Recent Advances in Cellulose-Based Biosensors for Medical Diagnosis
Abstract
1. Introduction
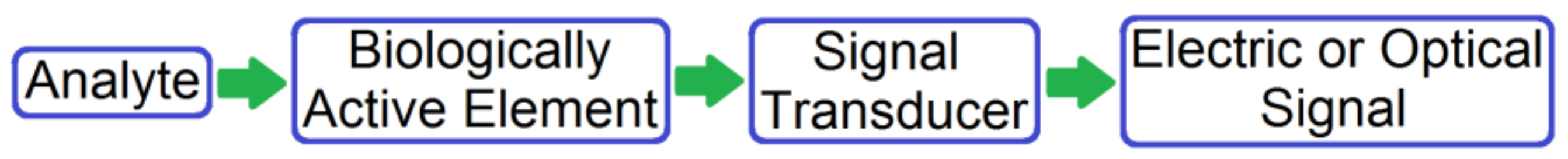
2. Classification of Biosensors
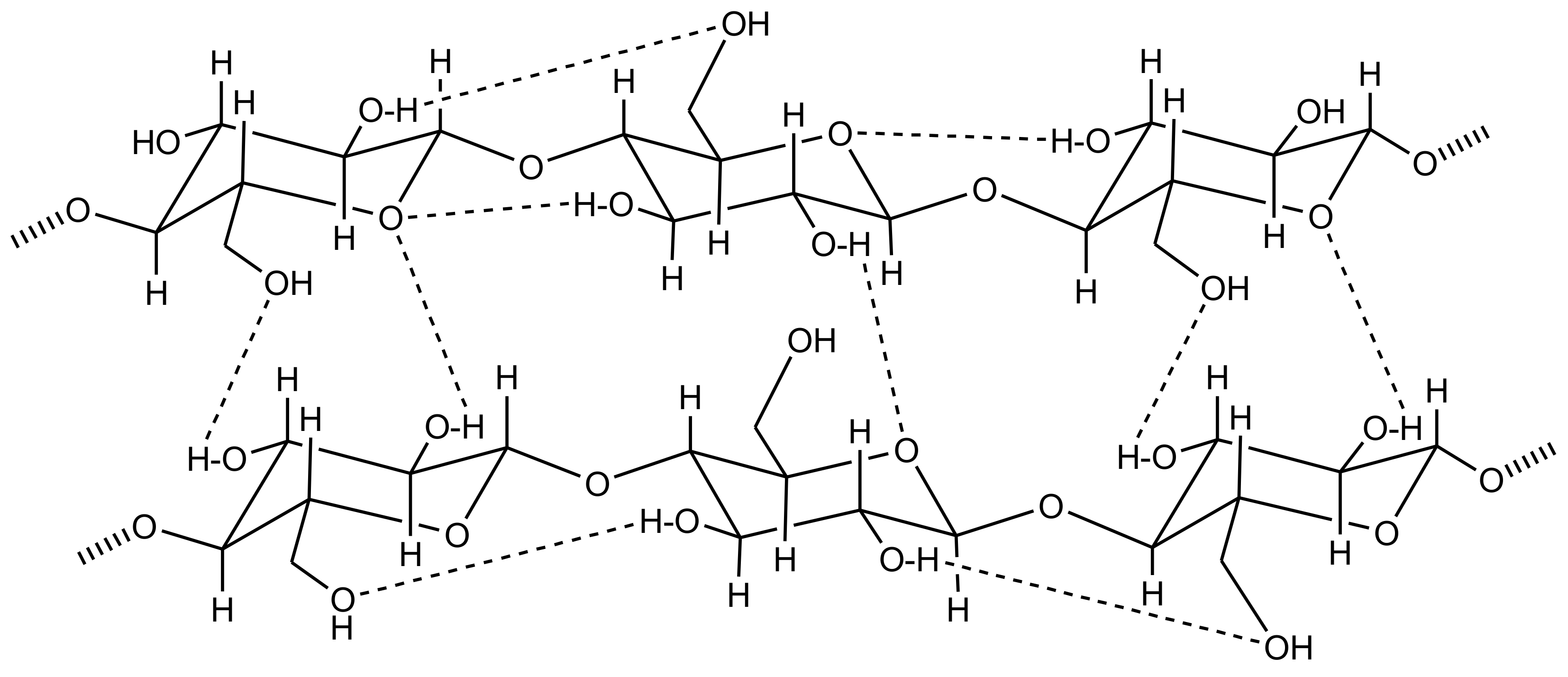
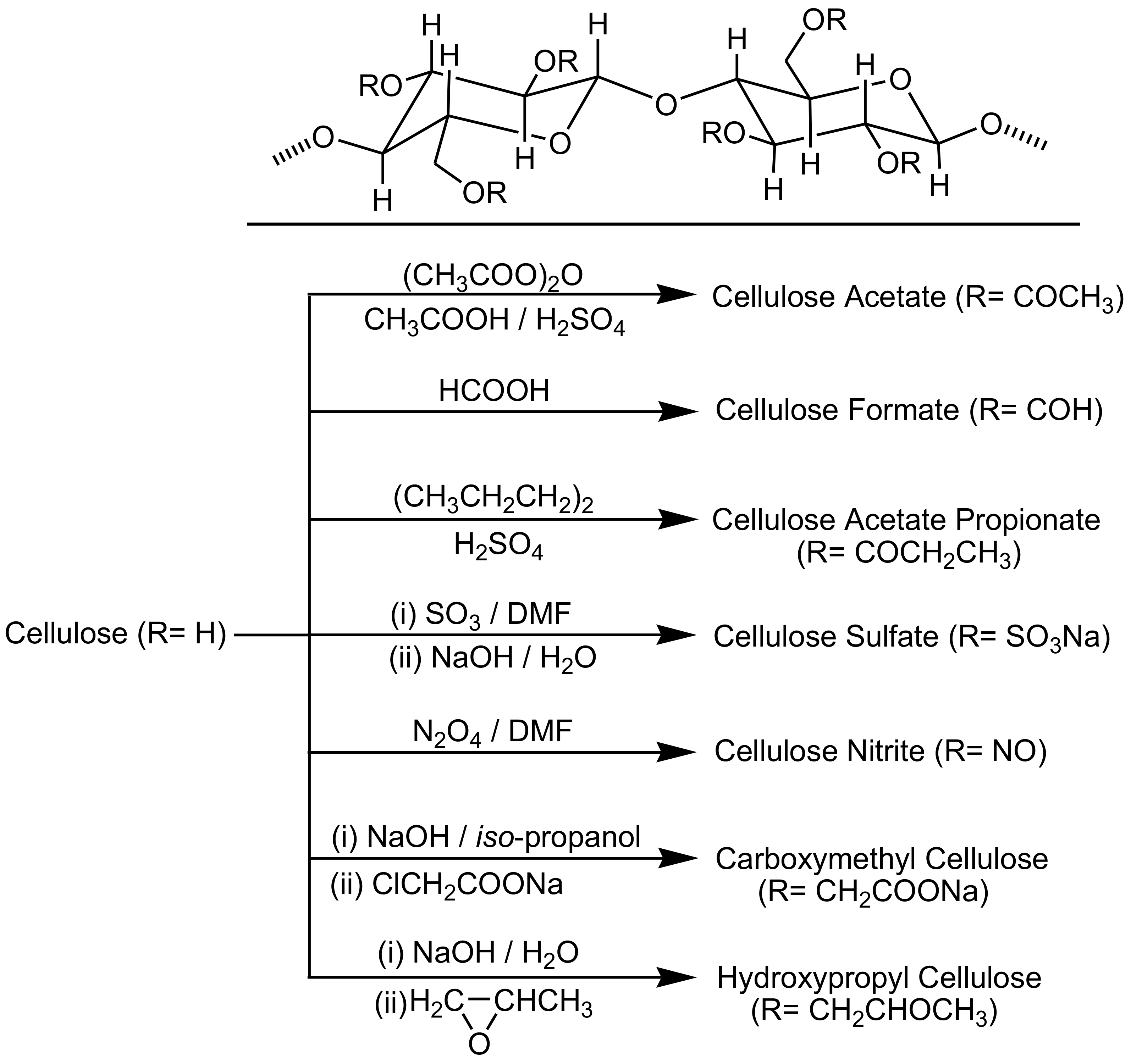
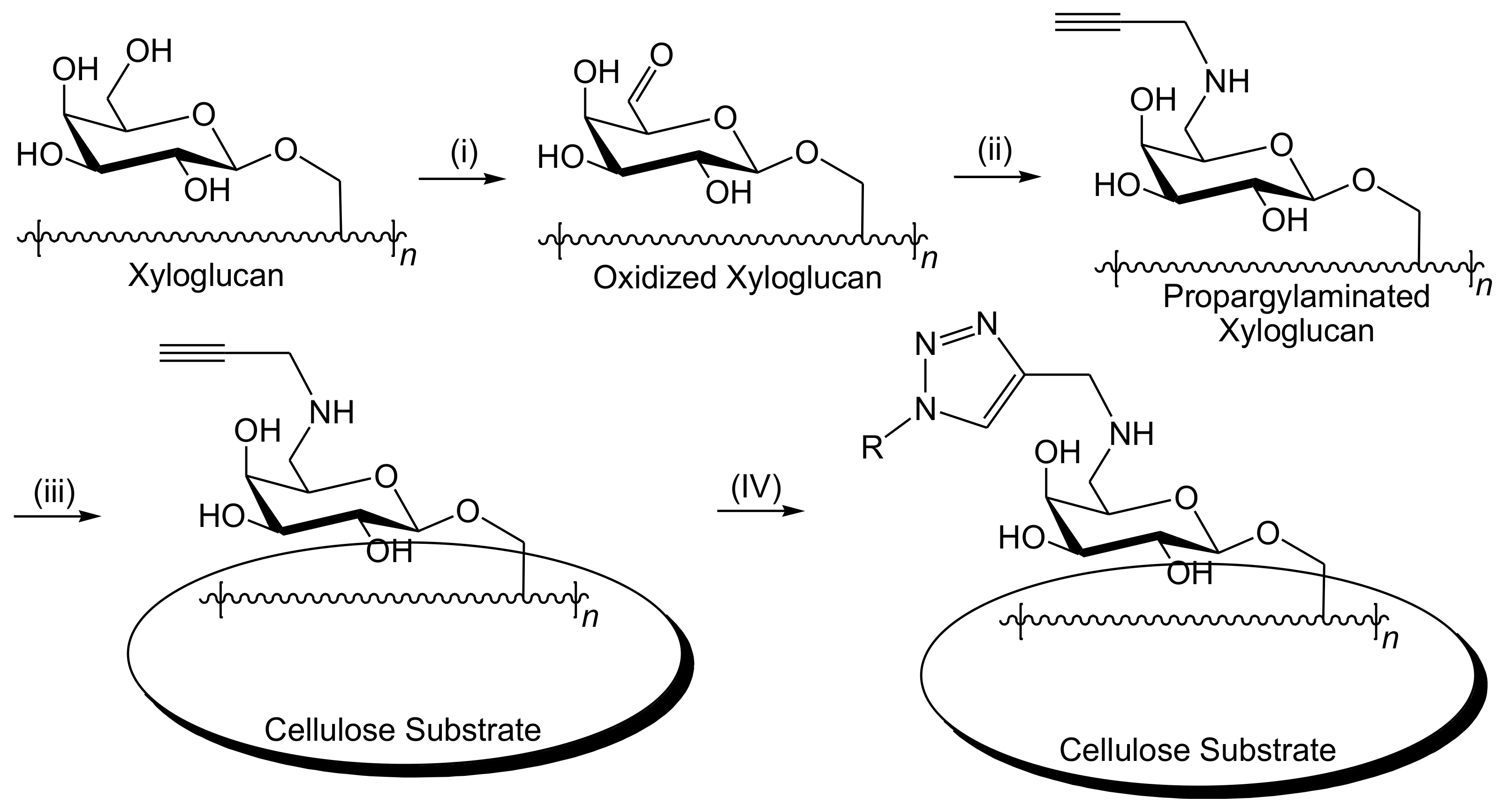
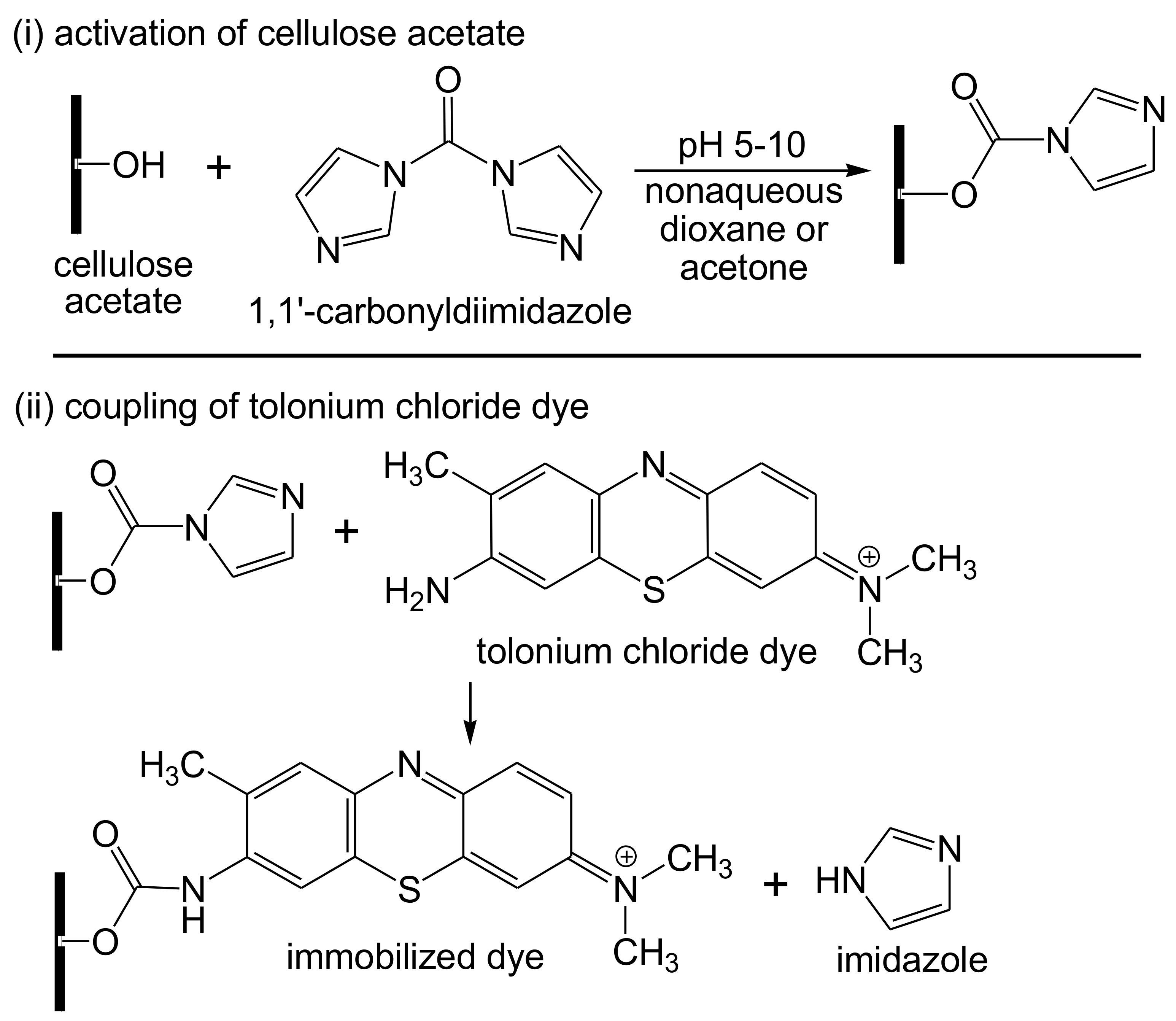
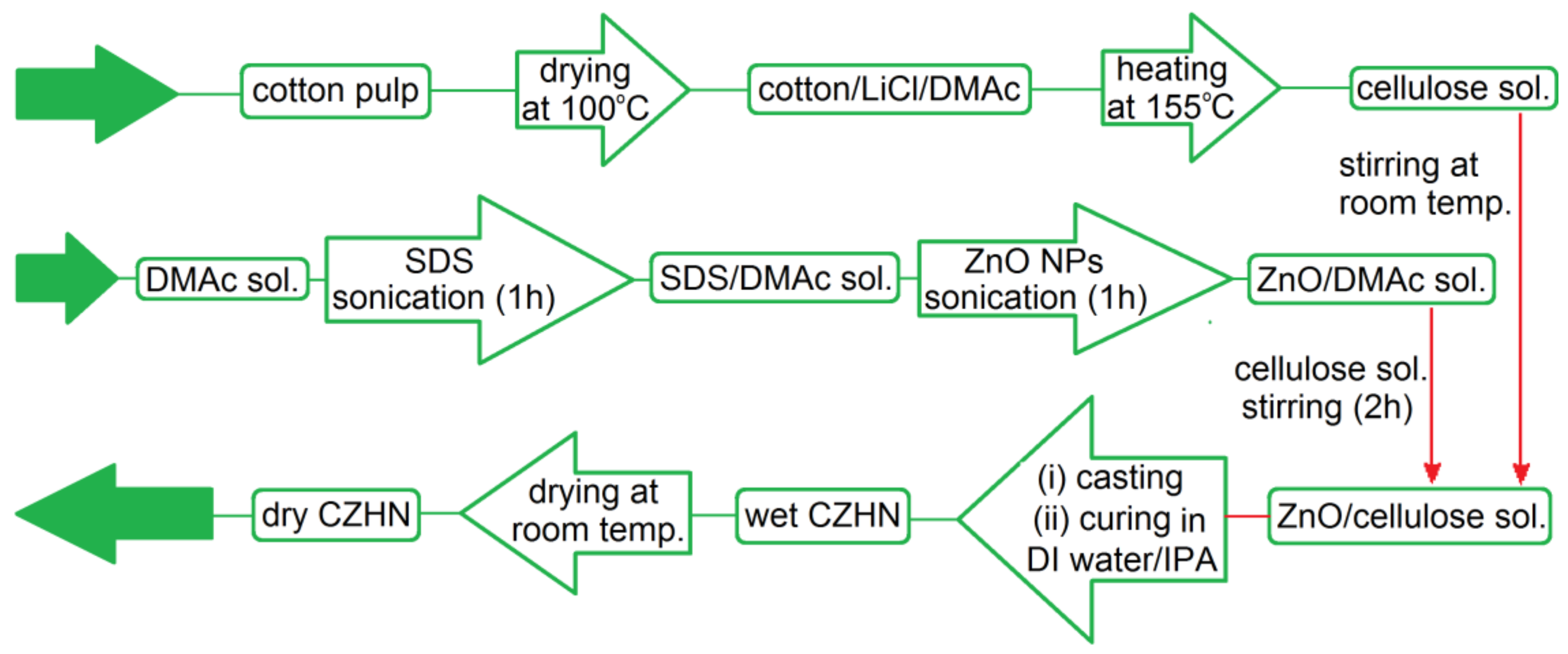
3. Cellulose Functionalization
4. Cellulose-Based Biosensors
4.1. Cellulose-Based Optical Biosensors
4.1.1. Label-Free Optical Biosensors
4.1.2. Label-Driven Optical Biosensors
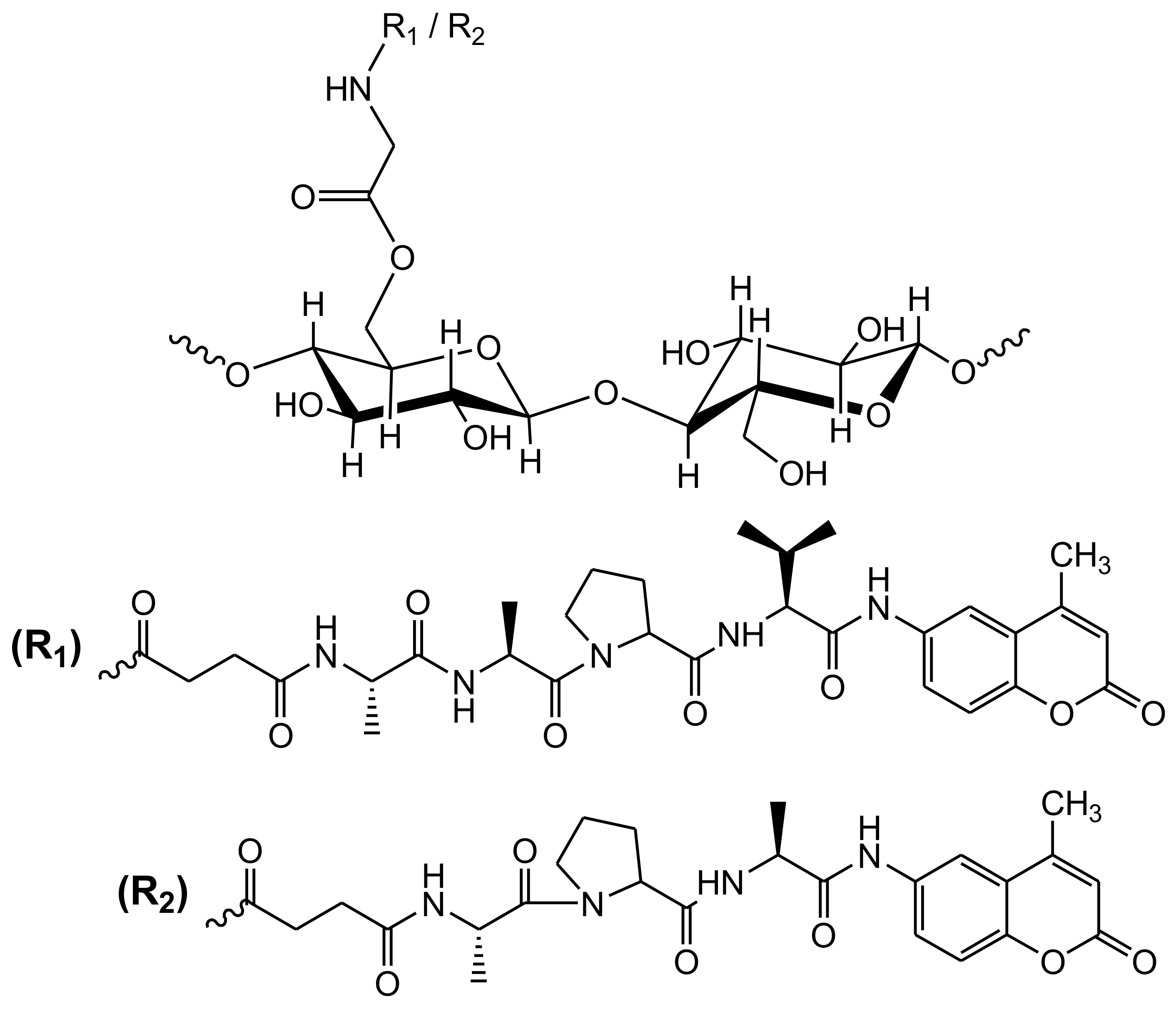
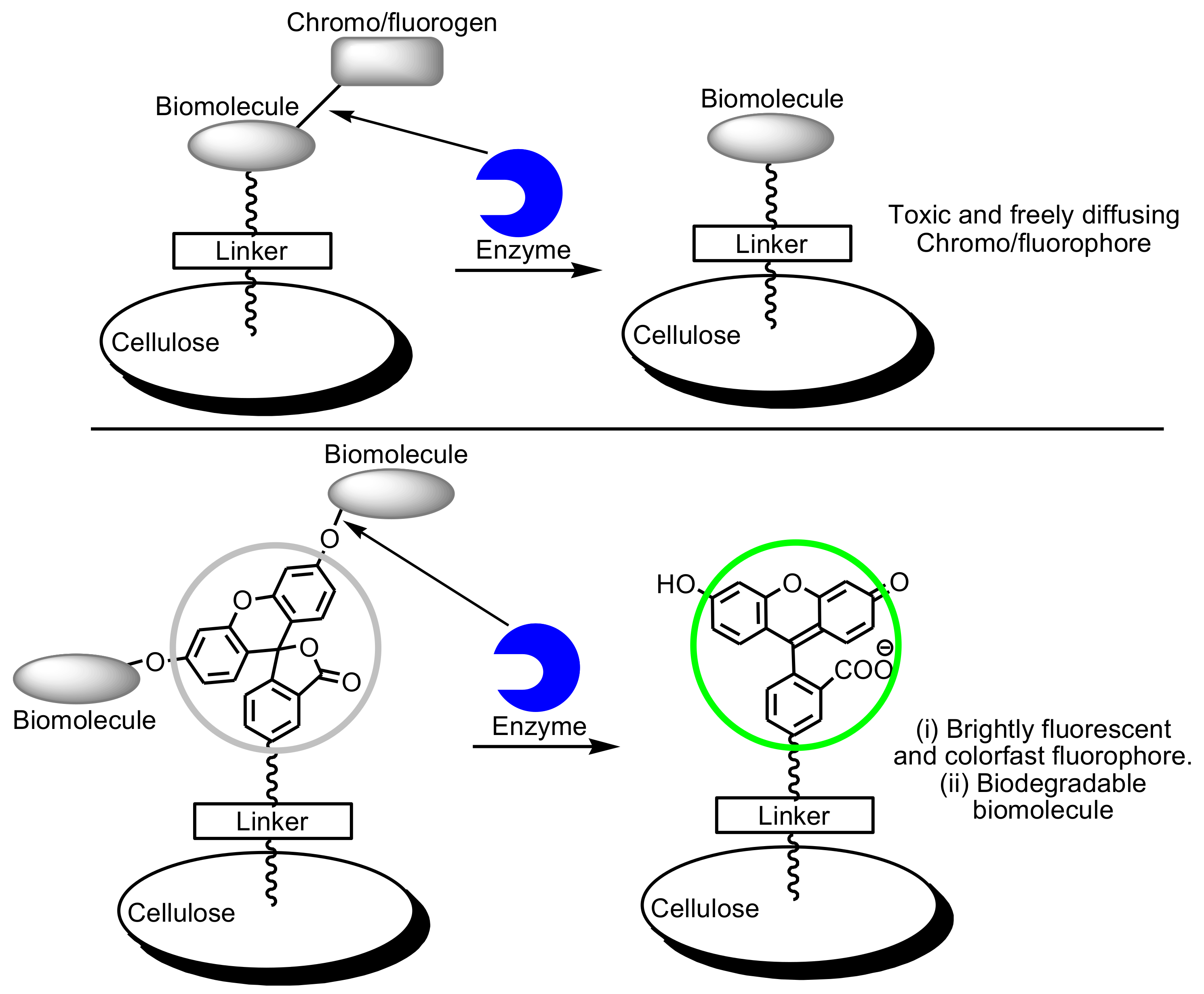
Cellulose-Based Fluorescent Biosensors
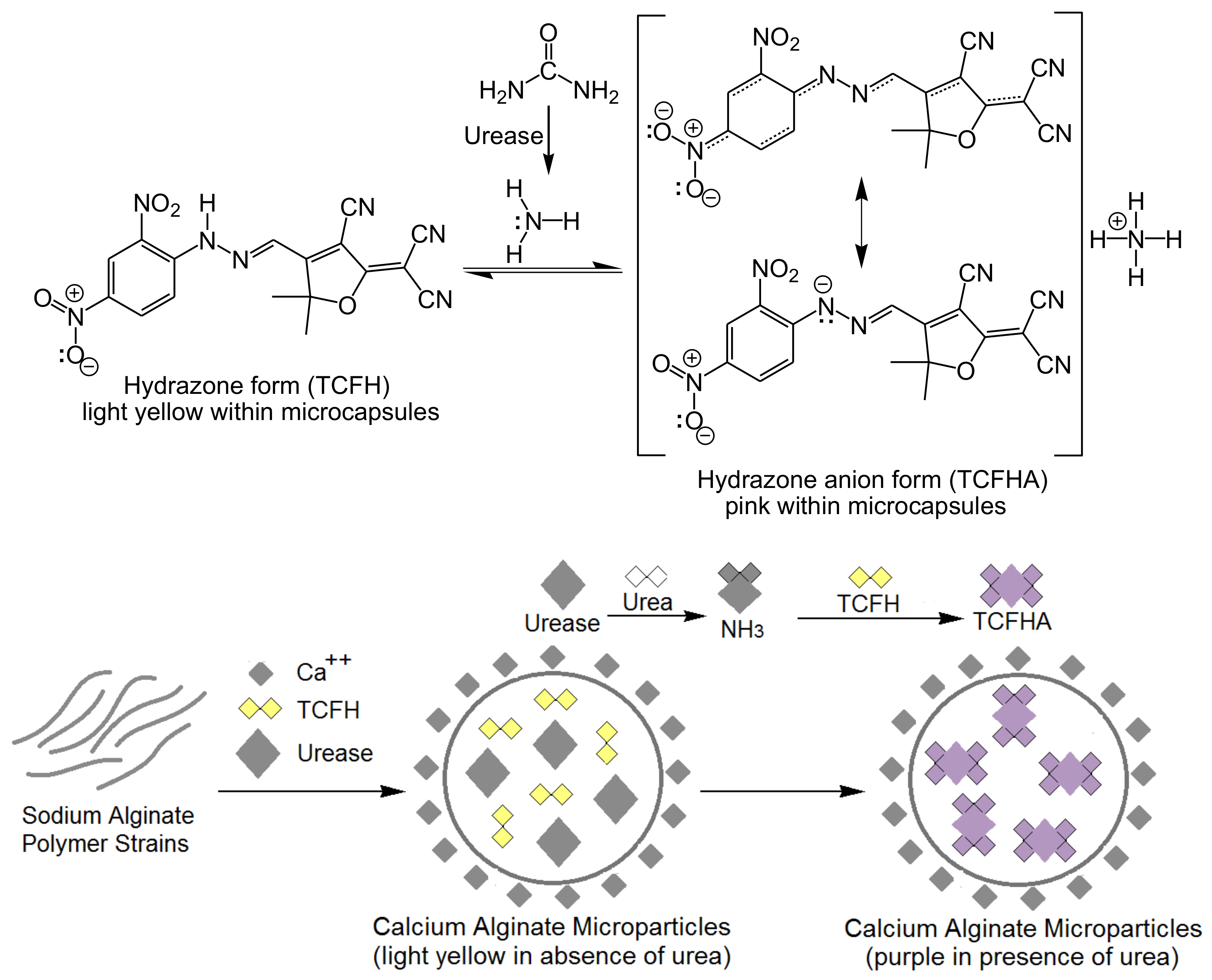
Cellulose-Based Colorimetric Biosensors
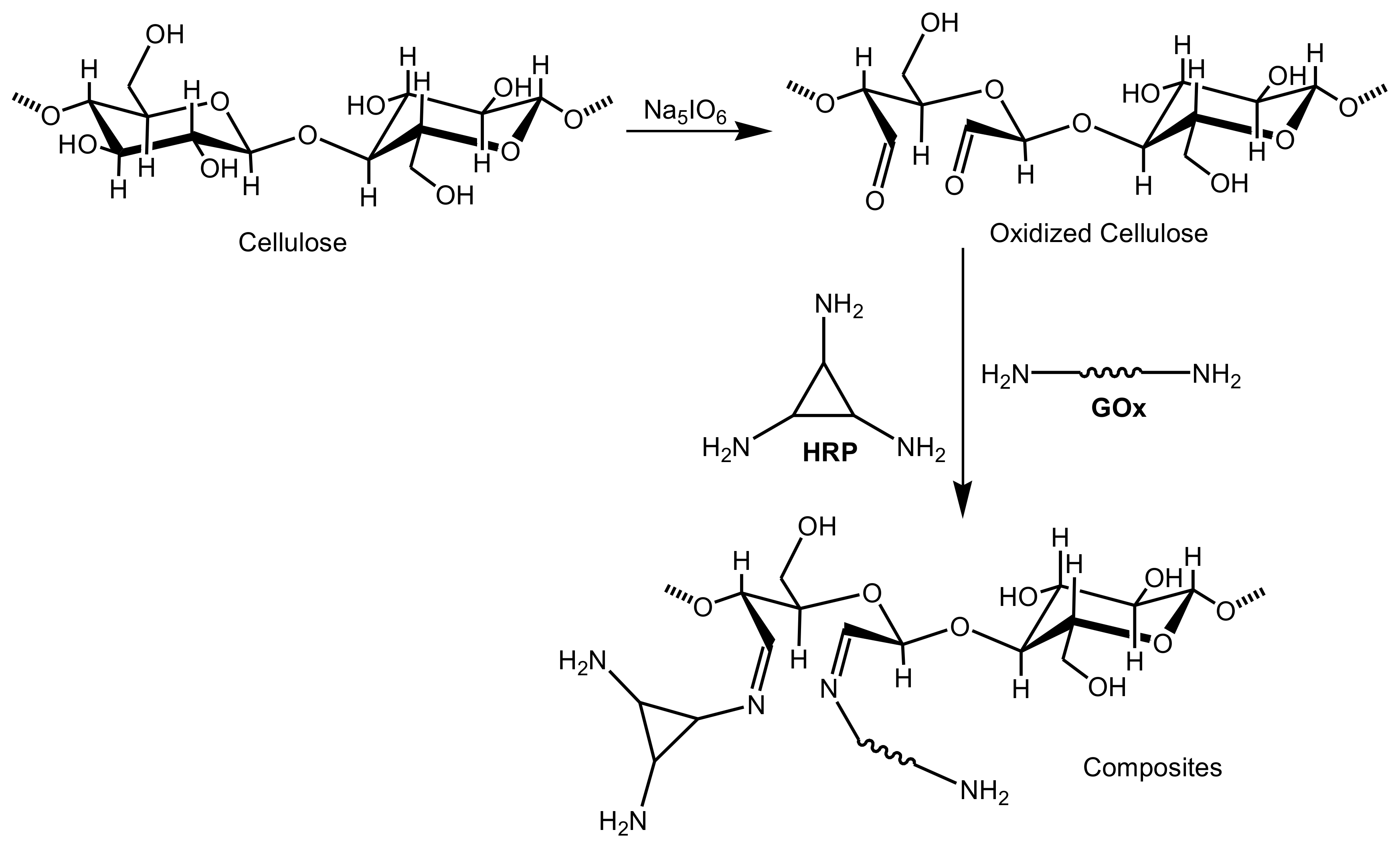
4.2. Cellulose-bBased Electrochemical Sensors
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, J.-H.; Mun, S.; Ko, H.-U.; Yun, G.-Y.; Kim, J. Disposable chemical sensors and biosensors made on cellulose paper. Nanotechnology 2014, 25, 092001. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.S.; Mostafa, A.M.; Mwafy, E.A.; Darwesh, O.M. Eco-friendly cellulose nano fibers via first reported Egyptian Humicola fuscoatra Egyptia X4: Isolation and characterization. Environ. Nanotechnol. Monit. Manag. 2018, 10, 409–418. [Google Scholar] [CrossRef]
- Khattab, T.A.; Mohamed, A.L.; Hassabo, A.G. Development of durable superhydrophobic cotton fabrics coated with silicone/stearic acid using different cross-linkers. Mater. Chem. Phys. 2020, 249, 122981. [Google Scholar] [CrossRef]
- El-Saied, H.; Mostafa, A.M.; Hasanin, M.S.; Mwafy, E.A.; Mohammed, A.A. Synthesis of antimicrobial cellulosic derivative and its catalytic activity. J. King Saud Univ.-Sci. 2020, 32, 436–442. [Google Scholar] [CrossRef]
- Ahmed, H.; Khattab, T.A.; Mashaly, H.M.; El-Halwagy, A.A.; Rehan, M. Plasma activation toward multi-stimuli responsive cotton fabric via in situ development of polyaniline derivatives and silver nanoparticles. Cellulose 2020, 27, 2913–2926. [Google Scholar] [CrossRef]
- Kamel, S.; Ali, N.; Jahangir, K.; Shah, S.M.; El-Gendy, A.A. Pharmaceutical significance of cellulose: A review. Express Polym. Lett. 2008, 2, 758–778. [Google Scholar] [CrossRef]
- Mwafy, E.A.; Hasanin, M.S.; Mostafa, A.M. Cadmium oxide/TEMPO-oxidized cellulose nanocomposites produced by pulsed laser ablation in liquid environment: Synthesis, characterization, and antimicrobial activity. Opt. Laser Technol. 2019, 120, 105744. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Awad, H.; El-Sayed, G.M.; Nagieb, Z.A.; Kamel, S. Synthesis and characterization of biocompatible hydrogel based on hydroxyethyl cellulose-g-poly (hydroxyethyl methacrylate). Polym. Bull. 2019, 1–15. [Google Scholar] [CrossRef]
- Rehan, M.; Ahmed-Farid, O.A.; Ibrahim, S.R.; Hassan, A.A.; Abdelrazek, A.M.; Khafaga, N.I.M.; Khattab, T.A. Green and sustainable encapsulation of Guava leaf extracts (Psidium guajava L.) into alginate/starch microcapsules for multifunctional finish over cotton gauze. ACS Sustain. Chem. Eng. 2019, 7, 18612–18623. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Lotfy, V.F.; Mwafy, E.A.; Basta, A.H. Influence of coating by Cu and Ag nanoparticles via pulsed laser deposition technique on optical, electrical and mechanical properties of cellulose paper. J. Mol. Struct. 2020, 1203, 127472. [Google Scholar] [CrossRef]
- Khattab, T.A.; Mowafi, S.; El-Sayed, H. Development of mechanically durable hydrophobic lanolin/silicone rubber coating on viscose fibers. Cellulose 2019, 26, 9361–9371. [Google Scholar] [CrossRef]
- Kim, J.; Seo, Y.B. Electro-active paper actuators. Smart Mater. Struct. 2002, 11, 355–360. [Google Scholar] [CrossRef]
- Deshpande, S.D.; Kim, J.; Yun, S.-R. Studies on conducting polymer electroactive paper actuators: Effect of humidity and electrode thickness. Smart Mater. Struct. 2005, 14, 876–880. [Google Scholar] [CrossRef]
- Kamel, S.; Haroun, A.A.; El-Nahrawy, A.M.; Diab, M.A. Electroconductive Composites Containing Nanocellulose, Nanopolypyrrole, and Silver Nano particles. J. Renew. Mater. 2019, 7, 193–203. [Google Scholar] [CrossRef]
- Yun, S.; Kim, J. Covalently bonded multi-walled carbon nanotubes-cellulose electro-active paper actuator. Sens. Actuators A Phys. 2009, 154, 73–78. [Google Scholar] [CrossRef]
- Kamel, S. Nanotechnology and its applications in lignocellulosic composites, a mini review. Express Polym. Lett. 2007, 1, 546–575. [Google Scholar] [CrossRef]
- Turky, G.; Moussa, M.A.; Hasanin, M.; El-Sayed, N.S.; Kamel, S. Carboxymethyl Cellulose-Based Hydrogel: Dielectric Study, Antimicrobial Activity and Biocompatibility. Arab. J. Sci. Eng. 2020, 1–14. [Google Scholar] [CrossRef]
- Siro, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Dacrory, S.; Moussa, M.; Turky, G.; Kamel, S. In situ synthesis of Fe3O4@ cyanoethyl cellulose composite as antimicrobial and semiconducting film. Carbohydr. Polym. 2020, 236, 116032. [Google Scholar] [CrossRef]
- Zakirov, A.S.; Yuldashev, S.U.; Cho, H.D.; Lee, J.C.; Kang, T.W.; Khamdamov, J.J.; Mamadalimov, A.T. Functional hybrid materials derived from natural cellulose. J. Korean Phys. Soc. 2012, 60, 1526–1530. [Google Scholar] [CrossRef]
- Gindl, W.; Keckes, J. All-cellulose nanocomposite. Polymer 2005, 46, 10221–10225. [Google Scholar] [CrossRef]
- Kumar, A.; Gullapalli, H.; Balakrishnan, K.; Botello-Mendez, A.; Vajtai, R.; Terrones, M.; Ajayan, P.M. Flexible ZnO—Cellulose nanocomposite for multisource energy conversion. Small 2011, 7, 2173–2178. [Google Scholar] [CrossRef]
- Khan, A.; Abas, Z.; Kim, H.S.; Kim, J. Recent progress on cellulose-based electro-active paper, its hybrid nanocomposites and applications. Sensors 2016, 16, 1172. [Google Scholar] [CrossRef] [PubMed]
- Khattab, T.A.; Fouda, M.M.G.; Abdelrahman, M.S.; Othman, S.I.; Bin-Jumah, M.; Alqaraawi, M.A.; Al Fassam, H.; Allam, A.A. Development of illuminant glow-in-the-dark cotton fabric coated by luminescent composite with antimicrobial activity and ultraviolet protection. J. Fluoresc. 2019, 29, 703–710. [Google Scholar] [CrossRef]
- El-Nahrawy, A.M.; Abou Hammad, A.B.; Khattab, T.A.; Haroun, A.; Kamel, S. Development of electrically conductive nanocomposites from cellulose nanowhiskers, polypyrrole and silver nanoparticles assisted with Nickel (III) oxide nanoparticles. React. Funct. Polym. 2020, 149, 104533. [Google Scholar] [CrossRef]
- Khattab, T.A.; Abou-Yousef, H.; Kamel, S. Photoluminescent spray-coated paper sheet: Write-in-the-dark. Carbohydr. Polym. 2018, 200, 154–161. [Google Scholar] [CrossRef]
- Abdelrahman, M.S.; Nassar, S.H.; Mashaly, H.; Mahmoud, S.; Maamoun, D.; El-Sakhawy, M.; Khattab, T.A.; Kamel, S. Studies of Polylactic Acid and Metal Oxide Nanoparticles-Based Composites for Multifunctional Textile Prints. Coatings 2020, 10, 58. [Google Scholar] [CrossRef]
- Khattab, T.A.; Aly, S.A.; Klapötke, T.M. Naked-eye facile colorimetric detection of alkylphenols using Fe (III)-impregnated silica-based strips. Chem. Pap. 2018, 72, 1553–1559. [Google Scholar] [CrossRef]
- Khattab, T.A. Synthesis and Self-assembly of Novel s-Tetrazine-based Gelator. Helv. Chim. Acta 2018, 101, e1800009. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Bandyopadhyay, D. Flexible paper touchpad for Parkinson’s hand tremor detection. Sens. Actuators A Phys. 2019, 294, 164–172. [Google Scholar] [CrossRef]
- Dutta, S.; Mandal, N.; Bandyopadhyay, D. Based α-amylase detector for point-of-care diagnostics. Biosens. Bioelectron. 2016, 78, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Parolo, C.; Merkoçi, A. Based nanobiosensors for diagnostics. Chem. Soc. Rev. 2013, 42, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, M.; Zhou, F.; Yang, G.; Qu, L.; Miao, X. Development of a paper-based, inexpensive, and disposable electrochemical sensing platform for nitrite detection. Electrochem. Commun. 2017, 81, 74–78. [Google Scholar] [CrossRef]
- Salentijn, G.I.J.; Grajewski, M.; Verpoorte, E. Reinventing (bio) chemical analysis with paper. Anal. Chem. 2018, 90, 13815–13825. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jung, E.; Kim, M.J.; Pyo, A.; Palani, T.; Eom, M.S.; Han, M.S.; Lee, S. A simple, fast, and easy assay for transition metal-catalyzed coupling reactions using a paper-based colorimetric iodide sensor. Chem. Commun. 2012, 48, 8751–8753. [Google Scholar] [CrossRef]
- Costa, S.V.; Gonçalves, A.S.; Zaguete, M.A.; Mazon, T.; Nogueira, A.F. ZnO nanostructures directly grown on paper and bacterial cellulose substrates without any surface modification layer. Chem. Commun. 2013, 49, 8096–8098. [Google Scholar] [CrossRef]
- Ikeda, M.; Fukuda, K.; Tanida, T.; Yoshii, T.; Hamachi, I. A supramolecular hydrogel containing boronic acid-appended receptor for fluorocolorimetric sensing of polyols with a paper platform. Chem. Commun. 2012, 48, 2716–2718. [Google Scholar] [CrossRef]
- Khazi, M.I.; Jeong, W.; Kim, J.-M. Functional materials and systems for rewritable paper. Adv. Mater. 2018, 30, 1705310. [Google Scholar] [CrossRef]
- Gorai, T.; Maitra, U. Eu/Tb luminescence for alkaline phosphatase and β-galactosidase assay in hydrogels and on paper devices. J. Mater. Chem. B 2018, 6, 2143–2150. [Google Scholar] [CrossRef]
- Credou, J.; Berthelot, T. Cellulose: From biocompatible to bioactive material. J. Mater. Chem. B 2014, 2, 4767–4788. [Google Scholar] [CrossRef]
- Rull-Barrull, J.; d’Halluin, M.; Le Grognec, E.; Felpin, F.-X. Chemically-modified cellulose paper as smart sensor device for colorimetric and optical detection of hydrogen sulfate in water. Chem. Commun. 2016, 52, 2525–2528. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Li, L.; Li, Z.; Zhang, Y.; Li, M.; Shi, J.; Wang, L.; Fan, C.; Yu, J.; Zuo, X. Molecular threading-dependent mass transport in paper origami for single-step electrochemical DNA sensors. Nano Lett. 2018, 19, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Güder, F.; Ainla, A.; Redston, J.; Mosadegh, B.; Glavan, A.; Martin, T.J.; Whitesides, G.M. Paper-based electrical respiration sensor. Angew. Chem. Int. Ed. 2016, 55, 5727–5732. [Google Scholar] [CrossRef]
- Lin, H.; Wang, X.; Wu, J.; Li, H.; Li, F. Equipment-free and visualized biosensor for transcription factor rapid assay based on dopamine-functionalized cellulose paper. J. Mater. Chem. B 2019, 7, 5461–5464. [Google Scholar] [CrossRef] [PubMed]
- Hepel, M.; Stobiecka, M. Detection of oxidative stress biomarkers using functional gold nanoparticles. In Fine Particles in Medicine and Pharmacy; Springer: Boston, MA, USA, 2012; pp. 241–281. [Google Scholar]
- Ratajczak, K.; Stobiecka, M. High-performance modified cellulose paper-based biosensors for medical diagnostics and early cancer screening: A concise review. Carbohydr. Polym. 2020, 229, 115463. [Google Scholar] [CrossRef] [PubMed]
- Mahadeva, S.K.; Kim, J. Hybrid nanocomposite based on cellulose and tin oxide: Growth, structure, tensile and electrical characteristics. Sci. Technol. Adv. Mater. 2011, 12, 055006. [Google Scholar] [CrossRef] [PubMed]
- Manan, F.A.A.; Hong, W.W.; Abdullah, J.; Yusof, N.A.; Ahmad, I. Nanocrystalline cellulose decorated quantum dots based tyrosinase biosensor for phenol determination. Mater. Sci. Eng. C 2019, 99, 37–46. [Google Scholar] [CrossRef]
- Liao, C.-W.; Chou, J.-C.; Sun, T.-P.; Hsiung, S.-K.; Hsieh, J.-H. Preliminary investigations on a glucose biosensor based on the potentiometric principle. Sens. Actuators B Chem. 2007, 123, 720–726. [Google Scholar] [CrossRef]
- Delvaux, M.; Demoustier-Champagne, S. Immobilisation of glucose oxidase within metallic nanotubes arrays for application to enzyme biosensors. Biosens. Bioelectron. 2003, 18, 943–951. [Google Scholar] [CrossRef]
- Ekanayake, E.M.I.M.; Preethichandra, D.M.G.; Kaneto, K. Polypyrrole nanotube array sensor for enhanced adsorption of glucose oxidase in glucose biosensors. Biosens. Bioelectron. 2007, 23, 107–113. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Anal. Lett. 2001, 34, 635–659. [Google Scholar] [CrossRef]
- Monošík, R.; Streďanský, M.; Šturdík, E. Biosensors-classification, characterization and new trends. Acta Chim. Slovaca 2012, 5, 109–120. [Google Scholar] [CrossRef]
- Baptista, A.C.; Ferreira, I.M.M.; Borges, J.P.M.R. Cellulose-based bioelectronic devices. In Cellulose-Medical, Pharmaceutical and Electronic Applications; InTech: London, UK, 2013; pp. 67–82. [Google Scholar]
- Monk, D.J.; Walt, D.R. Optical fiber-based biosensors. Anal. Bioanal. Chem. 2004, 379, 931–945. [Google Scholar] [CrossRef]
- Palanisamy, S.; Velusamy, V.; Chen, S.-W.; Yang, T.C.K.; Balu, S.; Banks, C.E. Enhanced reversible redox activity of hemin on cellulose microfiber integrated reduced graphene oxide for H2O2 biosensor applications. Carbohydr. Polym. 2019, 204, 152–160. [Google Scholar] [CrossRef]
- Erden, P.E.; Kılıç, E. A review of enzymatic uric acid biosensors based on amperometric detection. Talanta 2013, 107, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, A.R.; Liu, C.; Schafer, K.-H.; Saumer, M.; Yang, G.; Liu, Y. Poly (4-vinylaniline)/Polyaniline Bilayer-Functionalized Bacterial Cellulose for Flexible Electrochemical Biosensors. Langmuir 2019, 35, 10354–10366. [Google Scholar] [CrossRef]
- Thakur, V.K.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Deng, Q.; Wu, X. Synthesis and characterization of cellulose derivatives prepared in NaOH/urea aqueous solutions. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5911–5920. [Google Scholar] [CrossRef]
- Koschella, A.; Fenn, D.; Illy, N.; Heinze, T. Regioselectively functionalized cellulose derivatives: A mini review. In Macromolecular Symposia; WILEY-VCH Verlag: Weinheim, Germany, 2006; Volume 244, pp. 59–73. [Google Scholar]
- Desmet, G.; Takács, E.; Wojnárovits, L.; Borsa, J. Cellulose functionalization via high-energy irradiation-initiated grafting of glycidyl methacrylate and cyclodextrin immobilization. Radiat. Phys. Chem. 2011, 80, 1358–1362. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Yang, J.; Li, Z.; Wang, H. Functionalized bacterial cellulose derivatives and nanocomposites. Carbohydr. Polym. 2014, 101, 1043–1060. [Google Scholar] [CrossRef]
- Kang, H.; Liu, R.; Huang, Y. Cellulose derivatives and graft copolymers as blocks for functional materials. Polym. Int. 2013, 62, 338–344. [Google Scholar] [CrossRef]
- Heinze, T.; El Seoud, O.A.; Koschella, A. Cellulose Derivatives: Synthesis, Structure, and Properties; Springer: Berlin, Germany, 2018. [Google Scholar]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Male, K.B.; Chong, J.H.; Leung, A.C.W.; Luong, J.H.T. Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends Biotechnol. 2012, 30, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Reis, D.T.; dos Santos Pereira, A.K.; Scheidt, G.N.; Pereira, D.H. Plant and Bacterial Cellulose: Production, Chemical Structure, Derivatives and Applications. Orbital Electron. J. Chem. 2019, 11, 321–329. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Xie, Y.; Zhang, K. Functional nanomaterials through esterification of cellulose: A review of chemistry and application. Cellulose 2018, 25, 3703–3731. [Google Scholar] [CrossRef]
- Abdelrahman, M.S.; Khattab, T.A. Development of One-Step Water-Repellent and Flame-Retardant Finishes for Cotton. ChemistrySelect 2019, 4, 3811–3816. [Google Scholar] [CrossRef]
- Sternberg, R.; Bindra, D.S.; Wilson, G.S.; Thevenot, D.R. Covalent enzyme coupling on cellulose acetate membranes for glucose sensor development. Anal. Chem. 1988, 60, 2781–2786. [Google Scholar] [CrossRef]
- Yun, G.Y.; Kim, H.S.; Kim, J.; Kim, K.; Yang, C. Effect of aligned cellulose film to the performance of electro-active paper actuator. Sens. Actuators A Phys. 2008, 141, 530–535. [Google Scholar] [CrossRef]
- Tamayo, J.; Kosaka, P.M.; Ruz, J.J.; San Paulo, A.; Calleja, M. Biosensors based on nanomechanical systems. Chem. Soc. Rev. 2013, 42, 1287–1311. [Google Scholar] [CrossRef]
- Abou-Yousef, H.; Khattab, T.A.; Youssef, Y.A.; Al-Balakocy, N.; Kamel, S. Novel cellulose-based halochromic test strips for naked-eye detection of alkaline vapors and analytes. Talanta 2017, 170, 137–145. [Google Scholar] [CrossRef]
- Khattab, T.A.; Dacrory, S.; Abou-Yousef, H.; Kamel, S. Development of microporous cellulose-based smart xerogel reversible sensor via freeze drying for naked-eye detection of ammonia gas. Carbohydr. Polym. 2019, 210, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Khattab, T.A.; Kassem, N.F.; Adel, A.M.; Kamel, S. Optical recognition of ammonia and amine vapor using “turn-on” fluorescent chitosan nanoparticles imprinted on cellulose strips. J. Fluoresc. 2019, 29, 693–702. [Google Scholar] [CrossRef]
- Khattab, T.A.; Dacrory, S.; Abou-Yousef, H.; Kamel, S. Smart microfibrillated cellulose as swab sponge-like aerogel for real-time colorimetric naked-eye sweat monitoring. Talanta 2019, 205, 120166. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.S.; Fouda, M.M.G.; Ajarem, J.S.; Maodaa, S.N.; Allam, A.A.; Khattab, T.A. Development of colorimetric cotton swab using molecular switching hydrazone probe in calcium alginate. J. Mol. Struct. 2020, 1216, 128301. [Google Scholar] [CrossRef]
- Guo, X. Surface plasmon resonance based biosensor technique: A review. J. Biophotonics 2012, 5, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Rich, R.L.; Myszka, D.G. Survey of the 2009 commercial optical biosensor literature. J. Mol. Recognit. 2011, 24, 892–914. [Google Scholar] [CrossRef]
- Yoo, S.M.; Lee, S.Y. Optical biosensors for the detection of pathogenic microorganisms. Trends Biotechnol. 2016, 34, 7–25. [Google Scholar] [CrossRef]
- Mateescu, A.; Wang, Y.; Dostalek, J.; Jonas, U. Thin hydrogel films for optical biosensor applications. Membranes 2012, 2, 40–69. [Google Scholar] [CrossRef]
- Wang, L.; Ren, J.; Han, X.; Claes, T.; Jian, X.; Bienstman, P.; Baets, R.; Zhao, M.; Morthier, G. A label-free optical biosensor built on a low-cost polymer platform. IEEE Photonics J. 2012, 4, 920–930. [Google Scholar] [CrossRef]
- Long, F.; Zhu, A.; Shi, H.; Wang, H.; Liu, J. Rapid on-site/in-situ detection of heavy metal ions in environmental water using a structure-switching DNA optical biosensor. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef]
- Abdolrahim, M.; Rabiee, M.; Alhosseini, S.N.; Tahriri, M.; Yazdanpanah, S.; Tayebi, L. Development of optical biosensor technologies for cardiac troponin recognition. Anal. Biochem. 2015, 485, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Gu, H.; Ma, C. An aptamer-based fluorescent biosensor for potassium ion detection using a pyrene-labeled molecular beacon. Anal. Biochem. 2010, 400, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhu, L.; Wu, J.; Hou, Y.; Wang, P.; Wang, Z.; Yang, M. A fluorescent biosensor based on carbon dots-labeled oligodeoxyribonucleotide and graphene oxide for mercury (II) detection. Biosens. Bioelectron. 2015, 6, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Emrani, A.S.; Danesh, N.M.; Lavaee, P.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Colorimetric and fluorescence quenching aptasensors for detection of streptomycin in blood serum and milk based on double-stranded DNA and gold nanoparticles. Food Chem. 2016, 190, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Povlich, L.K.; Kim, J. Recent advances in fluorescent and colorimetric conjugated polymer-based biosensors. Analyst 2010, 135, 2179–2189. [Google Scholar] [CrossRef]
- Bañuls, M.-J.; Puchades, R.; Maquieira, A. Chemical surface modifications for the development of silicon-based label-free integrated optical (IO) biosensors: A review. Anal. Chim. Acta 2013, 777, 1–16. [Google Scholar] [CrossRef]
- Baharifar, H.; Honarvarfard, E.; Malek-kheili, M.H.; Maleki, H.; Barkhi, M.; Ghasemzadeh, A.; Khoshnevisan, K. The Potentials and Applications of Cellulose Acetate in biosensor technology. Nanomed. Res. J. 2017, 2, 216–223. [Google Scholar]
- Wang, S.; Li, S.; Yu, Y. Immobilization of cholesterol oxidase on cellulose acetate membrane for free cholesterol biosensor development. Artif. Cells Blood Substit. Biotechnol. 2004, 32, 413–425. [Google Scholar] [CrossRef]
- Yu, S.; Ding, L.; Lin, H.; Wu, W.; Huang, J. A novel optical fiber glucose biosensor based on carbon quantum dots-glucose oxidase/cellulose acetate complex sensitive film. Biosens. Bioelectron. 2019, 146, 111760. [Google Scholar] [CrossRef]
- An, K.; Duong, H.D.; Rhee, J.I. Ratiometric fluorescent l-arginine and l-asparagine biosensors based on the oxazine 170 perchlorate-ethyl cellulose membrane. Eng. Life Sci. 2017, 17, 847–856. [Google Scholar] [CrossRef]
- Duong, H.D.; Rhee, J.I. Development of a ratiometric fluorescent urea biosensor based on the urease immobilized onto the oxazine 170 perchlorate-ethyl cellulose membrane. Talanta 2015, 134, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Schyrr, B.; Pasche, S.; Voirin, G.; Weder, C.; Simon, Y.C.; Foster, E.J. Biosensors based on porous cellulose nanocrystal–poly (vinyl alcohol) scaffolds. ACS Appl. Mater. Interfaces 2014, 6, 12674–12683. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.W.; Niamnont, N.; Hare, C.D.; Sukwattanasinitt, M.; Cheng, Q. Nanofibers doped with dendritic fluorophores for protein detection. ACS Appl. Mater. Interfaces 2010, 2, 1798–1803. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Caston-Pierre, S.; Bopp, A.F.; Goynes, W. Detection of human neutrophil elastase with peptide-bound cross-linked ethoxylate acrylate resin analogs. J. Pept. Res. 2005, 66, 160–168. [Google Scholar] [CrossRef]
- Edwards, J.V.; Prevost, N.T.; French, A.D.; Concha, M.; Condon, B.D. Kinetic and structural analysis of fluorescent peptides on cotton cellulose nanocrystals as elastase sensors. Carbohydr. Polym. 2015, 116, 278–285. [Google Scholar] [CrossRef]
- Edwards, J.V.; Prevost, N.; Sethumadhavan, K.; Ullah, A.; Condon, B. Peptide conjugated cellulose nanocrystals with sensitive human neutrophil elastase sensor activity. Cellulose 2013, 20, 1223–1235. [Google Scholar] [CrossRef]
- Edwards, J.V.; Prevost, N.; French, A.; Concha, M.; DeLucca, A.; Wu, Q. Nanocellulose-based biosensors: Design, preparation, and activity of peptide-linked cotton cellulose nanocrystals having fluorimetric and colorimetric elastase detection sensitivity. Engineering 2013, 5, 20–28. [Google Scholar] [CrossRef]
- Fontenot, K.R.; Edwards, J.V.; Haldane, D.; Pircher, N.; Liebner, F.; Condon, B.D.; Qureshi, H.; Yager, D. Designing cellulosic and nanocellulosic sensors for interface with a protease sequestrant wound-dressing prototype: Implications of material selection for dressing and protease sensor design. J. Biomater. Appl. 2017, 32, 622–637. [Google Scholar] [CrossRef]
- Shadnia, H.; Wright, J.S. Understanding the toxicity of phenols: Using quantitative structure—Activity relationship and enthalpy changes to discriminate between possible mechanisms. Chem. Res. Toxicol. 2008, 21, 1197–1204. [Google Scholar] [CrossRef]
- Derikvand, F.; Yin, D.T.; Barrett, R.; Brumer, H. Cellulose-based biosensors for esterase detection. Anal. Chem. 2016, 88, 2989–2993. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Spadiut, O.; Araújo, A.C.; Nakhai, A.; Brumer, H. Chemo-enzymatic assembly of clickable cellulose surfaces via multivalent polysaccharides. ChemSusChem 2012, 5, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Chopra, A.; Arora, S.; Yadav, S.; Kumar, S.; Kaur, I. Amperometric sensing of urea using edge activated graphene nanoplatelets. RSC Adv. 2015, 5, 13278–13284. [Google Scholar] [CrossRef]
- Soldatkin, O.O.; Kucherenko, I.S.; Marchenko, S.V.; Kasap, B.O.; Akata, B.; Soldatkin, A.P.; Dzyadevych, S.V. Application of enzyme/zeolite sensor for urea analysis in serum. Mater. Sci. Eng. C 2014, 42, 155–160. [Google Scholar] [CrossRef]
- Kessler, A.; Siekmann, L. Measurement of urea in human serum by isotope dilution mass spectrometry: A reference procedure. Clin. Chem. 1999, 45, 1523–1529. [Google Scholar] [CrossRef]
- Nguyen, N.S.; Das, G.; Yoon, H.H. Nickel/cobalt oxide-decorated 3D graphene nanocomposite electrode for enhanced electrochemical detection of urea. Biosens. Bioelectron. 2016, 77, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Bisht, V.; Takashima, W.; Kaneto, K. An amperometric urea biosensor based on covalent immobilization of urease onto an electrochemically prepared copolymer poly (N-3-aminopropyl pyrrole-co-pyrrole) film. Biomaterials 2005, 26, 3683–3690. [Google Scholar]
- Khattab, T.A.; Fouda, M.M.G.; Abdelrahman, M.S.; Othman, S.I.; Bin-Jumah, M.; Alqaraawi, M.A.; Al Fassam, H.; Allam, A.A. Co-encapsulation of enzyme and tricyanofuran hydrazone into alginate microcapsules incorporated onto cotton fabric as a biosensor for colorimetric recognition of urea. React. Funct. Polym. 2019, 142, 199–206. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef]
- Khattab, T.A.; Fouda, M.M.G.; Rehan, M.; Okla, M.K.; Alamri, S.A.; Alaraidh, I.A.; AL-ghamdi, A.A.; Soufan, W.H.; Abdelsalam, E.M.; Allam, A.A. Novel halochromic cellulose nanowhiskers from rice straw: Visual detection of urea. Carbohydr. Polym. 2020, 231, 115740. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, L.; Li, Q.; Wang, S.; Luo, X.; Deng, H.; Liu, S. Porous structured cellulose microsphere acts as biosensor for glucose detection with “signal-and-color” output. Carbohydr. Polym. 2019, 205, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Imamura, A.H.; Segato, T.P.; de Oliveira, L.J.M.; Hassan, A.; Crespilho, F.N.; Carrilho, E. Monitoring cellulose oxidation for protein immobilization in paper-based low-cost biosensors. Microchim. Acta 2020, 187, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Kedari, C.; Tripathi, S. Spectrophotometric determination of trace amount of uranium (VI) in different aqueous and organic streams of nuclear fuel processing using 2-(5-bromo-2-pyridylazo-5-diethylaminophenol). J. Radioanal. Nucl. Chem. 2010, 285, 675–681. [Google Scholar] [CrossRef]
- Hu, L.; Yan, X.-W.; Li, Q.; Zhang, X.-J.; Shan, D. Br-PADAP embedded in cellulose acetate electrospun nanofibers: Colorimetric sensor strips for visual uranyl recognition. J. Hazard. Mater. 2017, 329, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xia, J.; Jiang, X.; Yang, M.; Liu, S. Cellulose-Based Strips Designed Based on a Sensitive Enzyme Colorimetric Assay for the Low Concentration of Glucose Detection. Anal. Chem. 2019, 91, 15461–15468. [Google Scholar] [CrossRef]
- Lawrence, C.S.K.; Tan, S.N.; Floresca, C.Z. A “green” cellulose paper based glucose amperometric biosensor. Sens. Actuators B Chem. 2014, 193, 536–541. [Google Scholar] [CrossRef]
- Cruys-Bagger, N.; Ren, G.; Tatsumi, H.; Baumann, M.J.; Spodsberg, N.; Andersen, H.D.; Gorton, L.; Borch, K.; Westh, P. An amperometric enzyme biosensor for real-time measurements of cellobiohydrolase activity on insoluble cellulose. Biotechnol. Bioeng. 2012, 109, 3199–3204. [Google Scholar] [CrossRef]
- Saeed, A.A.; Abbas, M.N.; Singh, B.; Abou-Zeid, R.E.; Kamel, S. Cellulose nanocrystals decorated with gold nanoparticles immobilizing GOx enzyme for non-invasive biosensing of human salivary glucose. Anal. Methods 2019, 11, 6073–6083. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Kim, J. Conductometric glucose biosensor made with cellulose and tin oxide hybrid nanocomposite. Sens. Actuators B Chem. 2011, 157, 177–182. [Google Scholar] [CrossRef]
- Ansari, S.G.; Ansari, Z.A.; Wahab, R.; Kim, Y.-S.; Khang, G.; Shin, H.-S. Glucose sensor based on nano-baskets of tin oxide templated in porous alumina by plasma enhanced CVD. Biosens. Bioelectron. 2008, 23, 1838–1842. [Google Scholar] [CrossRef] [PubMed]
- Gunasingham, H.; Teo, P.Y.T.; Lai, Y.-H.; Tan, S.-G. Chemically modified cellulose acetate membrane for biosensor applications. Biosensors 1989, 4, 349–359. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Kim, J. Porous tin-oxide-coated regenerated cellulose as disposable and low-cost alternative transducer for urea detection. IEEE Sens. J. 2013, 13, 2223–2228. [Google Scholar] [CrossRef]
- Ren, X.; Chen, D.; Meng, X.; Tang, F.; Du, A.; Zhang, L. Amperometric glucose biosensor based on a gold nanorods/cellulose acetate composite film as immobilization matrix. Colloids Surf. B Biointerfaces 2009, 72, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, H.; Akyilmaz, E.; Dinçkaya, E. Catalase immobilization in cellulose acetate beads and determination of its hydrogen peroxide decomposition level by using a catalase biosensor. Artif. Cells Blood Substit. Biotechnol. 2004, 32, 443–452. [Google Scholar] [CrossRef]
- Gilmartin, M.A.T.; Hart, J.P. Novel, reagentless, amperometric biosensor for uric acid based on a chemically modified screen-printed carbon electrode coated with cellulose acetate and uricase. Analyst 1994, 119, 833–840. [Google Scholar] [CrossRef]
- Tsiafoulis, C.G.; Prodromidis, M.I.; Karayannis, M.I. Development of amperometric biosensors for the determination of glycolic acid in real samples. Anal. Chem. 2002, 74, 132–139. [Google Scholar] [CrossRef]
- Moccelini, S.K.; Franzoi, A.C.; Vieira, I.C.; Dupont, J.; Scheeren, C.W. A novel support for laccase immobilization: Cellulose acetate modified with ionic liquid and application in biosensor for methyldopa detection. Biosens. Bioelectron. 2011, 26, 3549–3554. [Google Scholar] [CrossRef]
- Palanisamy, S.; Ramaraj, S.K.; Chen, S.-M.; Yang, T.C.K.; Yi-Fan, P.; Chen, T.-W.; Velusamy, V.; Selvam, S. A novel laccase biosensor based on laccase immobilized graphene-cellulose microfiber composite modified screen-printed carbon electrode for sensitive determination of catechol. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Vaidya, R.; Wilkins, E. Effect of interference on amperometric glucose biosensors with cellulose acetate membranes. Electroanalysis 1994, 6, 677–682. [Google Scholar] [CrossRef]
- Tsiafoulis, C.G.; Prodromidis, M.I.; Karayannis, M.I. Development of an amperometric biosensing method for the determination of L-fucose in pretreated urine. Biosens. Bioelectron. 2004, 20, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Tkáč, J.; Voštiar, I.; Gemeiner, P.; Šturdıík, E. Stabilization of ferrocene leakage by physical retention in a cellulose acetate membrane. The fructose biosensor. Bioelectrochemistry 2002, 55, 149–151. [Google Scholar] [CrossRef]
- Martins, G.V.; Tavares, A.P.M.; Fortunato, E.; Sales, M.G.F. Based sensing device for electrochemical detection of oxidative stress biomarker 8-hydroxy-2′-deoxyguanosine (8-OHdG) in point-of-care. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Cusenza, R.; Moscone, D.; Arduini, F. Based synthesis of Prussian Blue Nanoparticles for the development of whole blood glucose electrochemical biosensor. Talanta 2018, 187, 59–64. [Google Scholar] [CrossRef]
- Chen, X.; Lan, J.; Liu, Y.; Li, L.; Yan, L.; Xia, Y.; Wu, F.; Li, C.; Li, S.; Chen, J. A paper-supported aptasensor based on upconversion luminescence resonance energy transfer for the accessible determination of exosomes. Biosens. Bioelectron. 2018, 102, 582–588. [Google Scholar] [CrossRef]
- Ramadhan, L.O.A.N.; Jahiding, M. Analysis of diazinon pesticide using potentiometric biosensor based on enzyme immobilized cellulose acetate membrane in gold electrode. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016; Volume 107, p. 012013. [Google Scholar]
- Pandey, A.; Pandey, P.; Pandey, O.P.; Shukla, N.K. Fabrication of Potentiometric Cholesterol Biosensor by Crosslinking of Cholesterol Oxidase and Carbon Nanotubes Modified Cellulose Acetate Membrane. Sens. Lett. 2016, 14, 102–108. [Google Scholar] [CrossRef]
- Alpat, Ş.; Telefoncu, A. Development of an alcohol dehydrogenase biosensor for ethanol determination with toluidine blue O covalently attached to a cellulose acetate modified electrode. Sensors 2010, 10, 748–764. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Jang, S.-D.; Kim, J. Titanium dioxide–cellulose hybrid nanocomposite and its glucose biosensor application. Mater. Sci. Eng. B 2012, 177, 844–848. [Google Scholar] [CrossRef]
- Mun, S.; Maniruzzaman, M.; Ko, H.-U.; Kafy, A.; Kim, J. Preparation and characterisation of cellulose ZnO hybrid film by blending method and its glucose biosensor application. Mater. Technol. 2015, 30, B150–B154. [Google Scholar] [CrossRef]
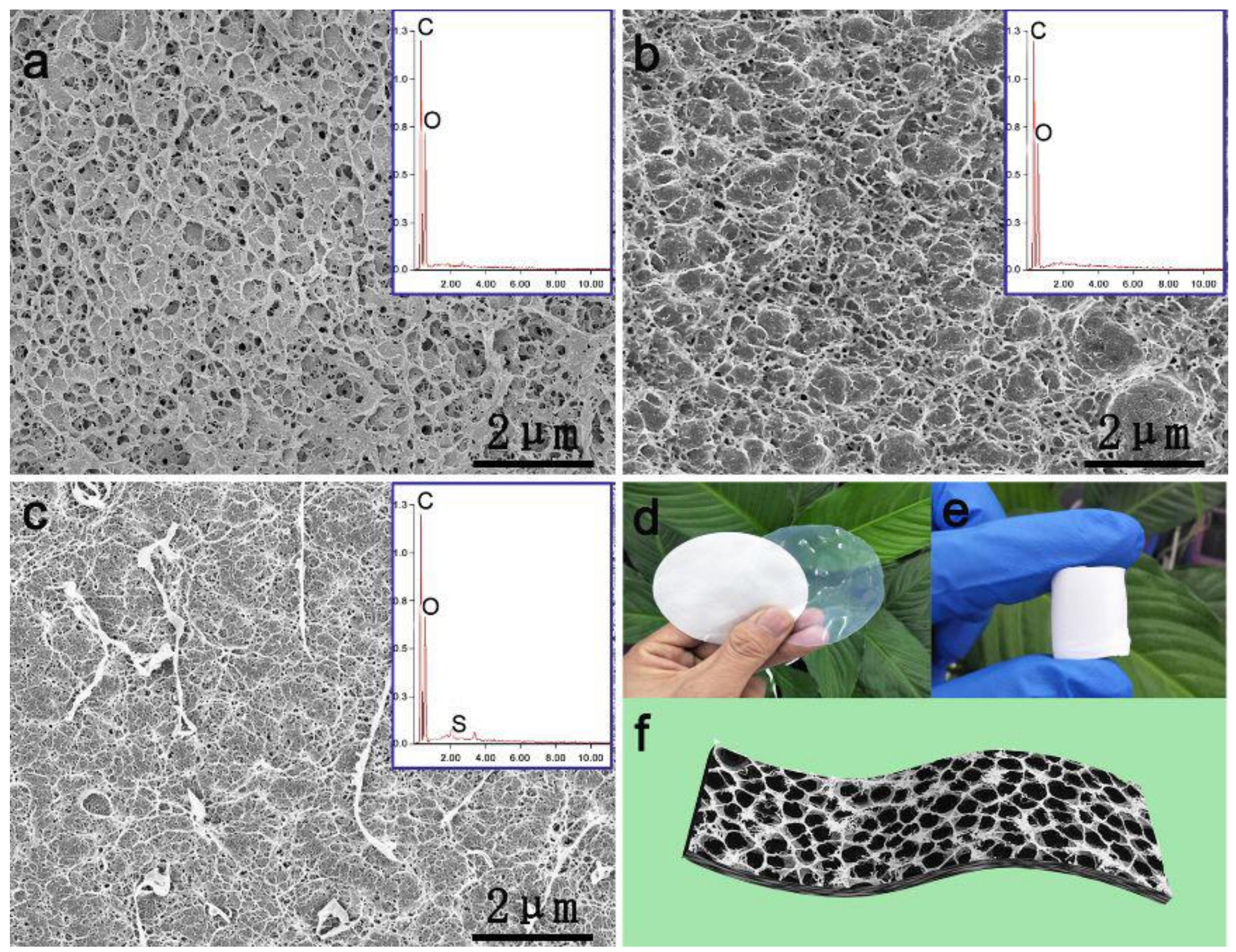
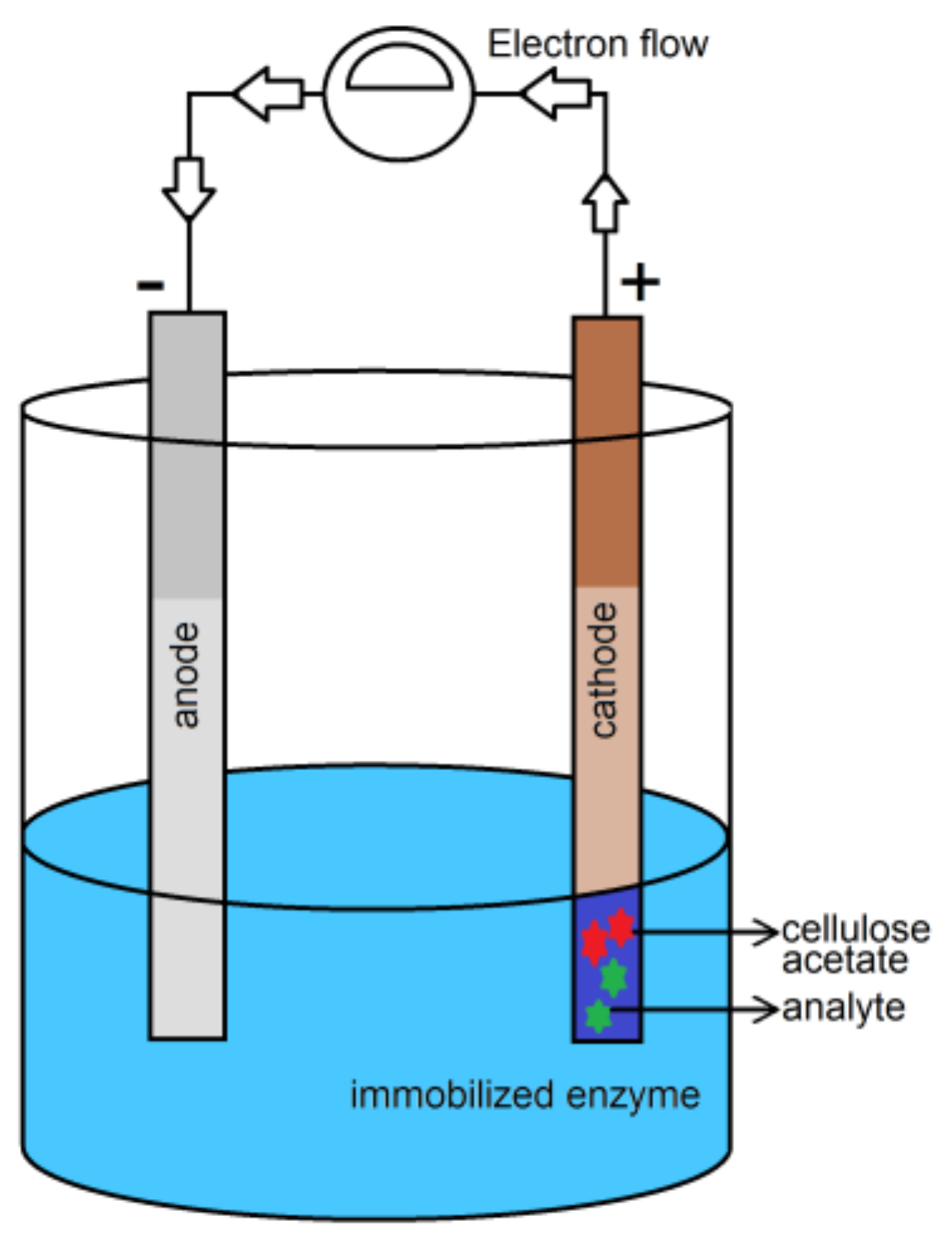
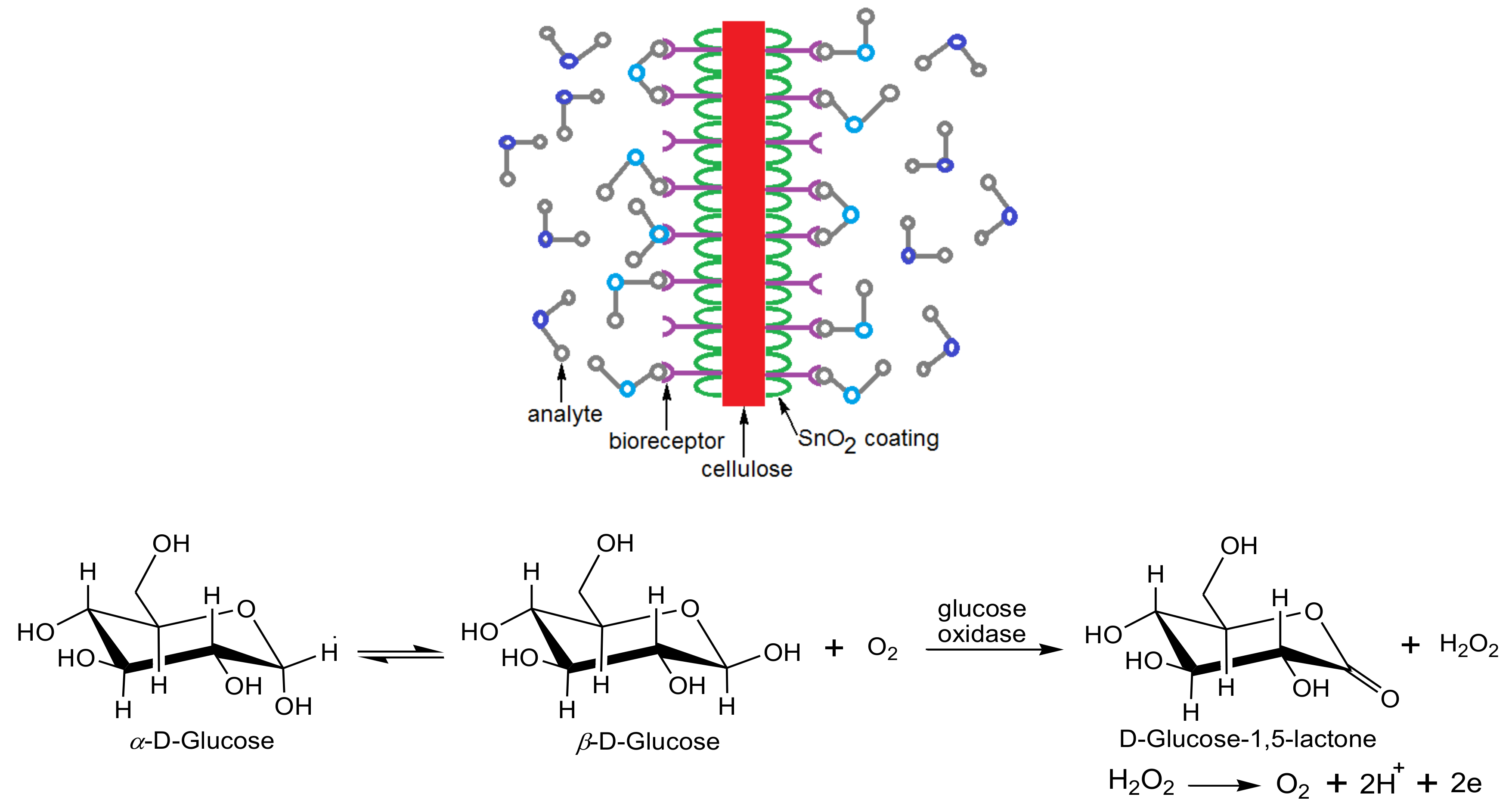
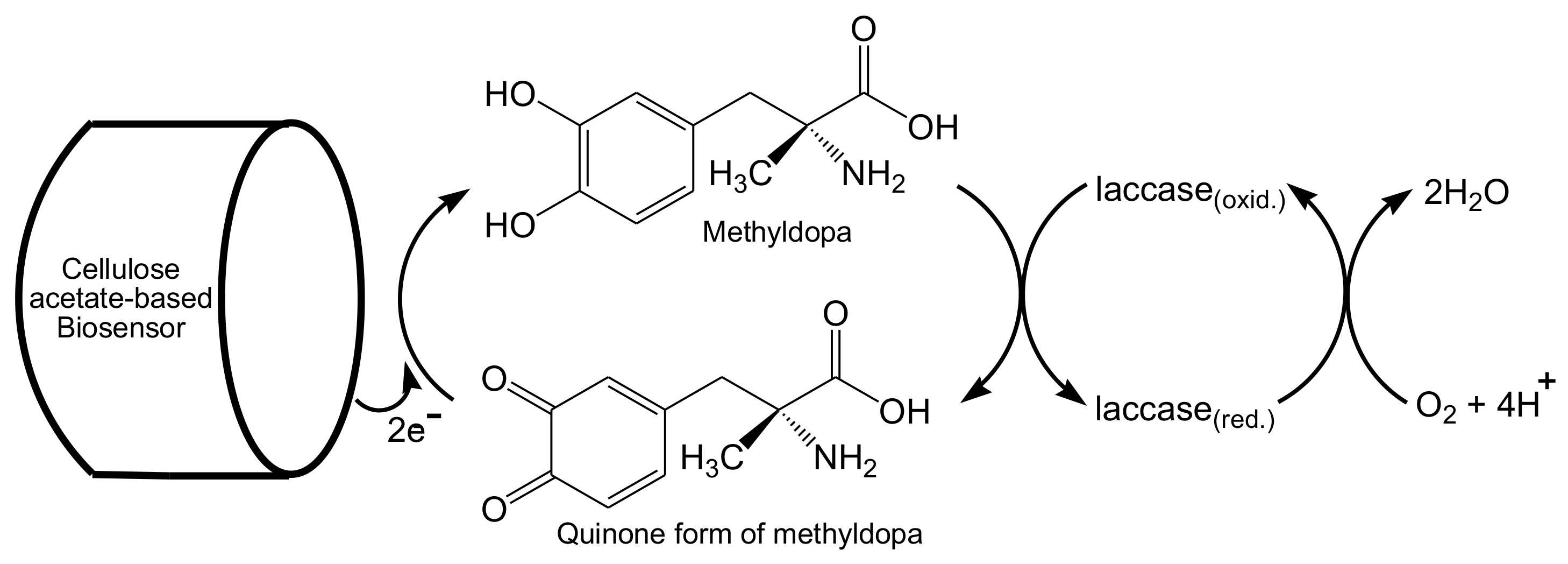
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamel, S.; A. Khattab, T. Recent Advances in Cellulose-Based Biosensors for Medical Diagnosis. Biosensors 2020, 10, 67. https://doi.org/10.3390/bios10060067
Kamel S, A. Khattab T. Recent Advances in Cellulose-Based Biosensors for Medical Diagnosis. Biosensors. 2020; 10(6):67. https://doi.org/10.3390/bios10060067
Chicago/Turabian StyleKamel, Samir, and Tawfik A. Khattab. 2020. "Recent Advances in Cellulose-Based Biosensors for Medical Diagnosis" Biosensors 10, no. 6: 67. https://doi.org/10.3390/bios10060067
APA StyleKamel, S., & A. Khattab, T. (2020). Recent Advances in Cellulose-Based Biosensors for Medical Diagnosis. Biosensors, 10(6), 67. https://doi.org/10.3390/bios10060067




