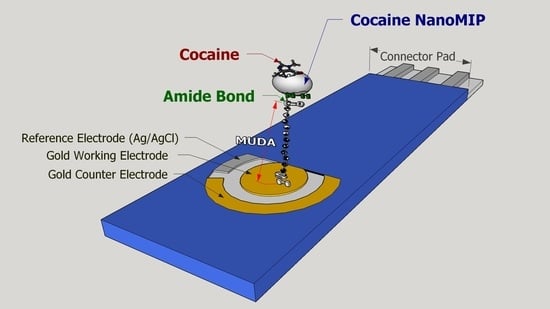
Molecularly Imprinted Nanoparticles Based Sensor for Cocaine Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus and Measurements
2.3. NanoMIP Sensor Fabrication and Characterization
2.4. Development and Optimization of the EIS NanoMIPs Affinity Sensor
2.4.1. Blocking Agent Optimization
2.4.2. Cocaine Assay
2.4.3. Specificity Assays
2.5. Data Processing and Analysis
3. Results and Discussion
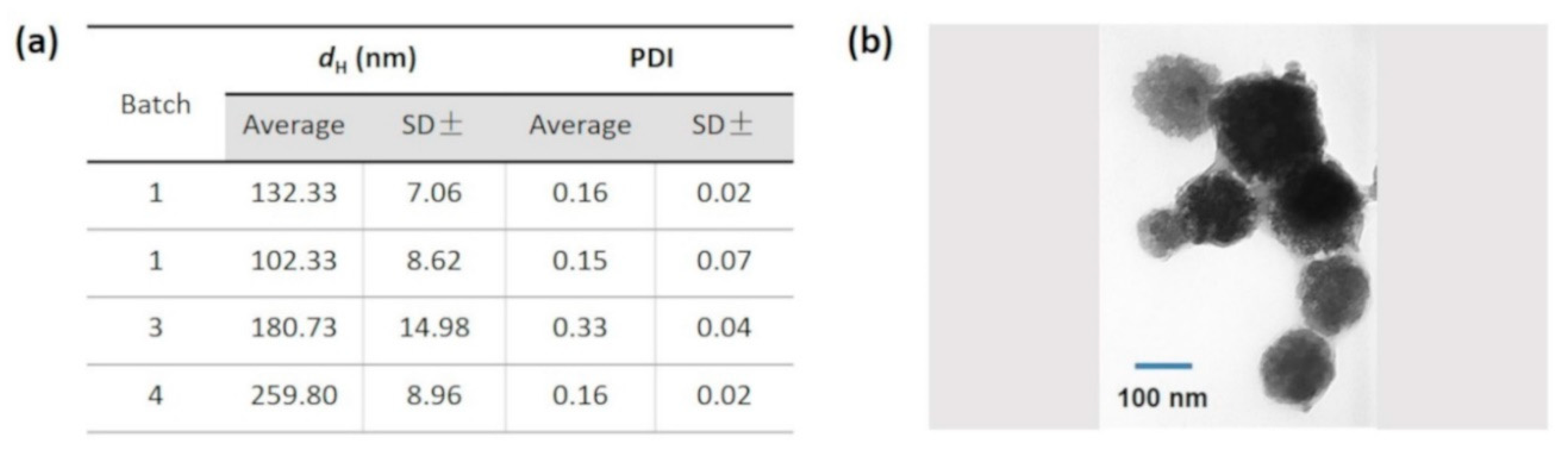
3.1. NanoMIPs Characterization Study
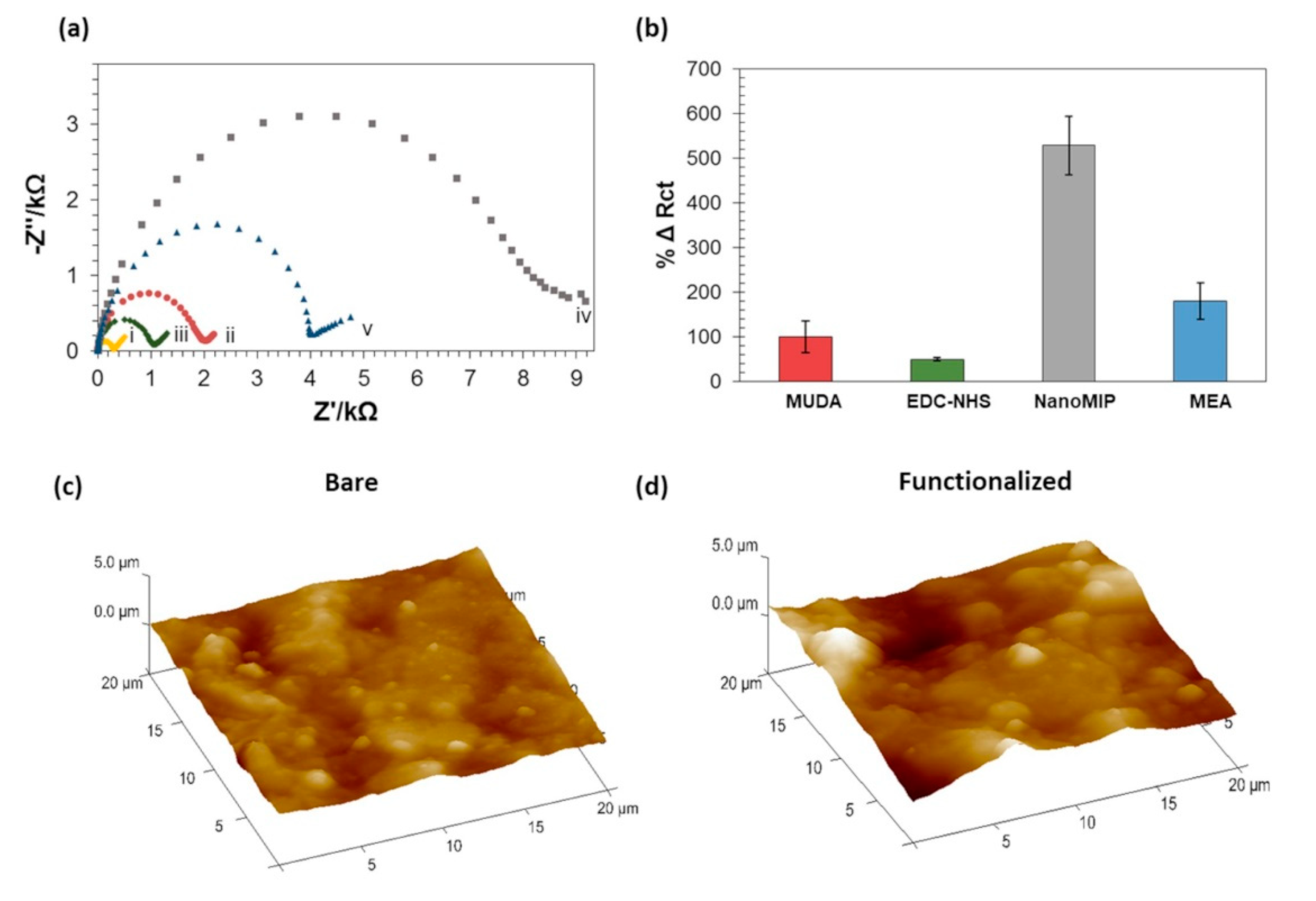
3.2. EIS NanoMIPs Sensor Construction
3.3. EIS NanoMIPs Sensor Optimization
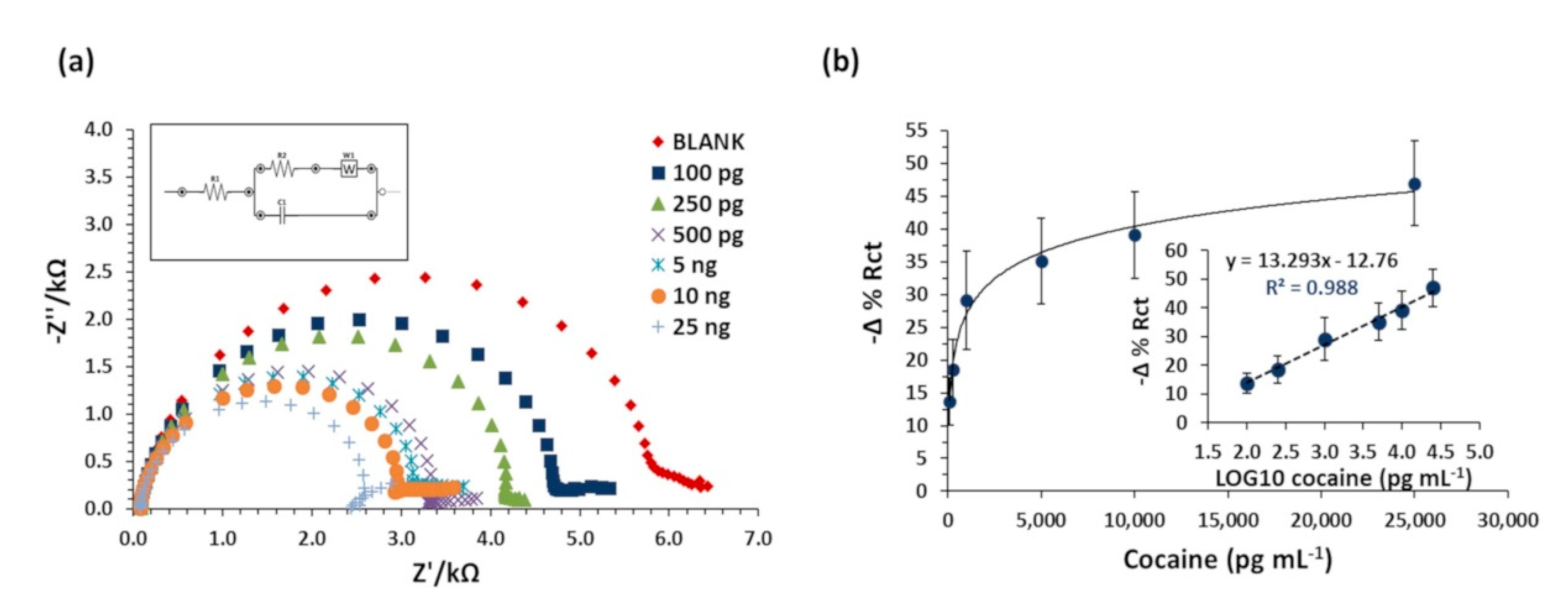
3.4. Sensor Sensitivity and Specificity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNODC. World Drug Report 2016; United Nations Office on Drugs and Crime: Vienna, Austria, 2016. [Google Scholar]
- EMCDDA. European Drug Report 2017: Trends and Developments; European Monitoring Centre for Drugs and Drug Addiction: Lisbon, Portugal, 2017. [Google Scholar]
- Cerreta, M.M.; Furton, K.G. An assessment of detection canine alerts using flowers that release methyl benzoate, the cocaine odorant, and an evaluation of their behavior in terms of the VOCs produced. Forensic Sci. Int. 2015, 251, 107–114. [Google Scholar] [CrossRef]
- Jezierski, T.; Adamkiewicz, E.; Walczak, M.; Sobczyńska, M.; Górecka-Bruzda, A.; Ensminger, J.; Papet, E. Efficacy of drug detection by fully-trained police dogs varies by breed, training level, type of drug and search environment. Forensic Sci. Int. 2014, 237, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Leitch, O.; Anderson, A.; Kirkbride, K.P.; Lennard, C. Biological organisms as volatile compound detectors: A review. Forensic Sci. Int. 2013, 232, 92–103. [Google Scholar] [CrossRef] [PubMed]
- UNODC. Recommended Methods for the Identification and Analysis of Cocaine in Seized Materials; United Nations Office on Drugs and Crime: Vienna, Austria, 2012. [Google Scholar]
- Harper, L.; Powell, J.; Pijl, E.M. An overview of forensic drug testing methods and their suitability for harm reduction point-of-care services. Harm Reduct. J. 2017, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Cumeras, R.; Figueras, E.; Davis, C.E.; Baumbach, J.I.; Gràcia, I. Review on ion mobility spectrometry. Part 1: Current instrumentation. Analyst 2015, 140, 1376–1390. [Google Scholar] [CrossRef] [PubMed]
- Musshoff, F.; Hokamp, E.G.; Bott, U.; Madea, B. Performance evaluation of on-site oral fluid drug screening devices in normal police procedure in Germany. Forensic Sci. Int. 2014, 238, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Cecinato, A.; Balducci, C.; Perilli, M. Illicit psychotropic substances in the air: The state-of-art. Sci. Total Environ. 2016, 539, 1–6. [Google Scholar] [CrossRef]
- Poltorak, L.; Sudhölter, E.J.R.; de Puit, M. Electrochemical cocaine (bio) sensing. From solid electrodes to soft junctions. TrAC Trends Anal. Chem. 2019, 114, 48–55. [Google Scholar] [CrossRef]
- Mokhtarzadeh, A.; Dolatabadi, J.E.N.; Abnous, K.; de la Guardia, M.; Ramezani, M. Nanomaterial-based cocaine aptasensors. Biosens. Bioelectron. 2015, 68, 95–106. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Danesh, N.M.; Emrani, A.S.; Ramezani, M.; Abnous, K. A novel electrochemical aptasensor based on single-walled carbon nanotubes, gold electrode and complimentary strand of aptamer for ultrasensitive detection of cocaine. Biosens. Bioelectron. 2015, 73, 245–250. [Google Scholar] [CrossRef]
- Goode, J.; Dillon, G.; Millner, P.A. The development and optimisation of nanobody based electrochemical immunosensors for IgG. Sens. Actuators B Chem. 2016, 234, 478–484. [Google Scholar] [CrossRef]
- Tran, T.B.; Son, S.J.; Min, J. Nanomaterials in label-free impedimetric biosensor: Current process and future perspectives. Biochip J. 2016, 10, 318–330. [Google Scholar] [CrossRef]
- Muñoz, J.; Montes, R.; Baeza, M. Trends in electrochemical impedance spectroscopy involving nanocomposite transducers: Characterization, architecture surface and bio-sensing. TrAC Trends Anal. Chem. 2017, 97, 201–215. [Google Scholar] [CrossRef]
- Fernandes, F.C.B.; Santos, A.; Martins, D.C.; Góes, M.S.; Bueno, P.R. Comparing label free electrochemical impedimetric and capacitive biosensing architectures. Biosens. Bioelectron. 2014, 57, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Gui, R.; Guo, H.; Jin, H. Preparation and applications of electrochemical chemosensors based on carbon-nanomaterial-modified molecularly imprinted polymers. Nanoscale Adv. 2019, 1, 3325–3363. [Google Scholar] [CrossRef]
- Ahmad, O.S.; Bedwell, T.S.; Esen, C.; Garcia-Cruz, A.; Piletsky, S.A. Molecularly Imprinted Polymers in Electrochemical and Optical Sensors. Trends Biotechnol. 2019, 37, 294–309. [Google Scholar] [CrossRef]
- Gui, R.; Jin, H.; Guo, H.; Wang, Z. Recent advances and future prospects in molecularly imprinted polymers-based electrochemical biosensors. Biosens. Bioelectron. 2018, 100, 56–70. [Google Scholar] [CrossRef]
- Abdin, M.J.; Altintas, Z.; Tothill, I.E. In silico designed nanoMIP based optical sensor for endotoxins monitoring. Biosens. Bioelectron. 2015, 67, 177–183. [Google Scholar] [CrossRef]
- Chianella, I.; Guerreiro, A.; Moczko, E.; Caygill, J.S.; Piletska, E.V.; De Vargas Sansalvador, I.M.P.; Whitcombe, M.J.; Piletsky, S.A. Direct replacement of antibodies with molecularly imprinted polymer nanoparticles in ELISA—Development of a novel assay for vancomycin. Anal. Chem. 2013, 85, 8462–8468. [Google Scholar] [CrossRef]
- Mazzotta, E.; Turco, A.; Chianella, I.; Guerreiro, A.; Piletsky, S.A.; Malitesta, C. Solid-phase synthesis of electroactive nanoparticles of molecularly imprinted polymers. A novel platform for indirect electrochemical sensing applications. Sens. Actuators B Chem. 2016, 229, 174–180. [Google Scholar] [CrossRef]
- Canfarotta, F.; Poma, A.; Guerreiro, A.; Piletsky, S. Solid-phase synthesis of molecularly imprinted nanoparticles. Nat. Protoc. 2016, 11, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Smolinska-Kempisty, K.; Ahmad, O.S.S.; Guerreiro, A.; Karim, K.; Piletska, E.; Piletsky, S. New potentiometric sensor based on molecularly imprinted nanoparticles for cocaine detection. Biosens. Bioelectron. 2017, 96, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ashley, J.; Shukor, Y.; D’Aurelio, R.; Trinh, L.; Rodgers, T.L.; Temblay, J.; Pleasants, M.; Tothill, I.E. Synthesis of MIP Nanoparticles for α-Casein Detection using SPR as a Milk Allergen Sensor. ACS Sens. 2018, 3, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Rushworth, J.V.; Wright, J.D.; Millner, P.A. Novel impedimetric immunosensor for detection of pathogenic bacteria Streptococcus pyogenes in human saliva. Anal. Chem. 2013, 85, 12118–12125. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.K.; Freire, R.S.; Fonseca, C.P.; Neves, S.; Kubota, L.T. Characterization of self-assembled thiols monolayers on gold surface by electrochemical impedance spectroscopy. J. Braz. Chem. Soc. 2004, 15, 849–855. [Google Scholar] [CrossRef]
- Sanders, W.; Vargas, R.; Anderson, M.R. Characterization of Carboxylic Acid-Terminated Self-Assembled Monolayers by Electrochemical Impedance Spectroscopy and Scanning Electrochemical Microscopy. Langmuir 2008, 24, 6133–6139. [Google Scholar] [CrossRef]
- Altintas, Z.; Uludag, Y.; Gurbuz, Y.; Tothill, I. Development of surface chemistry for surface plasmon resonance based sensors for the detection of proteins and DNA molecules. Anal. Chim. Acta 2012, 712, 138–144. [Google Scholar] [CrossRef]
- Hushegyi, A.; Bertok, T.; Damborsky, P.; Katrlik, J.; Tkac, J. An ultrasensitive impedimetric glycan biosensor with controlled glycan density for detection of lectins and influenza hemagglutinins. Chem. Commun. Camb 2015, 51, 7474–7477. [Google Scholar] [CrossRef]
- Riquelme, M.V.; Zhao, H.; Srinivasaraghavan, V.; Pruden, A.; Vikesland, P.; Agah, M. Optimizing blocking of nonspecific bacterial attachment to impedimetric biosensors. Sens. Bio-Sens. Res. 2016, 8, 47–54. [Google Scholar] [CrossRef]
- Atta, N.F.; Hassan, H.K.; Galal, A. Rapid and simple electrochemical detection of morphine on graphene-palladium-hybrid-modified glassy carbon electrode. Anal. Bioanal. Chem. 2014, 406, 6933–6942. [Google Scholar] [CrossRef]
- Parker, C.O.; Tothill, I.E. Development of an electrochemical immunosensor for aflatoxin M1 in milk with focus on matrix interference. Biosens. Bioelectron. 2009, 24, 2452–2457. [Google Scholar] [CrossRef] [PubMed]
- EMCDDA. European Drug Report 2012; European Monitoring Centre for Drugs and Drug Addiction: Luxembourg, 2012. [Google Scholar]
- Broséus, J.; Huhtala, S.; Esseiva, P. First systematic chemical profiling of cocaine police seizures in Finland in the framework of an intelligence-led approach. Forensic Sci. Int. 2015, 251, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Lapachinske, S.F.; Okai, G.G.; dos Santos, A.; de Bairros, A.V.; Yonamine, M. Analysis of cocaine and its adulterants in drugs for international trafficking seized by the Brazilian Federal Police. Forensic Sci. Int. 2015, 247, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Botelho, É.D.; Cunha, R.B.; Campos, A.F.C.; Maldaner, A.O. Chemical Profiling of Cocaine Seized by Brazilian Federal Police in 2009–2012: Major Components. J. Braz. Chem. Soc. 2014, 25, 611–618. [Google Scholar] [CrossRef]
- da Silva, A.F.; Grobério, T.S.; Zacca, J.J.; Maldaner, A.O.; Braga, J.W.B. Cocaine and adulterants analysis in seized drug samples by infrared spectroscopy and MCR-ALS. Forensic Sci. Int. 2018, 290, 169–177. [Google Scholar] [CrossRef]
- Magalhães, E.J.; Nascentes, C.C.; Pereira, L.S.A.; Guedes, M.L.O.; Lordeiro, R.A.; Auler, L.M.L.A.; Augusti, R.; de Queiroz, M.E.L.R. Evaluation of the composition of street cocaine seized in two regions of Brazil. Sci. Justice 2013, 53, 425–432. [Google Scholar] [CrossRef]
- Garcia, Y.; Smolinska-Kempisty, K.; Pereira, E.; Piletska, E.; Piletsky, S. Development of competitive ‘pseudo’-ELISA assay for measurement of cocaine and its metabolites using molecularly imprinted polymer nanoparticles. Anal. Methods 2017, 9, 4592–4598. [Google Scholar] [CrossRef]
- Waggoner, L.P.; Johnston, J.M.; Williams, M.; Jackson, J.; Jones, M.H.; Boussom, T.; Petrousky, J.A. Canine olfactory sensitivity to cocaine hydrochloride and methyl benzoate. In Chemistry-and Biology-Based Technologies for Contraband Detection, Proceedings of the Enabling Technologies for Law Enforcement and Security, Boston, MA, United States, 18–22 November 1996; Pilon, P., Burmeister, S., Eds.; SPIE Digital Library: Bellingham, WA, USA, 1997; Volume 2937, pp. 216–226. [Google Scholar]
- Weyermann, C.; Mimoune, Y.; Anglada, F.; Massonnet, G.; Esseiva, P.; Buzzini, P. Applications of a transportable Raman spectrometer for the in situ detection of controlled substances at border controls. Forensic Sci. Int. 2011, 209, 21–28. [Google Scholar] [CrossRef]
- Wille, S.M.R.; Samyn, N.; del Mar Ramírez-Fernández, M.; De Boeck, G. Evaluation of on-site oral fluid screening using Drugwipe-5+®, RapidSTAT ® and Drug Test 5000 ® for the detection of drugs of abuse in drivers. Forensic Sci. Int. 2010, 198, 2–6. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.; Jiang, J.; Zhang, J.; Gao, J.; Qiao, X. Rapid, in situ detection of cocaine residues based on paper spray ionization coupled with ion mobility spectrometry. Analyst 2014, 139, 1687–1691. [Google Scholar] [CrossRef]
- Yang, Z.; Castrignanò, E.; Estrela, P.; Frost, C.G.; Kasprzyk-Hordern, B. Community Sewage Sensors towards Evaluation of Drug Use Trends: Detection of Cocaine in Wastewater with DNA-Directed Immobilization Aptamer Sensors. Sci. Rep. 2016, 6, 21024. [Google Scholar] [CrossRef] [PubMed]
- Roushani, M.; Shahdost-fard, F. An aptasensor for voltammetric and impedimetric determination of cocaine based on a glassy carbon electrode modified with platinum nanoparticles and using rutin as a redox probe. Microchim. Acta 2016, 183, 185–193. [Google Scholar] [CrossRef]
- Vidal, J.C.; Bertolín, J.R.; Bonel, L.; Asturias, L.; Arcos-Martínez, M.J.; Castillo, J.R. A Multi-electrochemical Competitive Immunosensor for Sensitive Cocaine Determination in Biological Samples. Electroanalysis 2016, 28, 685–694. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Sun, T.; Grattan, K.T.V. Surface Plasmon Resonance Based Fibre Optic Chemical Sensor for the Detection of Cocaine. In Chemical, Environmental, Biological and Medical Sensors, Proceedings of the Sixth European Workshop on Optical Fibre Sensors, Limerick, Ireland, 31 May–3 June 2016; Lewis, E., Ed.; SPIE Digital Library: Bellingham, WA USA, 2016; Volume 9916, p. 991612. [Google Scholar]
- Wren, S.P.; Nguyen, T.H.; Gascoine, P.; Lacey, R.; Sun, T.; Grattan, K.T.V. Preparation of novel optical fibre-based Cocaine sensors using a molecular imprinted polymer approach. Sens. Actuators B Chem. 2014, 193, 35–41. [Google Scholar] [CrossRef]
- Neves, M.A.D.; Blaszykowski, C.; Bokhari, S.; Thompson, M. Ultra-high frequency piezoelectric aptasensor for the label-free detection of cocaine. Biosens. Bioelectron. 2015, 72, 383–392. [Google Scholar] [CrossRef]
- Oliveira, N.C.L.; El Khoury, G.; Versnel, J.M.; Moghaddam, G.K.; Leite, L.S.; Lima-Filho, J.L.; Lowe, C.R. A holographic sensor based on a biomimetic affinity ligand for the detection of cocaine. Sens. Actuators B Chem. 2018, 270, 216–222. [Google Scholar] [CrossRef]
- Adegoke, O.; McKenzie, C.; Daeid, N.N. Multi-shaped cationic gold nanoparticle-L-cysteine-ZnSeS quantum dots hybrid nanozyme as an intrinsic peroxidase mimic for the rapid colorimetric detection of cocaine. Sens. Actuators B Chem. 2019, 287, 416–427. [Google Scholar] [CrossRef]
- Poltorak, L.; Eggink, I.; Hoitink, M.; Sudhölter, E.J.R.; de Puit, M. Electrified Soft Interface as a Selective Sensor for Cocaine Detection in Street Samples. Anal. Chem. 2018, 90, 7428–7433. [Google Scholar] [CrossRef]
- Oueslati, R.; Cheng, C.; Wu, J.; Chen, J. Highly sensitive and specific on-site detection of serum cocaine by a low cost aptasensor. Biosens. Bioelectron. 2018, 108, 103–108. [Google Scholar] [CrossRef]
- Abnous, K.; Danesh, N.M.; Ramezani, M.; Taghdisi, S.M.; Emrani, A.S. A novel colorimetric aptasensor for ultrasensitive detection of cocaine based on the formation of three-way junction pockets on the surfaces of gold nanoparticles. Anal. Chim. Acta 2018, 1020, 110–115. [Google Scholar] [CrossRef]
- Yilmaz Sengel, T.; Guler, E.; Arslan, M.; Gumus, Z.P.; Sanli, S.; Aldemir, E.; Akbulut, H.; Odaci Demirkol, D.; Coskunol, H.; Timur, S.; et al. “Biomimetic-electrochemical-sensory-platform” for biomolecule free cocaine testing. Mater. Sci. Eng. C 2018, 90, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Guler, E.; Yilmaz Sengel, T.; Gumus, Z.P.; Arslan, M.; Coskunol, H.; Timur, S.; Yagci, Y. Mobile Phone Sensing of Cocaine in a Lateral Flow Assay Combined with a Biomimetic Material. Anal. Chem. 2017, 89, 9629–9632. [Google Scholar] [CrossRef]
- Abdelshafi, N.A.; Bell, J.; Rurack, K.; Schneider, R.J. Microfluidic electrochemical immunosensor for the trace analysis of cocaine in water and body fluids. Drug Test. Anal. 2018, 1, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Verkouteren, J.R.; Staymates, J.L. Reliability of ion mobility spectrometry for qualitative analysis of complex, multicomponent illicit drug samples. Forensic Sci. Int. 2011, 206, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, S.; Mellon, M.B.; Banuelos, S.; Arndt, C. Evaluation of commercial enzyme-linked immunosorbent assays to identify psychedelic phenethylamines. J. Anal. Toxicol. 2011, 35, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Suman, P.; Kumar, A.; Sharma, P.; Capalash, N.; Suri, C.R. Recent advances in immunosensor for narcotic drug detection. Bioimpacts 2015, 5, 207–213. [Google Scholar] [CrossRef]

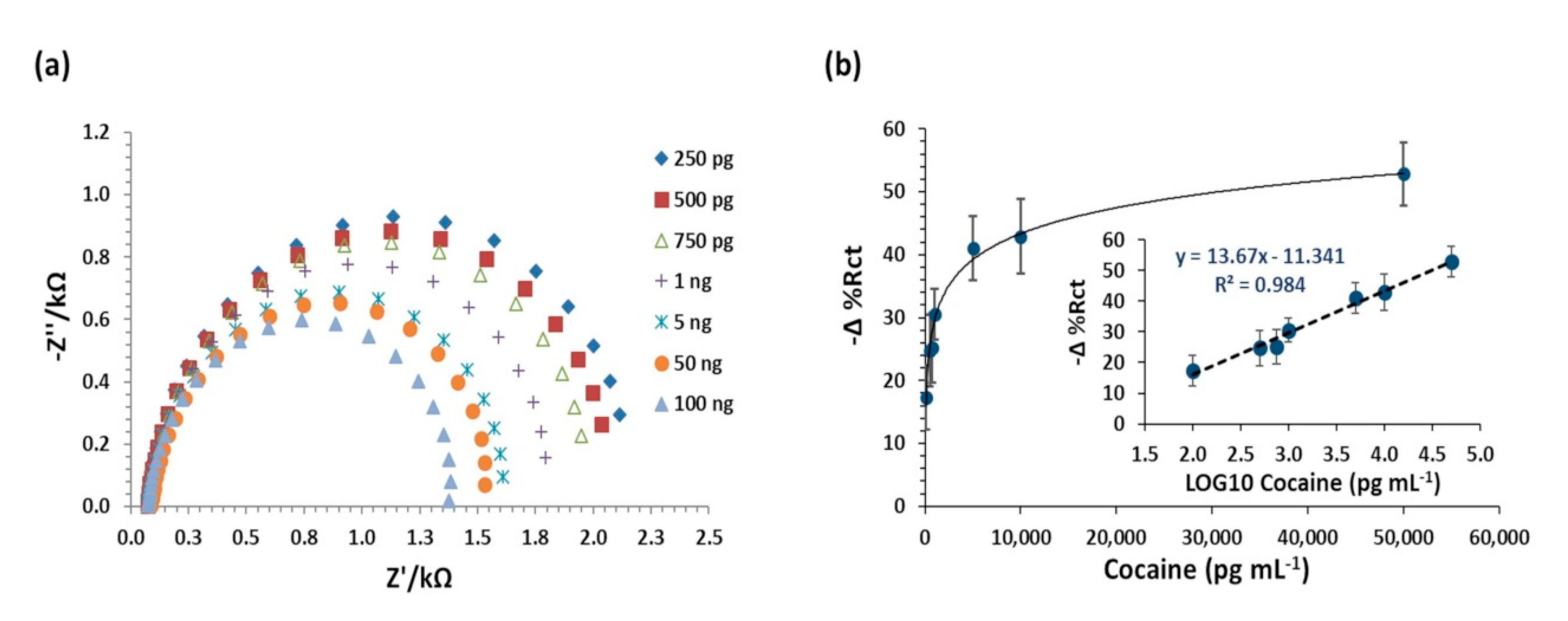
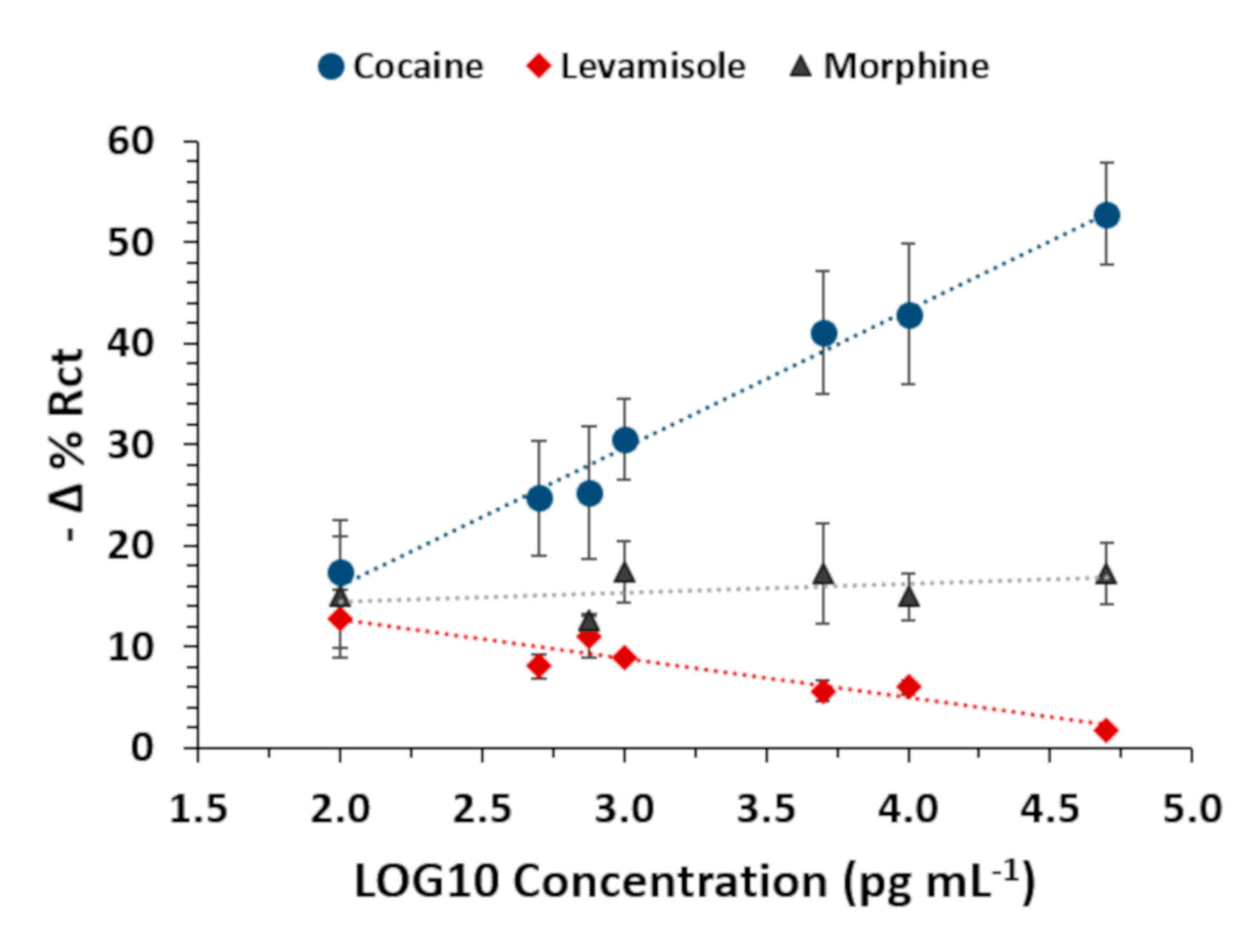
| Analyte | NanoMIPs (Yes/No) | Error % Rct (±SD 1) | Linear Equation | R2 | p-Value |
|---|---|---|---|---|---|
| Cocaine | Yes | 2.44 (±1.55) | 0.984 | <0.000 | |
| Morphine | Yes | 3.31 (±1.76) | 0.193 | 0.353 | |
| Levamisole | Yes | 1.47 (±1.33) | 0.879 | 0.001 |
| Detection Tools Description | Sample | LOD * | Linear Range | Reference |
| Dogs Olfactory system | Illicit cocaine vapor | 88.3 pM | - | [42] |
| Transportable Raman spectrometer | Mixture from street sample, seized materials | 14.7 mM | - | [43] |
| Immuno-based kit | Biological and environmental samples | 29.4–147 nM | - | [9,44] |
| Paper spray Ion Trap Mass Spectrometry (IMS) | Surface samples and bulk powder dissolved in liquid | 5.9 µM | 14.7–588 µM | [45] |
| Surface samples | 5 ng (sampled area) | - | ||
| Various Hyphenated MS Methods | Liquid sample | 5.8 µM | - | [10] |
| Outdoor and indoor air | 0.07–0.33 ng m−3 | - | ||
| EIS aptasensor | Wastewater | 10 nM | 10 nM–50 µM | [46] |
| Spiked serum | 200 pM | 1–8 nM | [47] | |
| Voltammetric aptasensor | Spiked serum | 1–8 nM | 1–8 nM | [47] |
| Amperometric immunosensor | Urine Saliva Serum | 42.38 nM 10.6 nM 74.16 nM | - | [48] |
| Potentiometric sensor MIP | Blood serum | - | 1 nM–1 mM | [25] |
| SPR–MIP film | Diluted cocaine | - | 0–400 µM | [49] |
| Optical fiber MIP | Diluted cocaine | - | 500–1000 µM | [50] |
| Electromagnetic piezoelectric acoustic sensor (EMPAS) aptasensor | Diluted cocaine | 0.9 µM | 2–50 µM | [51] |
| Holographic sensor—biomimetic receptor | Diluted cocaine | 7.1 mM | 10–50 mM | [52] |
| Colorimetric sensor—CTAB-AuNP-L-cyst-ZnSeS QDs hybrid nanozyme | Diluted cocaine | 128 nM | 20–100 µM | [53] |
| Voltammetry Sensor—no receptor | Diluted cocaine | 10.9 µM | 20 µM–100 mM | [54] |
| Capacitance sensor—Aptamer | Spiked serum Buffer | 1.34 pM 7.8 fM | 14.5 fM–1.45 pM | [55] |
| Colorimetric—Au NP Aptamer | Spiked serum | 830 pM | 2–100 nM (buffer) | [56] |
| Electrochemical Sensor—β CD | Diluted cocaine in synthetic urine | 28.62 nM | 25–200 nM | [57] |
| Electrochemical LFD—β CD Antibody | Synthetic saliva | - | 0.03–2.94 µM | [58] |
| Cyclic Voltammetry—Antibody | water, oral fluids and urine | 2.94 fM | 2.94 fM–2.9 µM | [59] |
| EIS-sensor nanoMIPs | Diluted cocaine | 0.70 nM | 0.30–147 nM | Our work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Aurelio, R.; Chianella, I.; Goode, J.A.; Tothill, I.E. Molecularly Imprinted Nanoparticles Based Sensor for Cocaine Detection. Biosensors 2020, 10, 22. https://doi.org/10.3390/bios10030022
D’Aurelio R, Chianella I, Goode JA, Tothill IE. Molecularly Imprinted Nanoparticles Based Sensor for Cocaine Detection. Biosensors. 2020; 10(3):22. https://doi.org/10.3390/bios10030022
Chicago/Turabian StyleD’Aurelio, Roberta, Iva Chianella, Jack A. Goode, and Ibtisam E. Tothill. 2020. "Molecularly Imprinted Nanoparticles Based Sensor for Cocaine Detection" Biosensors 10, no. 3: 22. https://doi.org/10.3390/bios10030022
APA StyleD’Aurelio, R., Chianella, I., Goode, J. A., & Tothill, I. E. (2020). Molecularly Imprinted Nanoparticles Based Sensor for Cocaine Detection. Biosensors, 10(3), 22. https://doi.org/10.3390/bios10030022