A Highly Efficient and Durable Fluorescent Paper Produced from Bacterial Cellulose/Eu Complex and Cellulosic Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of EuCl3·6H2O
2.3. Preparation of Eu-BC
2.4. Manufacture of Fluorescent Paper
2.5. Characterization of Eu-BC and Fluorescent Paper
3. Results and Discussion
3.1. Characterization of Eu-BC and Fluorescent Paper
3.2. Photofluorescent Properties of Eu-BC and Eu-BC Paper
3.3. Stability and Durability of Fluorescent Paper
3.4. Comparison of Eu-BC Fluorescent Paper with Other Composite Fluorescent Materials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tang, S.; Shao, C.; Liu, Y.; Mu, R. Electrospun nanofibers of poly(acrylonitrile)/Eu3+ and their photoluminescence properties. J. Phys. Chem. Solid. 2010, 71, 273–278. [Google Scholar] [CrossRef]
- Sabbatini, N.; Guardigli, M.; Lehn, J.M. Luminescent lanthanide complexes as photochemical supramolecular devices. Coord. Chem. Rev. 1993, 123, 201–228. [Google Scholar] [CrossRef]
- Wang, H.; He, P.; Yan, H.; Shi, J.; Gong, M. A novel europium(III)–imidazol–diketonate–phenanthroline complex as a red phosphor applied in LED. Inorg. Chem. Commun. 2011, 14, 1183–1185. [Google Scholar] [CrossRef]
- Reyes, R.; Cremona, M.; Teotonio, E.E.S.; Brito, H.F.; Malta, O.L. Voltage color tunable OLED with (Sm,Eu)-β-diketonate complex blend. Chem. Phys. Lett. 2004, 396, 54–58. [Google Scholar] [CrossRef]
- Teotonio, E.; Brito, H.F.; da Cunha Felinto, M.C.F.; Thompson, L.C.; Young, V.G.; Malta, O.L. Preparation, crystal structure and optical spectroscopy of the rare earth complexes (RE3+ = Sm, Eu, Gd and Tb) with 2-thiopheneacetate anion. J. Mol. Struct. 2005, 751, 85–94. [Google Scholar] [CrossRef]
- Reineke, T.M.; Eddaoudi, M.; Fehr, M.; Kelly, D.; Yaghi, O.M. From condensed lanthanide coordination solids to microporous frameworks having accessible metal sites. J. Am. Chem. Soc. 1999, 121, 1651–1657. [Google Scholar] [CrossRef]
- Ma, L.; Evans, O.R.; Foxman, B.M.; Lin, W. Luminescent lanthanide coordination polymers. Inorg. Chem. 1999, 38, 5837–5840. [Google Scholar] [CrossRef]
- Seo, J.S.; Whang, D.; Lee, H.; Jun, S.I.; Oh, J.; Jeon, Y.J.; Kim, K. A homochiral metal-organic porous material for enantioselective separation and catalysis. Nature 2000, 404, 982–986. [Google Scholar] [CrossRef]
- Verlan, V.I.; Iovu, M.S.; Culeac, I.; Nistor, Y.H.; Turta, C.I.; Zubareva, V.E. Photoluminescence properties of PVP/Tb(TTA)2(Ph3PO)2NO3 nanocomposites. J. Non-Crystalline Solid. 2013, 360, 21–25. [Google Scholar] [CrossRef]
- Cui, G.; Chen, S.; Jiang, B.; Zhang, Y.; Qiu, N.; Satoh, T.; Kakuchi, T.; Duan, Q. Synthesis and characterization of novel thermoresponsive fluorescence complexes based on copolymers with rare earth ions. Optic. Mater. 2013, 35, 2250–2256. [Google Scholar] [CrossRef]
- Ye, J.; Li, W.; Xiong, J. Relationship Between Size as Well as Size Distribution and Fluorescence Properties of CMC /Eu Complex Nanoparticles Synthesized at Different pH Values. J. South China Univ. Technol. 2014, 42, 73–78. [Google Scholar]
- Ye, J.; Wang, B.; Xiong, J. Effect of Eu3+ Concentration on the Structure and Fluorescence Quenching of Carboxymethyl Cellulose /Eu(III) Nanoparticles. Polym. Mater. Sci. Eng. 2016, 32, 32–37. [Google Scholar]
- Ye, J.; Li, W.; Xiong, J. Properties of Carboxyl-Containing Cellulose Derivatives/ Rare Earth Ions Nanocomposites. J. South China Univ. Technol. 2017, 45, 48–56. [Google Scholar]
- Ye, J.; Guo, Y.; Xiong, J. Effect of pH on size and dispersion of CMC/Eu nanocomposites with high fluorescence intensity. Funct. Mater. 2012, 18, 2541–2545. [Google Scholar]
- Dai, H.; Wong, E.W.; Lu, Y.Z.; Fan, S.; Lieber, C.M. Synthesis and characterization of carbide nanorods. Nature 1995, 375, 769–772. [Google Scholar] [CrossRef]
- Dong, S.; Roman, M. Fluorescently Labeled Cellulose Nanocrystals for Bioimaging Applications. J. Am. Chem. Soc. 2007, 129, 13810–13811. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Huang, F.; Pan, S.; Mu, W.; Meng, X.; Yang, H.; Xu, Z.; Ragauskas, A.J.; Deng, Y. Thermo-responsive and fluorescent cellulose nanocrystals grafted with polymer brushes. J. Mater. Chem. A 2015, 3, 1995–2005. [Google Scholar] [CrossRef]
- Díez, I.; Eronen, P.; Österberg, M.; Linder, M.B.; Ikkala, O.; Ras, R.H.A. Functionalization of Nanofibrillated Cellulose with Silver Nanoclusters: Fluorescence and Antibacterial Activity. Macromol. Biosci. 2011, 11, 1185–1191. [Google Scholar] [CrossRef]
- Niu, Q.; Gao, K.; Wu, W. Cellulose nanofibril based graft conjugated polymer films act as a chemosensor for nitroaromatic. Carbohydr. Polym. 2014, 110, 47–52. [Google Scholar] [CrossRef]
- Xiang, Z.; Jin, X.; Liu, Q.; Chen, Y.; Li, J.; Lu, F. The reinforcement mechanism of bacterial cellulose on paper made from woody and non-woody fiber sources. Cellulose 2017, 24, 5147–5156. [Google Scholar] [CrossRef]
- Xiang, Z.; Liu, Q.; Chen, Y.; Lu, F. Effects of physical and chemical structures of bacterial cellulose on its enhancement to paper physical properties. Cellulose 2017, 24, 3513–3523. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Lin, Z.; Yao, J.; Tan, S. Preparation and Photocatalysis Properties of Bacterial Cellulose/TiO2Composite Membrane Doped with Rare Earth Elements. Synth. React. Inorg. Metal-Organic Nano-Metal Chem. 2011, 41, 997–1004. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, S.; Hu, W.; Yin, N.; Zhang, W.; Xiang, C.; & Wang, H. Flexible luminescent CdSe/bacterial cellulose nanocomoposite membranes. Carbohydr. Polym. 2012, 88, 173–178. [Google Scholar] [CrossRef]
- Chen, L.F.; Huang, Z.H.; Liang, H.W.; Yao, W.T.; Yu, Z.-Y.; Yu, S.H. Flexible all-solid-state high-power supercapacitor fabricated with nitrogen-doped carbon nanofiber electrode material derived from bacterial cellulose. Energ. Environ. Sci. 2013, 6, 3331. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, T.; Ji, M.; Yang, J.; Zhu, C.; Sun, D. Simple preparation of carbonized bacterial cellulose—Pt composite as a high performance electrocatalyst for direct methanol fuel cells (DMFC). Mater. Lett. 2014, 128, 93–96. [Google Scholar] [CrossRef]
- Kwon, H.; Samain, F.; Kool, E.T. Fluorescent DNAs printed on paper: Sensing food spoilage and ripening in the vapor phase. Chem. Sci. 2012, 3, 2542. [Google Scholar] [CrossRef]
- Ma, Y.; Li, H.; Peng, S.; Wang, L. Highly Selective and Sensitive Fluorescent Paper Sensor for Nitroaromatic Explosive Detection. Anal. Chem. 2012, 84, 8415–8421. [Google Scholar] [CrossRef]
- Su, L.; Yang, L.; Sun, Q.; Zhao, T.; Liu, B.; Jiang, C.; Zhang, Z. A ratiometric fluorescent paper sensor for consecutive color change-based visual determination of blood glucose in serum. New J. Chem. 2018, 42, 6867–6872. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, X.; Zhu, Y.; Wei, X. Study on the Stability and Color Property of Fluorescent Ink-jet Ink. Lect. Notes Elect. Eng. 2015, 369, 919–925. [Google Scholar]
- Hou, X.; Ke, C.; Bruns, C.J.; McGonigal, P.R.; Pettman, R.B.; Stoddart, J.F. Tunable solid-state fluorescent materials for supramolecular encryption. Nat. Commun. 2015, 6, 6884. [Google Scholar] [CrossRef]
- Purington, E.; Bousfield, D.; Gramlich, W.M. Fluorescent dye adsorption in aqueous suspension to produce tagged cellulose nanofibers for visualization on paper. Cellulose 2019, 26, 5117–5131. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Chen, Y.; Liu, Q.; Lu, F. A highly recyclable dip-catalyst produced from palladium nanoparticle-embedded bacterial cellulose and plant fibers. Green Chem. 2018, 20, 1085–1094. [Google Scholar] [CrossRef]
- Wu, X.; Xiang, Z.; Song, T.; Qi, H. Wet-strength agent improves recyclability of dip-catalyst fabricated from gold nanoparticle-embedded bacterial cellulose and plant fibers. Cellulose 2019, 26, 3375–3386. [Google Scholar] [CrossRef]
- Xu, J.; Huang, X.; Zhou, N.; Zhang, J.; Bao, J.C.; Lu, T.; Li, C. Synthesis, XPS and fluorescence properties of Eu3+ complex with polydimethylsiloxane. Mater. Lett. 2004, 58, 1938–1942. [Google Scholar] [CrossRef]
- He, P.; Wang, H.H.; Liu, S.G.; Shi, J.X.; Wang, G.; Gong, M.L. Visible-Light Excitable Europium(III) Complexes with 2,7-Positional Substituted Carbazole Group-Containing Ligands. Inorg. Chem. 2009, 48, 11382–11387. [Google Scholar] [CrossRef] [PubMed]
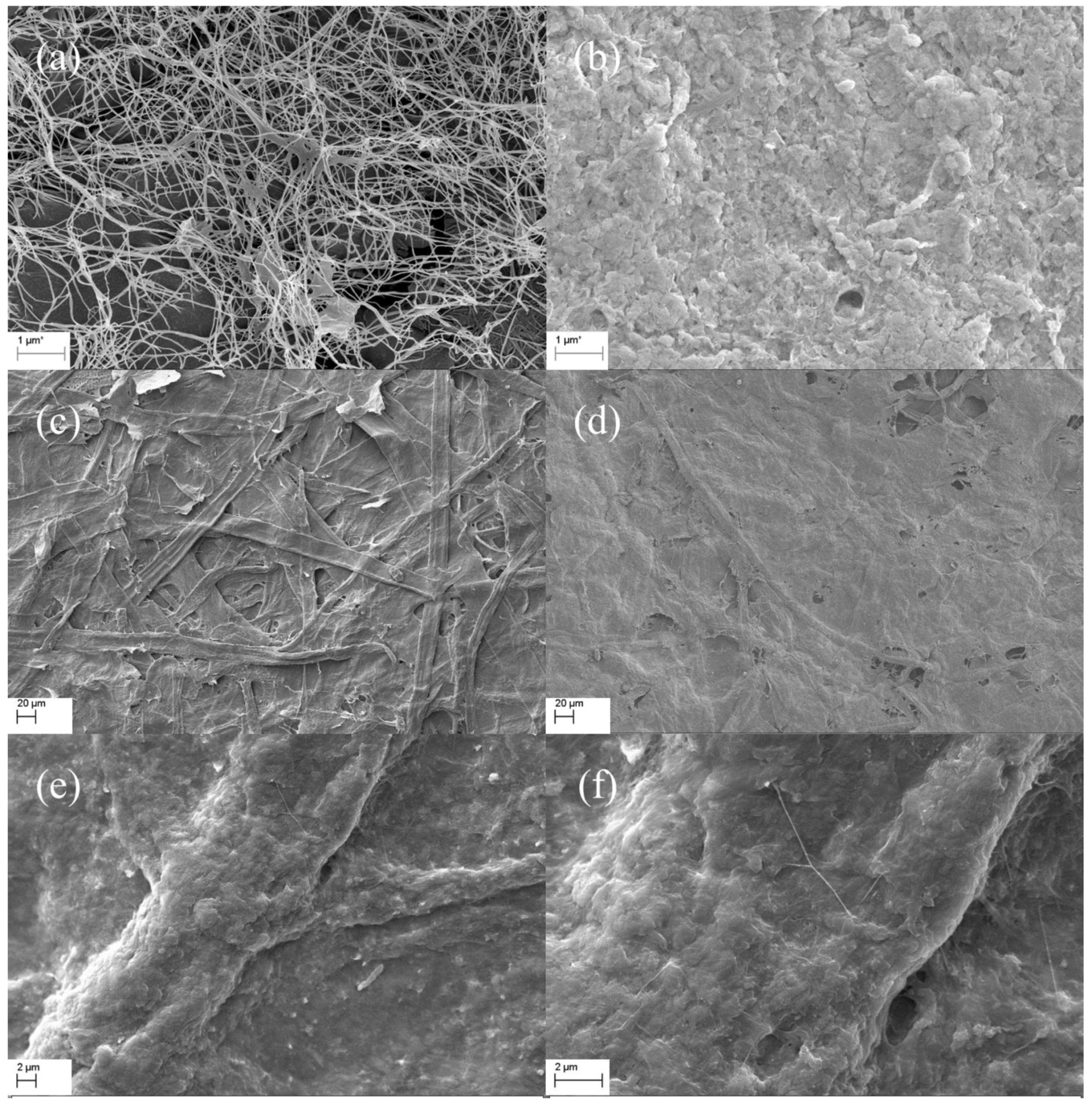
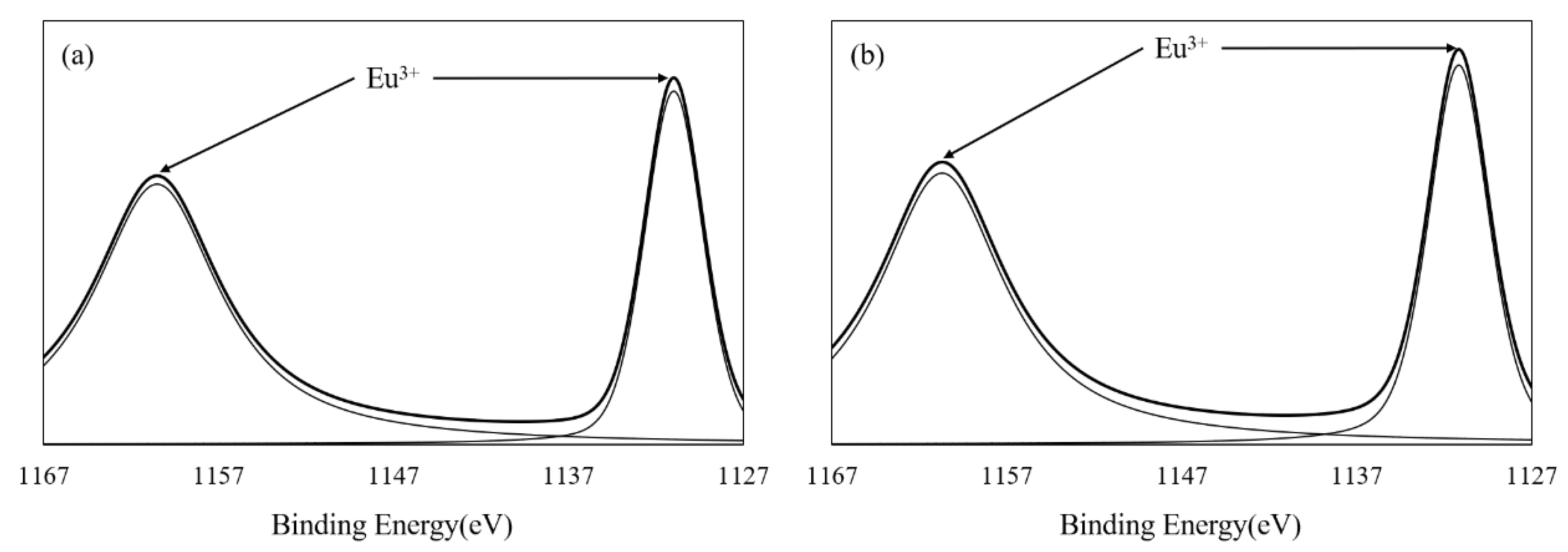
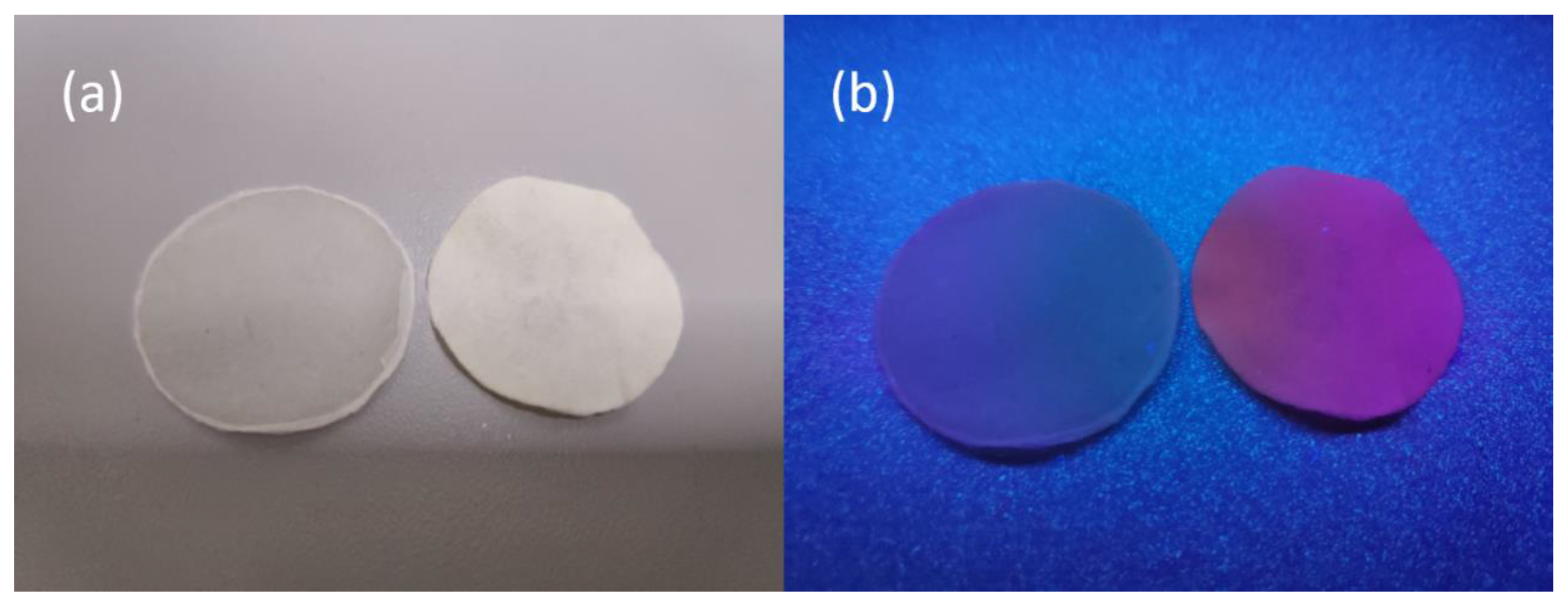
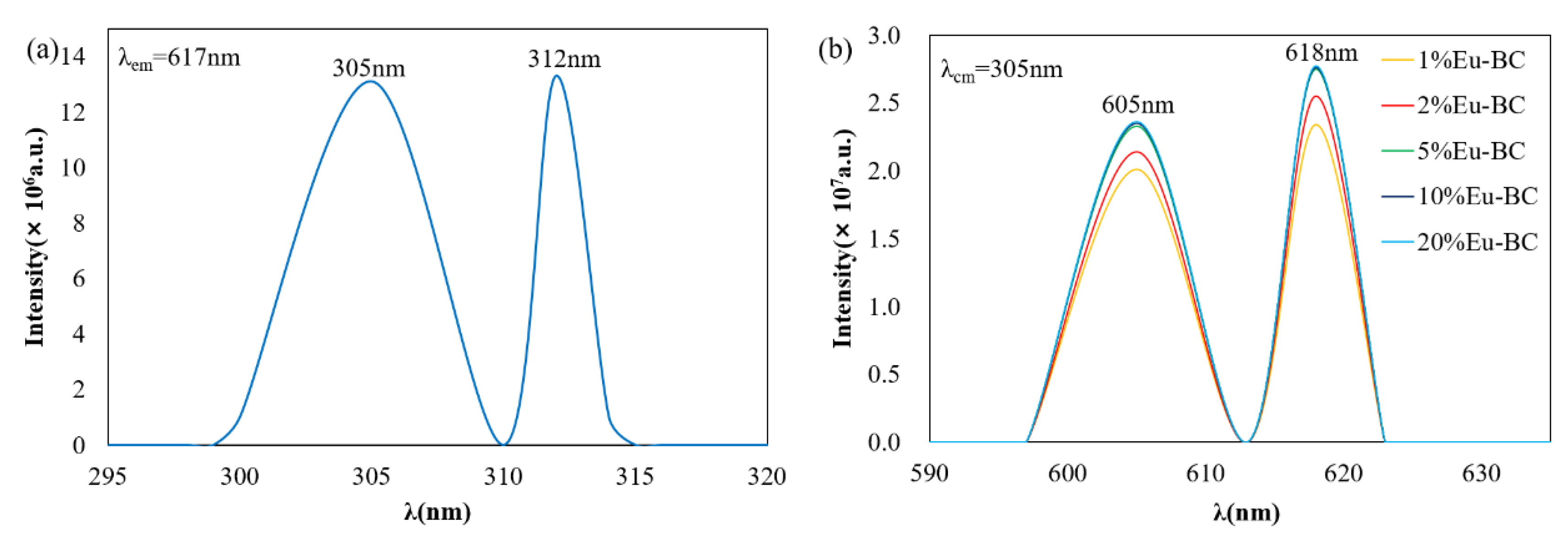
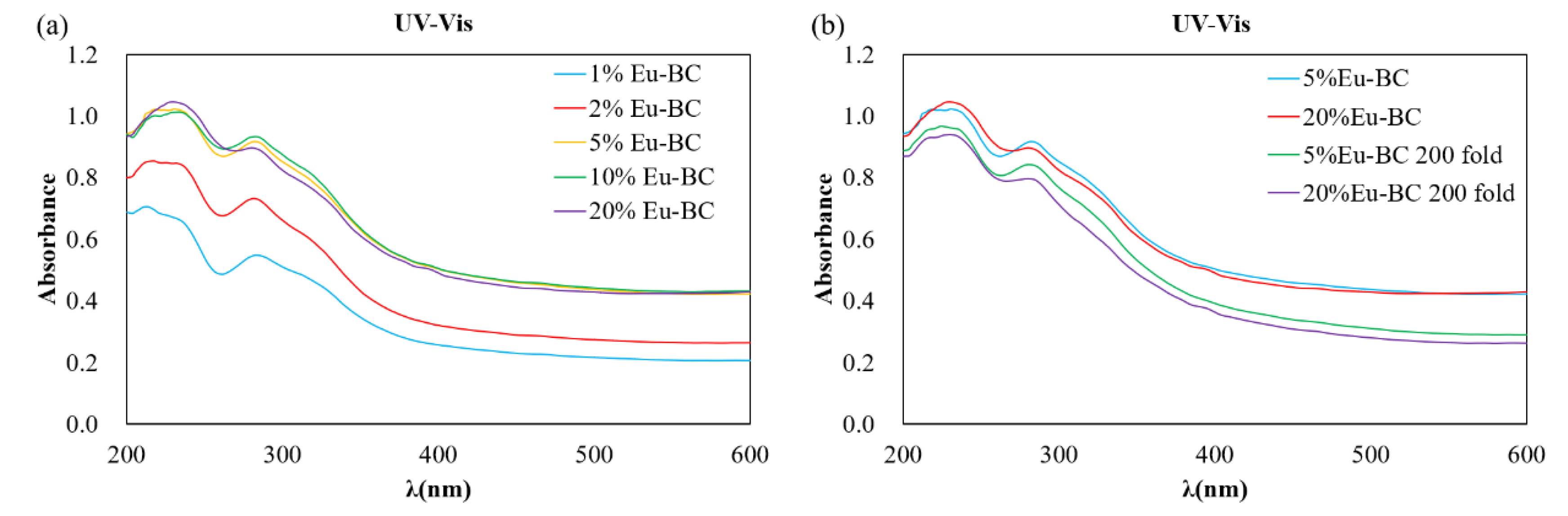

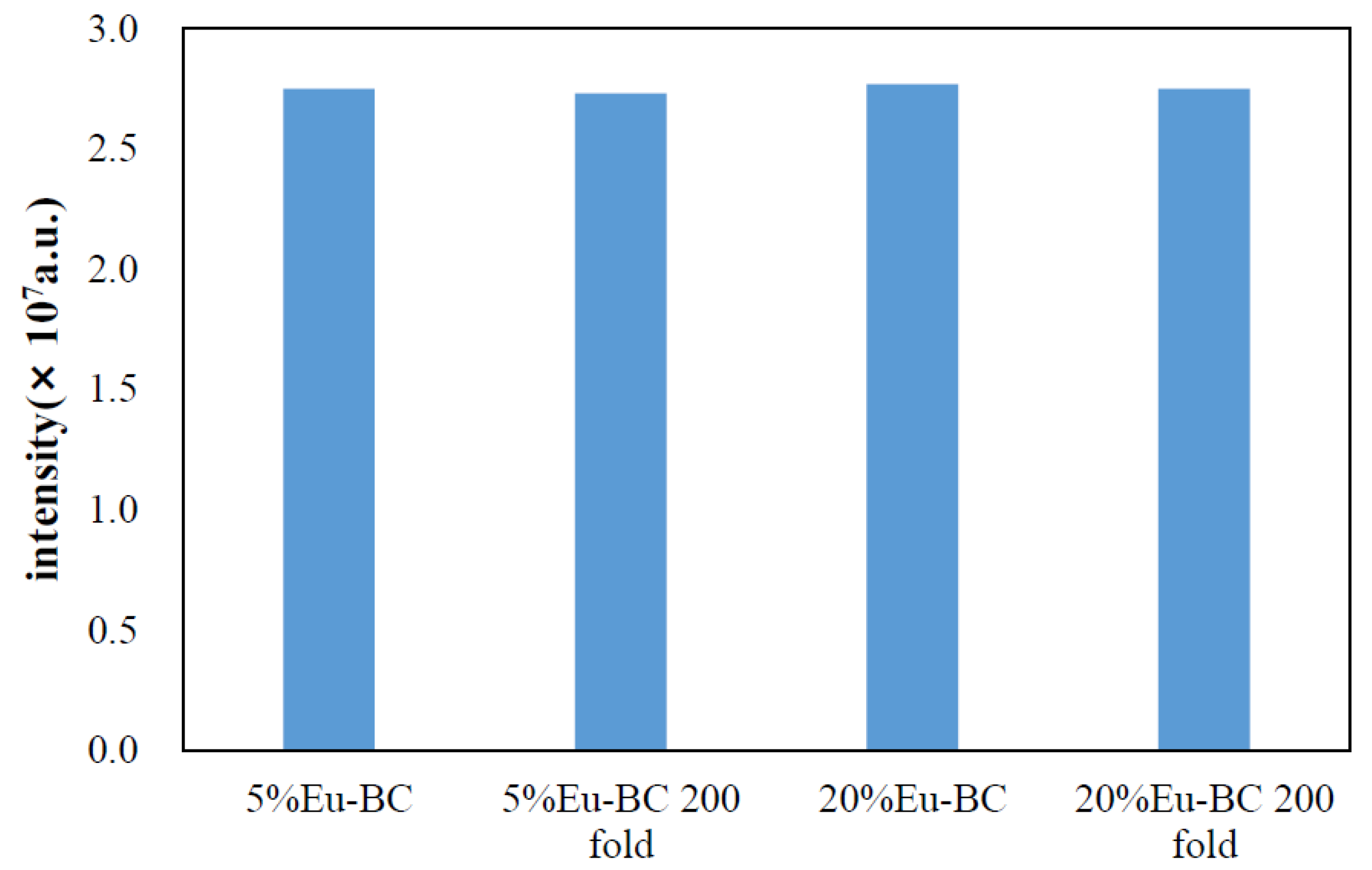
| Sample | Eu wt% | Specific Surface Area (m2/g) | Pore Volumn (cm3/g) | Average Pore Radius (nm) |
|---|---|---|---|---|
| BC | 0 | 41.69 | 0.1598 | 15.81 |
| Eu-BC | 32.30 | 6.84 | 0.0214 | 15.62 |
| 1% Eu-BC fluorescent paper | 0.32 | 6.81 | 0.0991 | 79.84 |
| 2% Eu-BC fluorescent paper | 0.88 | - | - | - |
| 5% Eu-BC fluorescent paper | 1.53 | 3.17 | 0.0024 | 5.81 |
| 10% Eu-BC fluorescent paper | 2.44 | - | - | - |
| 20% Eu-BC fluorescent paper | 7.74 | 12.12 | 0.0430 | 14.74 |
| Base | Reaction Temperature (°C) | Reaction Time (h) | Eu Mass Percentage | Fluorescent Emission Intensity (a.u.) | Reference |
|---|---|---|---|---|---|
| BC/Paper | 70 | 1.5 | 1.5% | 2.77 × 107 | this work |
| CMC | 70 | 0.25 | 4.4% | 1.05 × 107 | [12] |
| HIDTFBD | 60 | 6 | 18.0% | 4.21 × 106 | [3] |
| PDMS | 60 | 3 | 2% | 1.42 × 104 | [34] |
| 2-TFDBC | 60 | 6 | 16.7% | 6.90 × 106 | [35] |
| 2,7-BTFDBC | 60 | 6 | 24.4% | 6.20 × 106 | [35] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wu, X.; Hu, Z.; Xiang, Z.; Song, T.; Lu, F. A Highly Efficient and Durable Fluorescent Paper Produced from Bacterial Cellulose/Eu Complex and Cellulosic Fibers. Nanomaterials 2019, 9, 1322. https://doi.org/10.3390/nano9091322
Zhang M, Wu X, Hu Z, Xiang Z, Song T, Lu F. A Highly Efficient and Durable Fluorescent Paper Produced from Bacterial Cellulose/Eu Complex and Cellulosic Fibers. Nanomaterials. 2019; 9(9):1322. https://doi.org/10.3390/nano9091322
Chicago/Turabian StyleZhang, Mingquan, Xiao Wu, Zhenhua Hu, Zhouyang Xiang, Tao Song, and Fachuang Lu. 2019. "A Highly Efficient and Durable Fluorescent Paper Produced from Bacterial Cellulose/Eu Complex and Cellulosic Fibers" Nanomaterials 9, no. 9: 1322. https://doi.org/10.3390/nano9091322
APA StyleZhang, M., Wu, X., Hu, Z., Xiang, Z., Song, T., & Lu, F. (2019). A Highly Efficient and Durable Fluorescent Paper Produced from Bacterial Cellulose/Eu Complex and Cellulosic Fibers. Nanomaterials, 9(9), 1322. https://doi.org/10.3390/nano9091322






