Fecal Volatile Organic Compound Profiles are Not Influenced by Gestational Age and Mode of Delivery: A Longitudinal Multicenter Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Groups
2.3. Sample Size Calculation
2.4. Definitions
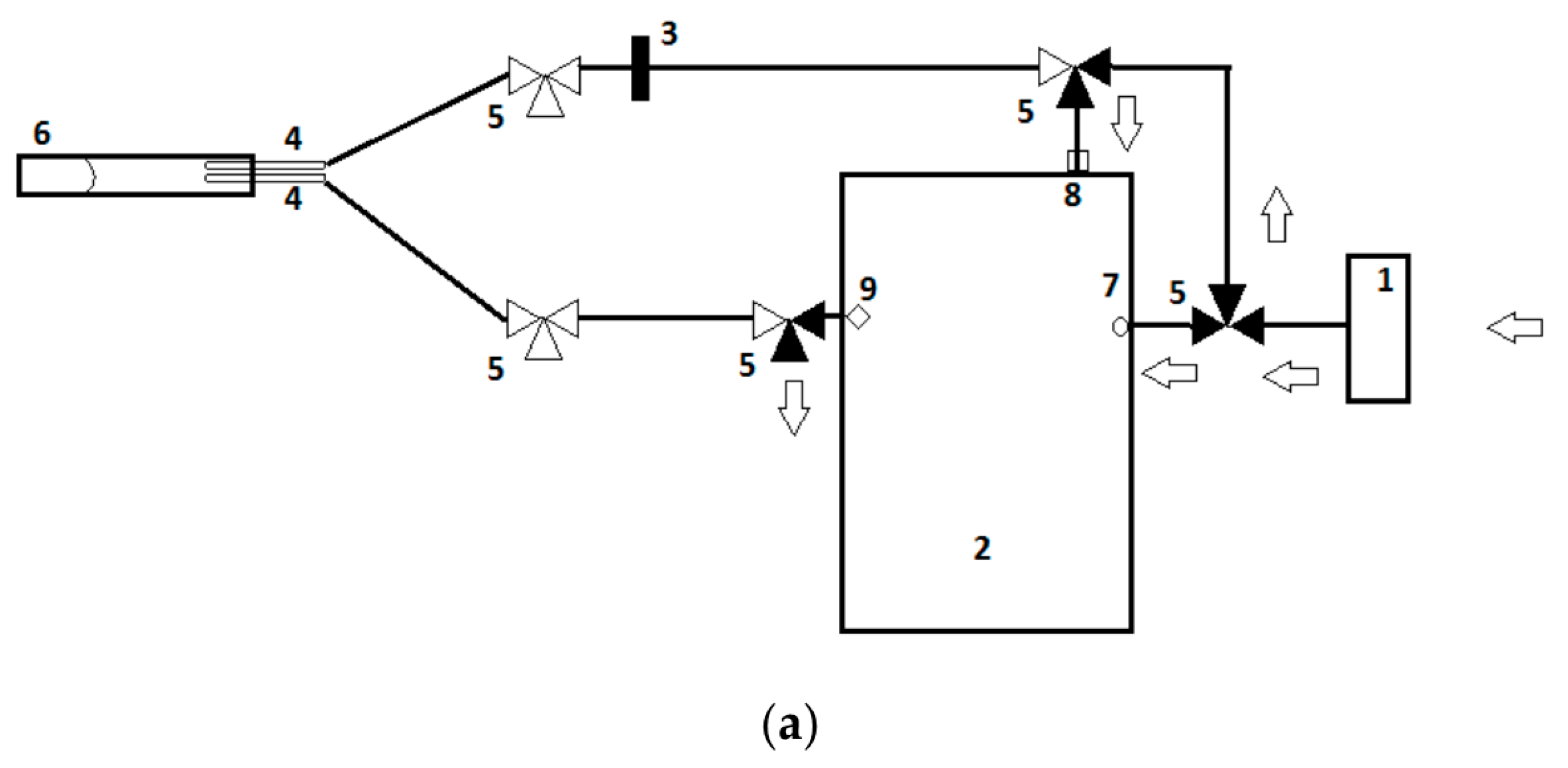
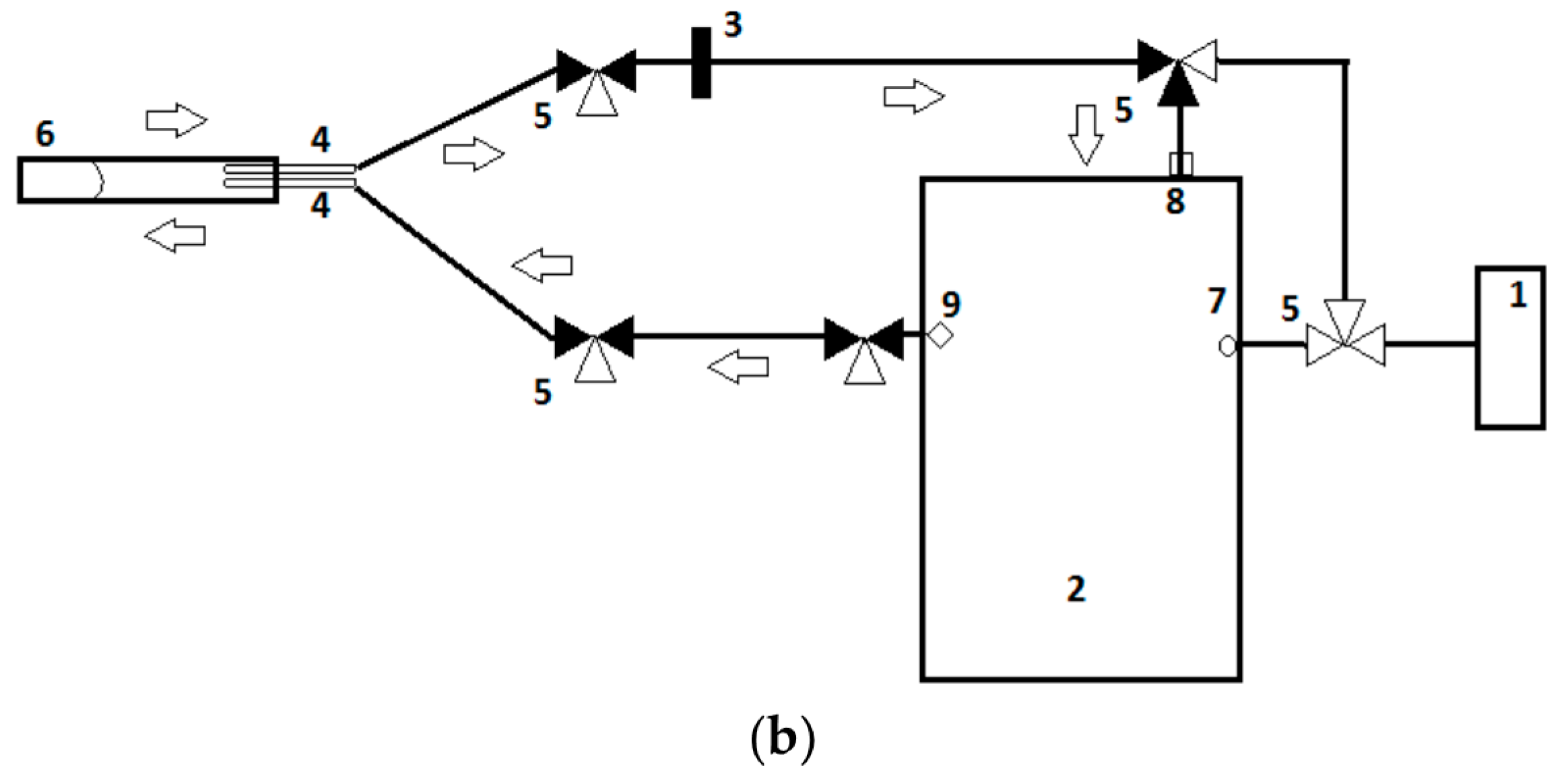
2.5. Sample Collection
2.6. VOC Analysis
2.7. Statistical Analysis
2.7.1. Demographic and Clinical Data
2.7.2. eNose Data
3. Results
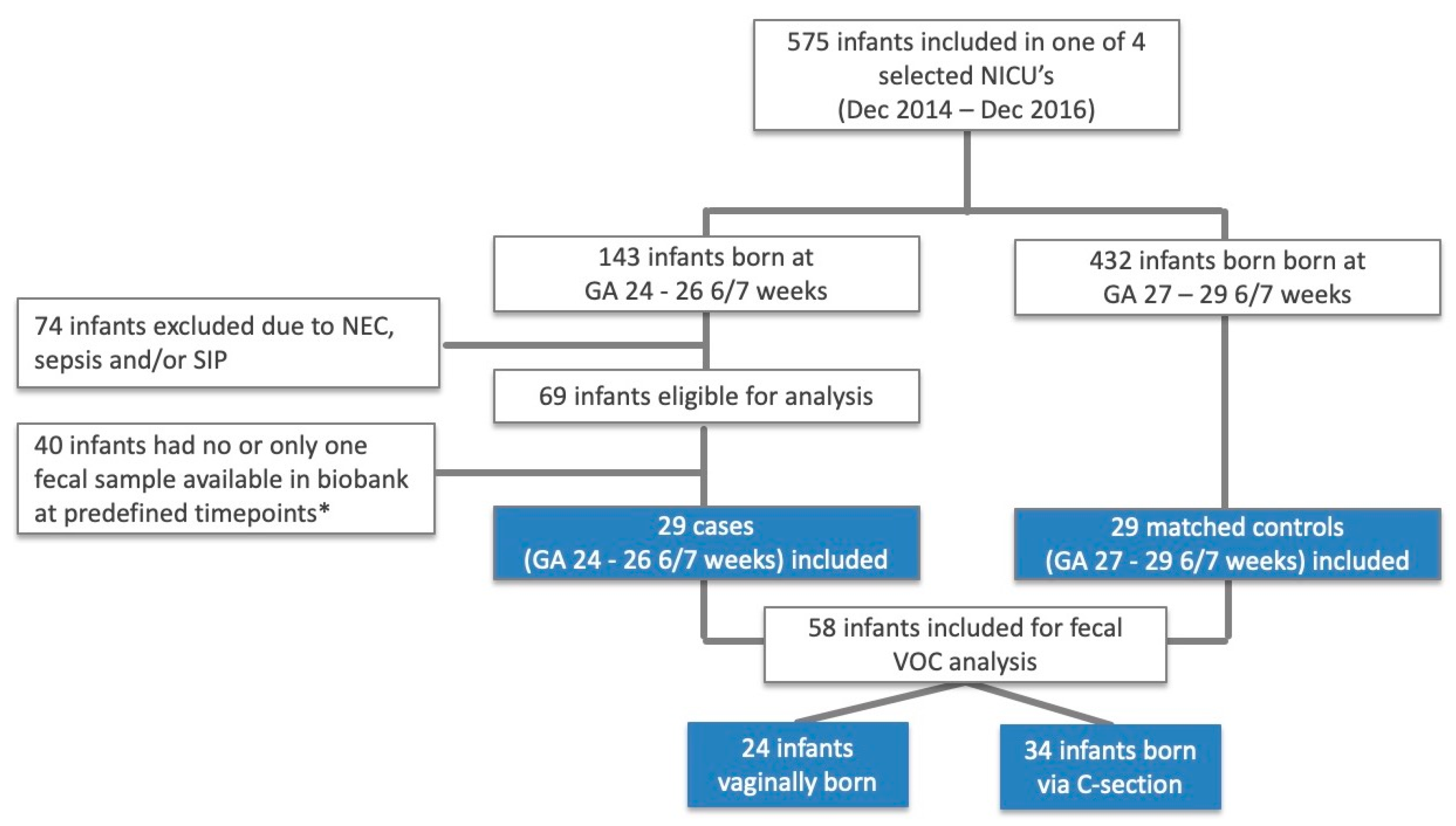
3.1. General Characteristics
3.2. Influence of Gestational Age and Delivery Mode on Fecal VOC
4. Discussion
4.1. Influence of Gestational Age on Fecal VOC
4.2. Influence of Mode of Delivery on Fecal VOC
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Te Pas, A.B. Improving Neonatal Care with Technology. Front. Pediatr. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Berrington, J.E.; Hearn, R.I.; Bythell, M.; Wright, C.; Embleton, N.D. Deaths in preterm infants: Changing pathology over 2 decades. J. Pediatr. 2012, 160, 49–53.e1. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.G.; Baer, R.J.; Partridge, J.C.; Kuppermann, M.; Franck, L.S.; Rand, L.; Jelliffe-Pawlowski, L.L.; Rogers, E.E. Survival and Major Morbidity of Extremely Preterm Infants: A Population-Based Study. Pediatrics 2016, 138, e20154434. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Bassler, D. Practice variations and rates of late onset sepsis and necrotizing enterocolitis in very preterm born infants, a review. Transl. Pediatr. 2019, 8, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Costeloe, K.; Hennessy, E.; Gibson, A.T.; Marlow, N.; Wilkinson, A.R. The EPICure study: Outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics 2000, 106, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Bizzarro, M.J.; Raskind, C.; Baltimore, R.S.; Gallagher, P.G. Seventy-five years of neonatal sepsis at Yale: 1928–2003. Pediatrics 2005, 116, 595–602. [Google Scholar] [CrossRef]
- Boghossian, N.S.; Page, G.P.; Bell, E.F.; Stoll, B.J.; Murray, J.C.; Cotten, C.M.; Shankaran, S.; Walsh, M.C.; Laptook, A.R.; Newman, N.S.; et al. Late-onset sepsis in very low birth weight infants from singleton and multiple-gestation births. J. Pediatr. 2013, 162, 1120–1124.e1. [Google Scholar] [CrossRef] [PubMed]
- Kliegman, R.M.; Walker, W.A.; Yolken, R.H. Necrotizing enterocolitis: Research agenda for a disease of unknown etiology and pathogenesis. Pediatr. Res. 1993, 34, 701–708. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kosloske, A.M. Epidemiology of necrotizing enterocolitis. Acta Paediatr. Suppl. 1994, 396, 2–7. [Google Scholar] [CrossRef]
- Thyoka, M.; De Coppi, P.; Eaton, S.; Khoo, K.; Hall, N.J.; Curry, J.; Kiely, E.; Drake, D.; Cross, K.; Pierro, A. Advanced necrotizing enterocolitis part 1: Mortality. Eur. J. Pediatric Surg. 2012, 22, 8–12. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N. Infections in VLBW infants: Studies from the NICHD neonatal research network. Semin. Perinatol. 2003, 27, 293–301. [Google Scholar] [CrossRef]
- Niemarkt, H.J.; de Meij, T.G.; van de Velde, M.E.; van der Schee, M.P.; Van Goudoever, J.B.; Kramer, B.W.; Andriessen, P.; de Boer, N.K. Necrotizing enterocolitis: A clinical review on diagnostic biomarkers and the role of the intestinal microbiota. Inflamm. Bowel Dis. 2015, 21, 436–444. [Google Scholar] [CrossRef]
- Gilfillan, M.; Bhandari, V. Neonatal sepsis biomarkers: Where are we now? Res. Rep. Neonatol. 2019, 9, 9–20. [Google Scholar] [CrossRef]
- Stoll, B.; Hansen, N.; Fanaroff, A.; Wright, L.; Carlo, W.; Ehrenkranz, R.; Lemons, J.; Donovan, E.; Stark, A.; Tyson, J.; et al. To Tap or Not to Tap: High Likelihood of Meningitis Without Sepsis Among Very Low Birth Weight Infants. Pediatrics 2004, 113, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, G.; Impellizzeri, P.; Marseglia, L.; Montalto, A.S.; Russo, T.; Salamone, I.; Falsaperla, R.; Corsello, G.; Romeo, C.; Gitto, E. Current status of laboratory and imaging diagnosis of neonatal necrotizing enterocolitis. Ital. J. Pediatr. 2018, 44, 84. [Google Scholar] [CrossRef] [PubMed]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef] [PubMed]
- Zetola, N.M. Diagnosis of pulmonary tuberculosis and assessment of treatment response through analyses of volatile compound patterns in exhaled breath samples. J. Infect. 2017, 74. [Google Scholar] [CrossRef]
- Wang, M.; Xie, R.; Jia, X.; Liu, R. Urinary volatile organic compounds as potential biomarkers in idiopathic membranous nephropathy. Med. Princ. Pract. 2017, 26. [Google Scholar] [CrossRef]
- Liu, D. Urine volatile organic compounds as biomarkers for minimal change type nephrotic syndrome. Biochem. Biophys. Res. Commun. 2018, 496. [Google Scholar] [CrossRef]
- El-Metwally, D. Urinary metabolites of volatile organic compounds of infants in the neonatal intensive care unit. Pediatr. Res. 2018, 83. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, D.J.C. Late-onset sepsis in preterm infants can be detected preclinically by fecal volatile organic compound analysis: A prospective, multicenter cohort study. Clin. Infect. Dis. 2019, 68, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Arasaradnam, R.P. Noninvasive diagnosis of pancreatic cancer through detection of volatile organic compounds in urine. Gastroenterology 2018, 154. [Google Scholar] [CrossRef] [PubMed]
- Arasaradnam, R.P. Non-invasive exhaled volatile organic biomarker analysis to detect inflammatory bowel disease (IBD). Dig. Liver Dis. 2016, 48. [Google Scholar] [CrossRef]
- Harrison, C.M.; Andersen, C.C. Exhaled breath measures of inflammation: Are they useful in neonatal chronic lung disease? Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F6. [Google Scholar] [CrossRef] [PubMed]
- Sankarganesh, D.; Suriyakalaa, U.; Ramachandran, R.; Achiraman, S.; Arunachalam, S.; Angayarkanni, J. Urinary volatile metabolomics as a viable alternative diagnostic tool for polycystic ovary syndrome: An exploratory hypothesis. Med. Hypotheses 2019, 124, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S.; Benton, H.P.; Casazza, K.; Cooper, S.J.; Cui, X.; Du, X.; Engler, J.; Kabarowski, J.H.; Li, S.; Pathmasiri, W.; et al. Training in metabolomics research. I. Designing the experiment, collecting and extracting samples and generating metabolomics data. J. Mass Spectrom. 2016, 51, 461–475. [Google Scholar] [CrossRef]
- Probert, C.; Greenwood, R.; Mayor, A.; Hughes, D.; Aggio, R.; Jackson, R.E.; Simcox, L.; Barrow, H.; Garcia-Finana, M.; Ewer, A.K. Faecal volatile organic compounds in preterm babies at risk of necrotising enterocolitis: The DOVE study. Arch. Dis. Child. Fetal Neonatal Ed. 2019. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; Ouaret, N.; Thomas, M.G.; Gold, P.; Quraishi, M.N.; Nwokolo, C.U.; Bardhan, K.D.; Covington, J.A. Evaluation of gut bacterial populations using an electronic e-nose and field asymmetric ion mobility spectrometry: Further insights into ‘fermentonomics’. J. Med. Eng. Technol. 2012, 36, 333–337. [Google Scholar] [CrossRef]
- D’Amico, A.; Pennazza, G.; Santonico, M.; Martinelli, E.; Roscioni, C.; Galluccio, G.; Paolesse, R.; Di Natale, C. An investigation on electronic nose diagnosis of lung cancer. Lung Cancer 2010, 68, 170–176. [Google Scholar] [CrossRef]
- Pavlou, A.K.; Magan, N.; McNulty, C.; Jones, J.; Sharp, D.; Brown, J.; Turner, A.P. Use of an electronic nose system for diagnoses of urinary tract infections. Biosens. Bioelectron. 2002, 17, 893–899. [Google Scholar] [CrossRef]
- Roine, A.; Saviauk, T.; Kumpulainen, P.; Karjalainen, M.; Tuokko, A.; Aittoniemi, J.; Vuento, R.; Lekkala, J.; Lehtimaki, T.; Tammela, T.L.; et al. Rapid and accurate detection of urinary pathogens by mobile IMS-based electronic nose: A proof-of-principle study. PLoS ONE 2014, 9, e114279. [Google Scholar] [CrossRef] [PubMed]
- Roine, A.; Veskimae, E.; Tuokko, A.; Kumpulainen, P.; Koskimaki, J.; Keinanen, T.A.; Hakkinen, M.R.; Vepsalainen, J.; Paavonen, T.; Lekkala, J.; et al. Detection of prostate cancer by an electronic nose: A proof of principle study. J. Urol. 2014, 192, 230–234. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Arasaradnam, R.P.; Covington, J.A.; Harmston, C.; Nwokolo, C.U. Review article: Next generation diagnostic modalities in gastroenterology—Gas phase volatile compound biomarker detection. Aliment. Pharmacol. Ther. 2014, 39, 780–789. [Google Scholar] [CrossRef]
- Covington, J.A.; van der Schee, M.P.; Edge, A.S.; Boyle, B.; Savage, R.S.; Arasaradnam, R.P. The application of FAIMS gas analysis in medical diagnostics. Analyst 2015, 140, 6775–6781. [Google Scholar] [CrossRef]
- Röck, F.; Barsan, N.; Weimar, U. Electronic Nose: Current Status and Future Trends. Chem. Rev. 2008, 108, 705–725. [Google Scholar] [CrossRef]
- Arshak, K.; Moore, E.; ÓLaighin, G.; Harris, J.; Clifford, S. A Review of Gas Sensors Employed in Electronic Nose Applications. Sens. Rev. 2004, 24, 181–198. [Google Scholar] [CrossRef]
- Berkhout, D.J.C.; Niemarkt, H.J.; Benninga, M.A.; Budding, A.E.; van Kaam, A.H.; Kramer, B.W.; Pantophlet, C.M.; van Weissenbruch, M.M.; de Boer, N.K.H.; de Meij, T.G.J. Development of severe bronchopulmonary dysplasia is associated with alterations in fecal volatile organic compounds. Pediatr. Res. 2018, 83, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, D.J.C.; Niemarkt, H.J.; Buijck, M.; van Weissenbruch, M.M.; Brinkman, P.; Benninga, M.A.; van Kaam, A.H.; Kramer, B.W.; Andriessen, P.; de Boer, N.K.H.; et al. Detection of Sepsis in Preterm Infants by Fecal Volatile Organic Compounds Analysis: A Proof of Principle Study. J. Pediatr. Gastroenterol. Nutr. 2017, 65, e47–e52. [Google Scholar] [CrossRef]
- Meij, T.G.J. Early detection of necrotizing enterocolitis by fecal volatile organic compounds analysis. J. Pediatr. 2015, 167. [Google Scholar] [CrossRef]
- Rogosch, T.; Herrmann, N.; Maier, R.F.; Domann, E.; Hattesohl, A.; Koczulla, A.R.; Zemlin, M. Detection of bloodstream infections and prediction of bronchopulmonary dysplasia in preterm neonates with an electronic nose. J. Pediatr. 2014, 165, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Nanda, R.; Chakraborty, T. Clinical application of volatile organic compound analysis for detecting infectious diseases. Clin. Microbiol. Rev. 2013, 26, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Campanella, B.; Onor, M.; Lomonaco, T.; Benedetti, E.; Bramanti, E. HS-SPME-GC-MS approach for the analysis of volatile salivary metabolites and application in a case study for the indirect assessment of gut microbiota. Anal. Bioanal. Chem. 2019, 411, 7551–7562. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; Blakstad, E.W.; Moltu, S.J.; Strømmen, K.; Nakstad, B.; Rønnestad, A.E.; Brække, K.; Iversen, P.O.; Drevon, C.A.; de Vos, W. Intestinal microbiota development and gestational age in preterm neonates. Sci. Rep. 2018, 8, 2453. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Parra-Llorca, A.; Gormaz, M.; Alcántara, C.; Cernada, M.; Nuñez-Ramiro, A.; Vento, M.; Collado, M.C. Preterm Gut Microbiome Depending on Feeding Type: Significance of Donor Human Milk. Front. Microbiol. 2018, 9, 1376. [Google Scholar] [CrossRef]
- Noto, A.; Fanos, V.; Dessì, A. Chapter Two—Metabolomics in Newborns. In Advances in Clinical Chemistry; Makowski, G.S., 1st, Ed.; Elsevier: Cambridge, MA, USA, 2016; Volume 74, pp. 35–61. [Google Scholar]
- El Manouni El Hassani, S.; Niemarkt, H.J.; Said, H.; Berkhout, D.J.C.; van Kaam, A.H.; van Lingen, R.A.; Benninga, M.A.; de Boer, N.K.H.; de Meij, T.G.J. Fecal Volatile Organic Compounds in Preterm Infants Are Influenced by Enteral Feeding Composition. Sensors 2018, 18, 3037. [Google Scholar] [CrossRef]
- Shirasu, M.; Touhara, K. The scent of disease: Volatile organic compounds of the human body related to disease and disorder. J. Biochem. 2011, 150, 257–266. [Google Scholar] [CrossRef]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef]
- Walsh, E.M.; Li, S.X.; Black, L.K.; Kuzniewicz, M. Incremental Cost of Prematurity by Week of Gestational Age. Am. J. Perinatol. Rep. 2019, 9, e76–e83. [Google Scholar] [CrossRef]
- Bosch, S.; El Manouni El Hassani, S.; Covington, J.A.; Wicaksono, A.N.; Bomers, M.K.; Benninga, M.A.; Mulder, C.J.J.; de Boer, N.K.H.; de Meij, T.G.J. Optimized Sampling Conditions for Fecal Volatile Organic Compound Analysis by Means of Field Asymmetric Ion Mobility Spectrometry. Anal. Chem. 2018, 90, 7972–7981. [Google Scholar] [CrossRef] [PubMed]
- Smiths Detection Inc. The Cyranose 320 Enose User’s Manual, 5th ed.; Smiths Detection Inc.: Pasadena, CA, USA, 2004. [Google Scholar]
- Berkhout, D.J.; Benninga, M.A.; van Stein, R.M.; Brinkman, P.; Niemarkt, H.J.; de Boer, N.K.; de Meij, T.G. Effects of Sampling Conditions and Environmental Factors on Fecal Volatile Organic Compound Analysis by an Electronic Nose Device. Sensors 2016, 16, 1967. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.; Hawken, S.; Ducharme, R.; Potter, B.K.; Little, J.; Thebaud, B.; Chakraborty, P. Metabolomics of prematurity: Analysis of patterns of amino acids, enzymes, and endocrine markers by categories of gestational age. Pediatr. Res. 2014, 75, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Grier, A.; Qiu, X.; Bandyopadhyay, S.; Holden-Wiltse, J.; Kessler, H.A.; Gill, A.L.; Hamilton, B.; Huyck, H.; Misra, S.; Mariani, T.J.; et al. Impact of prematurity and nutrition on the developing gut microbiome and preterm infant growth. Microbiome 2017, 5, 158. [Google Scholar] [CrossRef]
- Heida, F.; Beyduz, G.; Bulthuis, M.; Kooi, E.; Bos, A.; Timmer, A.; Hulscher, J. Paneth cells in the developing gut: When do they arise and when are they immune competent? Pediatr. Res. 2016, 80. [Google Scholar] [CrossRef]
- Esiaba, I.; Angeles, D.M.; Holden, M.S.; Tan, J.B.; Asmerom, Y.; Gollin, G.; Boskovic, D.S. Urinary Allantoin Is Elevated in Severe Intraventricular Hemorrhage in the Preterm Newborn. Transl. Stroke Res. 2016, 7, 97–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stewart, C.J.; Embleton, N.D.; Clements, E.; Luna, P.N.; Smith, D.P.; Fofanova, T.Y.; Nelson, A.; Taylor, G.; Orr, C.H.; Petrosino, J.F.; et al. Cesarean or Vaginal Birth Does Not Impact the Longitudinal Development of the Gut Microbiome in a Cohort of Exclusively Preterm Infants. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Itani, T.; Ayoub Moubareck, C.; Melki, I.; Rousseau, C.; Mangin, I.; Butel, M.J.; Karam Sarkis, D. Establishment and development of the intestinal microbiota of preterm infants in a Lebanese tertiary hospital. Anaerobe 2017, 43, 4–14. [Google Scholar] [CrossRef]
- Chu, D.M.; Ma, J.; Prince, A.L.; Antony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017, 23, 314–326. [Google Scholar] [CrossRef]
- Staude, B.; Oehmke, F.; Lauer, T.; Behnke, J.; Gopel, W.; Schloter, M.; Schulz, H.; Krauss-Etschmann, S.; Ehrhardt, H. The Microbiome and Preterm Birth: A Change in Paradigm with Profound Implications for Pathophysiologic Concepts and Novel Therapeutic Strategies. BioMed Res. Int. 2018, 2018, 7218187. [Google Scholar] [CrossRef]
- Atzori, L.; Barberini, L.; Lussu, M.; Murgia, F.; Noto, A.; Mercuro, G.; Bassareo, P.; Puddu, M.; Antonucci, R.; Neroni, P.; et al. Metabolomics and patent ductus arteriosus diagnosis: Is 1H-NMR (nuclear magnetic resonance) spectroscopy of urine at birth as predictive as ultrasound? In Proceedings of the Selected Abstracts of the 7th International Workshop on Neonatology, Cagliari, Italy, 22–26 October 2011. [Google Scholar]
- Voynow, J.A.; Fisher, K.; Sunday, M.E.; Cotten, C.M.; Hamvas, A.; Hendricks-Munoz, K.D.; Poindexter, B.B.; Pryhuber, G.S.; Ren, C.L.; Ryan, R.M.; et al. Urine gastrin-releasing peptide in the first week correlates with bronchopulmonary dysplasia and post-prematurity respiratory disease. Pediatr. Pulmonol. 2020, 55, 899–908. [Google Scholar] [CrossRef] [PubMed]
| 24–26 Weeks (n = 29) | 27–29 Weeks (n = 29) | p-Value | |
|---|---|---|---|
| Included neonates t1, t2, t3 (n) | 23, 27, 27 | 24, 27, 26 | 0.98 |
| GA days (mean [SD]) | 184 [5] | 199 [5] | <0.001 * |
| Gender female (n[%]) | 16 [55] | 15 [48] | 0.60 |
| Birth weight grams (mean [SD]) | 856 [140] | 1166 [198] | <0.001 * |
| Mode of delivery vaginal (n[%]) | 12 [41] | 12 [41] | 1.00 |
| Center of birth (n [%]) | 1.00 | ||
| 1 | 7 [24] | 7 [24] | |
| 2 | 6 [21] | 6 [21] | |
| 3 | 8 [28] | 8 [28] | |
| 4 | 8 [28] | 8 [28] | |
| Feeding mode prior to t1 (n[%]) | 0.28 | ||
| Mother’s milk | 12 [52] | 7 [29] | |
| Formula milk | 4 [17] | 6 [25] | |
| Combination MM/FM | 7 [30] | 11 [46] | |
| Feeding mode prior to t2 (n[%]) | 0.50 | ||
| Mother’s milk | 21 [78] | 24 [89] | |
| Formula milk | 3 [11] | 2 [7] | |
| Combination MM/FM | 3 [11] | 1 [4] | |
| Age at full enteral feeding days (mean [SD]) | 14 [3] | 14 [2] | 0.25 |
| Feeding mode prior to t3 (n[%]) | 0.58 | ||
| Mother’s milk | 20 [74] | 21 [84] | |
| Formula milk | 3 [11] | 1 [4] | |
| Combination MM/FM | 4 [15] | 3 [12] | |
| Parental feeding days (median [IQR]) | |||
| Prior to t1 | 7 [6–7] | 7 [6–7] | 0.53 |
| Prior to t2 | 11 [9–14] | 12 [9–13] | 0.99 |
| Prior to t3 | 11 [9–14] | 12 [11–13] | 0.27 |
| Antibiotic exposure prior to t3 (n[%]) | 27 [100] | 22 [85] | 0.05 |
| Antibiotic exposure days (median [IQR]) | |||
| Prior to t1 | 3 [2–4] | 3 [2–4] | 0.22 |
| Prior to t2 | 4 [2–6] | 3 [2–5] | 0.04 * |
| Prior to t3 | 5 [3–8] | 3 [2–6] | 0.03 * |
| Invasive ventilation prior to t3 (n[%]) | 13 [48] | 6 [23] | 0.09 |
| Invasive ventilation days (median [IQR]) | |||
| Prior to t1 | 5 [1–6] | 3 [2–4] | 0.33 |
| Prior to t2 | 6 [4–12] | 3 [2–5] | 0.10 |
| Prior to t3 | 7 [4–16] | 3 [2–6] | 0.11 |
| Sample weight grams (median [IQR]) | |||
| t1 | 149 [128–163] | 151 [137–163] | 0.22 |
| t2 | 154 [146–161] | 152 [137–158] | 0.68 |
| t3 | 155 [141–162] | 148 [137–157] | 0.34 |
| Sample age months (median [IQR]) | |||
| t1 | 35 [32–50] | 35 [33–44] | 0.82 |
| t2 | 35 [31–46] | 35 [33–44] | 0.73 |
| t3 | 35 [32–45] | 35 [33–45] | 0.78 |
| Vaginal (n = 24) | C-Section (n = 34) | p-Value | |
|---|---|---|---|
| Included neonates t1, t2, t3 (n) | 21, 24, 21 | 26, 30, 32 | 0.84 |
| GA in days (mean [SD]) | 190 [10] | 192 [9] | 0.29 |
| Gender female (n [%]) | 12 [50] | 18 [53] | 0.83 |
| Birth weight grams (mean [SD]) | 1055 [237] | 980 [225] | 0.23 |
| Center of birth (n [%]) | 0.05 | ||
| 1 | 8 [33] | 6 [18] | |
| 2 | 6 [25] | 6 [18] | |
| 3 | 2 [8] | 14 [41] | |
| 4 | 8 [33] | 8 [24] | |
| Feeding mode prior to t1 (n[%]) | 0.93 | ||
| Mother’s milk | 9 [43] | 10 [39] | |
| Formula milk | 4 [19] | 6 [23] | |
| Combination MM/FM | 8 [38] | 10 [39] | |
| Feeding mode prior to t2 (n[%]) | 0.23 | ||
| Mother’s milk | 18 [75] | 27 [90] | |
| Formula milk | 4 [17] | 1 [3] | |
| Combination MM/FM | 2 [8] | 2 [7] | |
| Feeding mode prior to t3 (n[%]) | 0.27 | ||
| Mother’s milk | 14 [70] | 27 [84] | |
| Formula milk | 3 [15] | 1 [3] | |
| Combination MM/FM | 3 [15] | 4 [13] | |
| Age at full enteral feeding days (median [IQR]) | 14 [12–17] | 14 [12–16] | 0.83 |
| Parental feeding days (median [IQR]) | |||
| Prior to t1 | 7 [7–7] | 7 [6–7] | 0.25 |
| Prior to t2 | 11 [10–13] | 11 [9–14] | 0.84 |
| Prior to t3 | 11 [11–13] | 11 [9–14] | 0.62 |
| Antibiotic exposure prior to t3 (n[%]) | 20 [95] | 29 [91] | 1.00 |
| Antibiotic exposure days (median [IQR]) | |||
| Prior to t1 | 3 [2–3] | 3 [2–4] | 0.67 |
| Prior to t2 | 3 [2–6] | 4 [2–5] | 0.70 |
| Prior to t3 | 4 [2–7] | 5 [2–6] | 0.60 |
| Invasive ventilation prior to t3 (n [%]) | 7 [33] | 12 [38] | 0.76 |
| Invasive ventilation days (median [IQR]) | |||
| Prior to t1 | 5 [2–5] | 4 [1–6] | 0.95 |
| Prior to t2 | 6 [4–12] | 4 [1–7] | 0.28 |
| Prior to t3 | 11 [4–19] | 5 [2–7] | 0.34 |
| Sample weight grams (median [IQR]) | |||
| t1 | 149 [128–159] | 151 [132–163] | 0.42 |
| t2 | 154 [139–162] | 152 [141–158] | 0.30 |
| t3 | 154 [131–162] | 149 [141–162] | 0.98 |
| Sample age months (median [IQR]) | |||
| t1 | 36 [32–46] | 34 [33–45] | 0.91 |
| t2 | 35 [31–44] | 35 [33–45] | 0.94 |
| t3 | 35 [32–44] | 35 [32–44] | 0.73 |
| p-Value (t1) | p-Value (t2) | p-Value (t3) | |
|---|---|---|---|
| Gestational age | |||
| F test for ANOVA * | 0.36 | 0.13 | 0.61 |
| Global test | 0.38 | 0.65 | 0.96 |
| Mode of delivery | |||
| F test for ANOVA * | 0.52 | 0.50 | 0.27 |
| Global test | 0.72 | 0.95 | 0.33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deianova, N.; el Manouni el Hassani, S.; Niemarkt, H.J.; Cossey, V.; van Kaam, A.H.; Jenken, F.; van Weissenbruch, M.M.; Doedes, E.M.; Baelde, K.; Menezes, R.; et al. Fecal Volatile Organic Compound Profiles are Not Influenced by Gestational Age and Mode of Delivery: A Longitudinal Multicenter Cohort Study. Biosensors 2020, 10, 50. https://doi.org/10.3390/bios10050050
Deianova N, el Manouni el Hassani S, Niemarkt HJ, Cossey V, van Kaam AH, Jenken F, van Weissenbruch MM, Doedes EM, Baelde K, Menezes R, et al. Fecal Volatile Organic Compound Profiles are Not Influenced by Gestational Age and Mode of Delivery: A Longitudinal Multicenter Cohort Study. Biosensors. 2020; 10(5):50. https://doi.org/10.3390/bios10050050
Chicago/Turabian StyleDeianova, Nancy, Sofia el Manouni el Hassani, Hendrik J. Niemarkt, Veerle Cossey, Anton H. van Kaam, Floor Jenken, Mirjam M. van Weissenbruch, Esmee M. Doedes, Kyra Baelde, Renee Menezes, and et al. 2020. "Fecal Volatile Organic Compound Profiles are Not Influenced by Gestational Age and Mode of Delivery: A Longitudinal Multicenter Cohort Study" Biosensors 10, no. 5: 50. https://doi.org/10.3390/bios10050050
APA StyleDeianova, N., el Manouni el Hassani, S., Niemarkt, H. J., Cossey, V., van Kaam, A. H., Jenken, F., van Weissenbruch, M. M., Doedes, E. M., Baelde, K., Menezes, R., Benninga, M. A., de Jonge, W. J., de Boer, N. K., & de Meij, T. G. (2020). Fecal Volatile Organic Compound Profiles are Not Influenced by Gestational Age and Mode of Delivery: A Longitudinal Multicenter Cohort Study. Biosensors, 10(5), 50. https://doi.org/10.3390/bios10050050





