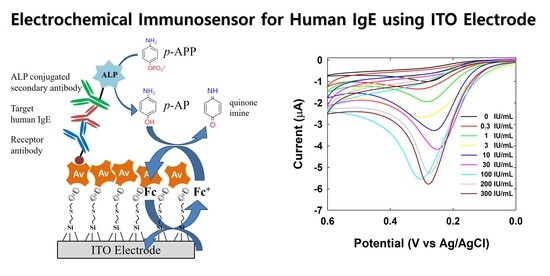
Electrochemical Immunosensor for Human IgE Using Ferrocene Self-Assembled Monolayers Modified ITO Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of ITO Electrode and an Electrochemical Sensing Layer
2.3. Electrochemical Cell and Measurements
3. Results and Discussion
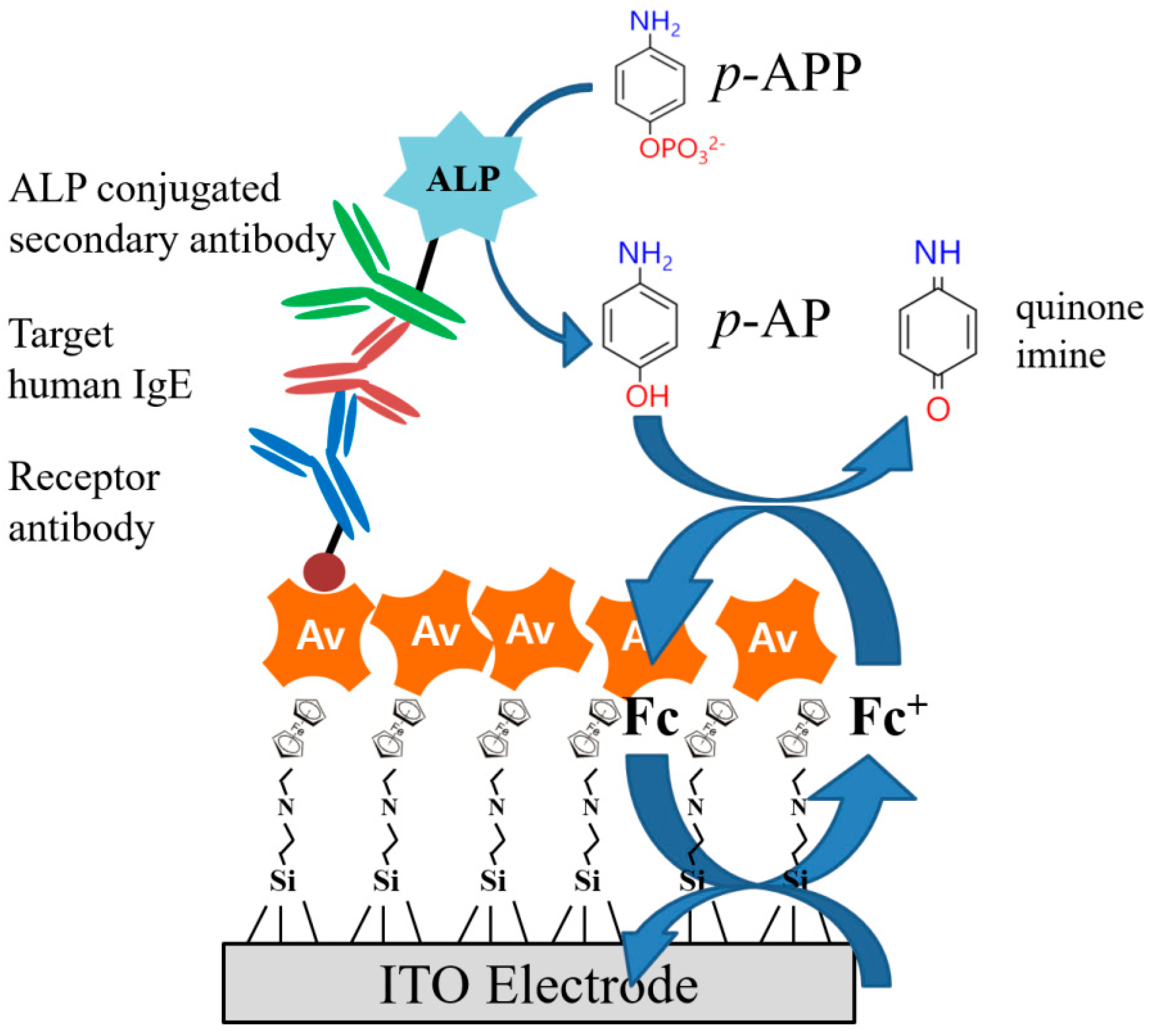
3.1. Preparation of Fc-Modified SAMs on the ITO Electrode
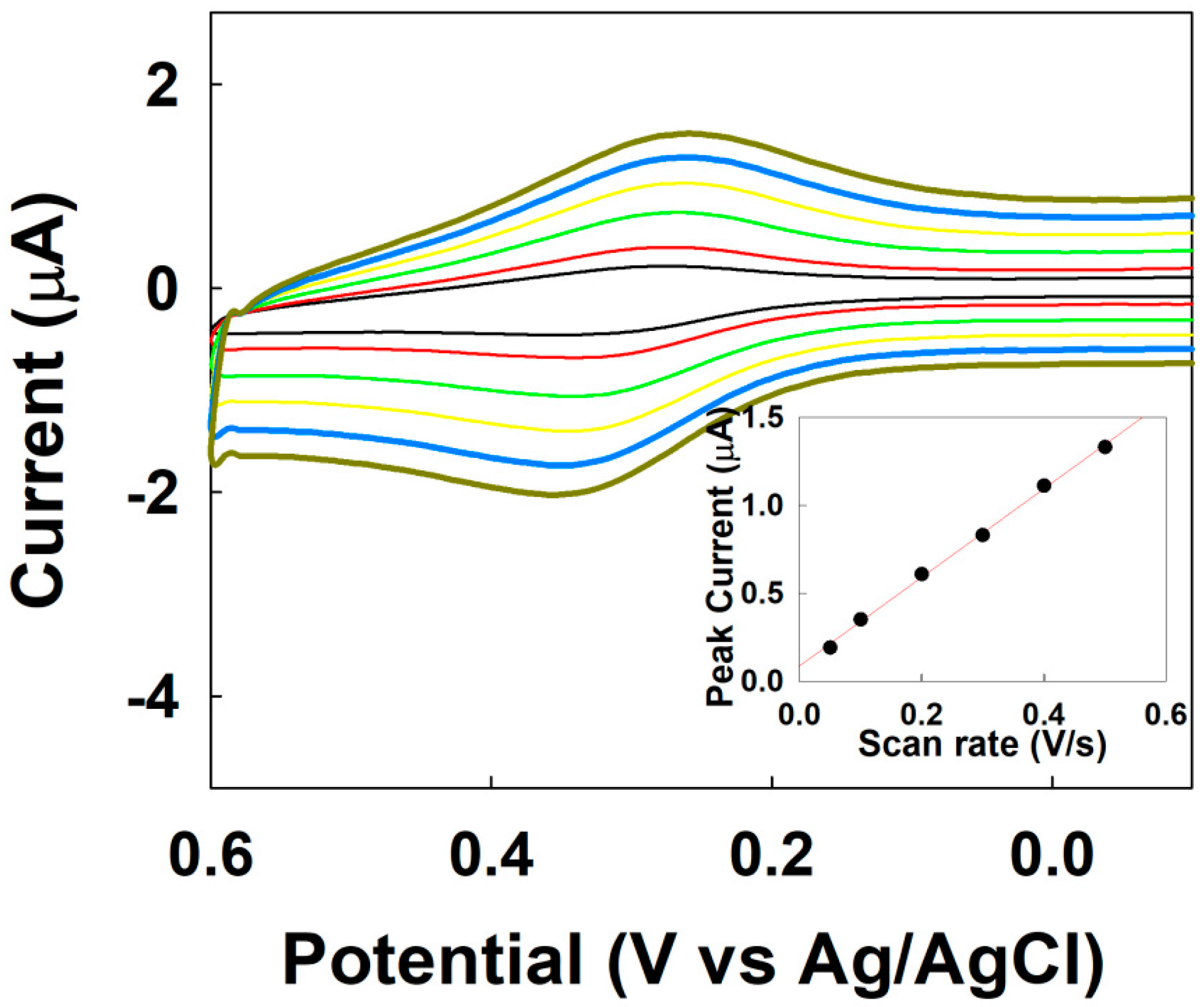
3.2. Electrocatalytic Amplification via Redox Cycling
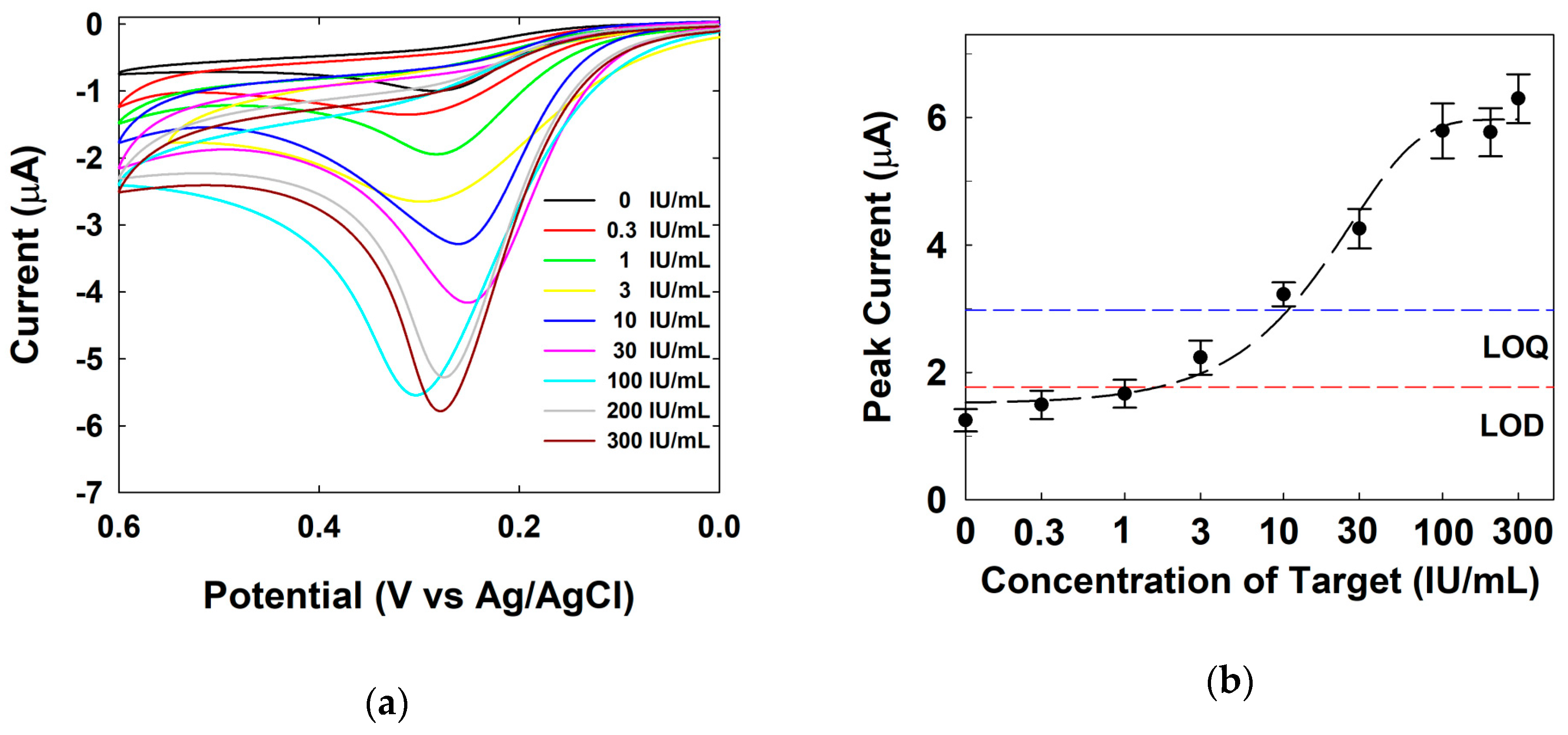
3.3. Electrochemical Detection of Human IgE via CV
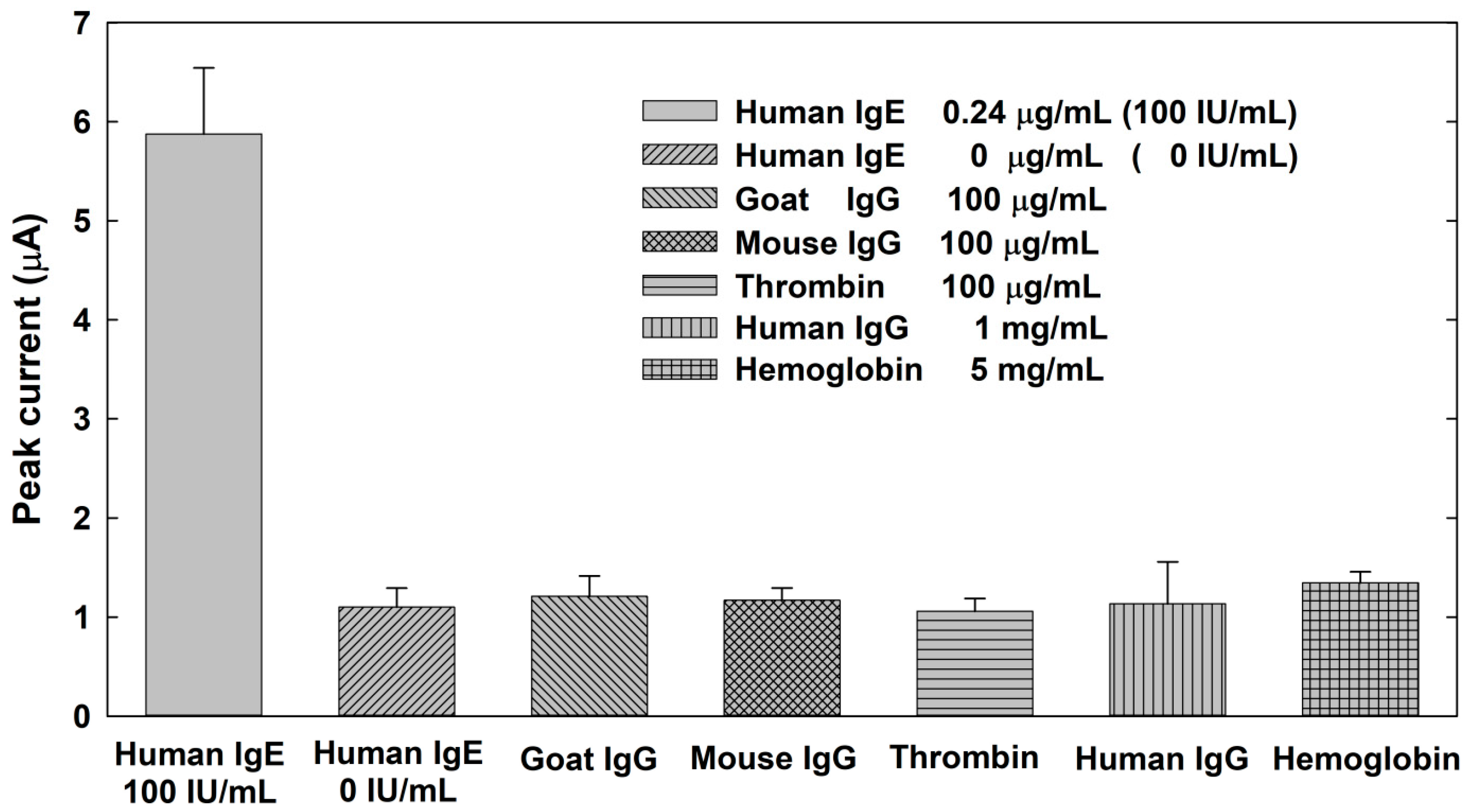
3.4. Selectivity Test for the Electrochemical Sensing
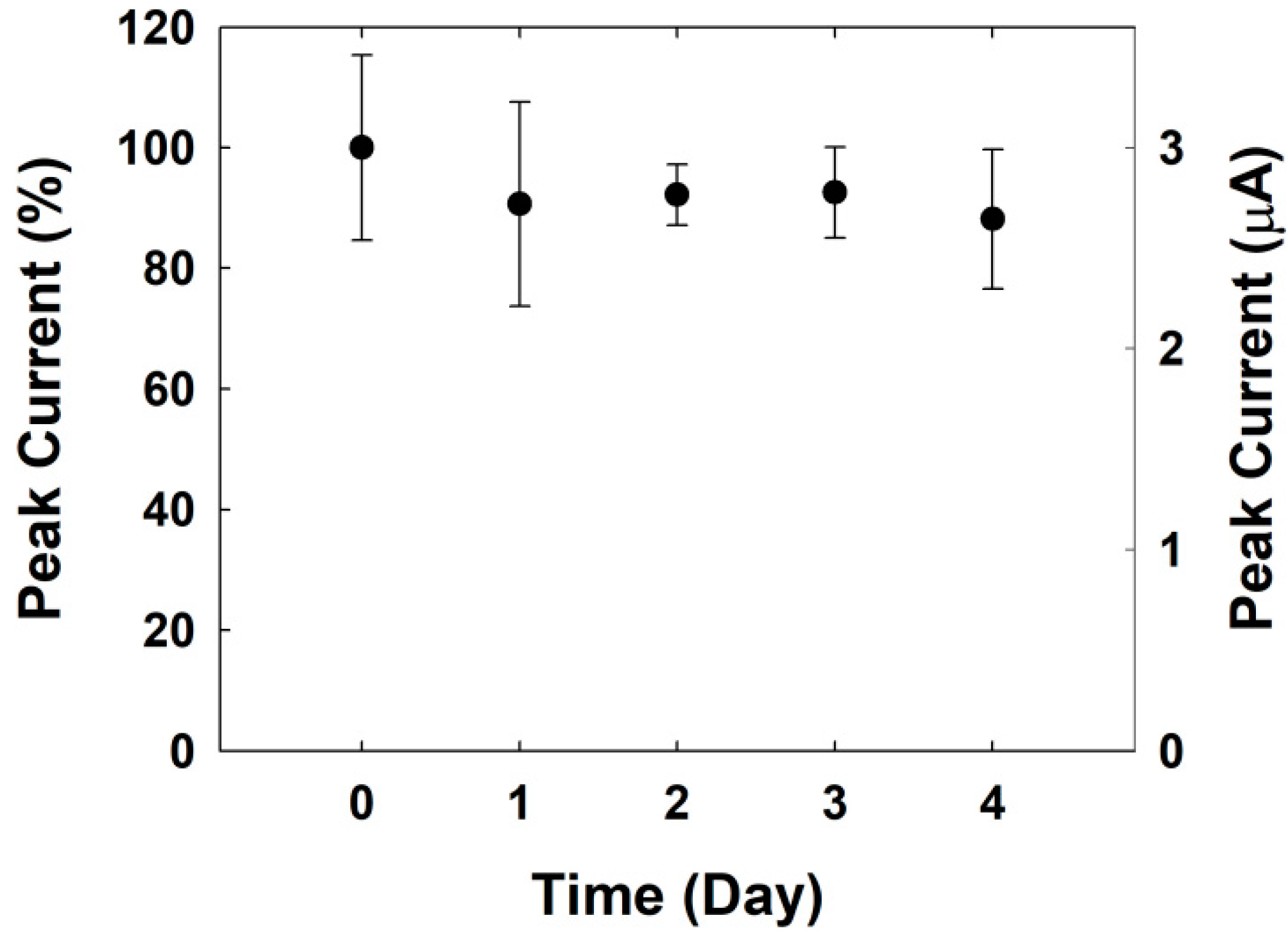
3.5. Stability Test for the Electrochemical Sensing
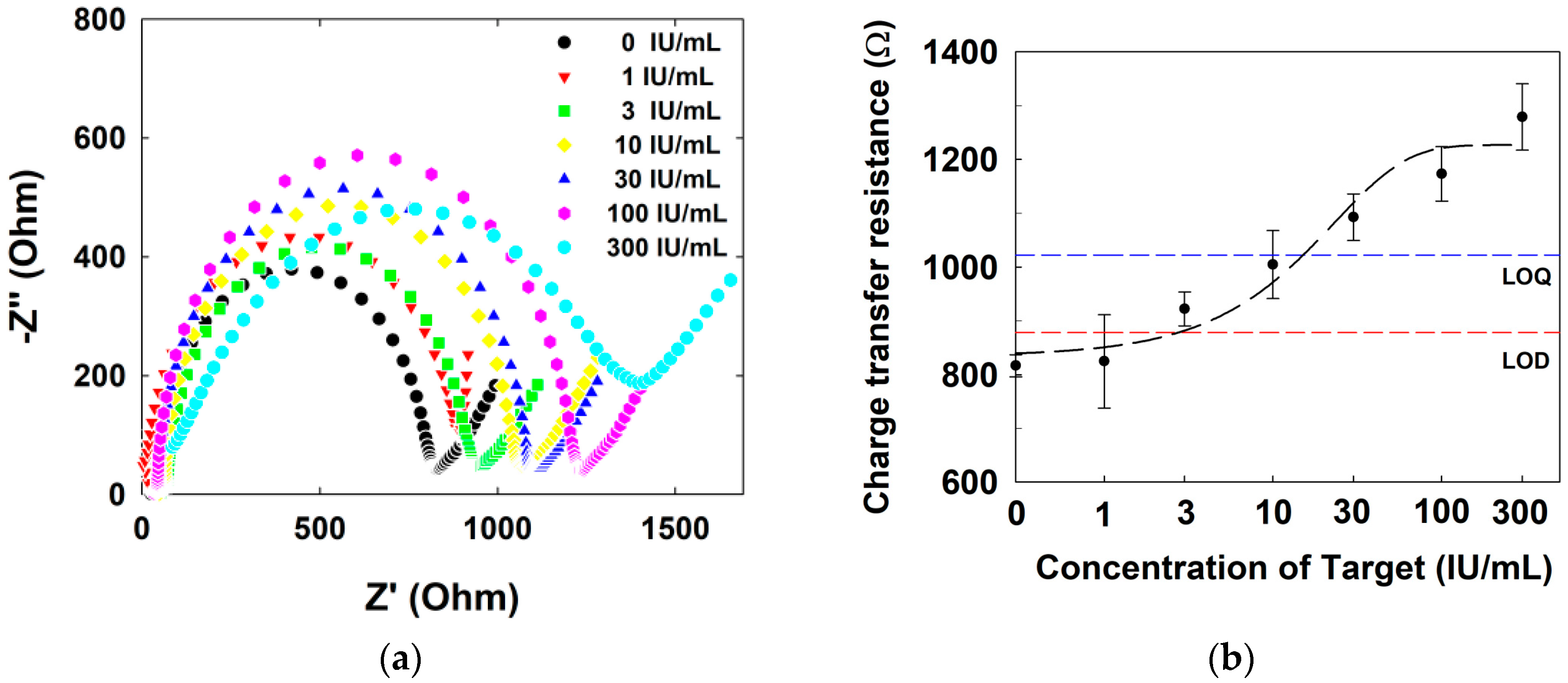
3.6. Electrochemical Detection of Human IgE via EIS
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Winter, W.E.; Hardt, N.S.; Fuhrman, S. Immunoglobulin E Importance in Parasitic Infections and Hypersensitivity Responses. Arch. Pathol. Lab. Med. 2000, 124, 1382–1385. [Google Scholar] [PubMed]
- Peavy, R.D.; Metcalfe, D.D. Understanding the mechanisms of anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Farid, S.; Meshik, X.; Xu, K.; Choi, M.; Ranginwala, S.; Wang, Y.Y.; Burke, P.; Dutta, M.; Stroscio, M.A. Detection of Immunoglobulin E with a Graphene-Based Field-Effect Transistor Aptasensor. J. Sens. 2018, 2018. [Google Scholar] [CrossRef]
- Cruz, A.A. Global Surveillance, Prevention and Control of Chronic Respiratory Diseases: A Comprehensive Approach; World Health Organization Press: Geneva, Switzerland, 2007. [Google Scholar]
- Leung, D.Y.M. Immunologic basis of chronic allergic disease: Clinical messages from the laboratory bench. Pediatr. Res. 1997, 42, 559–568. [Google Scholar] [CrossRef]
- Amarasekera, M. Immunoglobulin E in health and disease. Asia Pac. Allergy 2011, 1, 12–15. [Google Scholar] [CrossRef]
- Wachholz, P.A.; Dearman, R.J.; Kimber, I. Detection of Allergen-Specific IgE Antibody Responses. J. Immunotoxicol. 2004, 1, 189–199. [Google Scholar] [CrossRef]
- Burrows, B.; Martinez, F.D.; Halonen, M.; Barbee, R.A.; Cline, M.G. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N. Engl. J. Med. 1989, 320, 271–277. [Google Scholar] [CrossRef]
- Guilloux, L.; Ricard-Blum, S.; Ville, G.; Motin, J. A new radioimmunoassay using a commercially available solid support for the detection of IgE antibodies against muscle relaxants. J. Allergy Clin. Immunol. 1992, 90, 153–159. [Google Scholar] [CrossRef]
- Papamichael, K.I.; Kreuzer, M.P.; Guilbault, G.G. Viability of allergy (IgE) detection using an alternative aptamer receptor and electrochemical means. Sens. Actuators B 2007, 121, 178–186. [Google Scholar] [CrossRef]
- Kuby, J. Immunology, 3rd ed.; W.H. Freeman and Company: New York, NY, USA, 1997; Chapter 5. [Google Scholar]
- Kreuzer, M.P.; O’Sullivan, C.K.; Pravda, M.; Guilbault, G.G. Development of an immunosensor for the determination of allergy antibody (IgE) in blood samples. Anal. Chim. Acta 2001, 442, 45–53. [Google Scholar] [CrossRef]
- Grange, R.D.; Thompson, J.P.; Lambert, D.G. Radioimmunoassay, enzyme and non-enzyme-based immunoassays. Br. J. Anaesth. 2014, 112, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Cretich, M.; Carlo, G.D.; Giudici, C.; Pokoj, S.; Lauer, I.; Scheurer, S.; Chiari, M. Detection of allergen specific immunoglobulins by microarrays coupled to microfluidics. Proteomics 2009, 9, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Canio, M.D.; D’Aguanno, S.; Sacchetti, C.; Petrucci, F.; Cavagni, G.; Nuccetelli, M.; Federici, G.; Urbani, A.; Bernardini, S. Novel IgE Recognized Components of Lolium perenne Pollen Extract: Comparative Proteomics Evaluation of Allergic Patients Sensitization Profiles. J. Proteome Res. 2009, 8, 4383–4391. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.Y.; Lee, M.C. Development of a FPW allergy biosensor for human IgE detection by MEMS and cystamine-based SAM technologies. Sens. Actuators B 2008, 132, 340–348. [Google Scholar] [CrossRef]
- Liss, M.; Petersen, B.; Wolf, H.; Prohaska, E. An Aptamer-Based Quartz Crystal Protein Biosensor. Anal. Chem. 2002, 74, 4488–4495. [Google Scholar] [CrossRef]
- Nam, E.J.; Kim, E.J.; Wark, A.W.; Rho, S.; Kim, H.; Lee, H.J. Highly sensitive electrochemical detection of proteins using aptamer-coated gold nanoparticles and surface enzyme reactions. Analyst 2012, 137, 2011–2016. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Lee, S.J.; Lee, H.J. Ultra-sensitive detection of IgE using biofunctionalized nanoparticle-enhanced SPR. Talanta 2010, 81, 1755–1759. [Google Scholar] [CrossRef]
- Song, W.; Li, H.; Liu, H.; Wu, Z.; Qiang, W.; Xu, D. Fabrication of streptavidin functionalized silver nanoparticle decorated graphene and its application in disposable electrochemical sensor for immunoglobulin E. Electrochem. Commun. 2013, 31, 16–19. [Google Scholar] [CrossRef]
- Liang, Z.-Y.; Deng, Y.-Q.; Tao, Z.-Z. A quantum dot-based lateral flow immunoassay for the rapid, quantitative, and sensitive detection of specific IgE for mite allergens in sera from patients with allergic rhinitis. Anal. Bioanal. Chem. 2020, 412, 1785–1794. [Google Scholar] [CrossRef]
- Aydın, E.B.; Sezgintürk, M.K. Indium tin oxide (ITO): A promising material in biosensing technology. Trends Anal. Chem. 2017, 97, 309–315. [Google Scholar] [CrossRef]
- Huang, Y.; Bell, M.C.; Suni, I.I. Impedance Biosensor for Peanut Protein Ara h 1. Anal. Chem. 2008, 80, 9157–9161. [Google Scholar] [CrossRef] [PubMed]
- Khezrian, S.; Salimi, A.; Teymourian, H.; Hallaj, R. Label-free electrochemical IgE aptasensor based on covalent attachment of aptamer onto multiwalled carbon nanotubes/ionic liquid/chitosan nanocomposite modified electrode. Biosens. Bioelectron. 2013, 43, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Jo, K.; Yang, H. Effect of Different Pretreatments on Indium-Tin Oxide Electrodes. Bull. Korean Chem. Soc. 2013, 34, 421–425. [Google Scholar] [CrossRef][Green Version]
- Khan, Z.H. Effect of ITO surface properties on SAM modification: A review toward biosensor application. Cogent Eng. 2016, 3, 1170097. [Google Scholar] [CrossRef]
- Wei, Z.Q.; Wang, C.; Zhu, C.F.; Zhou, C.Q.; Xu, B.; Bai, C.L. Study on single-bond interaction between amino-terminated organosilane self-assembled monolayers by atomic force microscopy. Surf. Sci. 2000, 459, 401–412. [Google Scholar] [CrossRef]
- Yoon, H.C.; Hong, M.-Y.; Kim, H.-S. Functionalization of a Poly(amidoamine) Dendrimer with Ferrocenyls and Its Application to the Construction of a Reagentless Enzyme Electrode. Anal. Chem. 2000, 72, 4420–4427. [Google Scholar] [CrossRef]
- Xiao, Y.; Isaacs, S.N. Enzyme-linked immunosorbent assay (ELISA) and blocking with bovine serum albumin (BSA)-not all BSAs are alike. J. Immunol. Methods 2012, 384, 148–151. [Google Scholar] [CrossRef]
- Dubois, L.H.; Nuzzo, R.G. Synthesis, structure, and properties of model organic surfaces. Annu. Rev. Phys. Chem. 1992, 43, 437–463. [Google Scholar] [CrossRef]
- Ulman, A. An Introduction to Ultrathin Organic Films: From Langmuir-Blogett to Self-Assembly; Academic Press Inc: Boston, MA, USA, 1991. [Google Scholar]
- Aziz, M.A.; Jo, K.; Qaium, M.A.; Huh, C.-H.; Hong, I.S.; Yang, H. Platform for Highly Sensitive Alkaline Phosphatase-Based Immunosensors Using 1-Naphthyl Phosphate and an Avidin-Modified Indium Tin Oxide Electrode. Electroanalysis 2009, 21, 2160–2164. [Google Scholar] [CrossRef]
- Kwon, S.J.; Yang, H.; Jo, K.; Kwak, J. An electrochemical immunosensor using p-aminophenol redox cycling by NADH on a self-assembled monolayer and ferrocene-modified Au electrodes. Analyst 2008, 133, 1599–1604. [Google Scholar] [CrossRef]
- Oh, S.J.; Ahn, J.K.; Park, H.; Song, Y.; Kwon, S.J.; Shin, H.-B. An electrochemical immunosensing system on patterned electrodes for immunoglobulin E detection. Anal. Methods 2019, 11, 4410–4415. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, K.J.; Jung, S.Y.; Hwang, Y.J.; Kwon, S.J. Redox-Active Self-Assembled Monolayer on Au ultramicroelectrode and its Electrocatalytic Detection of p-aminophenol Oxidation. J. Electrochem. Sci. Technol. 2019, 10, 170–176. [Google Scholar]
- Zhu, G.; Lee, H.J. Electrochemical sandwich-type biosensors for α−1 antitrypsin with carbon nanotubes and alkaline phosphatase labeled antibody-silver nanoparticles. Biosens. Bioelectron. 2017, 89, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, K.; Yang, H.; Kim, Y.T.; Kwak, J. Enzyme-Amplified Electrochemical Detection of DNA Using Electrocatalysis of Ferrocenyl-Tethered Dendrimer. Anal. Chem. 2003, 75, 5665–5672. [Google Scholar] [CrossRef]
- Kwon, S.J.; Kim, E.; Yang, H.; Kwak, J. An electrochemical immunosensor using ferrocenyl-tethered dendrimer. Analyst 2006, 131, 402–406. [Google Scholar] [CrossRef]
- Kang, C.; Kang, J.; Lee, N.-S.; Yoon, Y.H.; Yang, H. DT-Diaphorase as a Bifunctional Enzyme Label That Allows Rapid Enzymatic Amplification and Electrochemical Redox Cycling. Anal. Chem. 2017, 89, 7974–7980. [Google Scholar] [CrossRef] [PubMed]
- Nandhakumar, P.; Kim, B.; Lee, N.-S.; Yoon, Y.H.; Lee, K.; Yang, H. Nitrosoreductase-Like Nanocatalyst for Ultrasensitive and Stable Biosensing. Anal. Chem. 2018, 90, 807–813. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.; Song, Y.; Kim, K.J.; Oh, S.J.; Ahn, J.K.; Park, H.; Shin, H.-B.; Kwon, S.J. Electrochemical Immunosensor for Human IgE Using Ferrocene Self-Assembled Monolayers Modified ITO Electrode. Biosensors 2020, 10, 38. https://doi.org/10.3390/bios10040038
Park M, Song Y, Kim KJ, Oh SJ, Ahn JK, Park H, Shin H-B, Kwon SJ. Electrochemical Immunosensor for Human IgE Using Ferrocene Self-Assembled Monolayers Modified ITO Electrode. Biosensors. 2020; 10(4):38. https://doi.org/10.3390/bios10040038
Chicago/Turabian StylePark, Myungsang, Yesol Song, Ki Jun Kim, Seung Jun Oh, Jun Ki Ahn, Hun Park, Hang-Beum Shin, and Seong Jung Kwon. 2020. "Electrochemical Immunosensor for Human IgE Using Ferrocene Self-Assembled Monolayers Modified ITO Electrode" Biosensors 10, no. 4: 38. https://doi.org/10.3390/bios10040038
APA StylePark, M., Song, Y., Kim, K. J., Oh, S. J., Ahn, J. K., Park, H., Shin, H.-B., & Kwon, S. J. (2020). Electrochemical Immunosensor for Human IgE Using Ferrocene Self-Assembled Monolayers Modified ITO Electrode. Biosensors, 10(4), 38. https://doi.org/10.3390/bios10040038