Composition―Nanostructure Steered Performance Predictions in Steel Wires
Abstract
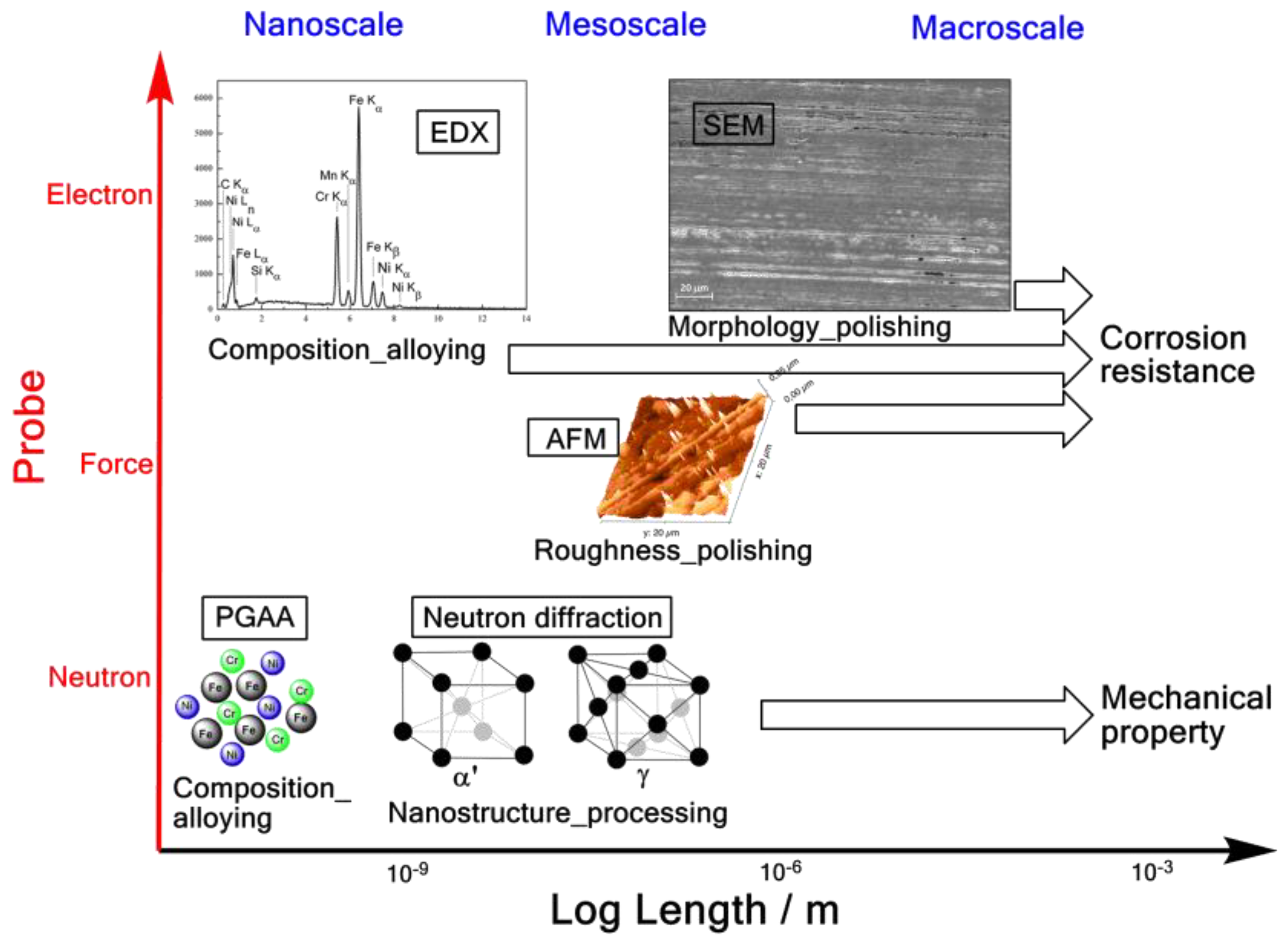
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Simultaneous Prompt Gamma Activation Alalysis (PGAA) and Neutron Diffraction Measurement
2.3. Simultaneous Elemental-Surface Morphology Measurement with SEM-EDX
2.4. Surface Roughness Measurement with AFM
2.5. Statistical Analysis
3. Results
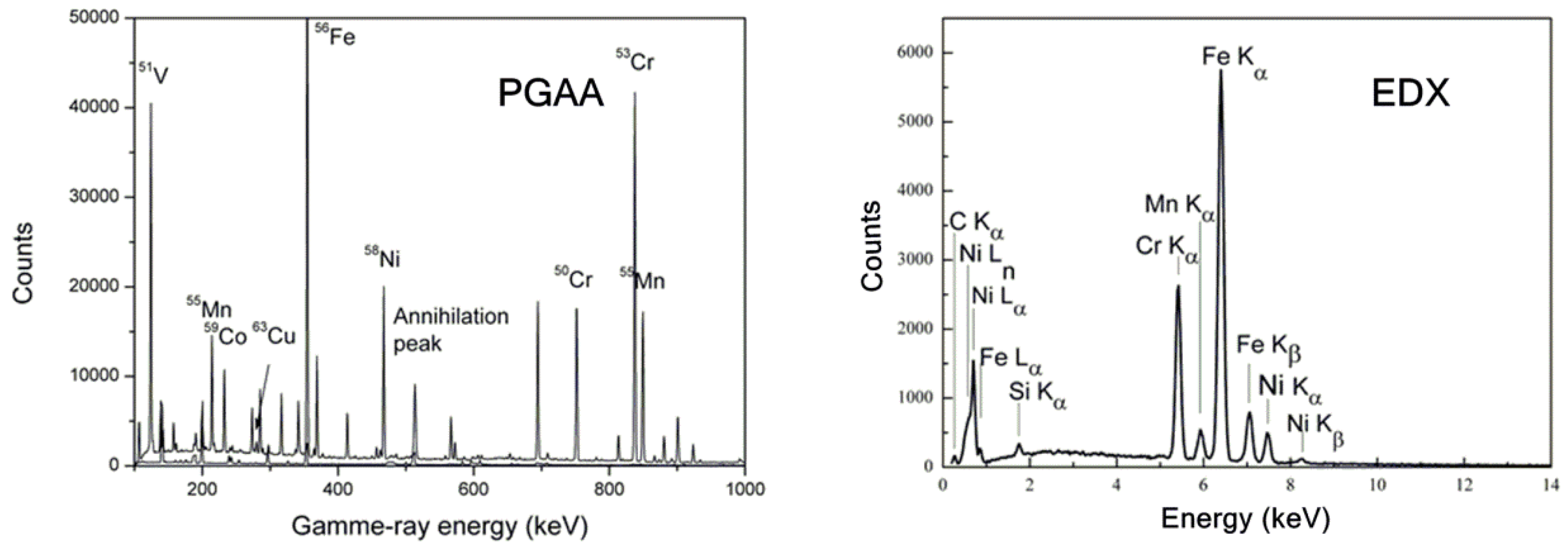
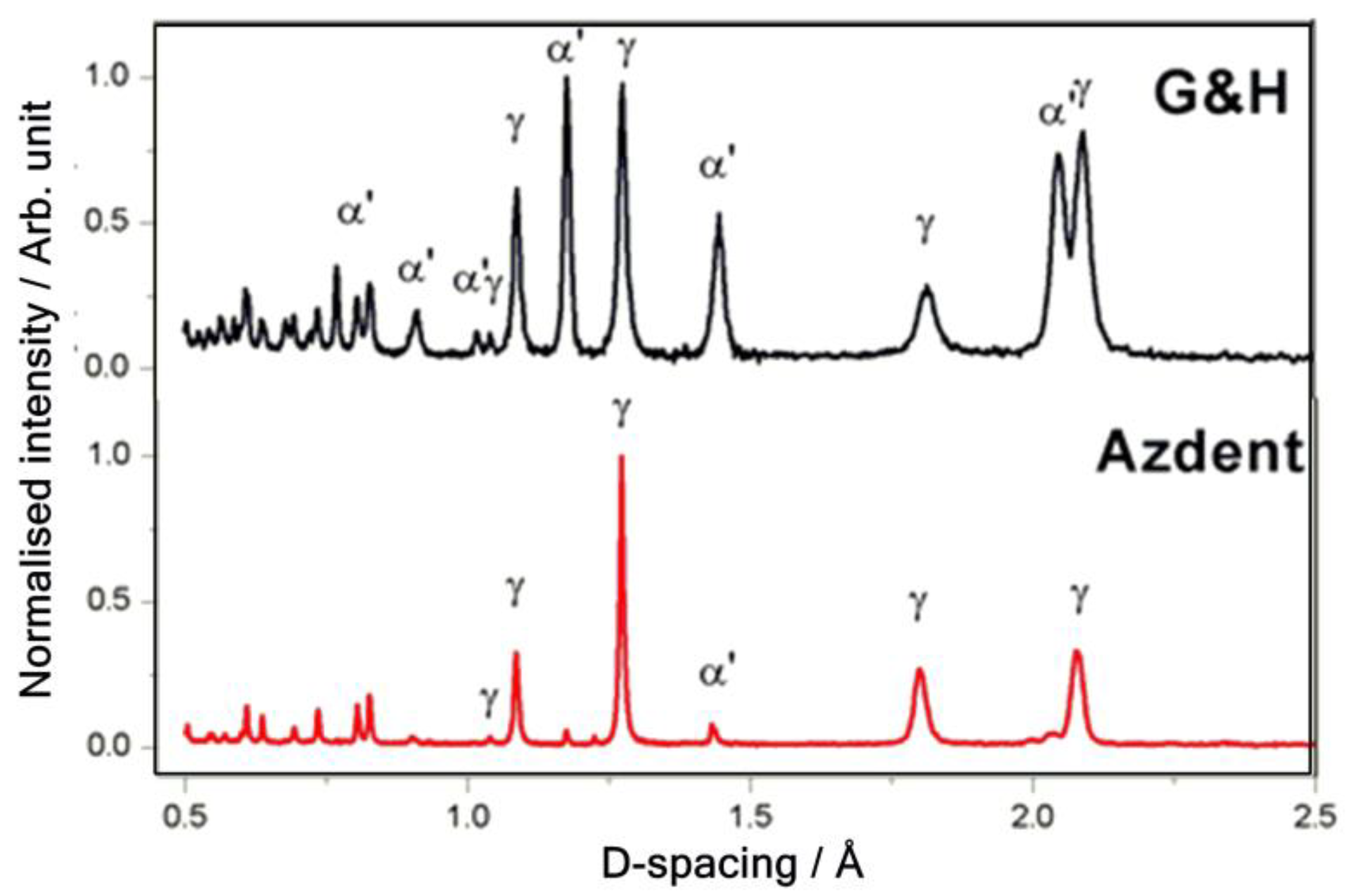
3.1. Simultaneous PGAA-Neutron Diffraction Results
3.1.1. PGAA Results
3.1.2. Neutron Diffraction Results
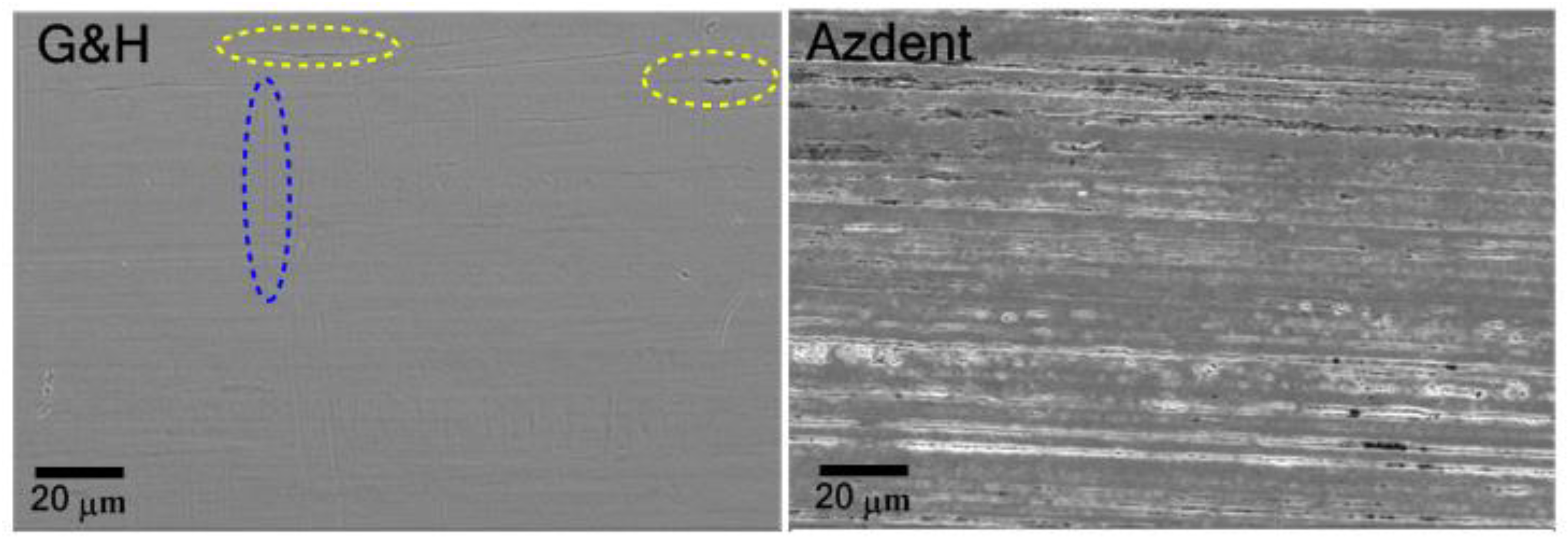
3.2. Simultaneous Elemental-Surface Morphology Measurement Results
3.2.1. Surface Elemental Composition
3.2.2. Surface Morphology
3.3. Surface Roughness Measurement Result
4. Discussion
4.1. Elemental Composition and Its Influence
4.2. Nanostructure and Its Influence
4.3. Surface Characteristics and Its Influence
4.4. Simultaneous Composition-Nanostructure Analyses with Neutron Spectroscopy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Proffit, W.R. Mechanical principals in orthodontic force control. In Contemporary Orthodontics, 5th ed.; Proffit, W.R., Fields, H.W., Sarver, D.M., Eds.; Mosby, Elsevier Inc.: St. Louis, MO, USA, 2013; pp. 312–346. ISBN 978-0-323-08317-1. [Google Scholar]
- Kusy, R.P. A review of contemporary archwires: Their properties and characteristics. Angle Orthod. 1997, 67, 197–208. [Google Scholar] [PubMed]
- Zinelis, S.; Brantley, W.A. An Overview of Research Methods on Orthodontic Alloys and Ceramics. In Research Methods in Orthodontics: A Guide to Understanding Orthodontic Research; Eliades, T., Ed.; Springer: Heidelberg, Germany, 2013; pp. 3–34. ISBN 978-3-642-31376-9. [Google Scholar]
- Darvell, B.W. Materials Science for Dentistry, 9th ed.; Woodhead publishing: Cambridge, UK, 2009; pp. 1–36. ISBN 978-1-84-569529-3. [Google Scholar]
- Eliades, T.; Zinelis, S.; Papadopoulos, M.A.; Eliades, G.; Athanasiou, A.E. Nickel content of as-received and retrieved NiTi and stainless steel archwires: Assessing the nickel release hypothesis. Angle Orthod. 2004, 74, 151–154. [Google Scholar] [PubMed]
- Tian, K.; Darvell, B.W. Determination of flexural modulus of elasticity of orthodontic archwires. Dent. Mater. 2010, 26, 821–829. [Google Scholar] [CrossRef]
- Tian, K.V.; Festa, G.; Basoli, F.; Laganà, G.; Scherillo, A.; Andreani, C.; Bollero, P.; Licoccia, S.; Senesi, R.; Cozza, P. Orthodontic archwire composition and phase analyses by neutron spectroscopy. Dent. Mater. J. 2017, 36, 282–288. [Google Scholar] [CrossRef]
- Juvvadi, S.R.; Kailasam, V.; Padmanabhan, S.; Chitharanjan, A.B. Physical, mechanical, and flexural properties of 3 orthodontic wires: An in-vitro study. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 623–630. [Google Scholar] [CrossRef]
- D’Antò, V.; Rongo, R.; Ametrano, G.; Spagnuolo, G.; Manzo, P.; Martina, R.; Paduano, S.; Valletta, R. Evaluation of surface roughness of orthodontic wires by means of atomic force microscopy. Angle Orthod. 2012, 82, 922–928. [Google Scholar] [CrossRef]
- Bennet, E.; Wilson, T.; Murphy, P.J.; Refson, K.; Hannon, A.C.; Imberti, S.; Callear, S.K.; Chass, G.A.; Parker, S.F. How the surface structure determines the properties of CuH. Inorg. Chem. 2015, 54, 2213–2220. [Google Scholar] [CrossRef]
- Tian, K.V.; Chass, G.A.; Di Tommaso, D. Simulations reveal the role of composition into the atomic-level flexibility of bioactive glass cements. Phys. Chem. Chem. Phys. 2016, 18, 837–845. [Google Scholar] [CrossRef]
- Khier, S.E.; Brantley, W.A.; Fournelle, R.A. Structure and mechanical properties of as-received and heat-treated stainless steel orthodontic wires. Am. J. Orthod. Dentofac. Orthop. 1988, 93, 206–212. [Google Scholar] [CrossRef]
- Davis, R.A.; Ardalan, S.; Mu, W.H.; Tian, K.; Farsaikiya, F.; Darvell, B.W.; Chass, G.A. Geometric, electronic and elastic properties of dental silver amalgam γ-(Ag3sn), γ1-(Ag2Hg3), γ2-(Sn8Hg) phases, comparison of experiment and theory. Intermetallics 2010, 18, 756–760. [Google Scholar] [CrossRef]
- Pedersen, M.T.; Tian, K.V.; Dobó-Nagy, C.; Chass, G.A.; Greaves, G.N.; Yue, Y.Z. Phase separation in an ionomer glass: Insight from calorimetry and phase transitions. J. Non-Cryst. Solids 2015, 415, 24–29. [Google Scholar] [CrossRef]
- Zhu, X.; Chass, G.A.; Kwek, L.C.; Rogach, A.L.; Su, H.B. Excitonic character in optical properties of tetrahedral CdX (X= S, Se, Te) clusters. J. Phys. Chem. C 2015, 119, 29171–29177. [Google Scholar] [CrossRef]
- Tian, K.V.; Mahmoud, M.Z.; Cozza, P.; Licoccia, S.; Fang, D.C.; Di Tommaso, D.; Chass, G.A.; Greaves, G.N. Periodic vs. molecular cluster approaches to resolving glass structure and properties: Anorthite a case study. J. Non-Cryst. Solids 2016, 451, 138–145. [Google Scholar] [CrossRef]
- Llewellyn, D.T. Work hardening effects in austenitic stainless steels. Mater. Sci. Technol. 1997, 13, 389–400. [Google Scholar] [CrossRef]
- Gbur, J.L.; Lewandowski, J.J. Fatigue and fracture of wires and cables for biomedical applications. Int. Mater. Rev. 2016, 61, 1–84. [Google Scholar] [CrossRef]
- Steel Research 80/81; British Steel Corporation: London, UK, 1981; pp. 34–37.
- NDT Resource Center. Available online: https://www.nde-ed.org (accessed on 25 April 2019).
- Cheary, R.W.; Ma-Sorrell, Y. Quantitative phase analysis by X-ray diffraction of martensite and austenite in strongly oriented orthodontic stainless steel wires. J. Mater. Sci. 2002, 35, 1105–1113. [Google Scholar] [CrossRef]
- Di Schino, A.; Salvatori, I.; Kenny, J.M. Effects of martensite formation and austenite reversion on grain refining of AISI 304 stainless steel. J. Mater. Sci. 2002, 37, 4561–4565. [Google Scholar] [CrossRef]
- Okamoto, G. Passive film of 18-8 stainless steel structure and its function. Corros. Sci. 1973, 13, 471–489. [Google Scholar] [CrossRef]
- Ryan, M.P.; Williams, D.E.; Chater, R.J.; Hutton, B.M.; McPhail, D.S. Why stainless steel corrodes. Nature 2002, 415, 770–774. [Google Scholar] [CrossRef]
- Dillon, C.P. Corrosion Resistance of Stainless Steels, 1st ed.; Marcel Dekker Inc.: New York, NY, USA, 1995; p. 104. ISBN 978-0-824-79629-7. [Google Scholar]
- Price, D.; Fernandez-Alonso, F. An Introduction to Neutron Scattering. In Experimental Methods in the Physical Sciences, Neutron Scattering—Fundamentals; Fernandez-Alonso, F., Price, D., Eds.; Academic Press: Cambridge, UK, 2013; Volume 44, pp. 1–136. ISBN 978-0-123-98374-9. [Google Scholar]
- Rèvay, Z.; Belgya, Z.T. Principles of PGAA Method. In Handbook of Prompt Gamma Activation Analysis with Neutron Beams; Molnàr, G.L., Ed.; Kluwer Academic Publishers: New York, NY, USA, 2004; pp. 1–30. ISBN 978-0-387-23359-8. [Google Scholar]
- Tian, K.V.; Festa, G.; Szentmiklósi, L.; Maróti, B.; Arcidiacono, L.; Laganà, G.; Andreani, C.; Licoccia, S.; Senesi, R.; Cozza, P. Compositional studies of functional orthodontic archwires using prompt-gamma activation analysis at a pulsed neutron source. J. Anal. At. Spectrom. 2017, 32, 1420–1427. [Google Scholar] [CrossRef]
- Tian, K.V.; Yang, B.; Yue, Y.Z.; Bowron, D.T.; Mayers, J.; Donnan, R.S.; Dobó-Nagy, C.; Nicholson, J.W.; Fang, D.C.; Greer, A.L.; et al. Atomic and vibrational origins of mechanical toughness in bioactive cement during setting. Nat. Commun. 2015, 6, 8631. [Google Scholar] [CrossRef]
- Larson, A.C.; von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report LAUR: Los Alamos, NM, USA, 1994; pp. 86–748. [Google Scholar]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Rèvay, Z.; Firestone, R.B.; Belgya, T.; Molnár, G.L. Prompt Gamma-Ray Spectrum Catalog. In Handbook of Prompt Gamma Activation Analysis with Neutron Beams; Molnàr, G.L., Ed.; Kluwer Academic Publishers: New York, NY, USA, 2004; pp. 173–364. ISBN 978-0-387-23359-8. [Google Scholar]
- Szentmiklósi, L.; Kis, Z.; Belgya, T.; Berlizov, A.N. On the design and installation of a Compton–suppressed HPGe spectrometer at the Budapest neutron-induced prompt gamma spectroscopy (NIPS) facility. J. Radioanal. Nucl. Chem. 2013, 298, 1605–1611. [Google Scholar] [CrossRef]
- Kis, Z.; Szentmiklósi, L.; Belgya, T. NIPS–NORMA station—A combined facility for neutron-based nondestructive element analysis and imaging at the Budapest Neutron Centre. Nucl. Instrum. Methods Phys. Res. Sect. A 2015, 779, 116–123. [Google Scholar] [CrossRef]
- Fazekas, B.; Molnár, G.; Belgya, T.; Dabolczi, L.; Simonits, A. Introducing HYPERMET-PC for automatic analysis of complex gamma-ray spectra. J. Radioanal. Nucl. Chem. 1997, 215, 271–277. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Central Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Taylor, J.R. An Introduction to Error Analysis: The Study of Uncertainties in Physical Measurements, 1st ed.; University Science Books: Sausalito, CA, USA, 1982; pp. 93–120. ISBN 978-0-935-70275-0. [Google Scholar]
- Student. The Probable Error of a Mean. Biometrika 1908, 6, 1–25. [Google Scholar] [CrossRef]
- Charlton, M.; Humberston, J.W. Positron Physics; Cambridge University Press: Cambridge, UK, 2000; p. 6. ISBN 978-0-511-53520-8. [Google Scholar]
- Miceli, A.; Festa, G.; Senesi, R.; Perelli Cippo, E.; Giacomelli, L.; Tardocchi, M.; Scherillo, A.; Schooneveld, E.; Frost, C.; Gorini, G.; et al. Measurements of gamma-ray background spectra at spallation neutron source beamlines. J. Anal. At. Spectrom. 2014, 29, 1897–1903. [Google Scholar] [CrossRef]
- Kasztovszky, Z.; Kockelmann, W.A.; Perelli Cippo, E.; Gorini, G.; Tardocchi, M. Prompt gamma activation studies on archaeological objects at a pulsed neutron source. Il Nuovo. Cimento. 2008, 31, 143–155. [Google Scholar] [CrossRef]
- ASTM A240/A240M-18, Standard Specification for Chromium and Chromium-Nickel Stainless Steel Plate, Sheet, and Strip for Pressure Vessels and for General Applications; ASTM International: West Conshohocken, PA, USA, 2018.
- Chieruzzi, M.; Pagano, S.; Cianetti, S.; Lombardo, G.; Kenny, J.M.; Torre, L. Effect of fibre posts, bone losses and fibre content on the biomechanical behaviour of endodontically treated teeth: 3D-finite element analysis. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017, 1, 334–346. [Google Scholar] [CrossRef]
- Backofen, W.A.; Gales, G.F. The low temperature heat-treatment of stainless steel for orthodontics. Angle Orthod. 1951, 21, 117–124. [Google Scholar]
- Kusy, R.P.; Whitley, J.Q.; Mayhew, M.J.; Buckthal, J.E. Surface roughness of orthodontic archwires via laser spectroscopy. Angle Orthod. 1988, 58, 33–45. [Google Scholar]
- Bourauel, C.; Fries, C.T.; Drescher, D.; Plietsch, R. Surface roughness of orthodontic wires via atomic force microscopy, laser specular reflectance, and profilometry. Eur. J. Orthodo 1998, 20, 79–92. [Google Scholar] [CrossRef]
- Widu, F.; Drescher, D.; Junker, R.; Bourauel, C. Corrosion and biocompatibility of orthodontic wires. J. Mater. Sci. Mater. Med. 1999, 10, 275–281. [Google Scholar] [CrossRef]
- Oshida, Y.; Sachdeva, R.C.L.; Miyazaki, S. Microanalytical characterization and surface modification of TiNi orthodontic archwires. Biomed. Mater. Eng. 1992, 2, 51–69. [Google Scholar] [CrossRef]
- Brantley, W.A. Orthodontic wires. In Orthodontic Materials: Scientific and Clinical Aspects; Brantley, W.A., Eliades, T., Eds.; Thieme: Stuttgart, Germany, 2001; pp. 77–103. ISBN 978-0-865-77929-7. [Google Scholar]


| Fe | Cr | Ni | Mn | Cu | Mo | Co | Si | C | N | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G&H | PGAA | Bal. (0.60) | 18.20 # (0.50) | 9.60 (0.20) | 1.28 (0.04) | 0.39 (0.01) | 0.18 (0.004) | 0.033 (0.002) | 0.75 a | 0.08 a | 0.1 a |
| EDX | Bal. | 19.17 | 8.25 | 1.37 | ― | ― | ― | 0.77 | 3.30 | ― | |
| (1.08) | (0.42) | (0.02) | (0.21) | (0.01) | (1.33) | ||||||
| Azdent | PGAA | Bal. * (0.06) | 17.60 * (0.50) | 8.90 * (0.20) | 2.50 .003 (0.09) | 1.10 .001 (0.03) | 0.03 .001 (0.002) | 0.28 .001 (0.008) | 0.75 a | 0.08 a | 0.1 a |
| EDX | Bal. * | 19.04 * | 7.79 * | 2.56 .009 | ― | ― | ― | 0.41 .001 | 3.28 * | ― | |
| (0.42) | (0.01) | (0.17) | (0.06) | (0.02) | (0.57) |
| Composition Effect | Phase Composition | Surface Roughness | ||||
|---|---|---|---|---|---|---|
| CF | PREN | γ Austenite | A’ Martensite | RMS (nm) | Ra (nm) | |
| G&H | 17.37 (0.06) | 18.79 (0.04) | 53.80 vol% | 46.20 vol% | 33 (3) | 14 (3) |
| Azdent | 17.76 (0.09) .03 | 17.70 (0.07) .003 | 93.25 vol% | 6.75 vol% | 67 (8) .03 | 50 (7) .02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, K.V.; Passaretti, F.; Nespoli, A.; Placidi, E.; Condò, R.; Andreani, C.; Licoccia, S.; Chass, G.A.; Senesi, R.; Cozza, P. Composition―Nanostructure Steered Performance Predictions in Steel Wires. Nanomaterials 2019, 9, 1119. https://doi.org/10.3390/nano9081119
Tian KV, Passaretti F, Nespoli A, Placidi E, Condò R, Andreani C, Licoccia S, Chass GA, Senesi R, Cozza P. Composition―Nanostructure Steered Performance Predictions in Steel Wires. Nanomaterials. 2019; 9(8):1119. https://doi.org/10.3390/nano9081119
Chicago/Turabian StyleTian, Kun V., Francesca Passaretti, Adelaide Nespoli, Ernesto Placidi, Roberta Condò, Carla Andreani, Silvia Licoccia, Gregory A. Chass, Roberto Senesi, and Paola Cozza. 2019. "Composition―Nanostructure Steered Performance Predictions in Steel Wires" Nanomaterials 9, no. 8: 1119. https://doi.org/10.3390/nano9081119
APA StyleTian, K. V., Passaretti, F., Nespoli, A., Placidi, E., Condò, R., Andreani, C., Licoccia, S., Chass, G. A., Senesi, R., & Cozza, P. (2019). Composition―Nanostructure Steered Performance Predictions in Steel Wires. Nanomaterials, 9(8), 1119. https://doi.org/10.3390/nano9081119







