Tuning the Supramolecular Structures of Metal-Free Porphyrin via Surfactant Assisted Self-Assembly to Enhance Photocatalytic Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of the Materials
2.3. Synthesis of Self-Assembled TCPP
2.3.1. The Self-Assembly Method through Acid-Base Neutralization in the Presence of CTAB
2.3.2. The Self-Assembly Method through Acid-Base Neutralization without CTAB
2.3.3. The Self-Assembly Method through Mixing Dual-Solvents under Ethylene Glycols (EG)
2.4. Evaluation of Photocatalytic Activity
2.5. Electrochemical Measurement
3. Results and Discussion
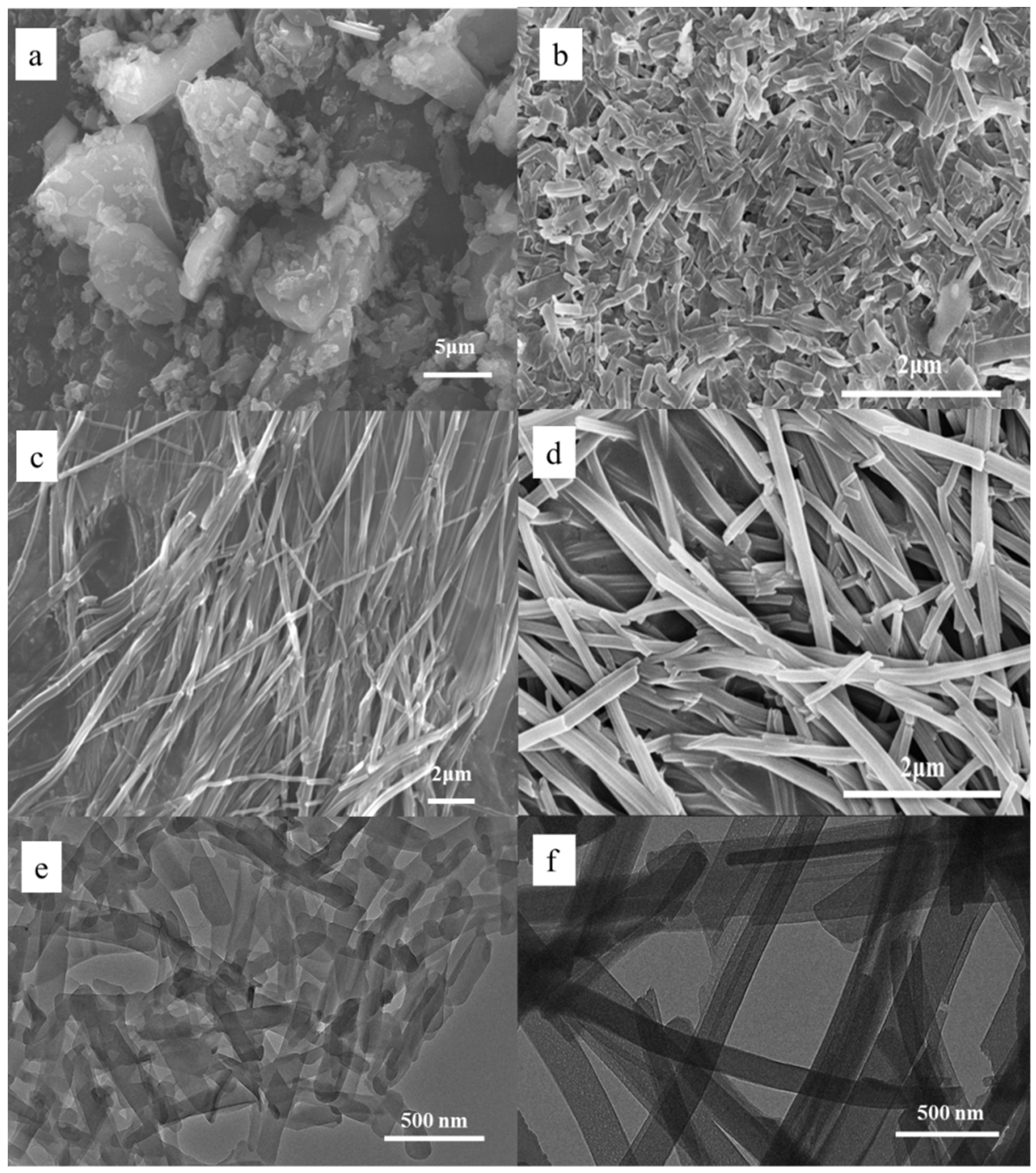
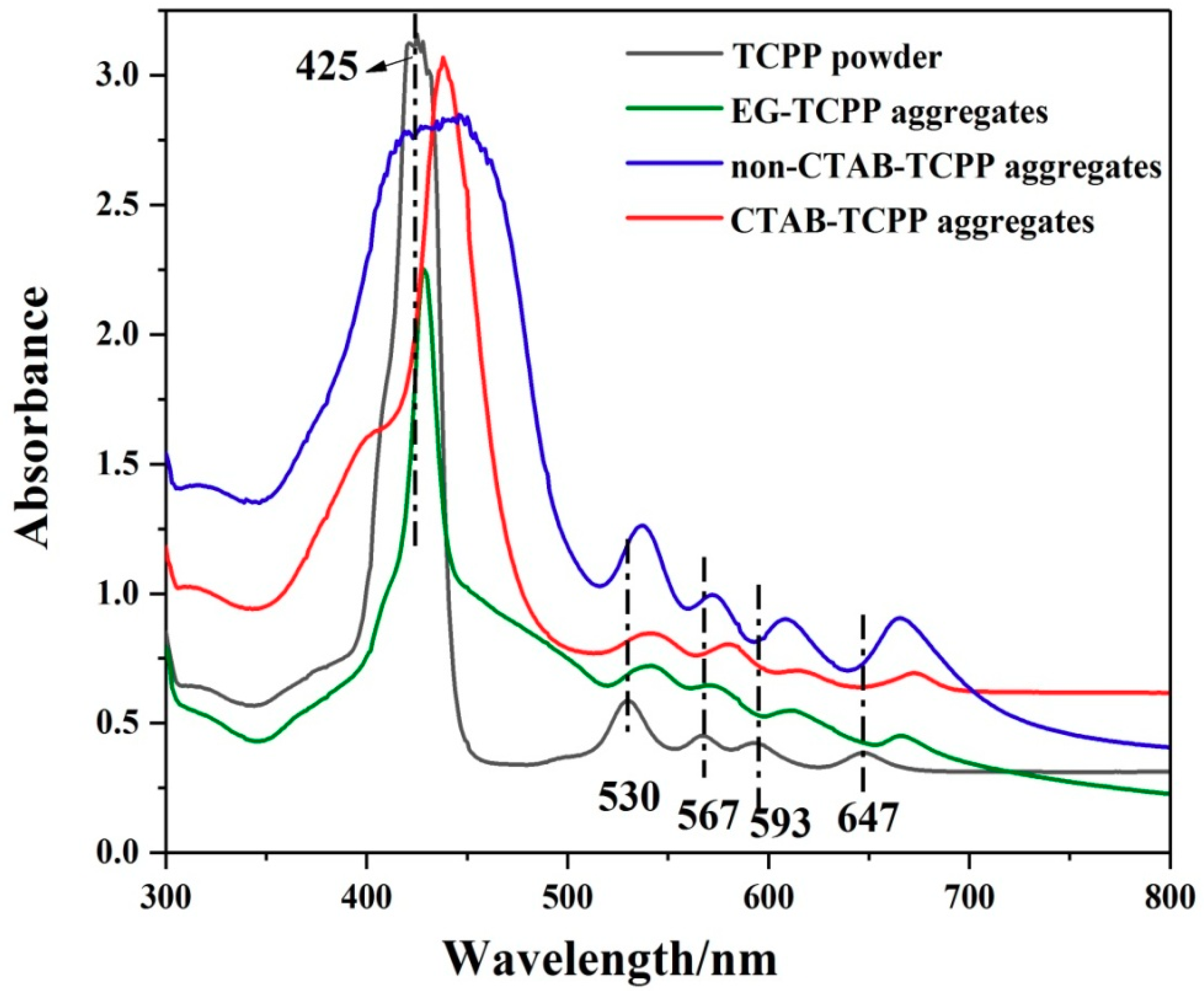
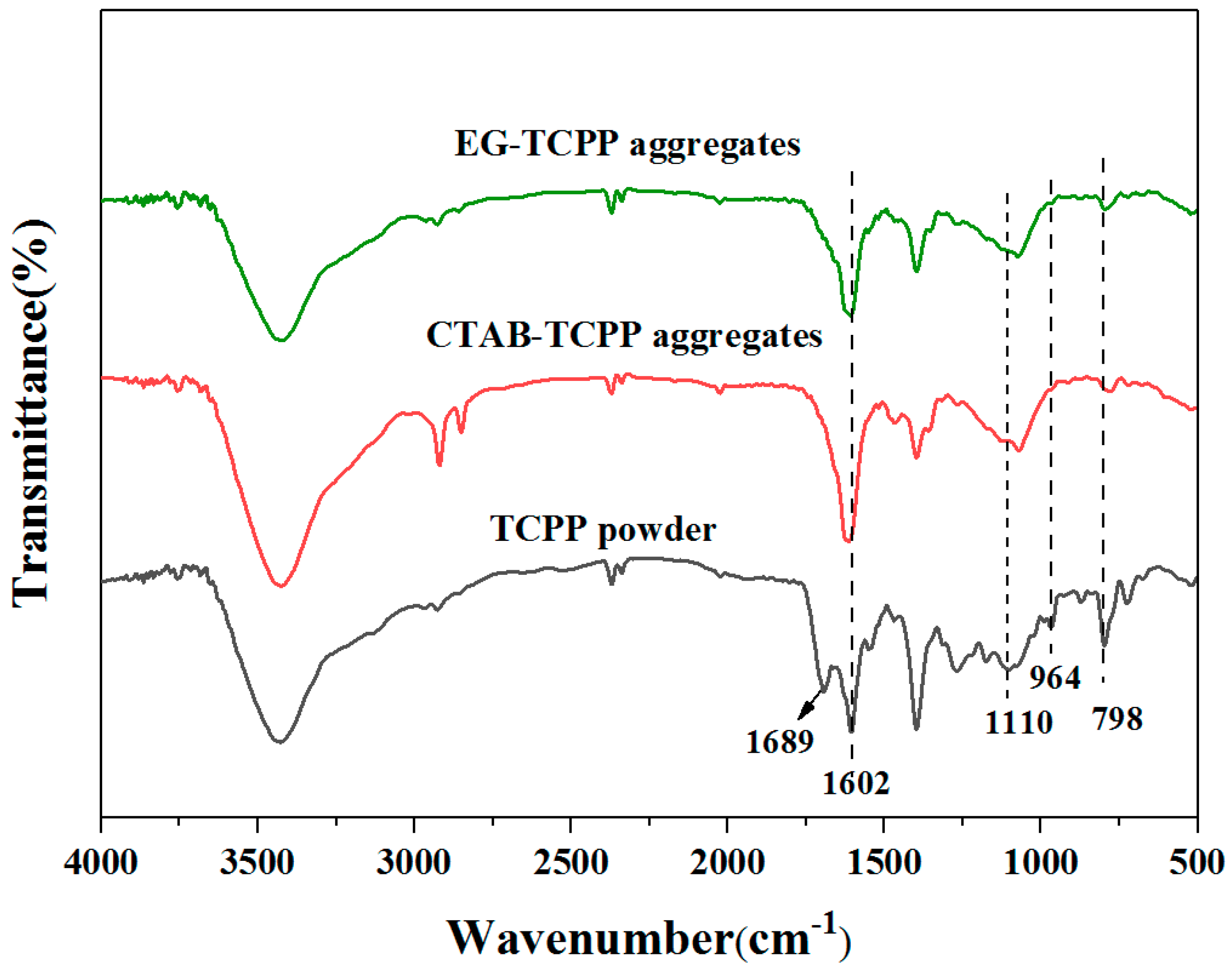
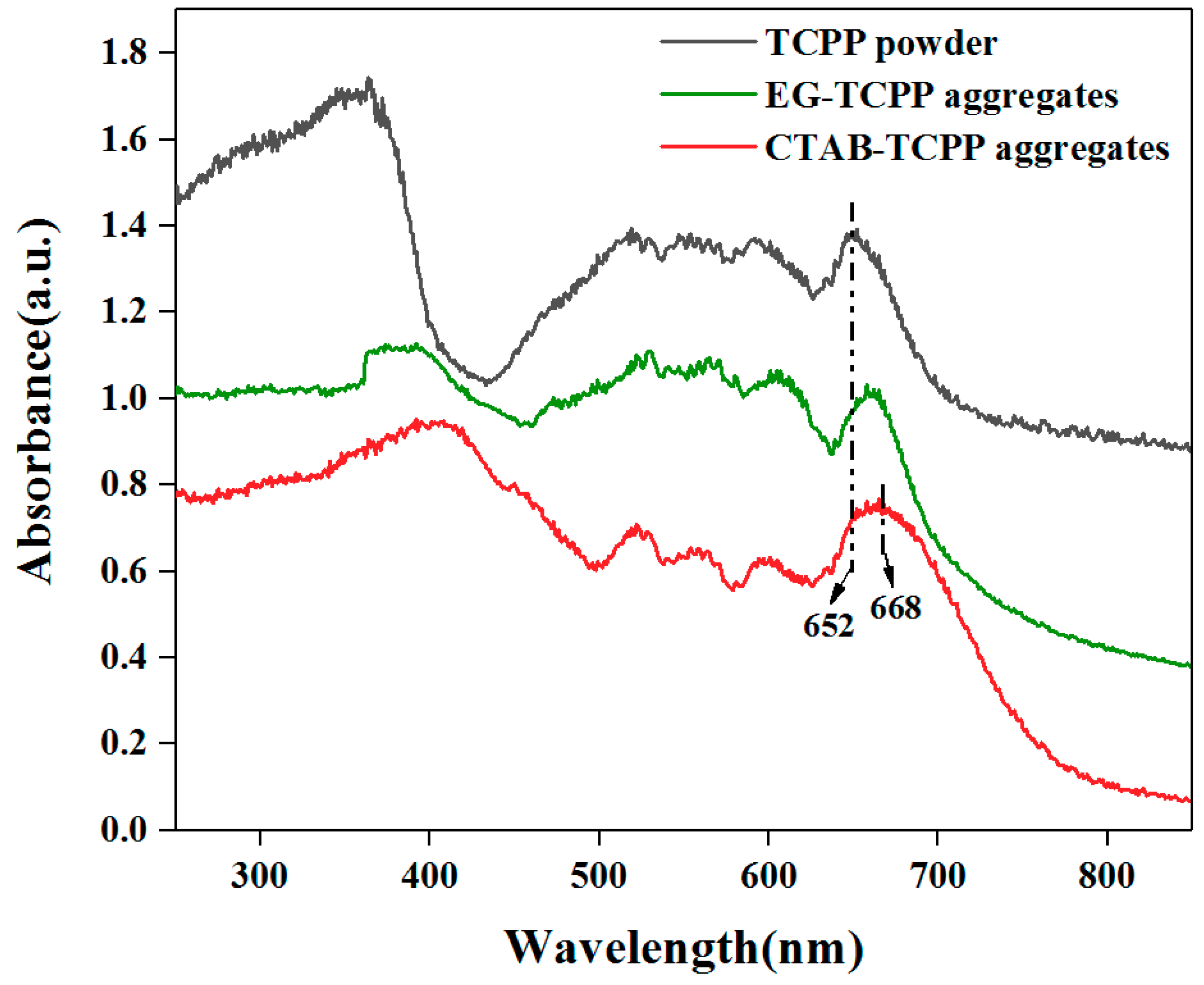
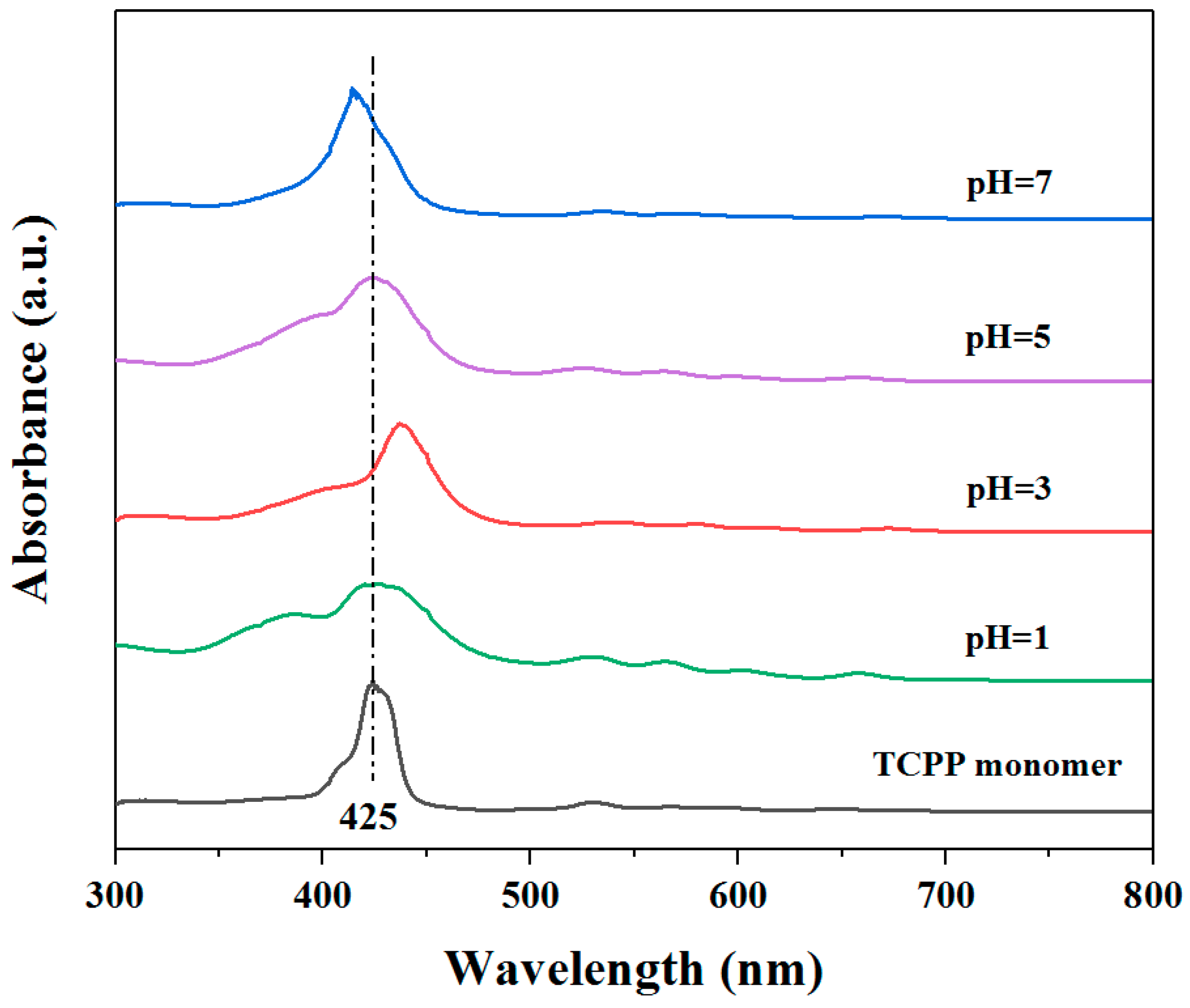
3.1. Self-Assembly Properties of TCPP to Form Different Aggregates and the Characterization of Their Structures
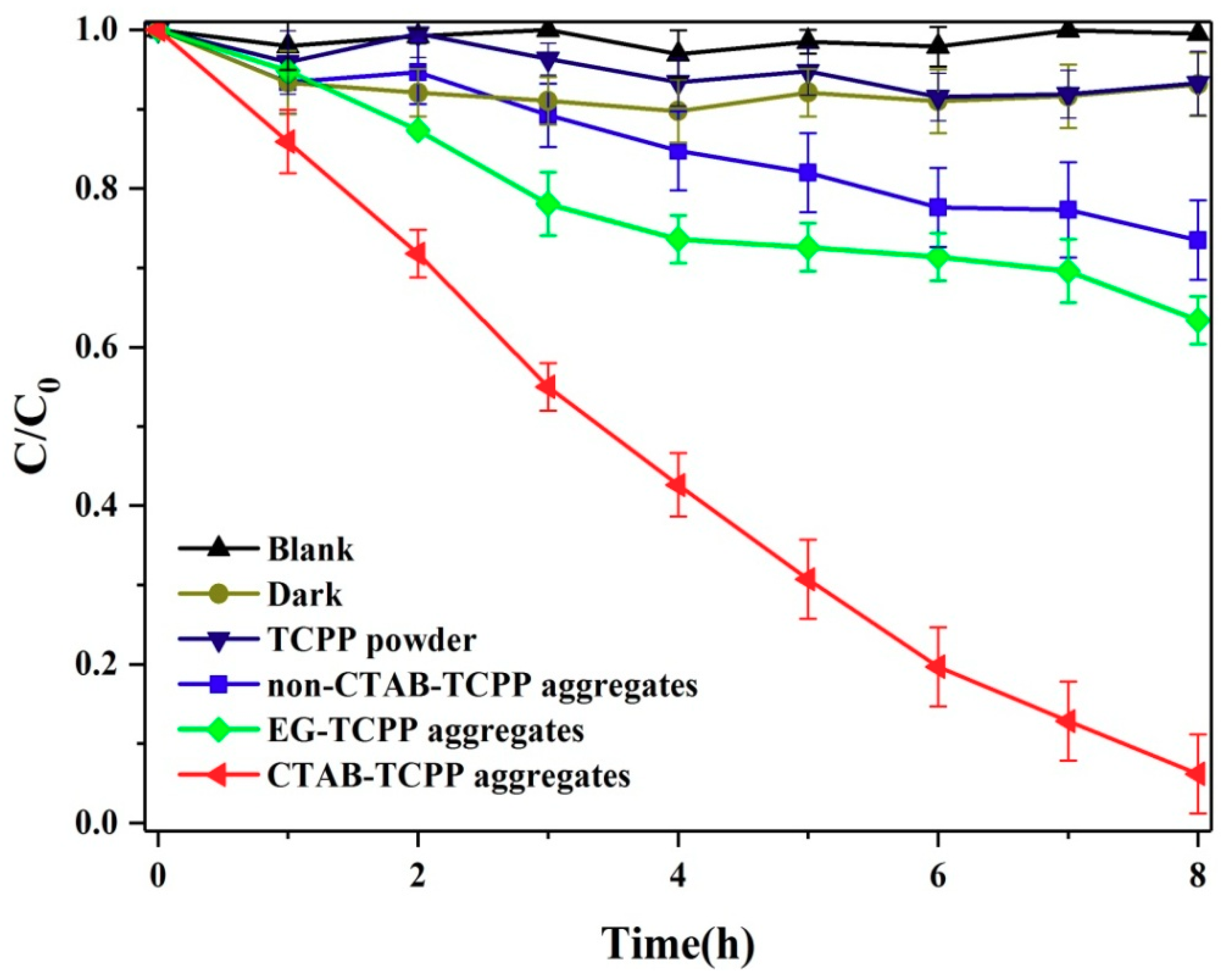
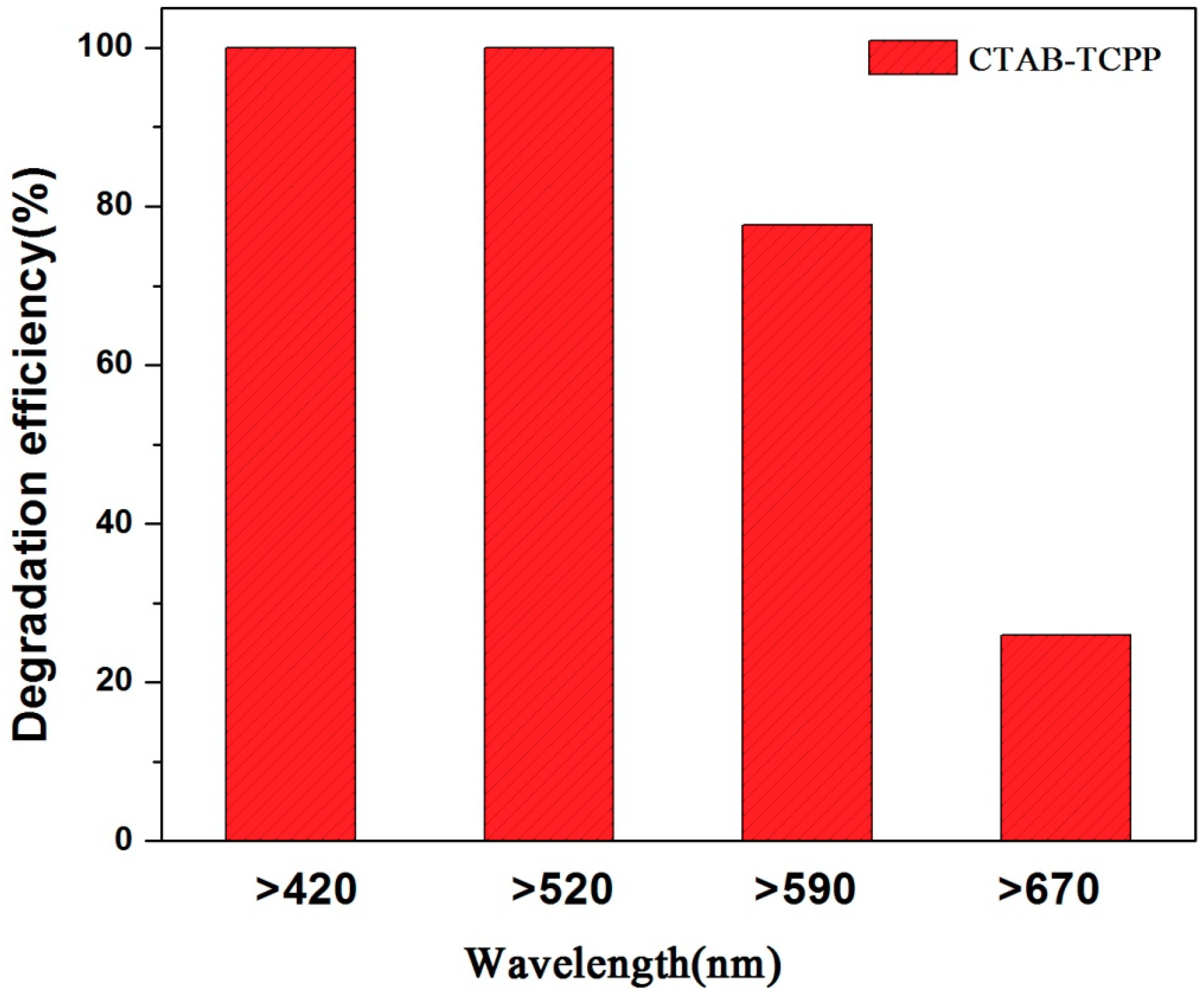
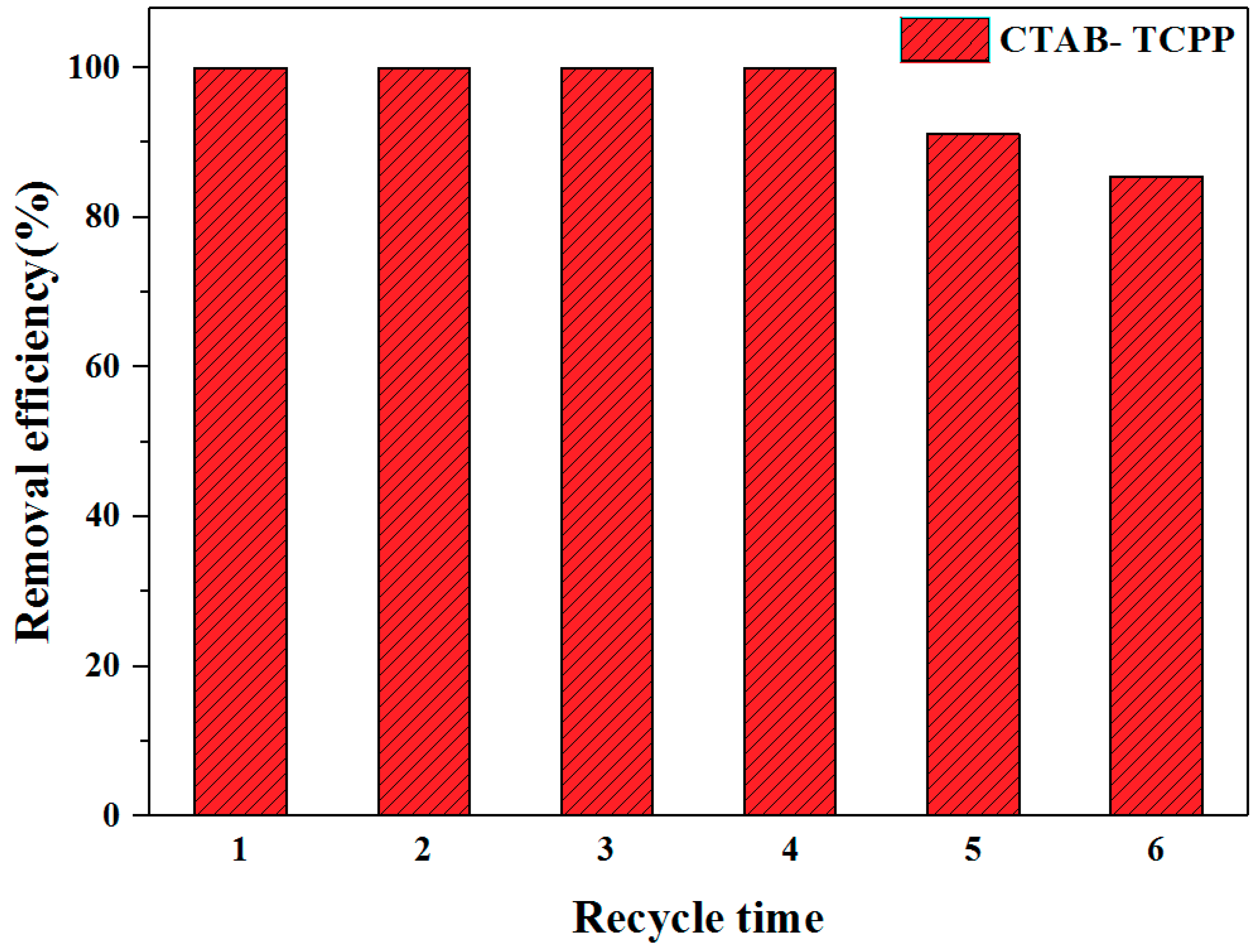
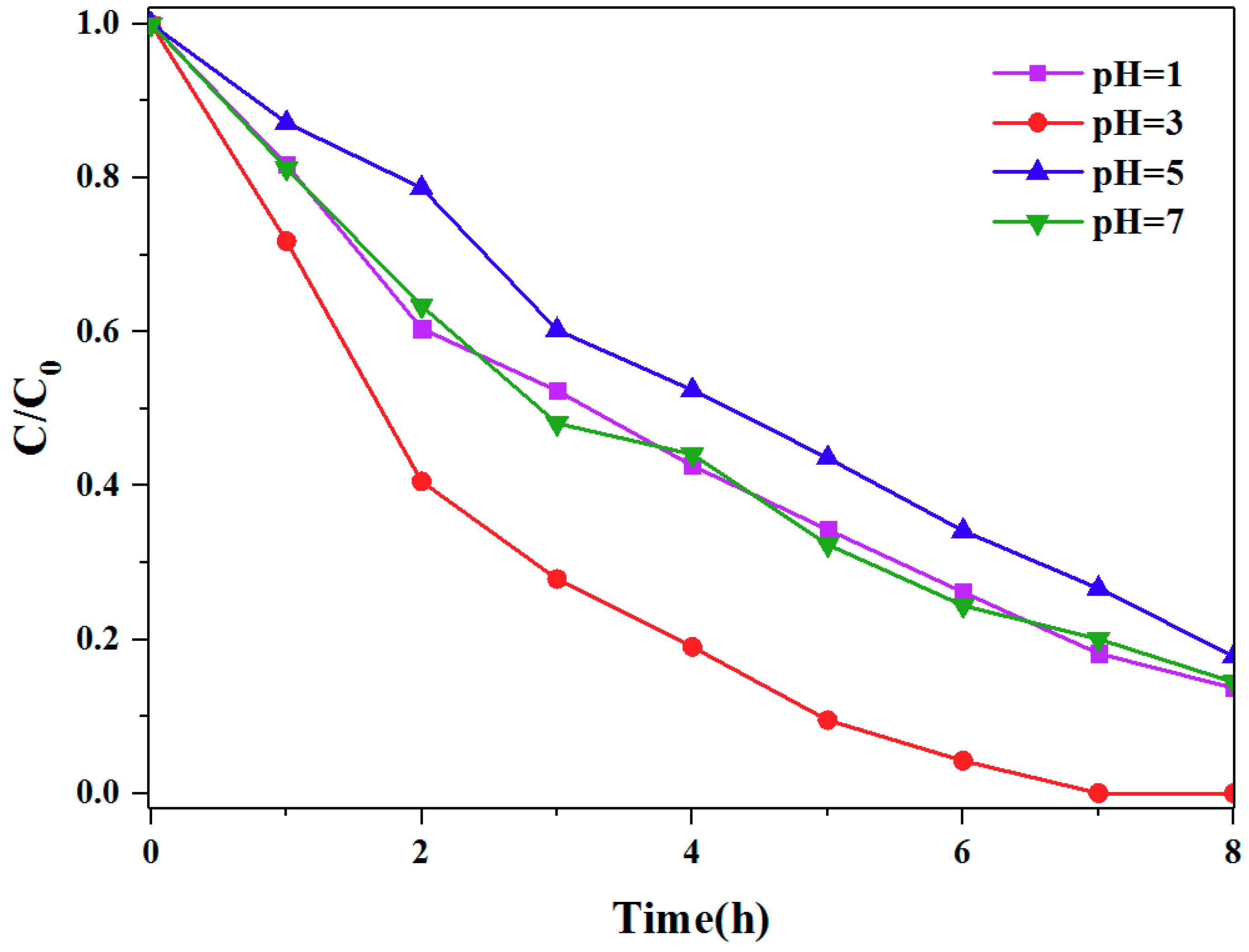
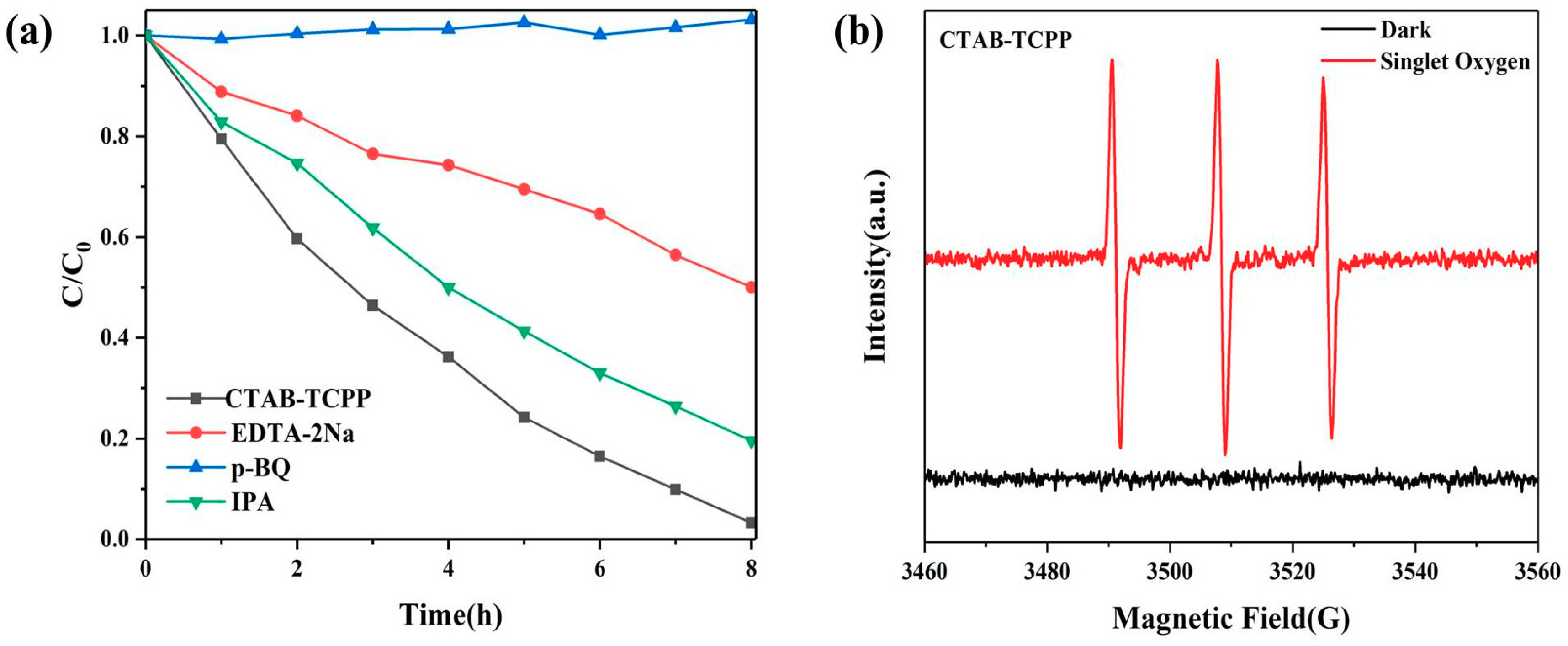
3.2. The Photocatalytic Activities of Different TCPP Aggregates
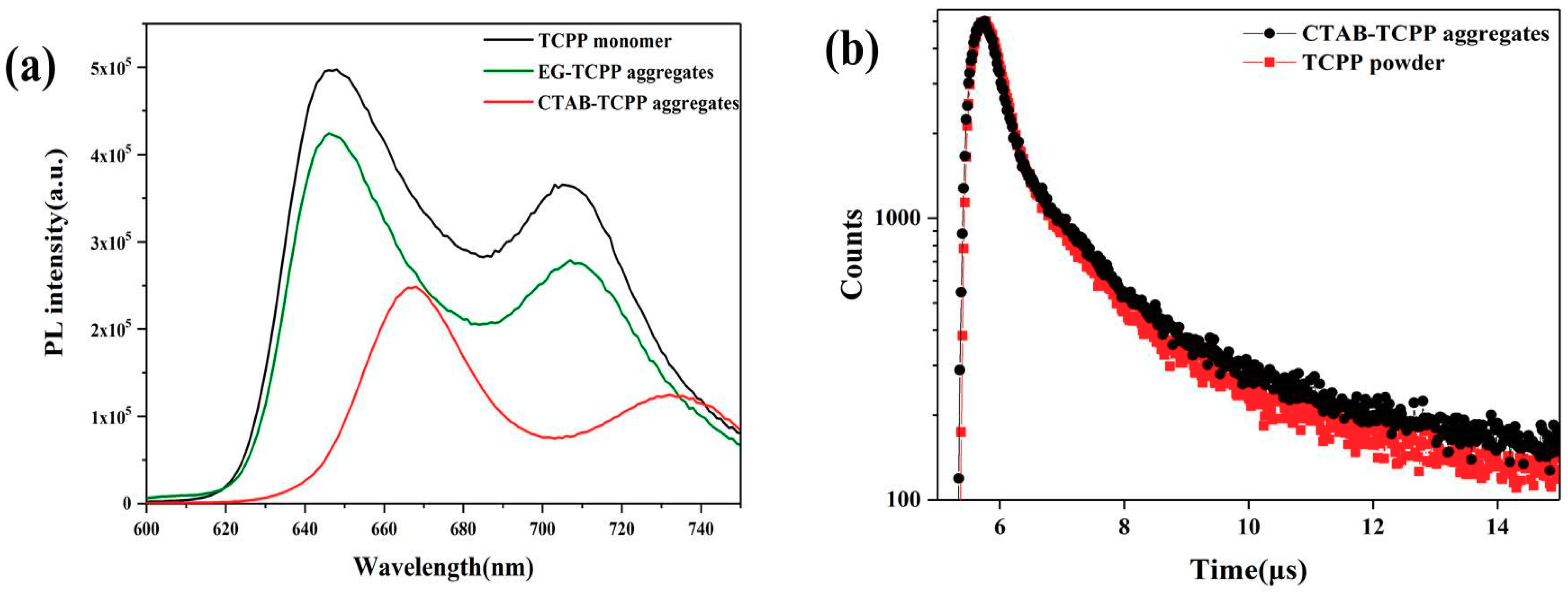
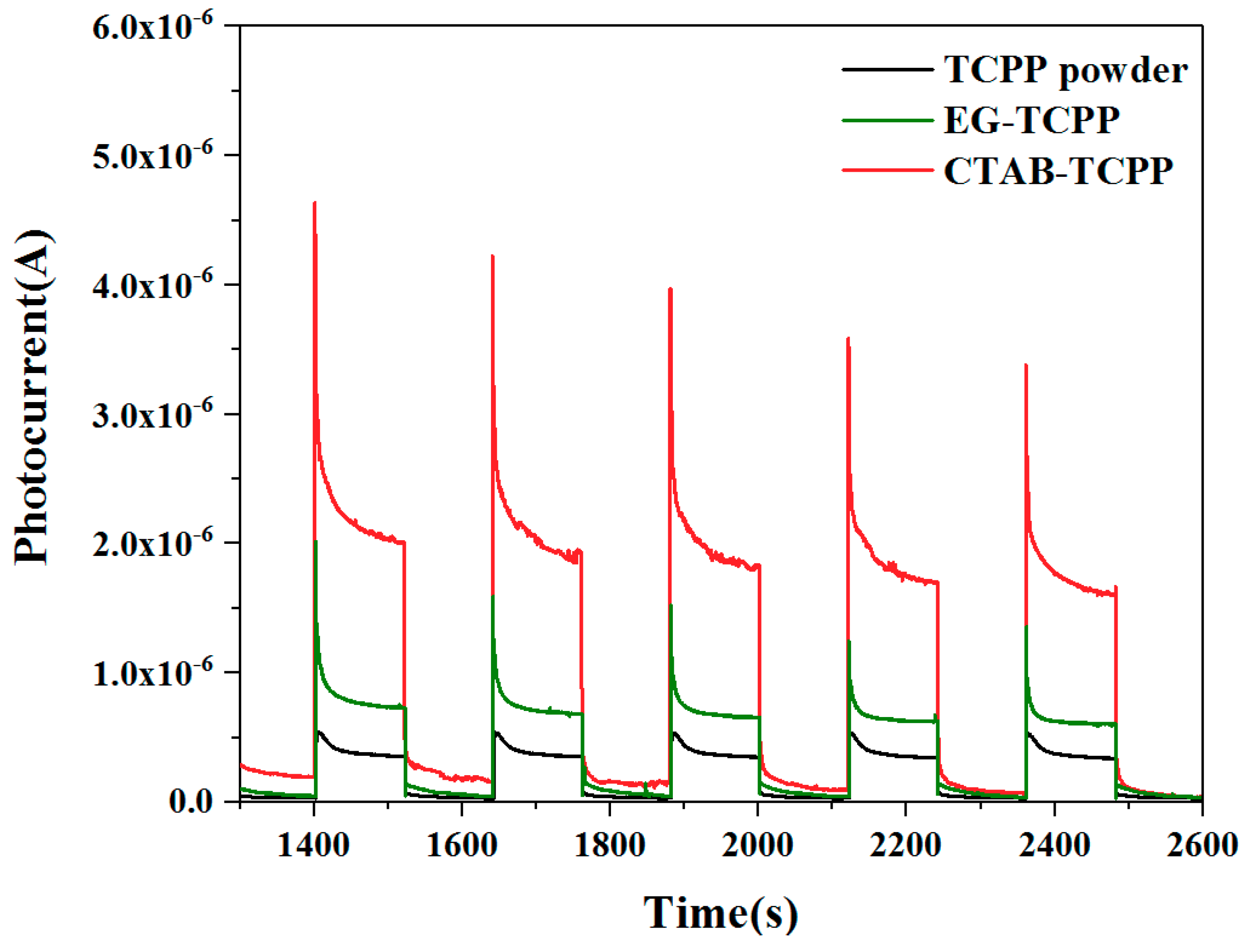
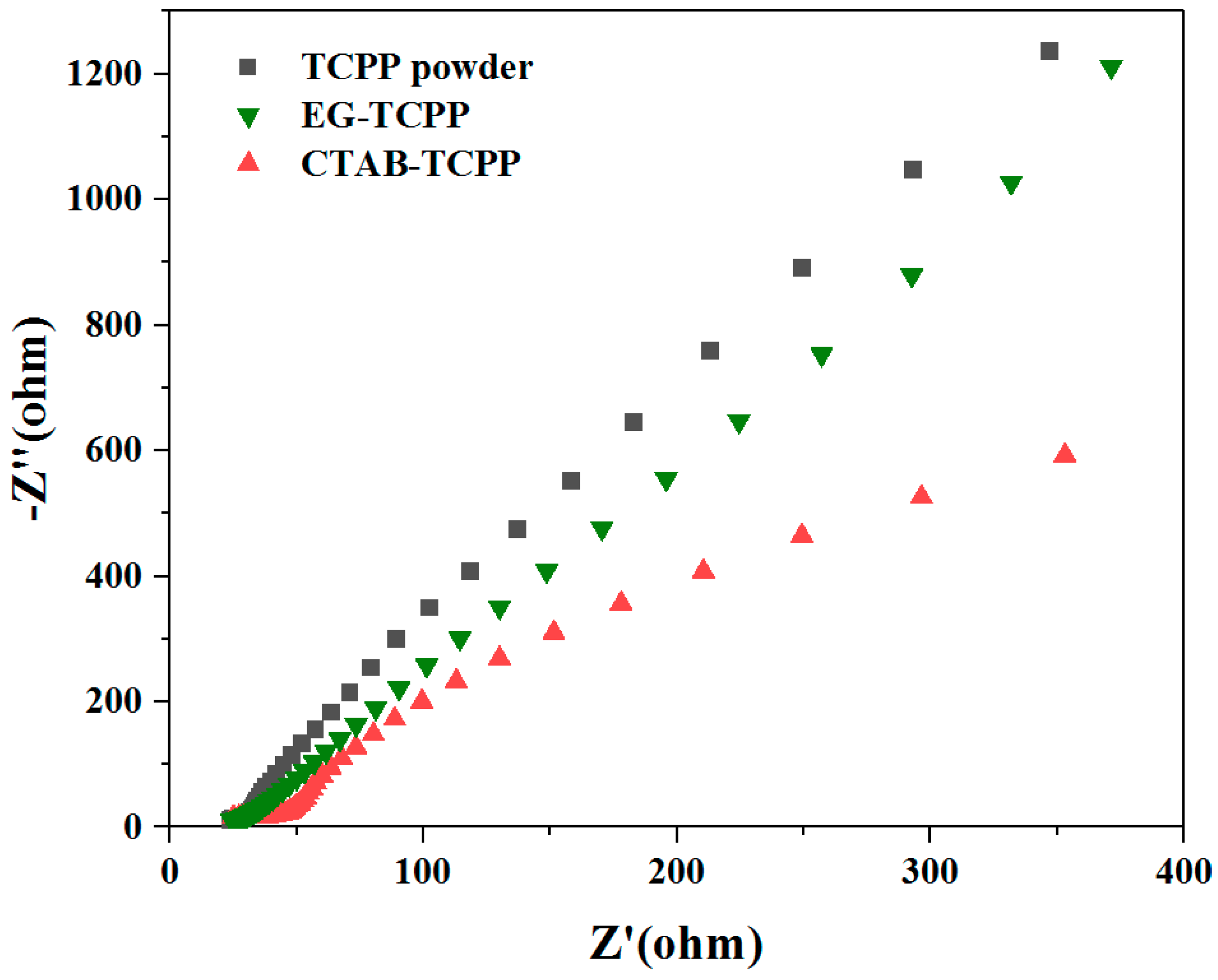
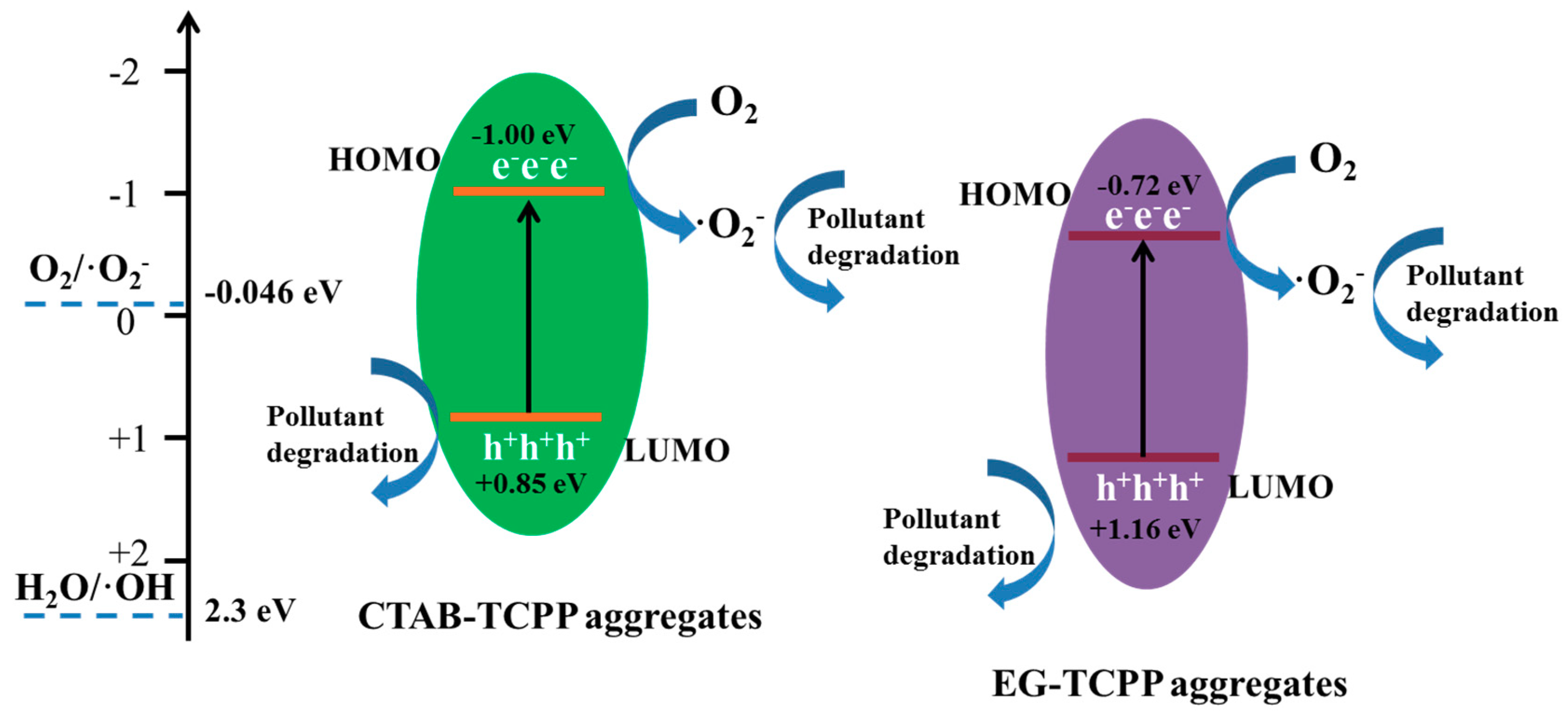
3.3. Mechanism of the Different Photocatalytic Activity of TCPP Aggregates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Crabtree, G.W.; Lewis, N.S. Solar Energy Conversion. Phys. Today 2007, 60, 37–43. [Google Scholar] [CrossRef]
- Ghosh, I.; Khamrai, J.; Savateev, A.; Shlapakov, N.; Antonietti, M.; König, B. Organic semiconductor photocatalyst can bifunctionalize arenes and heteroarenes. Science 2019, 365, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, L.; Chong, S.Y.; Little, M.A.; Wu, Y.; Zhu, W.H.; Clowes, R.; Yan, Y.; Zwijnenburg, M.A.; Sprick, R.S. Sulfone-containing covalent organic frameworks for photocatalytic hydrogen evolution from water. Nat. Chem. 2018, 10, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Xie, J.; Chen, X.; Lia, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Bai, X.; Zong, R.; Zhu, Y. Self-Assembled PDINH Supramolecular System for Photocatalysis under Visible Light. Adv. Mater. 2016, 28, 7284–7290. [Google Scholar] [CrossRef] [PubMed]
- Hasobe, T. Porphyrin-Based Supramolecular Nanoarchitectures for Solar Energy Conversion. J. Phys. Chem. Lett. 2013, 4, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Weingarten, A.S.; Kazantsev, R.V.; Palmer, L.C.; McClendon, M.; Koltonow, A.R.; Samuel, A.P.S.; Kiebala, D.J.; Wasielewski, M.R.; Stupp, S.I. Self-assembling hydrogel scaffolds for photocatalytic hydrogen production. Nat. Chem. 2014, 6, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.X.; Zhao, G.J. Modification of n-Type Organic Semiconductor Performance of Perylene Diimides by Substitution in Different Positions: Two-Dimensional π-Stacking and Hydrogen Bonding. ChemSusChem 2012, 5, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Liu, T.; He, C.; Shi, D.; Zhang, F.; Duan, C. Organized aggregation makes insoluble perylene diimide efficient for the reduction of aryl halides via consecutive visible light-induced electron-transfer processes. J. Am. Chem. Soc. 2016, 138, 3958–3961. [Google Scholar] [CrossRef]
- Wang, J.; Shi, W.; Liu, D.; Zhang, Z.J.; Zhu, Y.F.; Wang, D. Supramolecular organic nanofibers with highly efficient and stable visible light photooxidation performance. Appl. Catal. B Environ. 2017, 202, 289–297. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, J.; Jiang, W.; Yao, W.; Yang, H.; Zhu, Y. Self-assembled Perylene Diimide Based Supramolecular Heterojunction with Bi2WO6, for Efficient Visible-Light-Driven Photocatalysis. Appl. Catal. B Environ. 2018, 232, 175–181. [Google Scholar] [CrossRef]
- Tian, Y.; Martin, K.; Shelnutt, J.; Evans, L.; Busani, T.; Miller, J.E.; Medforth, C.J.; Shelnutt, J.A. Morphological families of self-assembled porphyrin structures and their photosensitization of hydrogen generation. Chem. Commun. 2011, 47, 6069–6071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, P.; Dong, H.; Zhen, Y.; Liu, M.; Hu, W. Porphyrin Supramolecular 1D Structures via Surfactant-Assisted Self-Assembly. Adv. Mater. 2015, 27, 5379–5387. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, A.; Huang, Z.H.; Wang, L.N.; Kang, F. Porphyrin-Based Nanostructures for Photocatalytic Applications. Nanomaterials 2016, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.; Kalyankar, M.; Nalage, S.; Lalander, C.; Bhosale, S.; Langford, S.; Oliver, R. pH Dependent Molecular Self-Assembly of Octaphosphonate Porphyrin of Nanoscale Dimensions: Nanosphere and Nanorod Aggregates. Int. J. Mol. Sci. 2011, 12, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Geng, G.; Chen, P.; Guan, B.; Jiang, L.; Xu, Z.; Di, D.; Tu, Z.; Hao, W.; Yi, Y.; Chen, C. Shape-Controlled Metal-Free Catalysts: Facet-Sensitive Catalytic Activity Induced by the Arrangement Pattern of Noncovalent Supramolecular Chains. ACS Nano 2017, 11, 4866–4876. [Google Scholar] [CrossRef]
- Hasobe, T. Photo- and electro-functional self-assembled architectures of porphyrins. Phys. Chem. Chem. Phys. 2012, 14, 15975–15987. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, L.; Wang, H.; Cao, R.; Wang, J.; Bai, F.; Fan, H. Self-Assembled One-Dimensional Porphyrin Nanostructures with Enhanced Photocatalytic Hydrogen Generation. Nano Lett. 2018, 18, 560–566. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Chen, X.; Zhang, H.; Wang, J. A Full-Spectrum Metal-Free Porphyrin Supramolecular Photocatalyst for Dual Functions of Highly Efficient Hydrogen and Oxygen Evolution. Adv. Mater. 2018, 31, 1806626. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, J.; Zhang, R.; Wei, W.; Wang, H.; Lü, X.; Bai, F.; Wu, H.; Haddad, R.; Fan, H. Morphology-controlled self-assembly and synthesis of photocatalytic nanocrystals. Nano Lett. 2014, 14, 7175–7179. [Google Scholar] [CrossRef]
- Guo, P.; Chen, P.; Liu, M. Porphyrin Assemblies via a Surfactant-Assisted Method: From Nanospheres to Nanofibers with Tunable Length. Langmuir 2012, 28, 15482–15490. [Google Scholar] [CrossRef] [PubMed]
- Sandanayaka, A.S.D.; Araki, Y.; Wada, T.; Hasobe, T. Structural and Photophysical Properties of Self-Assembled Porphyrin Nanoassemblies Organized by Ethylene Glycol Derivatives. J. Phys. Chem. C 2008, 112, 19209–19216. [Google Scholar] [CrossRef]
- Xu, W.; Guo, H.; Akins, D.L. Aggregation of Tetrakis(p-sulfonatophenyl)porphyrin within Modified Mesoporous MCM-41. J. Phys. Chem. B 2001, 105, 1543–1546. [Google Scholar] [CrossRef]
- McHale, J.L. Hierarchal Light-Harvesting Aggregates and Their Potential for Solar Energy Applications. J. Phys. Chem. Lett. 2012, 3, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Chen, P.; Ma, W.; Liu, M. Morphology-Dependent Supramolecular Photocatalytic Performance of Porphyrin Nanoassemblies: From Molecule to Artificial Supramolecular Nanoantenna. J. Mater. Chem. 2012, 22, 20243–20249. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Z.; Xiao, B.; Lu, Y.; Du, Y.; Yang, P.; Wang, X. Surfactant Assistance in Improvement of Photocatalytic Hydrogen Production with the Porphyrin Noncovalently Functionalized Graphene Nanocomposite. ACS Appl. Mater. Interfaces 2013, 5, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Nayak, S.K.; Mallampalli, S.; Patra, A. Surfactant-Assisted Porphyrin Based Hierarchical Nano/Micro Assemblies and Their Efficient Photocatalytic Behavior. ACS Appl. Mater. Interfaces 2014, 6, 130–136. [Google Scholar] [CrossRef]
- Patra, A.; Mandal, S.; Mondal, B.; Jana, B.; Nayak, S.K.; Bera, R. Graphene oxide–porphyrin nanorod composites for solar light harvesting. ACS Sustain. Chem. Eng. 2016, 4, 1562–1568. [Google Scholar]
- Maiti, N.C.; Azumdar, S.; Periasamy, N. J- and H-Aggregates of Porphyrin−Surfactant Complexes: Time-Resolved Fluorescence and Other Spectroscopic Studies. J. Phys. Chem. B 1998, 102, 1528–1538. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, H.; Li, Q.; Yu, L.; Zhang, H. Hydrogen-bond-linked photocatalyst of g-C3N4/3, 4, 9, 10-perylenetetracarboxylic acid anhydride with different bay-substitutents. Catal. Commun. 2018, 111, 90–94. [Google Scholar] [CrossRef]
- Liu, L.; Yue, M.; Lu, J.; Hu, J.S.; Liang, Y.H.; Cui, W.Q. The enrichment of photocatalysis via self-assembly perylene tetracarboxylic acid diimide polymer nanostructures incorporating TiO2 nanoparticles. Appl. Surf. Sci. 2018, 456, 645–656. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Y.; Wang, L.; Zhang, N.; Cao, R.; Bian, K.; Alarid, L.; Haddad, R.E.; Bai, F.; Fan, H. Morphology controlled synthesis and metalation of porphyrin nanoparticles with enhanced photocatalytic performance. Nano Lett. 2016, 16, 6523–6528. [Google Scholar] [CrossRef] [PubMed]
- Devaramani, S.; Shinger, M.I.; Ma, X.; Yao, M.; Zhang, S.; Qin, D.; Lu, X. Porphyrin aggregates decorated MWCNT film for solar light harvesting: Influence of J- and H-aggregation on the charge recombination resistance, photocatalysis, and photoinduced charge transfer kinetics. Chem. Phys. 2017, 19, 18232–18242. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Abbas, M.; Zhao, L.; Li, S.; Shen, G.; Yan, X. Biological Photothermal Nanodots Based on Self-Assembly of Peptide-Porphyrin Conjugates for Antitumor Therapy. J. Am. Chem. Soc. 2017, 139, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xing, R.; Li, Y.; Zou, Q.; Möhwald, H.; Yan, X. Mimicking Primitive Photobacteria: Sustainable Hydrogen Evolution Based on Peptide-Porphyrin Co-Assemblies with a Self-Mineralized Reaction Center. Angew. Chem. Int.Ed. 2016, 55, 12503–12507. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Li, X.; Zhuang, B.; Kang, S.Z.; Qin, L.X.; Li, G.D. Assembly mechanism and photoproduced electron transfer for a novel cubic Cu2O/tetrakis(4-hydroxyphenyl)porphyrin hybrid with visible photocatalytic activity for hydrogen evolution. Appl. Catal. B Environ. 2017, 211, 296–304. [Google Scholar] [CrossRef]
- Xie, J.; Shevlin, S.A.; Ruan, Q.; Moniz, S.J.A.; Liu, Y.R.; Liu, X.; Li, Y.M.; Lau, C.C.; Guo, Z.X.; Tang, J.W. Efficient visible light-driven water oxidation and proton reduction by an ordered covalent triazine-based framework. Energy Environ. Sci. 2018, 11, 1617–1624. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Feng, H.; Ren, X.; Ji, J.; Bai, F.; Fan, H. Microemulsion-Assisted Self-Assembly and Synthesis of Size Controlled Porphyrin Nanocrystals with Enhanced Photocatalytic Hydrogen Evolution. Nano Lett. 2019, 19, 2614–2619. [Google Scholar] [CrossRef]
- Liang, Y.; Shang, R.; Lu, J.; Liu, L.; Cui, W.Q. Ag3PO4@UMOFNs Core-Shell Structure: Two-Dimensional MOFs Promoted Photoinduced Charge Separation and Photocatalysis. ACS Appl. Mater. Interfaces 2018, 10, 8758–8769. [Google Scholar] [CrossRef]
- Yin, M.; Li, Z.; Kou, J.; Zou, Z. Mechanism investigation of visible light-induced degradation in a heterogeneous TiO2/Eosin Y/ Rhodamine B system. Environ. Sci. Technol. 2009, 43, 8361–8366. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.W.; Choi, W. Platinized WO3 as an Environmental Photocatalyst that Generates OH Radicals under Visible Light. Environ. Sci. Technol. 2010, 44, 6849–6854. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Li, Z.; An, W.; Liu, L.; Cui, W. Tuning the Supramolecular Structures of Metal-Free Porphyrin via Surfactant Assisted Self-Assembly to Enhance Photocatalytic Performance. Nanomaterials 2019, 9, 1321. https://doi.org/10.3390/nano9091321
Lu J, Li Z, An W, Liu L, Cui W. Tuning the Supramolecular Structures of Metal-Free Porphyrin via Surfactant Assisted Self-Assembly to Enhance Photocatalytic Performance. Nanomaterials. 2019; 9(9):1321. https://doi.org/10.3390/nano9091321
Chicago/Turabian StyleLu, Jinrong, Zihan Li, Weijia An, Li Liu, and Wenquan Cui. 2019. "Tuning the Supramolecular Structures of Metal-Free Porphyrin via Surfactant Assisted Self-Assembly to Enhance Photocatalytic Performance" Nanomaterials 9, no. 9: 1321. https://doi.org/10.3390/nano9091321
APA StyleLu, J., Li, Z., An, W., Liu, L., & Cui, W. (2019). Tuning the Supramolecular Structures of Metal-Free Porphyrin via Surfactant Assisted Self-Assembly to Enhance Photocatalytic Performance. Nanomaterials, 9(9), 1321. https://doi.org/10.3390/nano9091321




