–1718. [Google Scholar] [CrossRef]
- Yadav, P.K.; Singh, V.K.; Chandra, S.; Bano, D.; Kumar, V.; Talat, M.; Hasan, S.H. Green synthesis of fluorescent carbon quantum dots from azadirachta indica leaves and their peroxidase-mimetic activity for the detection of H2O2 and ascorbic acid in common fresh fruits. ACS Biomater. Sci. Eng. 2018, 5, 623–632. [Google Scholar] [CrossRef]
- Chen, K.; Qing, W.; Hu, W.; Lu, M.; Wang, Y.; Liu, X. On-off-on fluorescent carbon dots from waste tea: Their properties, antioxidant and selective detection of CrO42−, Fe3+, ascorbic acid and L-cysteine in real samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 228–234. [Google Scholar] [CrossRef]
- Raveendran, V.; Babu, A.R.S.; Renuka, N. Mint leaf derived carbon dots for dual analyte detection of Fe(iii) and ascorbic acid. RSC Adv. 2019, 9, 12070–12077. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921. [Google Scholar] [CrossRef]
- Chan, K.K.; Yap, S.H.K.; Yong, K. Biogreen synthesis of carbon dots for biotechnology and nanomedicine applications. NanoMicro Lett. 2018, 10, 72. [Google Scholar] [CrossRef]
- Long, R.; Guo, Y.; Xie, L.; Shi, S.; Xu, J.; Tong, C.; Lin, Q.; Li, T. White pepper-derived ratiometric carbon dots for highly selective detection and imaging of coenzyme A. Food Chem. 2020, 315, 126171. [Google Scholar] [CrossRef]
- Farshbaf, M.; Davaran, S.; Rahimi, F.; Annabi, N.; Salehi, R.; Akbarzadeh, A. Carbon quantum dots: Recent progresses on synthesis, surface modification and applications. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1331–1348. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, M.; Na, N.; Chen, Z.; Li, S.; Liu, S.; Li, J. Review of natural product derived carbon dots: From natural products to functional materials. ChemSusChem 2018, 11, 11–24. [Google Scholar] [CrossRef]
- Huang, Q.; Li, Q.; Chen, Y.; Tong, L.; Lin, X.; Zhu, J.; Tong, Q.-X. High quantum yield nitrogen-doped carbon dots: Green synthesis and application as “off-on” fluorescent sensors for the determination of Fe3+ and adenosine triphosphate in biological samples. Sens. Actuators B Chem. 2018, 276, 82–88. [Google Scholar] [CrossRef]
- Monte-Filho, S.S.; De Andrade, S.I.E.; Lima, M.; Araujo, M.C.U. Synthesis of highly fluorescent carbon dots from lemon and onion juices for determination of riboflavin in multivitamin/mineral supplements. J. Pharm. Anal. 2019, 9, 209–216. [Google Scholar] [CrossRef]
- Feng, Y.; Zhong, D.; Miao, H.; Yang, X. Carbon dots derived from rose flowers for tetracycline sensing. Talanta 2015, 140, 128–133. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, N.; Gong, N.; Wang, H.; Shi, X.; Gu, W.; Ye, L. One-step microwave-assisted polyol synthesis of green luminescent carbon dots as optical nanoprobes. Carbon 2014, 68, 258–264. [Google Scholar] [CrossRef]
- Purbia, R.; Paria, S. A simple turn on fluorescent sensor for the selective detection of thiamine using coconut water derived luminescent carbon dots. Biosens. Bioelectron. 2016, 79, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 24–92. [Google Scholar] [CrossRef] [PubMed]
- Pourreza, N.; Ghomi, M. Green synthesized carbon quantum dots from prosopis juliflora leaves as a dual off-on fluorescence probe for sensing mercury (II) and chemet drug. Mater. Sci. Eng. C 2019, 98, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xu, J.; Arsalan, M.; Cheng, N.; Sheng, Q.; Zheng, J.; Cao, W.; Yue, T. Nitrogen doped carbon dots derived from natural seeds and their application for electrochemical sensing. J. Electrochem. Soc. 2019, 166, B56–B62. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Kasouni, A.; Sygellou, L.; Avgeropoulos, A.; Troganis, A.; Stalikas, C. Two of a kind but different: Luminescent carbon quantum dots from citrus peels for iron and tartrazine sensing and cell imaging. Talanta 2017, 175, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Prathumsuwan, T.; Jaiyong, P.; In, I.; Paoprasert, P. Label-free carbon dots from water hyacinth leaves as a highly fluorescent probe for selective and sensitive detection of borax. Sens. Actuators B Chem. 2019, 299, 126936. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, X.; Ruan, H.; Yin, K.; Li, H. Production of yellow-emitting carbon quantum dots from fullerene carbon soot. Sci. China Mater. 2017, 60, 141–150. [Google Scholar] [CrossRef]
- Shen, P.; Xia, Y. Synthesis-modification integration: One-step fabrication of boronic acid functionalized carbon dots for fluorescent blood sugar sensing. Anal. Chem. 2014, 86, 5323–5329. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.-A.; Wang, Y.; Gao, Y.; Li, H.; Dai, T.; Liu, Y.-L.; Huo, Q. Commercially activated carbon as the source for producing multicolor photoluminescent carbon dots by chemical oxidation. Chem. Commun. 2010, 46, 8812. [Google Scholar] [CrossRef]
- Gunjal, D.B.; Gore, A.H.; Naik, V.M.; Pawar, S.P.; Anbhule, P.V.; Shejwal, R.V.; Kolekar, G.B. Carbon dots as a dual sensor for the selective determination of d-penicillamine and biological applications. Opt. Mater. 2019, 88, 134–142. [Google Scholar] [CrossRef]
- Gunjal, D.B.; Gurav, Y.M.; Gore, A.H.; Naik, V.M.; Waghmare, R.D.; Patil, C.S.; Sohn, D.; Anbhule, P.V.; Shejwal, R.V.; Kolekar, G.B. Nitrogen doped waste tea residue derived carbon dots for selective quantification of tetracycline in urine and pharmaceutical samples and yeast cell imaging application. Opt. Mater. 2019, 98, 109484. [Google Scholar] [CrossRef]
- Desai, M.L.; Jha, S.; Basu, H.; Singhal, R.K.; Park, T.J.; Kailasa, S.K. Acid oxidation of muskmelon fruit for the fabrication of carbon dots with specific emission colors for recognition of Hg2+ ions and cell imaging. ACS Omega 2019, 4, 19332–19340. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Ha, S.; Baek, S.H.; Phan, L.M.T.; Kim, S.; Kwak, K.; Park, T.J. Tuning of carbon dots emission color for sensing of Fe3+ ion and bioimaging applications. Mater. Sci. Eng. C 2019, 98, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Anilbhai, G.D.; Desai, M.L.; Malek, N.I.; Kailasa, S.K. Fluorescence detection of Fe3+ ion using ultra-small fluorescent carbon dots derived from pineapple (Ananas comosus): Development of miniaturized analytical method. J. Mol. Struct. 2020, 1216, 128343. [Google Scholar]
- Chu, K.-W.; Lee, S.L.; Chang, C.-J.; Liu, L. Recent progress of carbon dot precursors and photocatalysis applications. Polymers 2019, 11, 689. [Google Scholar] [CrossRef]
- Briscoe, J.; Marinovic, A.; Sevilla, M.; Dunn, S.; Titirici, M.; Solis, M.S. Biomass-derived carbon quantum dot sensitizers for solid-state nanostructured solar cells. Angew. Chem. Int. Ed. 2015, 54, 4463–4468. [Google Scholar] [CrossRef]
- Abdelhamidab, H.N.; El-Bery, H.M.; Metwally, A.A.; Elshazly, M.; Hathout, R.M. Synthesis of CdS-modified chitosan quantum dots for the drug delivery of Sesamol. Carbohydr. Polym. 2019, 214, 90–99. [Google Scholar] [CrossRef]
- Abdelhamidab, H.N.; Wu, H.-F. Probing the interactions of chitosan capped CdS quantum dots with pathogenic bacteria and their biosensing application. J. Mater. Chem. B 2013, 1, 6094–6106. [Google Scholar] [CrossRef]
- Wu, H.-F.; Gopal, J.; Abdelhamidab, H.N.; Hasan, N. Quantum dot applications endowing novelty to analytical proteomics. Proteomics 2012, 12, 2949–2961. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230. [Google Scholar] [CrossRef]
- Ansi, V.; Renuka, N. Table sugar derived Carbon dot—A naked eye sensor for toxic Pb2+ ions. Sens. Actuators B Chem. 2018, 264, 67–75. [Google Scholar] [CrossRef]
- Ding, Y.-Y.; Gong, X.; Liu, Y.; Lu, W.-J.; Gao, Y.-F.; Xian, M.; Shuang, S.; Dong, C. Facile preparation of bright orange fluorescent carbon dots and the constructed biosensing platform for the detection of pH in living cells. Talanta 2018, 189, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Shih, Z.-Y.; Lee, C.-H.; Chang, H.-T. Synthesis and analytical applications of photoluminescent carbon nanodots. Green Chem. 2012, 14, 917. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Chen, C.-Y.; Wang, C.-M.; Tan, Y.Z.; Liao, W.-S. Multicolor functional carbon dots via one-step refluxing synthesis. ACS Sens. 2017, 2, 354–363. [Google Scholar] [CrossRef]
- Yang, X.; Zhuo, Y.; Zhu, S.; Luo, Y.; Feng, Y.; Dou, Y. Novel and green synthesis of high-fluorescent carbon dots originated from honey for sensing and imaging. Biosens. Bioelectron. 2014, 60, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; He, X.; Ya, D.; Zhong, J.; Deng, B. One step hydrothermal synthesis of nitrogen-doped graphitic quantum dots as a fluorescent sensing strategy for highly sensitive detection of metacycline in mice plasma. Sens. Actuators B Chem. 2017, 249, 256–264. [Google Scholar] [CrossRef]
- Xua, Z.; Yang, Q.-Q.; Lan, J.-Y.; Zhang, J.-Q.; Peng, W.; Jin, J.-C.; Jiang, F.-L.; Liu, Y. Interactions between carbon nanodots with human serum albumin and γ-globulins: The effects on the transportation function. J. Hazard Mater. 2016, 301, 242–249. [Google Scholar] [CrossRef]
- Lin, H.; Yan, H.; Luo, M. Enrichment of Nicotine in Human Plasma and Urine with Ionic Lliquid Based Liquid Phase Microextraction. In Proceedings of the 2010 3rd International Conference on Biomedical Engineering and Informatics, Yantai, China, 16–18 October 2010; Volume 5, pp. 2038–2040. [Google Scholar]
- Li, K.; Xie, X.; Zhang, W. De porous graphitic carbon nitride derived from melamine–Ammonium oxalate stacking sheets with excellent photocatalytic hydrogen evolution activity. ChemCatChem 2016, 8, 2128–2135. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Kulinich, S.A.; Liu, Y.; Zeng, H. Engineering surface states of carbon dots to achieve controllable luminescence for solid-luminescent composites and sensitive Be2+ detection. Sci. Rep. 2014, 4, 4976. [Google Scholar] [CrossRef]
- Dong, Y.; Pang, H.; Bin Yang, H.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-based dots Co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chem. Int. Ed. 2013, 52, 7800–7804. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Lin, Y.-Y.; Mattevi, C.; Yamaguchi, H.; Chen, H.-A.; Chen, I.-S.; Chen, C.-W.; Chhowalla, M. Blue photoluminescence from chemically derived graphene oxide. Adv. Mater. 2010, 22, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, P.; Wang, X.; Cao, L.; Sahu, S.; Liu, J.-H.; Wang, P.; Korch, K.; Ii, K.N.T.; Parenzan, A.; Sun, Y.-P. Toward quantitatively fluorescent carbon-based “quantum” dots. Nanoscale 2011, 3, 2023. [Google Scholar] [CrossRef]
- Guo, K.; Peng, M.; Tong, C.; Shi, S.; Liu, Y. Direct from solid natural products to pure compounds in a single step: Coupling online extraction with high-speed counter-current chromatography. J. Sep. Sci. 2018, 41, 3607–3613. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Wang, X.; Lu, Q.; Liu, X.; Wang, L. A biocompatible fluorescent ink based on water? Soluble luminescent carbon nanodots. Angew. Chem. 2012, 124, 12381–12384. [Google Scholar] [CrossRef]
- Long, Y.-M.; Zhou, C.-H.; Zhang, Z.; Tian, Z.-Q.; Bao, L.; Lin, Y.; Pang, D.-W. Shifting and non-shifting fluorescence emitted by carbon nanodots. J. Mater. Chem. 2012, 22, 5917–5920. [Google Scholar] [CrossRef]
- Nie, H.; Li, M.; Li, Q.; Liang, S.; Tan, Y.; Sheng, L.; Shi, W.; Zhang, S.X.-A. Carbon dots with continuously tunable full-color emission and their application in ratiometric pH sensing. Chem. Mater. 2014, 26, 3104–3112. [Google Scholar] [CrossRef]
- Wen, X.; Yu, P.; Toh, Y.-R.; Lee, Y.-C.; Hsu, A.-C.; Tang, J. Near-infrared enhanced carbon nanodots by thermally assisted growth. Appl. Phys. Lett. 2012, 101, 163107. [Google Scholar] [CrossRef]
- Feng, T.; Zeng, Q.; Lu, S.; Yan, X.; Liu, J.; Tao, S.; Yang, M.; Yang, B. Color-tunable carbon dots possessing solid-state emission for full-color light-emitting diodes applications. ACS Photon. 2017, 5, 502–510. [Google Scholar] [CrossRef]
- Wang, H.; Sun, C.; Chen, X.; Zhang, Y.; Colvin, V.L.; Rice, Q.; Seo, J.; Feng, S.; Wang, S.; Yu, W.W. Excitation wavelength independent visible color emission of carbon dots. Nanoscale 2017, 9, 1909–1915. [Google Scholar] [CrossRef]
- Yan, F.; Sun, Z.; Zhang, H.; Sun, X.; Jiang, Y.; Bai, Z. The fluorescence mechanism of carbon dots, and methods for tuning their emission color: A review. Microchim. Acta 2019, 186, 583. [Google Scholar] [CrossRef]
- Jiang, K.; Feng, X.; Gao, X.; Wang, Y.; Cai, C.; Li, Z.; Lin, H. Preparation of multicolor photoluminescent carbon dots by tuning surface states. Nanomaterials 2019, 9, 529. [Google Scholar] [CrossRef]
- Huo, F.; Liang, W.; Tang, Y.; Zhang, W.; Liu, X.; Pei, D.-S.; Wang, H.; Jia, W.; Jia, P.; Yang, F. Full-color carbon dots with multiple red-emission tuning: On/off sensors, in vitro and in vivo multicolor bioimaging. J. Mater. Sci. 2019, 54, 6815–6825. [Google Scholar] [CrossRef]
- Zhu, J.; Bai, X.; Bai, J.; Pan, G.; Zhu, Y.; Zhai, Y.; Shao, H.; Chen, X.; Dong, B.; Zhang, H.; et al. Emitting color tunable carbon dots by adjusting solvent towards light-emitting devices. Nanotechnology 2018, 29, 085705. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, green, and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef]
- Yu, P.; Wen, X.; Toh, Y.-R.; Tang, J. Temperature-dependent fluorescence in carbon dots. J. Phys. Chem. C 2012, 116, 25552–25557. [Google Scholar] [CrossRef]
- Ho, J.-A.A.; Chang, H.-C.; Su, W.-T. DOPA-mediated reduction allows the facile synthesis of fluorescent gold nanoclusters for use as sensing probes for ferric ions. Anal. Chem. 2012, 84, 3246–3253. [Google Scholar]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene quantum dots derived from carbon fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, W. Nitrogen-doped carbon quantum dots: Facile synthesis and application as a “turn-off” fluorescent probe for detection of Hg2+ ions. Biosens. Bioelectron. 2014, 55, 83–90. [Google Scholar] [CrossRef]
- Zheng, M.; Xie, Z.; Qu, D.; Li, D.; Du, P.; Jing, X.; Sun, Z. On–off–on fluorescent carbon dot nanosensor for recognition of Chromium(VI) and ascorbic acid based on the inner filter effect. ACS Appl. Mater. Interfaces 2013, 5, 13242–13247. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, H.; Tang, J.; Deng, S.; Yan, F.; Li, W.; Qu, M. Carbon dots doped with heteroatoms for fluorescent bioimaging: A review. Microchim. Acta 2016, 184, 343–368. [Google Scholar] [CrossRef]
- Xu, Q.; Mi, H.-Y.; Liu, Y.; Cai, L.; Peng, X.; Sreeprasad, T.S.; Zhao, P.; Yu, Z.; Li, N. Heteroatom-doped carbon dots: Synthesis, characterization, properties, photoluminescence mechanism and biological applications. J. Mater. Chem. B 2016, 4, 7204–7219. [Google Scholar] [CrossRef]
- Kandasamy, G. Recent advancements in doped/Co-doped carbon quantum dots for multi-potential applications. J. Carbon Res. 2019, 5, 24. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Sun, Y.; Geng, X.; Hu, Y.; Meng, H.; Ge, J.; Qu, L. Synthesis of luminescent carbon dots with ultrahigh quantum yield and inherent folate receptor-positive cancer cell targetability. Sci. Rep. 2018, 8, 1086. [Google Scholar] [CrossRef]
| Methods | Advantages | Disadvantages |
|---|---|---|
| Hydrothermal/Solvothermal | Low cost, eco-friendly, non-toxic, simple | Poor control of size, long synthesis duration |
| Microwave/Microwave-Hydrothermal | Facile, rapid, scalable, low cost, eco-friendly, treamlined process | Poor control of size |
| Pyrolysis | Simple, short synthesis duration, ecofriendly | Difficult to scale up, broad size distribution |
| Chemical Oxidation | Cheap, large-scale production, effective | Tedious steps, toxic acid/base reagents, expensive oxidants, complicated post-treatment |
| Precursor | Part | Form | Amount | Solvent (mL) | Temp (°C) | Time (h) | Ref. |
|---|---|---|---|---|---|---|---|
| Aloe | Stem | Powder | 5.0 g | 25 | 180 | 11 | [48] |
| Sargassum fluitans | Stem | Extract | 10 mL | 50 | 180 | 5 | [49] |
| Mushroom | Stem | Powder | 0.6 g | 6 | 200 | 6 | [50] |
| Shiitake mushroom | Stem | Powder | 0.5 g | 10 | 200 | 12 | [51] |
| Saffron | Flower | Powder | 0.5 g | 100 | 200 | 14 | [52] |
| Cauliflower juice | Flower | Extract | 60 mL | - | 120 | 5 | [53] |
| Groundnut | Seed | Powder | 1.0 g | 30 | 250 | 6 | [54] |
| Mustard | Seed | Powder | 2.0 g | 50 | 180 | 4 | [55] |
| Water chestnut/Onion | Root | Powder | 2.0/3.0 g | 30 | 180 | 4 | [56] |
| Tomato juice | Fruit | Extract | 10 mL | - | 150 | 2 | [57] |
| Papaya flesh | Fruit | Powder | 0.4 g | 10 | 200 | 5 | [58] |
| Lemon juice | Fruit | Extract | 15 mL | 10 | 240 | 12 | [59] |
| Lemon/grape/turmeric + EDA | Fruit | Extract | 1.0 g | 40 | 180 | 6 | [60] |
| Lantana camara berry + EDA | Fruit | Extract | 5.0 g | 50 | 180 | 3 | [61] |
| Lycii fructus + NH3 | Fruit | Powder | 1.0 g | 30 | 200 | 5 | [62] |
| Date kernel | Fruit | Powder | 2.0 g | 10 | 200 | 8 | [63] |
| Pineapple peel juice | Fruit | Extract | 10 mL | 10 | 150 | 2 | [64] |
| Watermelon juice | Fruit | Extract | 50 mL | 5 | 180 | 3 | [65] |
| Ginkgo leaf | Leaf | Powder | 1.0 g | 25 | 200 | 10 | [66] |
| Henna | Leaf | Powder | 0.5 g | 40 | 180 | 12 | [67] |
| Scallion | Leaf | Powder | 4.0 g | 20 | 180 | 12 | [68] |
| Azadirachta indica (neem) | Leaf | Powder | 10 g | 100 | 150 | 4 | [69] |
| Waste tea + EDA | Leaf | Extract | 1.5 g | 30 | 150 | 6 | [70] |
| Mint | Leaf | Extract | 5.0 g | 40 | 200 | 5 | [71] |
| Precursors | λEx (nm) | λEm (nm) | QY (%) | Abs. (nm) | FL Color | Ref. |
|---|---|---|---|---|---|---|
| Aloe | 441 | 503 | 10.37 | 278 | Bright blue | [48] |
| Sargassum fluitans | 340 | 450 | 18.2 | 226/280 | Blue | [49] |
| Mushroom | 370 | 455 | 15.3 | 285 | Blue | [50] |
| Shiitake mushroom | 330 | 410 | 5.5 | 240–290/420–500 | Bright blue | [51] |
| Saffron | 400 | 485 | 23.6 | 275 | Green-blue | [52] |
| Cauliflower | 325 | 400 | 43 | 280 | Blue | [53] |
| Bauhinia flower | 355 | 442 | 27 | 228/282 | Blue | [77] |
| Rose flower | 390 | 435 | 13.45 | - | Blue | [79] |
| Mahogany fruit shell | 320 | 430 | 1.9 | 300 | - | [90] |
| Coconut water | 390 | 450 | 2.8 | 290 | Blue/green | [81] |
| Tomato juice | 367 | 440 | 13.9 | - | Blue | [57] |
| Tomato | 360/420/460 | 450/520/560 | 12.7 | 260/280/285 | B/G/Y | [93] |
| Papaya flesh | 370 | 450 | 18.98 | 250–290 | Blue | [58] |
| Lemon/grape/turmeric/EDA | - | - | 20 | 350 | Blue | [60] |
| L. camara berry/EDA | 360 | 450 | 33.15 | 285/356 | Blue | [61] |
| Muskmelon | 342/415/425 | 432/515/554 | 14.3 | 314/414/467 | B/G/Y | [92] |
| Lemon juice | 420 | 540 | 21 | 280 | Bright green | [59] |
| Lycii fructus + NH3 | 350 | 430 | 17.2 | 271/300 | Blue | [62] |
| Date kernel | 340 | 430 | 12.5 | 275 | Blue | [63] |
| Pineapple Peel | 380 | 435 | 42 | 280 | Blue | [64] |
| Pineapple | 318/395/393 | 438/516/543 | 44.7 | 318/395/393 | B/G/Y | [94] |
| Watermelon juice | 355 | 439 | 10.6 | 282/355 | Blue | [65] |
| Citrus sinensis/limon peels | 365/330 | 455/390, 435 | 16.8/15.5 | 270 | Blue | [85] |
| Lemon/Onion/NH3 | 340 | 425 | 23.6 | 280/340 | Bright blue | [78] |
| Ginkgo leaf | 350 | 436 | 22.8 | 230/280 | Bright blue | [66] |
| Waste tea residue | 310 | 430 | 2.47 | 302 | - | [91] |
| Henna | 360 | 440 | 28.7 | 270–380 | Green | [67] |
| Scallion | 320 | 418 | 3.2 | 281 | Blue | [68] |
| Azadirachta indica (neem) | 340 | 467 | 27.2 | 276/340 | Blue | [69] |
| Prosopis juliflora | 325/350 | 396/437 | 5 | 300–500 | Bright blue | [83] |
| Waste tea/EDA | 350 | 445 | 7.1 | 270/330 | Blue | [70] |
| Mint | 360 | 441 | 7.64 | 225/281/323 | Cyan | [71] |
| Water hyacinth | 400 | 486 | 27 | 285/350 | Blue | [86] |
| White pepper | 420 | 520/668 | 10.4 | 261/310/343/665 | - | [74] |
| Groundnut | 360 | 443 | 7.87 | 279 | - | [54] |
| Kiwi, white & black sesame | - | - | - | 275/325 | - | [84] |
| Mustard | 330 | 423 | 4.6 | 245/312 | Blue | [55] |
| Water Chestnut/Onion | 370 | 475 | 12 | 242/333 | Green-blue | [56] |
| Sources | Parts | Target Analytes | LOD (µM) | Ref. |
|---|---|---|---|---|
| Pineapple Peel | Fruit | Cys | 0.87 | [64] |
| Watermelon juice | Fruit | Cys | 0.27 | [65] |
| Waste tea + EDA | Leaf | Cys & AA | 8.785/153.5 & 87.02/19.78 | [70] |
| Groundnut | Seed | GSH | - | [54] |
| Mahogany fruit shell | Fruit | D-PA | 49.59/39.27 | [90] |
| Azadirachta indica | Leaf | AA | 1.773 | [69] |
| Mustard | Seed | AA | 3.26 | [55] |
| Mint | Leaf | AA | 0.079 | [71] |
| Coconut water | Fruit | Thiamine | 0.28 | [81] |
| Lemon/Onion/NH3 | F + R | Riboflavin | 0.003 | [78] |
| White pepper | Seed | CoA | 0.00875 | [74] |
| Water Chestnut/Onion | Root | CoA | 0.01 | [56] |
| Mushroom | Stem | HA/HAase | 0.3 × 10−5/0.1 U mL−1 | [50] |
| Papaya flesh | Fruit | E. coli | 9.5 × 104 cfu mL−1 | [58] |
| Lemon/grape/turmeric+EDA | Fruit | E. coli | - | [60] |
| Scallion | Leaf | Hemin | 0.1 | [68] |
| Shiitake mushroom | Stem | Hemin | 0.12 | [51] |
| Sargassum fluitans | Stem | DNA | 15.15 | [49] |
| Bauhinia flower | Flower | ATP | 0.005 | [77] |
| Tomato juice | Fruit | CEA & aptamer | 0.3 ng mL−1/0.00188 | [57] |
| Sources | Parts | Analytes | LOD (µM) | Biological Samples | Ref. |
|---|---|---|---|---|---|
| L. camara berry + EDA | Fruit | Pb2+ | 0.00964 | Serum/Urine | [61] |
| Muskmelon | Fruit | Hg2+ | 0.33 | Serum | [92] |
| Lemon juice | Fruit | V5+ | 27.36 | Fetal Bovine Serum | [59] |
| Lycii fructus + NH3 | Fruit | Fe3+ | 0.021 | Urine | [62] |
| Tomato | Fruit | Fe3+ | 0.016, 0.072, 0.065 | Plasma/urine | [93] |
| Pineapple | Fruit | Fe3+ | 0.03 | Plasma/urine | [94] |
| Citrus sinensis/limon peels | Fruit | Fe3+/Tartrazine | 0.003/0.2 | Blood/urine | [85] |
| Date kernel | Fruit | ZA | 0.04 | Serum | [63] |
| Prosopis juliflora | Leaf | Chemet | 0.0077 | Serum | [83] |
| Ginkgo leaf | Leaf | SASP | 0.04 | Mouse plasma | [66] |
| Henna | Leaf | MTX | 0.007 | Plasma | [67] |
| Saffron | Flower | PC | 0.0018 | Plasma | [52] |
| Waste tea residue | Leaf | TC | 0.09 | Urine | [91] |
| Rose flower | Flower | TC | 0.0033 | Urine | [79] |
| Aloe | Stem | Tartrazine | 0.073 | Foods | [48] |
| Cauliflower | Flower | Pesticides | 0.00082, 0.0021, 0.012 | Cherry tomato | [53] |
| Kiwi/white & black sesame | Seed | NO2− | 0.23 | Ham sausage | [84] |
| Water hyacinth | Leaf | Borax | 1.5/11.85 | Fishball | [86] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulfajri, M.; Abdelhamid, H.N.; Sudewi, S.; Dayalan, S.; Rasool, A.; Habib, A.; Huang, G.G. Plant Part-Derived Carbon Dots for Biosensing. Biosensors 2020, 10, 68. https://doi.org/10.3390/bios10060068
Zulfajri M, Abdelhamid HN, Sudewi S, Dayalan S, Rasool A, Habib A, Huang GG. Plant Part-Derived Carbon Dots for Biosensing. Biosensors. 2020; 10(6):68. https://doi.org/10.3390/bios10060068
Chicago/Turabian StyleZulfajri, Muhammad, Hani Nasser Abdelhamid, Sri Sudewi, Sandhiya Dayalan, Akhtar Rasool, Ahsan Habib, and Genin Gary Huang. 2020. "Plant Part-Derived Carbon Dots for Biosensing" Biosensors 10, no. 6: 68. https://doi.org/10.3390/bios10060068
APA StyleZulfajri, M., Abdelhamid, H. N., Sudewi, S., Dayalan, S., Rasool, A., Habib, A., & Huang, G. G. (2020). Plant Part-Derived Carbon Dots for Biosensing. Biosensors, 10(6), 68. https://doi.org/10.3390/bios10060068








