Biocompatibility of Blank, Post-Processed and Coated 3D Printed Resin Structures with Electrogenic Cells
Abstract
1. Introduction
2. Materials and Methods
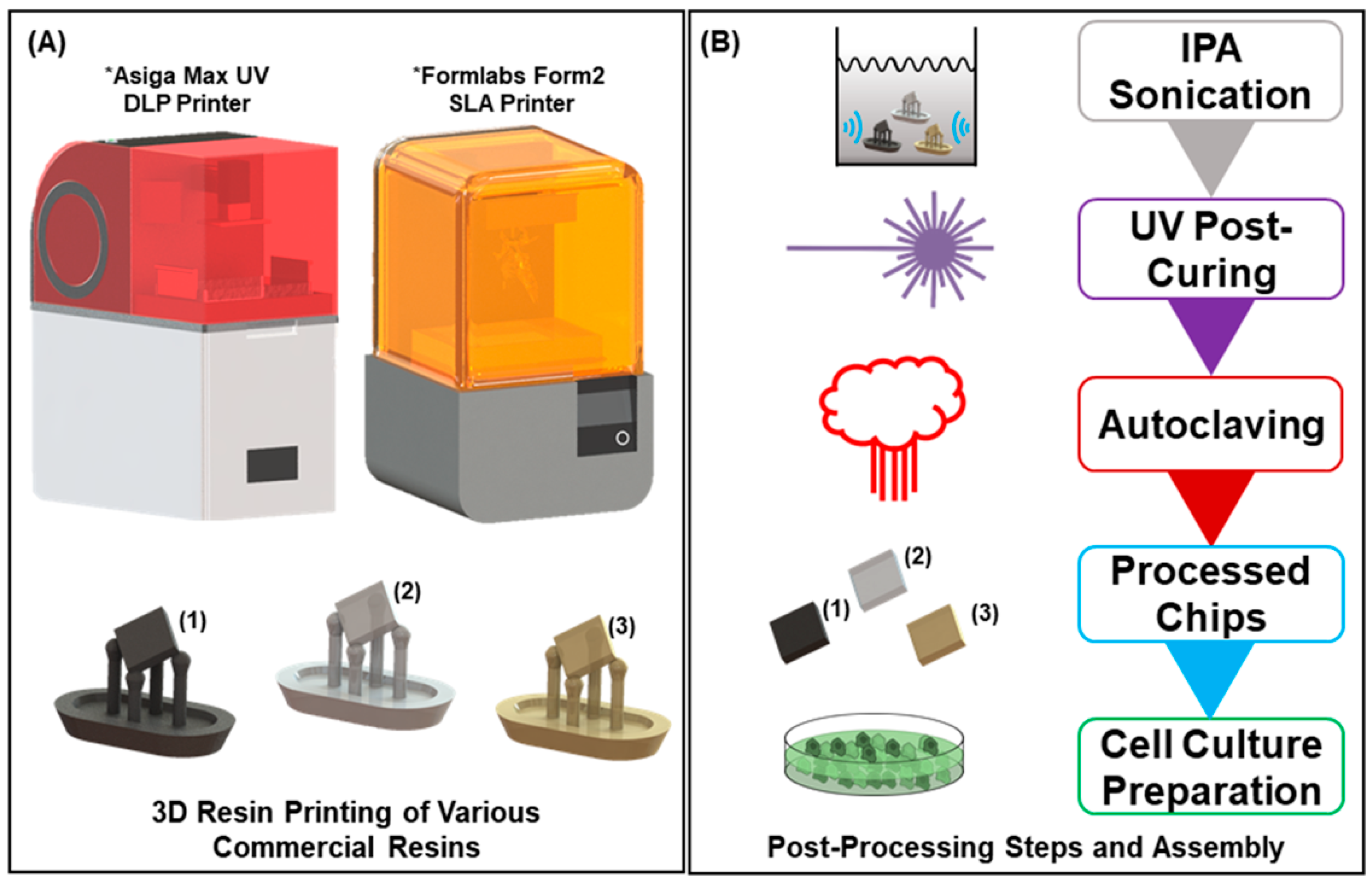
2.1. 3D Printed Resin Chips
2.2. HL-1 Cell Culture
2.3. Negative and Positive Controls
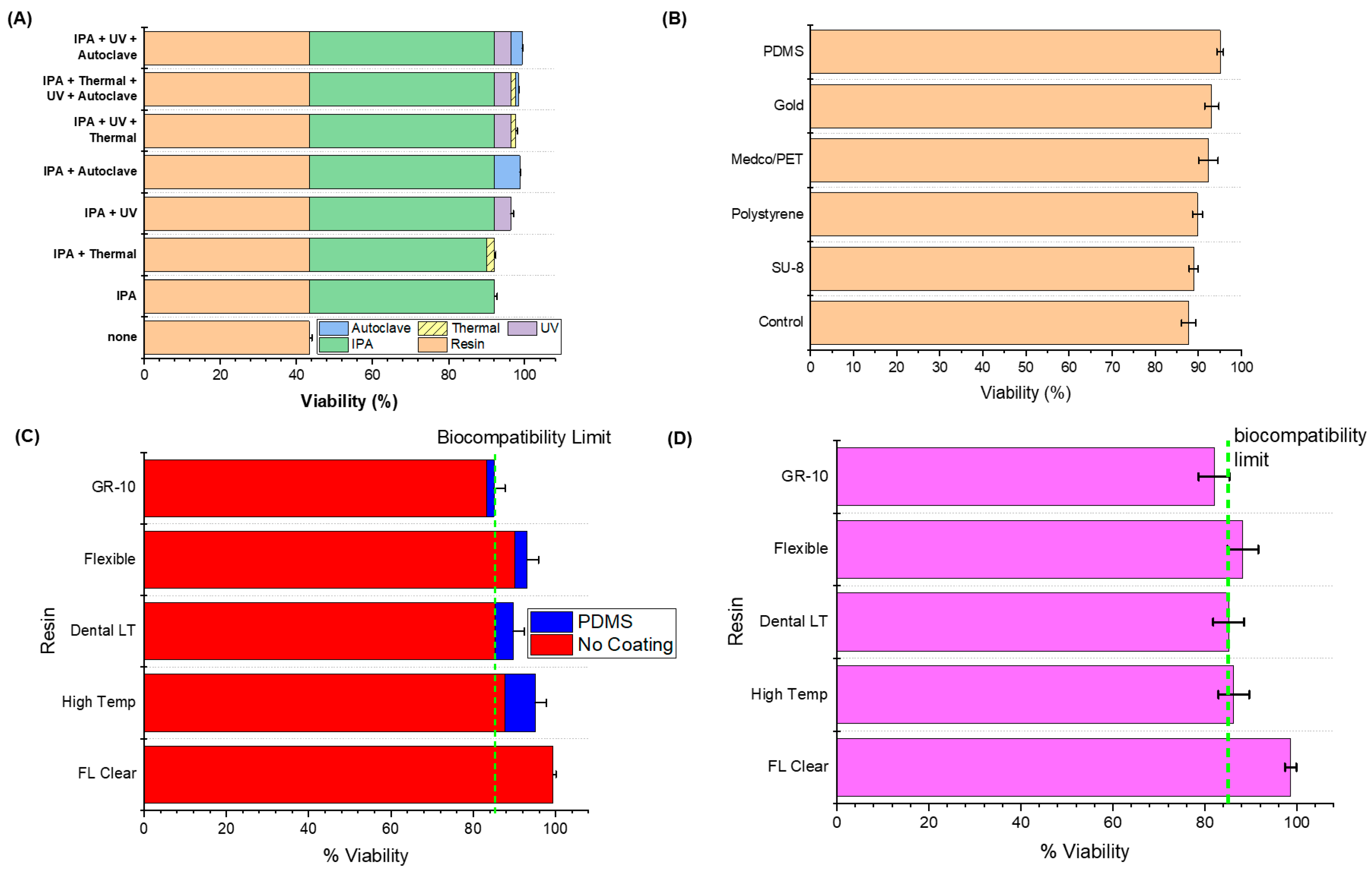
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Amin, R.; Knowlton, S.; Hart, A.; Yenilmez, B.; Ghaderinezhad, F.; Katebifar, S.; Messina, M.; Khademhosseini, A.; Tasoglu, S. 3D-printed microfluidic devices. Biofabrication 2016, 8, 022001. [Google Scholar] [CrossRef]
- Au, A.K.; Huynh, W.; Horowitz, L.F.; Folch, A. 3D-Printed Microfluidics. Angew. Chem. Int. Ed. 2016, 55, 3862–3881. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. Evaluation of 3D Printing and Its Potential Impact on Biotechnology and the Chemical Sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Ni, Y.; Ji, R.; Long, K.; Bu, T.; Chen, K.; Zhuang, S. A review of 3D-printed sensors. Appl. Spectrosc. Rev. 2017, 52, 623–652. [Google Scholar] [CrossRef]
- Pucci, J.U.; Christophe, B.R.; Sisti, J.A.; Connolly, E.S. Three-dimensional printing: Technologies, applications, and limitations in neurosurgery. Biotechnol. Adv. 2017, 35, 521–529. [Google Scholar] [CrossRef]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef]
- Moreno-Rivas, O.; Hernández-Velázquez, D.; Piazza, V.; Marquez, S. Rapid prototyping of microfluidic devices by SL 3D printing and their biocompatibility study for cell culturing. Mater. Today Proc. 2019, 13, 436–445. [Google Scholar] [CrossRef]
- Grigalevičiūtė, G.; Baltriukienė, D.; Balčiūnas, E.; Jonusauskas, L.; Malinauskas, M. Fabrication of flexible microporous 3D scaffolds via stereolithography and optimization of their biocompatibility. SPIE 2018, 10544. [Google Scholar] [CrossRef]
- Asghar, W.; Kim, Y.-T.; Ilyas, A.; Sankaran, J.; Wan, Y.; Iqbal, S.M. Synthesis of nano-textured biocompatible scaffolds from chicken eggshells. Nanotechnology 2012, 23, 475601. [Google Scholar] [CrossRef] [PubMed]
- Babaliari, E.; Kavatzikidou, P.; Angelaki, D.; Chaniotaki, L.; Manousaki, A.; Siakouli-Galanopoulou, A.; Ranella, A.; Stratakis, E. Engineering Cell Adhesion and Orientation via Ultrafast Laser Fabricated Microstructured Substrates. Int. J. Mol. Sci. 2018, 19, 2053. [Google Scholar] [CrossRef] [PubMed]
- Shirtcliffe, N.J.; Toon, R.; Roach, P. Surface Treatments for Microfluidic Biocompatibility. In Microfluidic Diagnostics: Methods and Protocols; Jenkins, G., Mansfield, C.D., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 241–268. [Google Scholar]
- Jang, T.-S.; Jung, H.-D.; Pan, H.M.; Han, W.T.; Chen, S.; Song, J. 3D printing of hydrogel composite systems: Recent advances in technology for tissue engineering. Int. J. Bioprint. 2018, 4. [Google Scholar] [CrossRef]
- Luis, E.; Pan, H.M.; Sing, S.L.; Bastola, A.K.; Goh, G.D.; Tan, H.K.J.; Bajpai, R.; Song, J.; Yeong, W.Y. Silicone 3D Printing: Process Optimization, Product Biocompatibility, and Reliability of Silicone Meniscus Implants. 3D Print. Addit. Manuf. 2019, 6, 319–332. [Google Scholar] [CrossRef]
- Luis, E.; Pan, H.M.; Sing, S.L.; Bajpai, R.; Song, J.; Yeong, W.Y. 3D Direct Printing of Silicone Meniscus Implant Using a Novel Heat-Cured Extrusion-Based Printer. Polymers 2020, 12, 1031. [Google Scholar] [CrossRef]
- Alifui-Segbaya, F.; George, R. Biocompatibility of 3D-Printed Methacrylate for Hearing Devices. Inventioms 2018, 3, 52. [Google Scholar] [CrossRef]
- Carve, M.; Wlodkowic, D. 3D-Printed Chips: Compatibility of Additive Manufacturing Photopolymeric Substrata with Biological Applications. Micromachines 2018, 9, 91. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 1–14. [Google Scholar] [CrossRef]
- Gautam, R.; Singh, R.D.; Sharma, V.P.; Siddhartha, R.; Chand, P.; Kumar, R. Biocompatibility of polymethylmethacrylate resins used in dentistry. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1444–1450. [Google Scholar] [CrossRef]
- Kurzmann, C.; Janjić, K.; Shokoohi-Tabrizi, H.; Edelmayer, M.; Pensch, M.; Moritz, A.; Agis, H. Evaluation of Resins for Stereolithographic 3D-Printed Surgical Guides: The Response of L929 Cells and Human Gingival Fibroblasts. BioMed Res. Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Rimington, R.P.; Capel, A.J.; Player, D.J.; Bibb, R.; Christie, S.D.R.; Lewis, M.P. Feasibility and Biocompatibility of 3D-Printed Photopolymerized and Laser Sintered Polymers for Neuronal, Myogenic, and Hepatic Cell Types. Macromol. Biosci. 2018, 18, e1800113. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Turecek, C.; Kaiser, B.; Stampfl, J.; Liska, R.; Varga, F. Evaluation of Biocompatible Photopolymers I: Photoreactivity and Mechanical Properties of Reactive Diluents. J. Macromol. Sci. Part A 2007, 44, 547–557. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Jones, A.; Rusling, J.F. 3D-Printed Biosensor Arrays for Medical Diagnostics. Micromachines 2018, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Takenaga, S.; Schneider, B.; Erbay, E.; Biselli, M.; Schnitzler, T.; Schöning, M.J.; Wagner, T. Fabrication of biocompatible lab-on-chip devices for biomedical applications by means of a 3D-printing process. Phys. Status Solidi 2015, 212, 1347–1352. [Google Scholar] [CrossRef]
- Zhu, F.; Friedrich, T.; Nugegoda, D.; Kaslin, J.; Wlodkowic, D. Assessment of the biocompatibility of three-dimensional-printed polymers using multispecies toxicity tests. Biomicrofluidics 2015, 9, 061103. [Google Scholar] [CrossRef]
- Zhu, F.; Skommer, J.; Friedrich, T.; Kaslin, J.; Wlodkowic, D. 3D printed polymers toxicity profiling: A caution for biodevice applications (SPIE Micro+Nano Materials, Devices, and Applications). SPIE 2015. [Google Scholar] [CrossRef]
- Claycomb, W.C.; Lanson, J.N.A.; Stallworth, B.S.; Egeland, D.B.; Delcarpio, J.B.; Bahinski, A.; Izzo, J.N.J. HL-1 cells: A cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl. Acad. Sci. USA 1998, 95, 2979–2984. [Google Scholar] [CrossRef]
- White, S.M.; Constantin, P.E.; Claycomb, W.C. Cardiac physiology at the cellular level: Use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. Am. J. Physiol. Circ. Physiol. 2004, 286, H823–H829. [Google Scholar] [CrossRef]
- Clear Resin; SDS No. 1801037; FormLabs: Somerville, MA, USA, 2019; Available online: https://formlabs-media.formlabs.com/datasheets/Safety_Data_Sheet_EN_-_Clear.pdf (accessed on 1 February 2020).
- High Temp Resin V2; SDS No. 2001047; FormLabs: Somerville, MA, USA, 2019; Available online: https://formlabs-media.formlabs.com/datasheets/Safety_Data_Sheet_EN_-_High_Temp.pdf (accessed on 1 February 2020).
- Flexible Resin; SDS No. 1801044; FormLabs: Somerville, MA, USA, 2019; Available online: https://formlabs-media.formlabs.com/datasheets/Safety_Data_Sheet_EN_-_Flexible.pdf (accessed on 1 February 2020).
- Dental LT Resin; SDS No. 2001421; FormLabs: Somerville, MA, USA, 2019; Available online: https://formlabs-media.formlabs.com/datasheets/2001421-SDS-ENUS-0.pdf (accessed on 1 February 2020).
- GR-10; SDS No. 1000800; Pro3dure Medical: Iserlohn, Germany, 2019; Available online: https://www.pro3dure.com/media/pdf/80/57/9e/GR-10guide-E.pdf (accessed on 1 February 2020).
- Kundu, A.; Nattoo, C.; Fremgen, S.; Springer, S.; Ausaf, T.; Rajaraman, S. Optimization of makerspace microfabrication techniques and materials for the realization of planar, 3D printed microelectrode arrays in under four days. RSC Adv. 2019, 9, 8949–8963. [Google Scholar] [CrossRef]
- Azim, N.; Hart, C.; Sommerhage, F.; Aubin, M.; Hickman, J.J.; Rajaraman, S. Precision Plating of Human Electrogenic Cells on Microelectrodes Enhanced With Precision Electrodeposited Nano-Porous Platinum for Cell-Based Biosensing Applications. J. Microelectromech. Syst. 2019, 28, 50–62. [Google Scholar] [CrossRef]
- Hart, C.; Kundu, A.; Kumar, K.; Varma, S.J.; Thomas, J.; Rajaraman, S. Rapid Nanofabrication of Nanostructured Interdigitated Electrodes (nIDEs) for Long-Term In Vitro Analysis of Human Induced Pluripotent Stem Cell Differentiated Cardiomyocytes. Biosensors 2018, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.; Kumar, K.S.; Li, J.; Thomas, J.; Rajaraman, S. Investigation of the Enhanced Sensitivity of Interdigitated Electrodes for Cellular Biosensing with Geometric, Nanostructured Surface Area, and Surface Plasmon Resonance Modes. J. Microelectromechan. Syst. 2020, 29, 1109–1111. [Google Scholar] [CrossRef]
- Didier, C.M.; Kundu, A.; Rajaraman, S. Facile, Packaging Substrate-Agnostic, Microfabrication and Assembly of Scalable 3D Metal Microelectrode Arrays for in Vitro Organ-on-a-Chip and Cellular Disease Modeling. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019. [Google Scholar]
- Kundu, A.; Ausaf, T.; Rajaraman, S. 3D Printing, Ink Casting and Micromachined Lamination (3D PICLμM): A Makerspace Approach to the Fabrication of Biological Microdevices. Micromachines 2018, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Lacour, S.P.; Benmerah, S.; Tarte, E.; Fitzgerald, J.; Serra, J.; McMahon, S.; Fawcett, J.; Graudejus, O.; Yu, Z.; Morrison, B. Flexible and stretchable micro-electrodes for in vitro and in vivo neural interfaces. Med. Biol. Eng. Comput. 2010, 48, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Sakhaei, A.H.; Layani, M.; Zhang, B.; Ge, Q.; Magdassi, S. Highly Stretchable and UV Curable Elastomers for Digital Light Processing Based 3D Printing. Adv. Mater. 2017, 29, 1606000. [Google Scholar] [CrossRef]
- Sivashankar, S.; Agambayev, S.; AlAmoudi, K.; Buttner, U.; Khashab, N.M.; Salama, K.N. Compatibility analysis of 3D printer resin for biological applications. Micro Nano Lett. 2016, 11, 654–659. [Google Scholar] [CrossRef]
- Sivashankar, S.; Agambayev, S.; Buttner, U.; Salama, K.N. Characterization of solid UV curable 3D printer resins for biological applications. In Proceedings of the 2016 IEEE 11th Annual International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Sendai, Japan, 17–20 April 2016; pp. 305–309. [Google Scholar] [CrossRef]
- Rutala, W.A.; Stiegel, M.M.; Sarubbi, F.A. Decontamination of laboratory microbiological waste by steam sterilization. Appl. Environ. Microbiol. 1982, 43, 1311–1316. [Google Scholar] [CrossRef]
- Chan, W.-M.; Gloor, P.E.; Hamielec, A.E. A kinetic model for olefin polymerization in high-pressure autoclave reactors. AIChE J. 1993, 39, 111–126. [Google Scholar] [CrossRef]
- Durkan, R.; Ozel, M.B.; Bagis, B.; Usanmaz, A. In vitro comparison of autoclave polymerization on the transverse strength of denture base resins. Dent. Mater. J. 2008, 27, 640–642. [Google Scholar] [CrossRef][Green Version]
- Kwag, B.G.; Choi, K.Y. Effect of initiator characteristics on high-pressure ethylene polymerization in autoclave reactors. Ind. Eng. Chem. Res. 1994, 33, 211–217. [Google Scholar] [CrossRef]
- EPO-TEK 353ND; SDS No. 353NDGHS; Epoxy Technology Inc.: Billerica, MA, USA, 2018; Available online: https://www.epotek.com/site/administrator/components/com_products/assets/files/File_Uploads/353ND%20GHS.pdf (accessed on 1 February 2020).
- CellTiter-Glo Luminescent Cell Viability Assay Technical Bulletin; Cat. No. G7570; Promega: Madison, WI, USA, 2015; Available online: https://www.promega.com/-/media/files/resources/protocols/technical-bulletins/0/celltiter-glo-luminescent-cell-viability-assay-protocol.pdf?la=en (accessed on 1 February 2020).
- Tipton, D.A.; Lewis, J.W. Effects of a hindered amine light stabilizer and a UV light absorber used in maxillofacial elastomers on human gingival epithelial cells and fibroblasts. J. Prosthet. Dent. 2008, 100, 220–231. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Krifka, S.; Hiller, K.-A.; Bolay, C.; Waha, C.; Van Meerbeek, B.; Schmalz, G.; Schweikl, H. Evaluation of cell responses toward adhesives with different photoinitiating systems. Dent. Mater. 2015, 31, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Popal, M.; Volk, J.; Leyhausen, G.; Geurtsen, W. Cytotoxic and genotoxic potential of the type I photoinitiators BAPO and TPO on human oral keratinocytes and V79 fibroblasts. Dent. Mater. 2018, 34, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Al-Namnam, N.; Kim, K.H.; Chai, W.; Ha, K.; Siar, C.; Ngeow, W.C.; Al-Namnam, N. A biocompatibility study of injectable poly(caprolactone-trifumarate) for use as a bone substitute material. Front. Life Sci. 2015, 8, 215–222. [Google Scholar] [CrossRef]
- Azim, N.; Kunduet, A.; Royse, M.; Li Sip, Y.Y.; Young, M.; Santra, S.; Zhai, L.; Rajaraman, S. Fabrication and Characterization of a 3D Printed, MicroElectrodes Platform With Functionalized Electrospun Nano-Scaffolds and Spin Coated 3D Insulation Towards Multi- Functional Biosystems. J. Microelectromech. Syst. 2019, 28, 606–618. [Google Scholar] [CrossRef]
- Kadimisetty, K.; Song, J.; Doto, A.M.; Hwang, Y.; Peng, J.; Mauk, M.G.; Bushman, F.D.; Gross, R.; Jarvis, J.N.; Liu, C. Fully 3D printed integrated reactor array for point-of-care molecular diagnostics. Biosens. Bioelectron. 2018, 109, 156–163. [Google Scholar] [CrossRef]
- Kundu, A.; Ausaf, T.; Rajasekaran, P.; Rajaraman, S. Multimodal Microfluidic Biosensor with Interdigitated Electrodes (IDE) And Microelectrode Array (MEA) For Bacterial Detection And Identification. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019; pp. 1199–1202. [Google Scholar] [CrossRef]
- Liu, Y.; Smela, E.; Nelson, N.M.; Abshire, P. Cell-lab on a chip: A CMOS-based microsystem for culturing and monitoring cells. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; Volume 1, pp. 2534–2537. [Google Scholar] [CrossRef]
- Macdonald, N.P.; Zhu, F.; Hall, C.J.; Reboud, J.; Crosier, P.S.; Patton, E.E.; Wlodkowic, D.; Cooper, J.M. Assessment of biocompatibility of 3D printed photopolymers using zebrafish embryo toxicity assays. Lab Chip 2016, 16, 291–297. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, B.; Hojaiji, H.; Lin, S.; Lin, H.; Zhu, J.; Yeung, C.; Emaminejad, S. JMEMS Letters an Adhesive and Corrosion-Resistant Biomarker Sensing Film for Biosmart Wearable Consumer Electronics. J. MicroelectroMech. Syst. 2020, 29, 1114–1115. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 1997, 21, A.3B.1–A.3B.2. [Google Scholar]
- Eimre, M.; Paju, K.; Pelloux, S.; Beraud, N.; Roosimaa, M.; Kadaja, L.; Gruno, M.; Peet, N.; Orlova, E.; Remmelkoor, R.; et al. Distinct organization of energy metabolism in HL-1 cardiac cell line and cardiomyocytes. Biochim. Biophys. Acta (BBA) Gen. Subj. 2008, 1777, 514–524. [Google Scholar] [CrossRef]
- Didier, C.; Kundu, A.; Rajaraman, S. Capabilities and limitations of 3D printed microserpentines and integrated 3D electrodes for stretchable and conformable biosensor applications. Microsyst. Nanoeng. 2020, 6, 1–15. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hart, C.; Didier, C.M.; Sommerhage, F.; Rajaraman, S. Biocompatibility of Blank, Post-Processed and Coated 3D Printed Resin Structures with Electrogenic Cells. Biosensors 2020, 10, 152. https://doi.org/10.3390/bios10110152
Hart C, Didier CM, Sommerhage F, Rajaraman S. Biocompatibility of Blank, Post-Processed and Coated 3D Printed Resin Structures with Electrogenic Cells. Biosensors. 2020; 10(11):152. https://doi.org/10.3390/bios10110152
Chicago/Turabian StyleHart, Cacie, Charles M. Didier, Frank Sommerhage, and Swaminathan Rajaraman. 2020. "Biocompatibility of Blank, Post-Processed and Coated 3D Printed Resin Structures with Electrogenic Cells" Biosensors 10, no. 11: 152. https://doi.org/10.3390/bios10110152
APA StyleHart, C., Didier, C. M., Sommerhage, F., & Rajaraman, S. (2020). Biocompatibility of Blank, Post-Processed and Coated 3D Printed Resin Structures with Electrogenic Cells. Biosensors, 10(11), 152. https://doi.org/10.3390/bios10110152




