Significantly Enhanced Electrical Performances of Eco-Friendly Dielectric Liquids for Harsh Conditions with Fullerene
Abstract
1. Introduction
2. Experimental
2.1. Preparation of C60 Nanofluid
2.2. Electrical Test
2.3. Thermal Aging Test
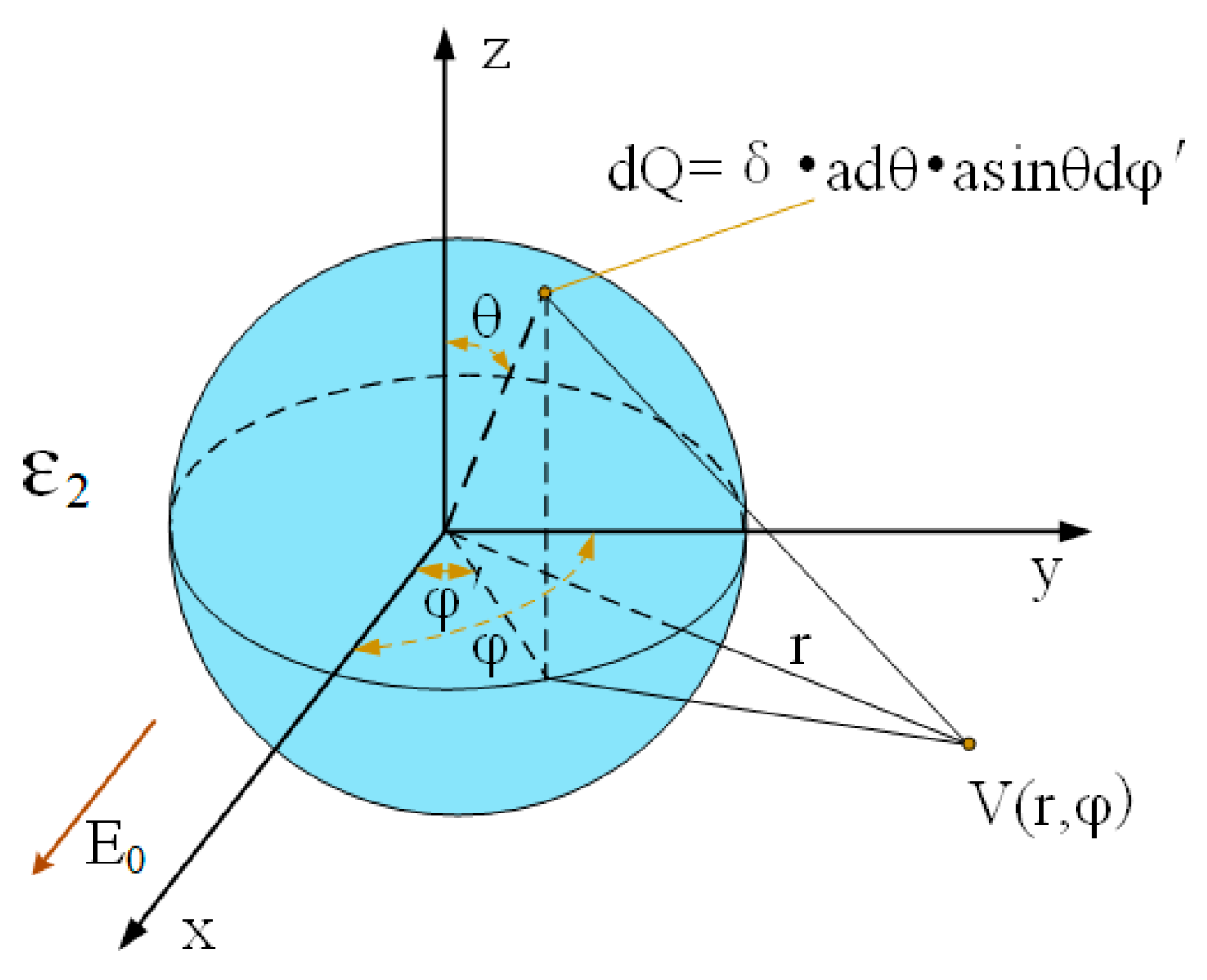
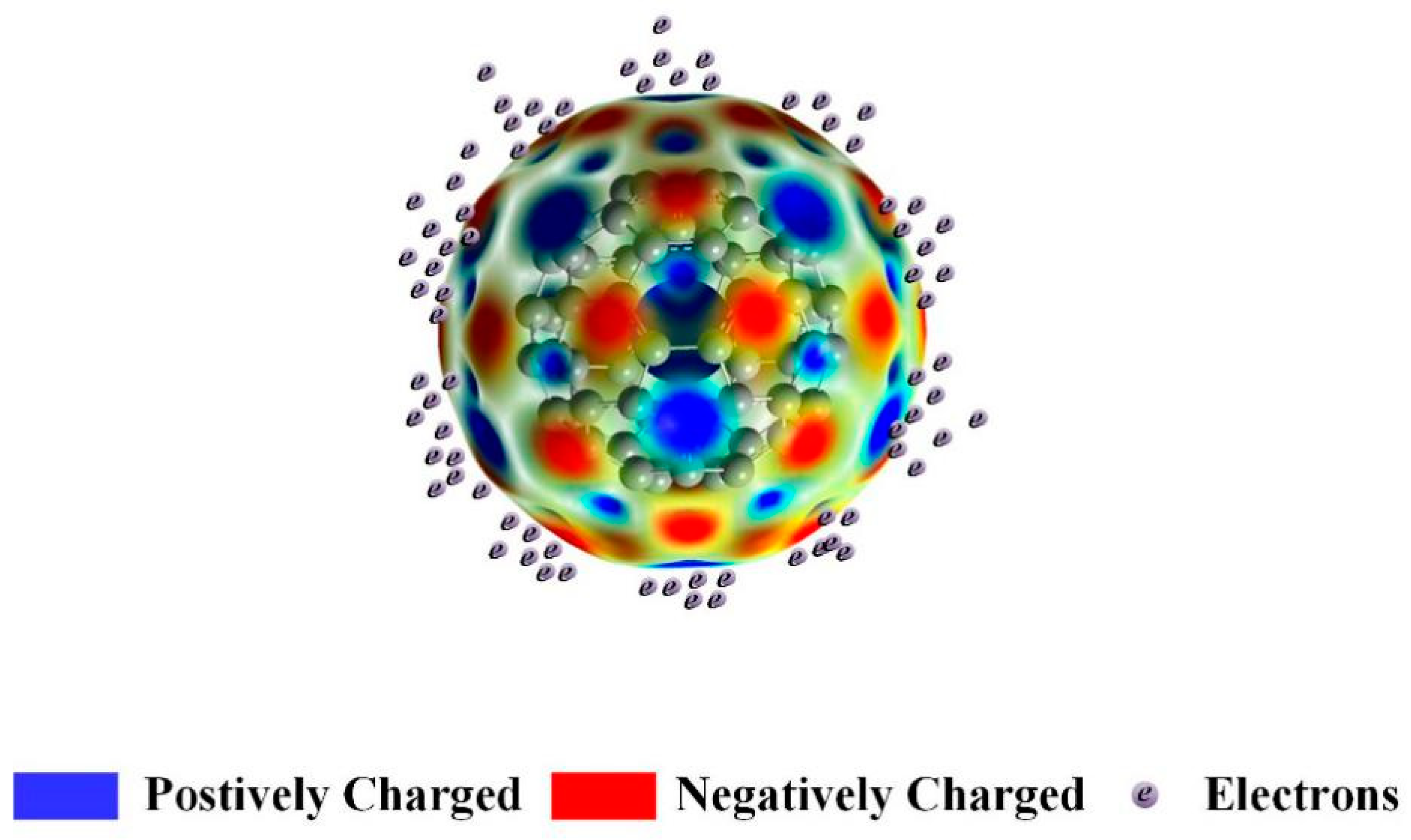
2.4. Simulation of Electron Density Distribution of C60
3. Results and Discussion
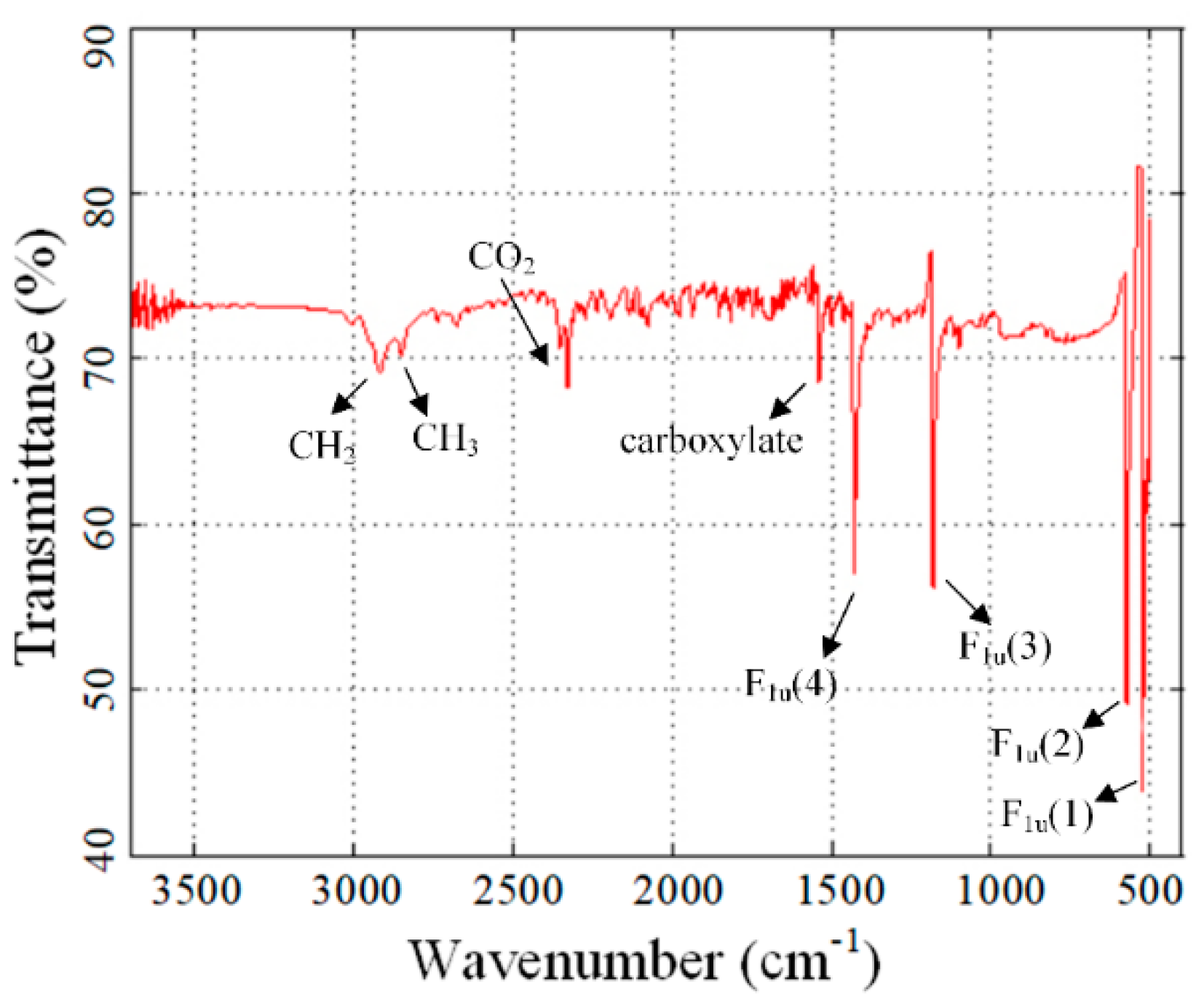
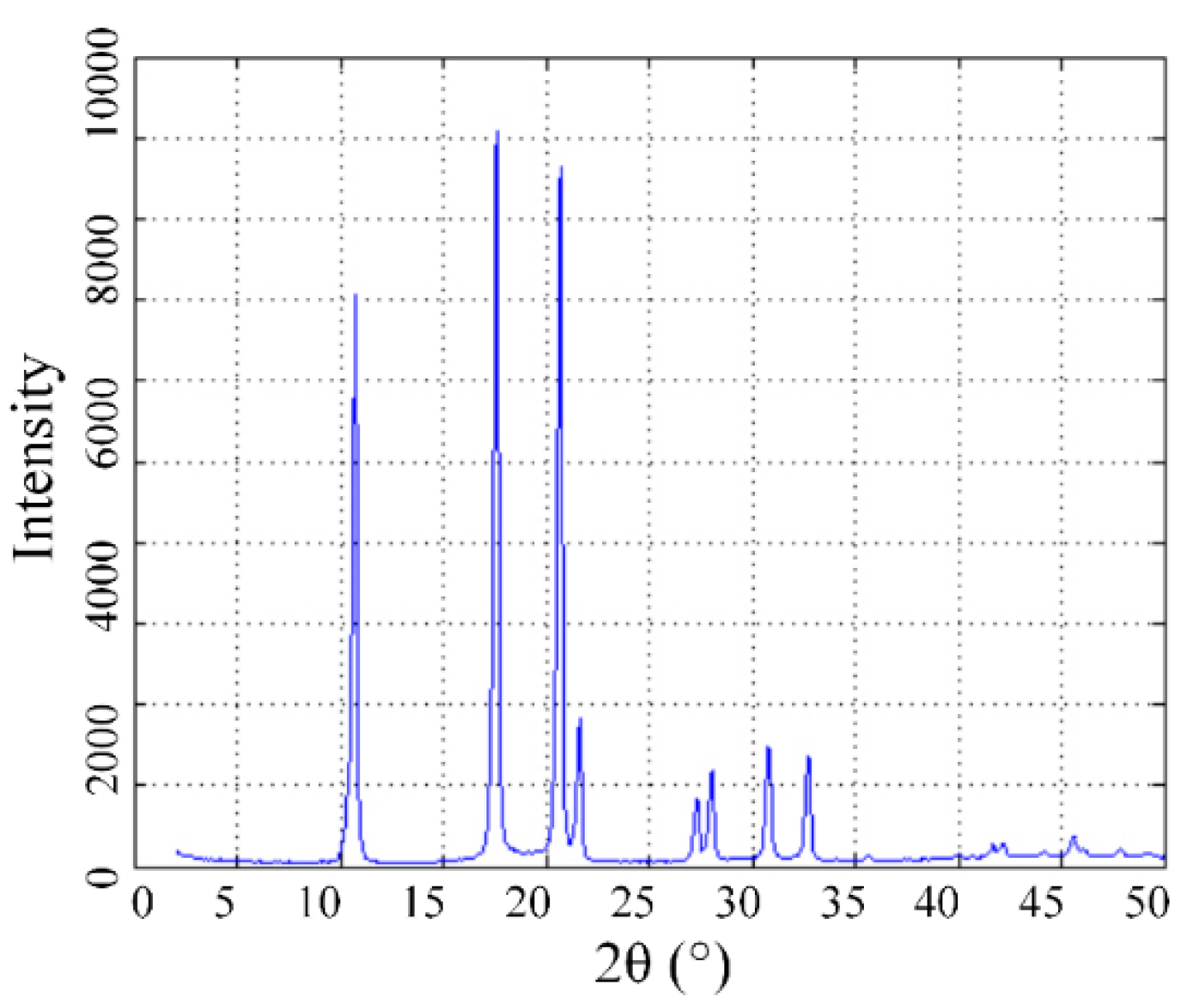
3.1. Characterization of Nanofluids
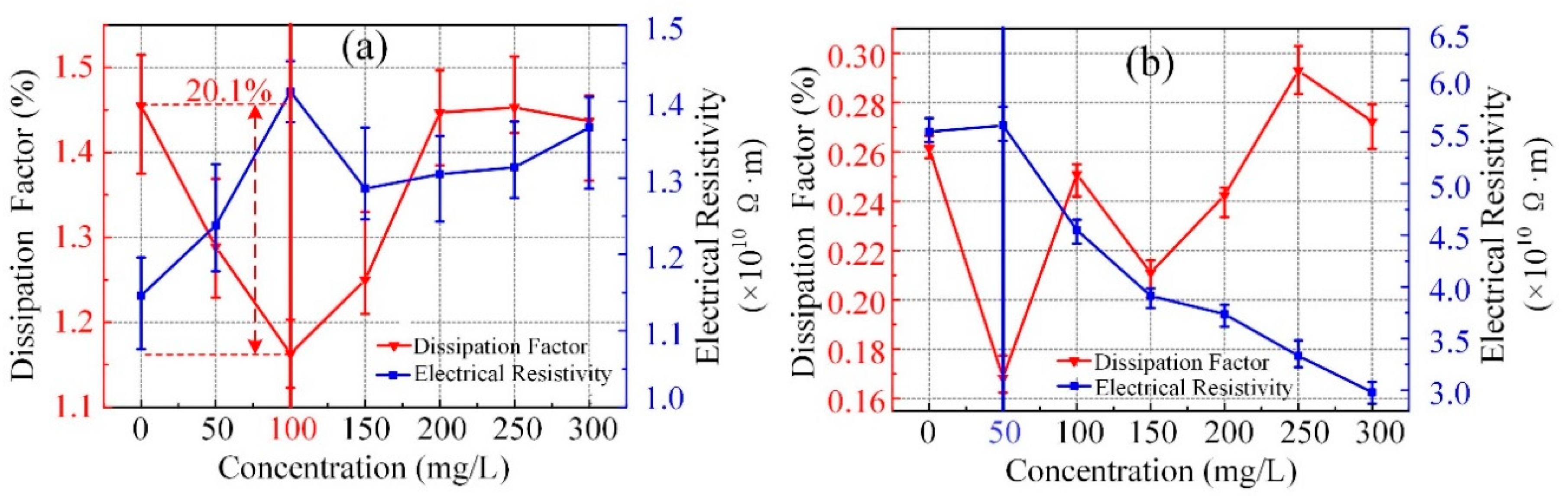
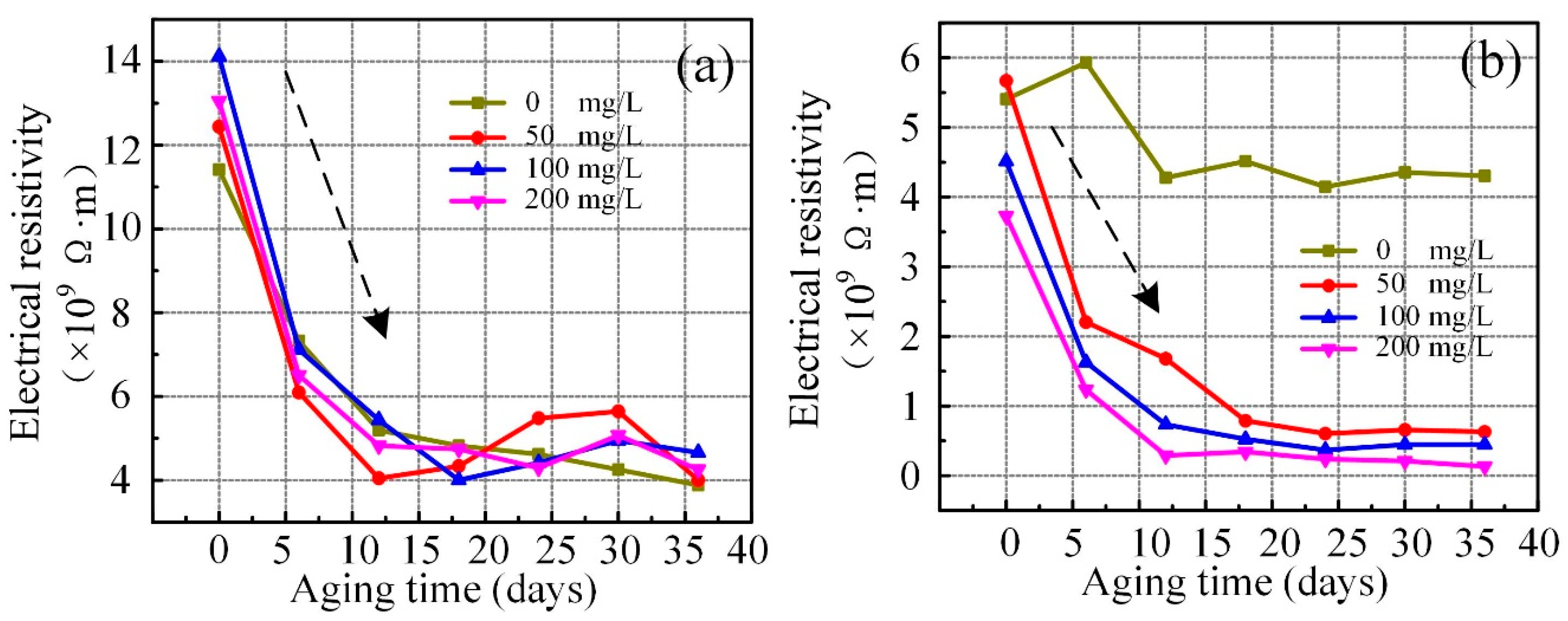
3.2. Dielectric Loss and Electrical Resistivity
3.3. Dielectric Breakdown Strength
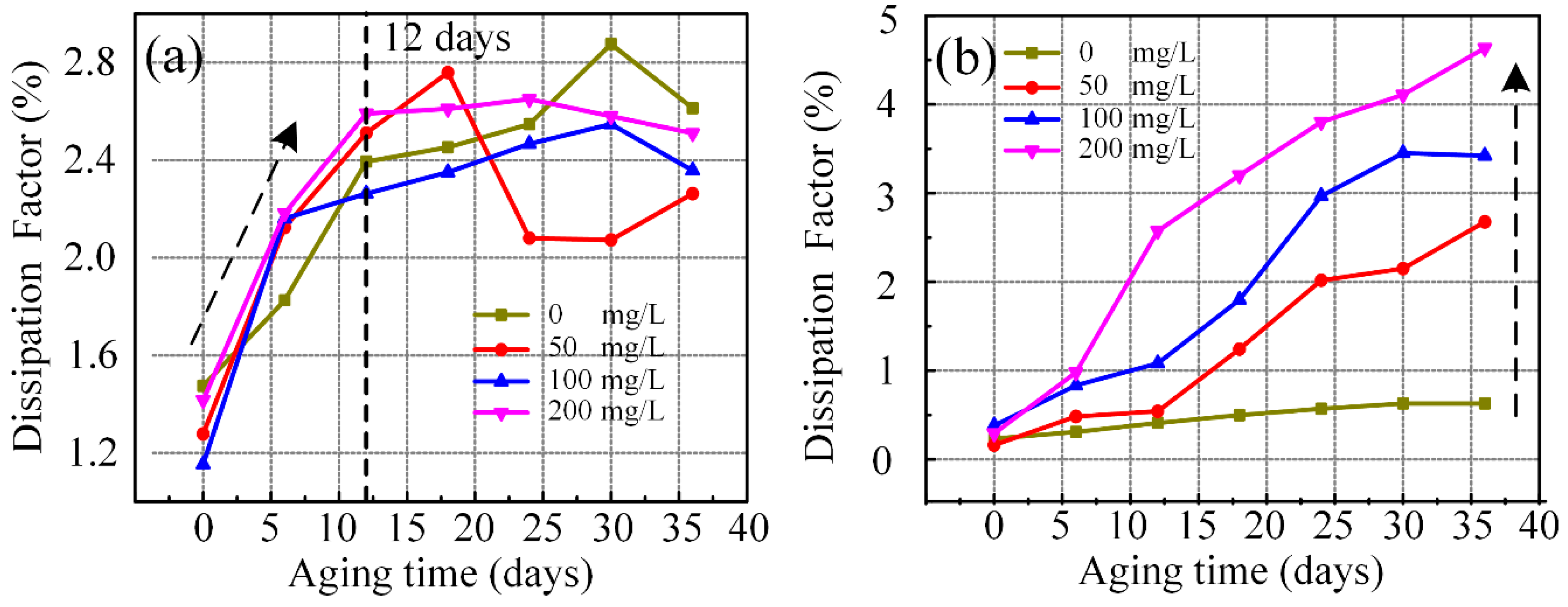
3.4. Dielectric Properties of Aging Nanofluid
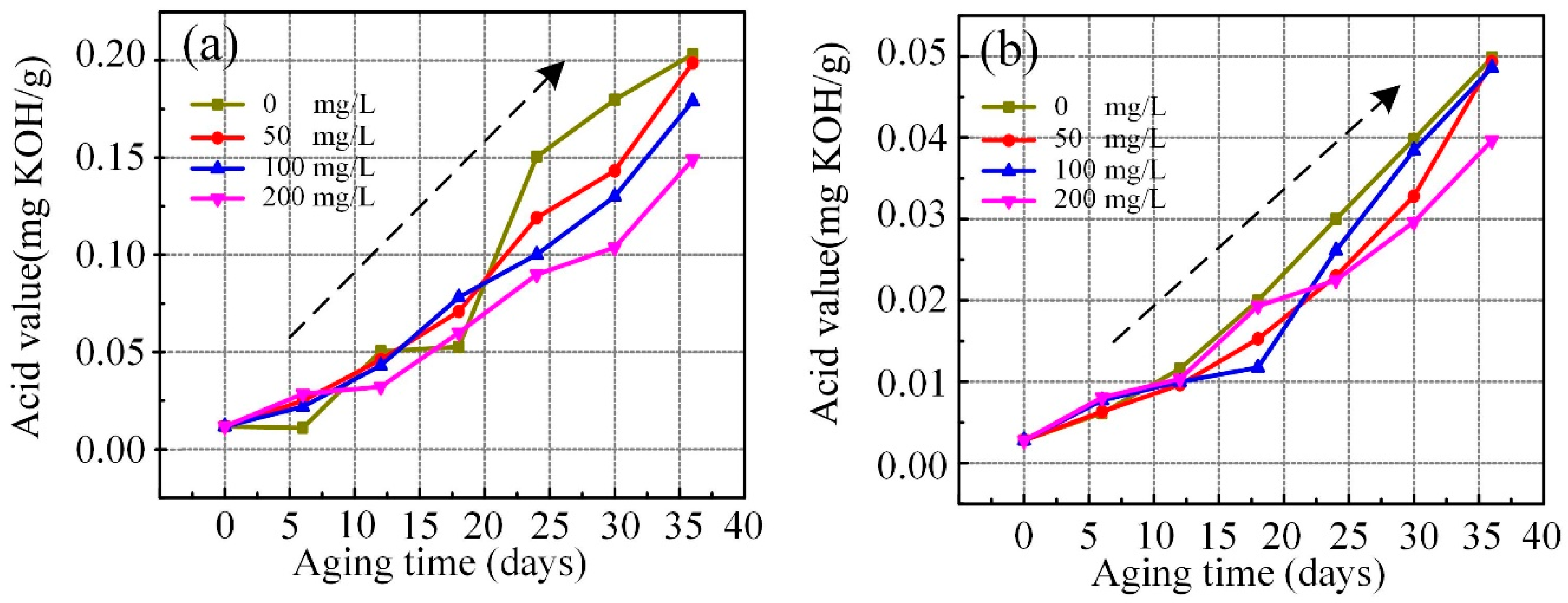
3.5. Acid Values of Aged Nanofluid
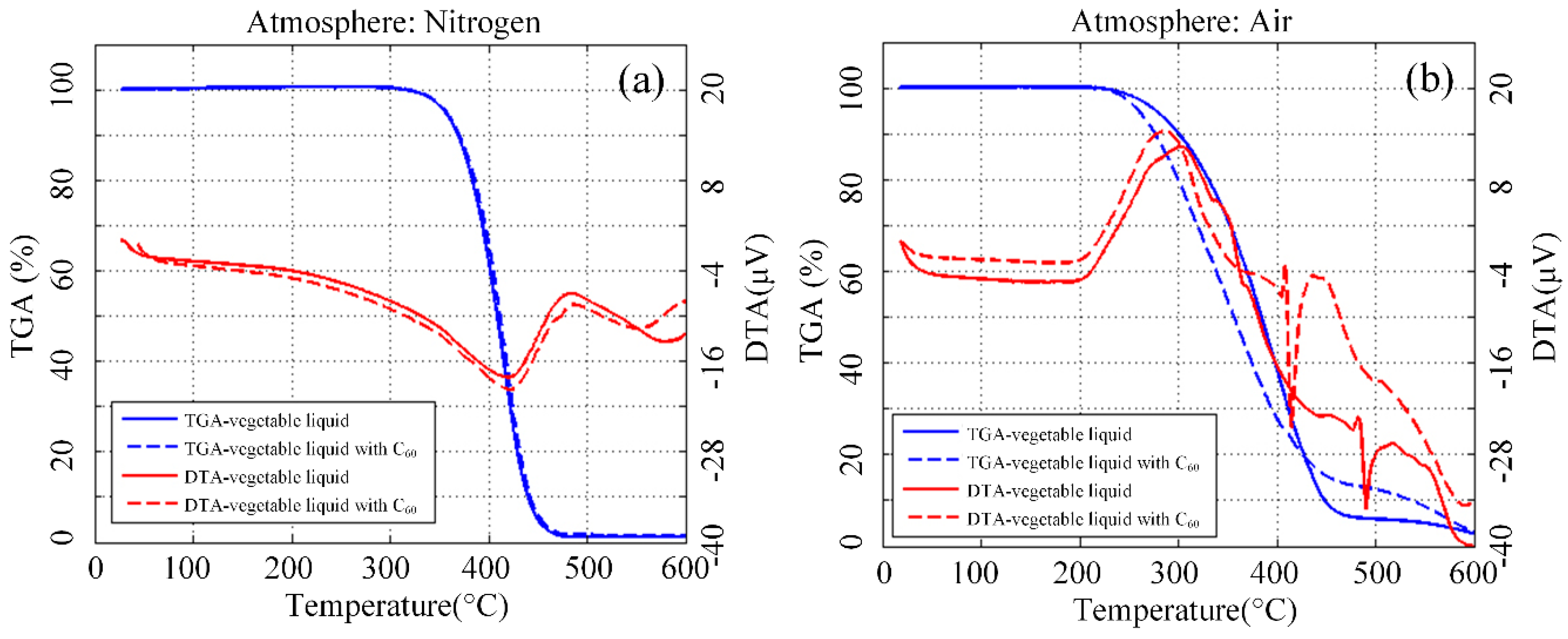
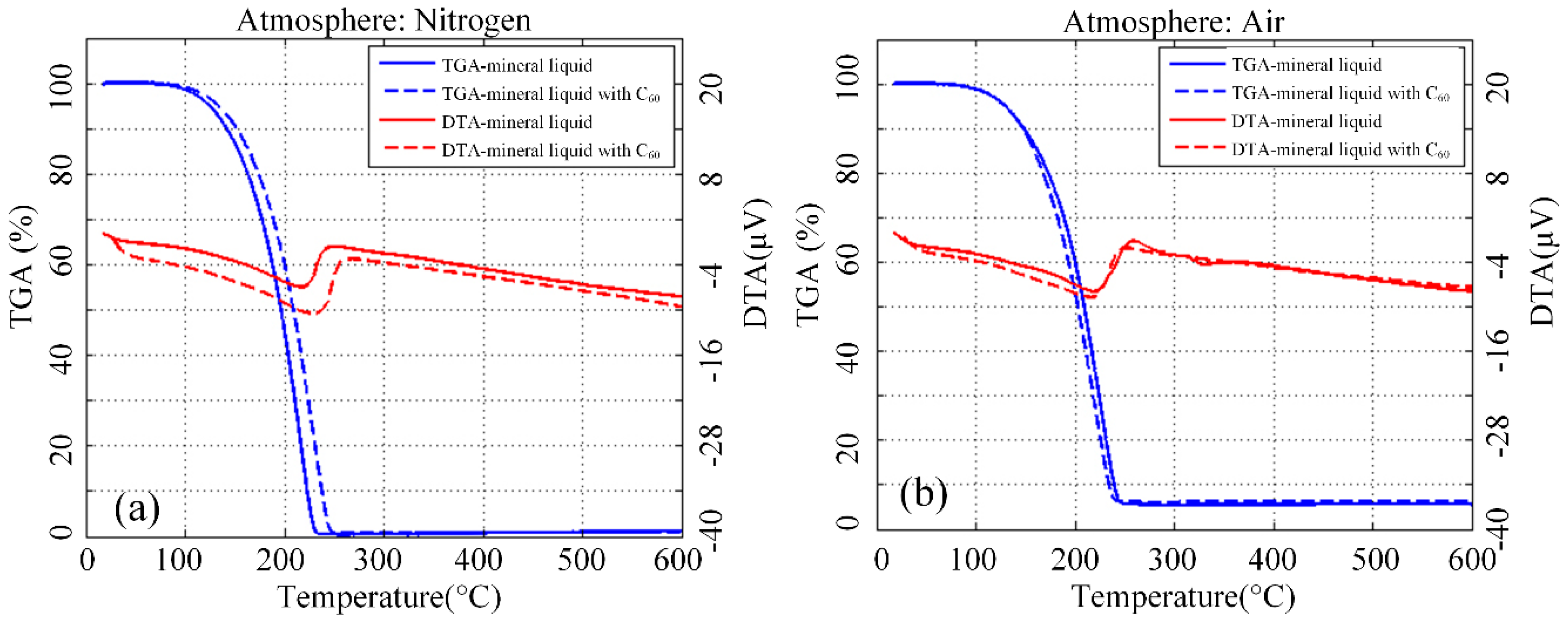
3.6. Thermal Analysis of Aged Nanofluid
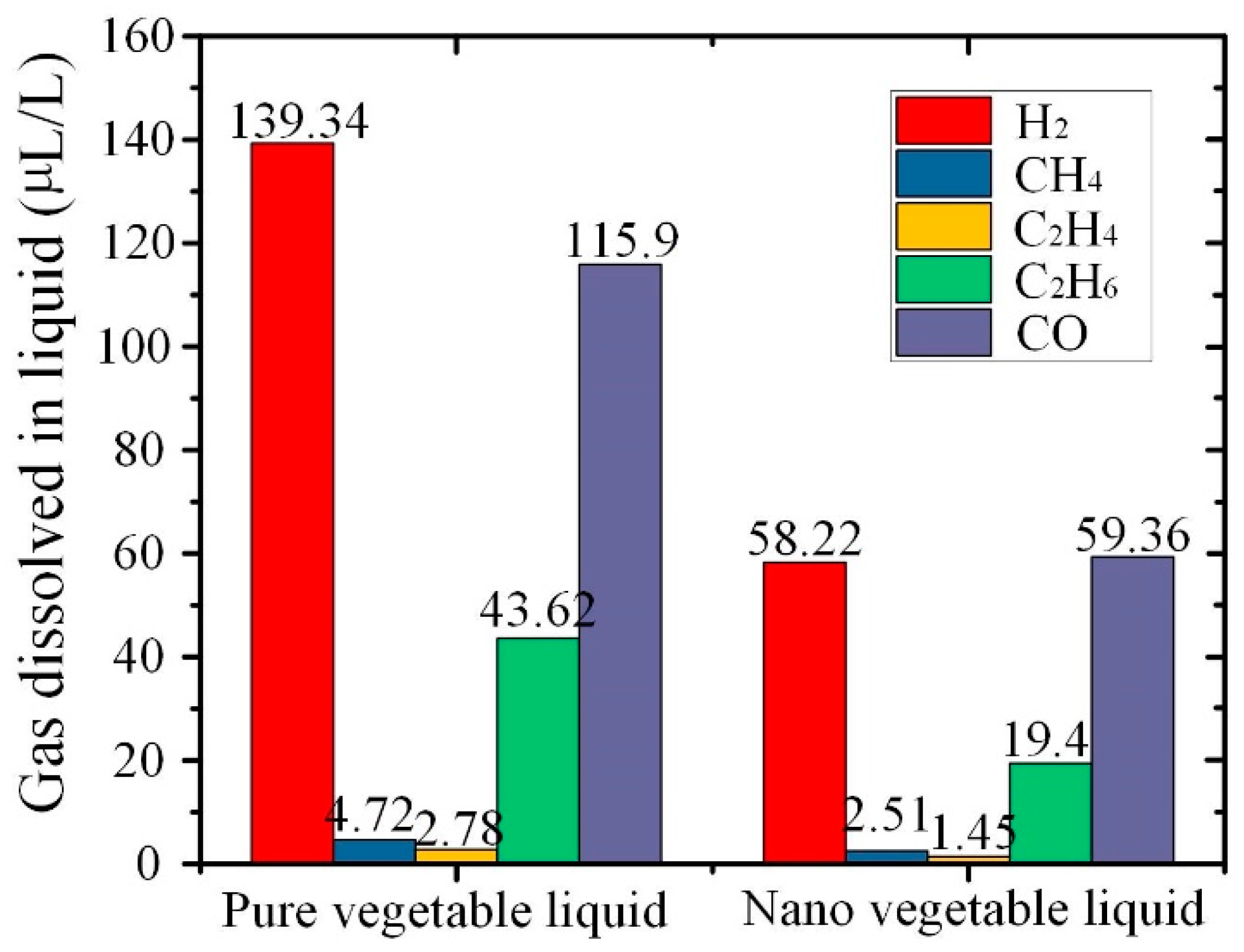
3.7. Dissolved Gas Analysis of Aged Nanofluid
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Romann, T.; Oll, O.; Pikma, P.; Kirsimäe, K.; Lust, E. 4–10 V Capacitors With Graphene-Based Electrodes and Ionic Liquid Electrolyte. J. Power Sources 2015, 280, 606–611. [Google Scholar] [CrossRef]
- Dinh Tiep, D.; My, B.N.; Tuan, V.Q.; Thinh, P.Q.; Cuong, T.M.; Tung, B.T.; Trinh, C.D. Tilt sensor based on three electrodes dielectric liquid capacitive sensor. In Proceedings of the 2016 IEEE 6th International Conference on Communications and Electronics, Ha Long Bay, Vietnam, 27–29 July 2016; pp. 172–175. [Google Scholar] [CrossRef]
- Hosier, I.L.; Koilraj, J.E.A.; Vaughan, A.S. Effect of Aging on the Physical, Chemical and Dielectric Properties of Dodecylbenzene Cable Oil. In Proceedings of the 2016 IEEE International Conference on Dielectrics (ICD), Montpellier, France, 3–7 July 2016; Volume 23, pp. 3389–3396. [Google Scholar] [CrossRef]
- Ravulapalli, S.; Kunta, R.; Ramamoorty, M. Preparation, characterization and feasibility analysis of methyl ester of Sesbania seeds oil (MESSO) as alternate liquid dielectrics in distribution transformers. RSC Adv. 2019, 9, 3311–3319. [Google Scholar] [CrossRef]
- Junaid, M.; Xiang, B.; Wang, J.; Liu, Z.; Geng, Y. Experimental Test of Superconductor Fault-Current Switchgear Using Liquid Nitrogen as the Insulation and Arc-Quenching Medium. IEEE Trans. Appl. Supercond. 2019, 29, 1–4. [Google Scholar] [CrossRef]
- Dix, L.; McShane, C.P.; Moore, H.R.; Moore, S.; Murphy, J.; Prevost, T.; Smith, S.D. Progress report on natural esters for distribution and power transformers. In Proceedings of the IEEE Power Engineering Society Transmission and Distribution Conference, Calgary, AB, Canada, 26–30 July 2006; pp. 15–17. [Google Scholar] [CrossRef]
- Islam, S.; Hossain, M.S.; Reza, M.F.; Rashid, M.M. Experimental Investigation of Insulating Properties of Vegetable Oil under High Voltage. Eur. J. Eng. Res. Sci. 2019, 4, 17–23. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Li, J.; Liang, S.; Zhou, J. Electronic properties of typical molecules and the discharge mechanism of vegetable and mineral insulating oils. Energies 2018, 11, 523. [Google Scholar] [CrossRef]
- Singha, S.; Asano, R., Jr.; Frimpong, G.; Claiborne, C.; Cherry, D. Comparative aging characteristics between a high oleic natural ester dielectric liquid and mineral oil. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 149–158. [Google Scholar] [CrossRef]
- Raymon, A.; Sakthibalan, S.; Cinthal, C.; Subramaniaraja, R.; Yuvaraj, M. Enhancement and comparison of nano-ester insulating fluids. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 892–900. [Google Scholar] [CrossRef]
- Jin, H.; Andritsch, T.; Tsekmes, I.A.; Kochetov, R.; Morshuis, P.H.F.; Smit, J.J. Properties of mineral oil based silica nanofluids. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1100–1108. [Google Scholar] [CrossRef]
- Dhar, P.; Katiyar, A.; Maganti, L.S.; Pattamatta, A.; Das, S.K. Superior dielectric breakdown strength of graphene and carbon nanotube infused nano-oils. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 943–956. [Google Scholar] [CrossRef]
- Fal, J.; Mahian, O.; Żyła, G. Nanofluids in the Service of High Voltage Transformers: Breakdown Properties of Transformer Oils with Nanoparticles, a Review. Energies 2018, 11, 2942. [Google Scholar] [CrossRef]
- Rabinovich, A.; Raj, K.; Nattrass, D.; Hjortsberg, A.; Segal, V. AC (60 Hz) and Impulse Breakdown Strength of a Colloidal Fluid Based on Transformer Oil and Magnetite Nanoparticles. In Proceedings of the 1998 IEEE International Symposium on Electrical Insulation (Cat. No.98CH36239), Arlington, VA, USA, 7–10 June 1998; Volume 2, pp. 619–622. [Google Scholar] [CrossRef]
- Zeng, J.; Xuan, Y. Tunable full-spectrum photo-thermal conversion features of magnetic-plasmonic Fe3O4/TiN nanofluid. Nano Energy 2018, 51, 754–763. [Google Scholar] [CrossRef]
- Kopčanský, P.; Marton, K.; Tomčo, L.; Koneracká, M.; Timko, M.; Potočová, I.; Herchl, F. The DC- and AC-dielectric breakdown strength of magnetic fluids based on transformer oil. Magnetohydrodynamics 2005, 41, 391–395. [Google Scholar] [CrossRef]
- Chen, M.; Zhong, Y.; Lv, Y.; Zhou, Y.; Zhang, S.; Du, Y.; Li, C.; Chen, L. Insulating properties and charge characteristics of natural ester fluid modified by TiO2 semiconductive nanoparticles. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 135–140. [Google Scholar] [CrossRef]
- Li, J.; Du, B.; Wang, F.; Yao, W.; Yao, S. The effect of nanoparticle surfactant polarization on trapping depth of vegetable insulating oil-based nanofluids. Phys. Lett. Sect. A Gen. At. Solid State Phys. 2016, 380, 604–608. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhou, J.; Chen, M.; Li, C.; Du, Y.; Li, X.; Zhou, Y.; Lv, Y. Effect of semiconductive nanoparticles on insulating performances of transformer oil. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 770–776. [Google Scholar] [CrossRef]
- Stockton, D.P.; Bland, J.R.; McClanahan, T.; Wilson, J.; Harris, D.L.; McShane, P. Natural Ester Transformer Fluids: Safety, Reliability & Environmental Performance. In Proceedings of the 2007 IEEE Petroleum and Chemical Industry Technical Conference, Calgary, AB, Canada, 17–19 September 2007; pp. 1–7. [Google Scholar] [CrossRef]
- Liao, R.-J.; Sun, C.-X.; Li, J.; Zou, P.; Zhang, Z.-T. Dielectric Properties and Electrodynamic Process of Natural Ester-Based Insulating Nanofluid. Mod. Phys. Lett. B 2011, 25, 2021–2031. [Google Scholar] [CrossRef]
- Semenov, K.N.; Charykov, N.A.; Murin, I.V.; Pukharenko, Y.V. Physico-chemical properties of the C60-tris-malonic derivative water solutions. J. Mol. Liq. 2015, 201, 50–58. [Google Scholar] [CrossRef]
- Spicer, P.P.; Tran, L.A.; Sitharaman, B.; Mikos, A.G.; Shi, X.; Rusakova, I.; Wilson, L.J. Injectable in situ cross-linkable nanocomposites of biodegradable polymers and carbon nanostructures for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2007, 18, 655–671. [Google Scholar] [CrossRef]
- Yoo, J.; Jeong, J.; Jung, K.; Hyun, G.; Kim, M.; Lee, H.; Yi, Y. Electronic structure of Ba(OH)2 interface in inverted organic photovoltaics: Improved electron transport by charged state of C60. Appl. Surf. Sci. 2019, 476, 435–441. [Google Scholar] [CrossRef]
- Omacrsawa, E. Perspectives of Fullerene Nanotechnology; Springer Science & Business Media: Chiba, Japan, 2012; ISBN 978-94-010-9600-3. [Google Scholar]
- Lalwani, G.; Sitharaman, B. Multifunctional Fullerene- and Metallofullerene-Based Nanobiomaterials. Nano Life 2013, 3, 1342003. [Google Scholar] [CrossRef]
- Aksamit, P.; Zmarzły, D. Dielectric properties of Fullerene-doped insulation liquids. In Proceedings of the 2009 IEEE Conference on Electrical Insulation and Dielectric Phenomena, Virginia Beach, VA, USA, 18–21 October 2009; pp. 212–215. [Google Scholar]
- Aksamit, P.; Zmarzły, D.; Boczar, T.; Szmechta, M. Aging properties of fullerene doped transformer oils. In Proceedings of the 2010 IEEE International Symposium on Electrical Insulation, San Diego, CA, USA, 6–9 June 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Boczar, T.; Zmarzły, D.; Aksamit, P.; Lorenc, M. Electrostatic properties of fullerene-doped hydrocarbons. In Proceedings of the International Symposium on Electrical Insulating Materials, Mie, Japan, 7–11 September 2008; pp. 295–298. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Zou, P.; Grzybowski, S.; Zahn, M. Preparation of a vegetable oil-based nanofluid and investigation of its breakdown and dielectric properties. IEEE Electr. Insul. Mag. 2012, 28, 43–50. [Google Scholar] [CrossRef]
- Kuzmany, H.; Winkler, R.; Pichler, T. Infrared spectroscopy of fullerenes. J. Phys. Condens. Matter 1995, 7, 6601–6624. [Google Scholar] [CrossRef]
- Vassallo, A.M.; Pang, L.S.K.; Cole-Clarke, P.A.; Wilson, M.A. Emission FTIR study of C60 thermal stability and oxidation. J. Am. Chem. Soc. 1991, 113, 7820–7821. [Google Scholar] [CrossRef]
- Patterson, A.L. The scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Rao, A.M.; Bi, X.-X.; Amster, I.J.; Hager, G.T.; Eklund, P.C.; Holden, J.M.; Wang, Y.; Cornett, D.S.; Lee, W.-T.; Zhou, P.; et al. Photoinduced Polymerization of Solid C60 Films. Science. 1993, 955–957. [Google Scholar] [CrossRef]
- Hwang, J.G.; O’Sullivan, F.; Zahn, M.; Hjortstam, O.; Pettersson, L.A.A.; Liu, R. Modeling of streamer propagation in transformer oil-based nanofluids. In Proceedings of the 2008 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Quebec, QC, Canada, 26–29 October 2008; pp. 361–366. [Google Scholar]
- Liu, R.; Hjortstam, O.; Hwang, J.G.; Pettersson, L.A.A.; Zahn, M.; O’Sullivan, F.M. Effects of nanoparticle charging on streamer development in transformer oil-based nanofluids. J. Appl. Phys. 2010, 107, 014310. [Google Scholar] [CrossRef]
- Zou, P.; Li, J.; Liao, R.-J.; Zhang, Z.-T.; Du, B. Lightning Impulse Breakdown Characteristics and Electrodynamic Process of Insulating Vegetable Oil-Based Nanofluid. Mod. Phys. Lett. B 2012, 26, 1250095. [Google Scholar] [CrossRef]
- Du, B.X.; Li, X.L. Dielectric and thermal characteristics of vegetable oil filled with BN nanoparticles. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 956–963. [Google Scholar] [CrossRef]
- Xiang, C.; Zhou, Q.; Li, J.; Huang, Q.; Song, H.; Zhang, Z. Comparison of Dissolved Gases in Mineral and Vegetable Insulating Oils under Typical Electrical and Thermal Faults. Energies 2016, 9, 312. [Google Scholar] [CrossRef]

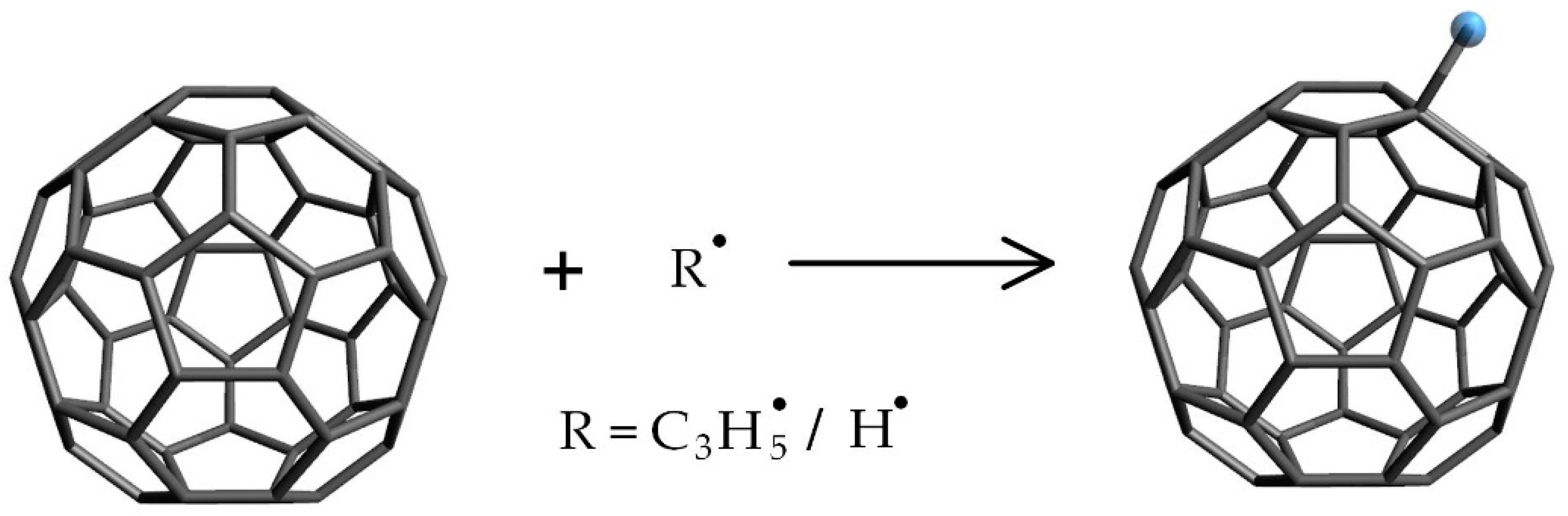
| Parameters | Unit Symbol | Typical Value | |
|---|---|---|---|
| Vegetable Liquid | Mineral Liquid | ||
| Kinematic Viscosity at 40 °C | mm2·s−1 | 41.0 | 10.0 |
| Density at 20 °C | kg·m−3 | 0.90 | 0.83–0.89 |
| Flash Point | °C | 320 | ≥135 |
| Pour Point | °C | −18 | −22 |
| Acid Value | mgKOH·g−1 | ≤0.03 | ≤0.01 |
| Liquid Types | Breakdown Voltage (kV) | Breakdown Time (μs) | ||
|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |
| Vegetable Liquid | 78.2 | 83.7 | 10.3 | 11.9 |
| Nano Vegetable Liquid | 83.9 | 89.9 | 10.9 | 12.3 |
| Mineral Liquid | 60.8 | 103.3 | 8.7 | 11.1 |
| Nano Mineral Liquid | 66.9 | 111.2 | 9.2 | 11.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Wang, F.; Wang, Q.; Yao, W.; Sun, K.; Zhang, R.; Zhao, J.; Lou, Z.; Li, J. Significantly Enhanced Electrical Performances of Eco-Friendly Dielectric Liquids for Harsh Conditions with Fullerene. Nanomaterials 2019, 9, 989. https://doi.org/10.3390/nano9070989
Huang Z, Wang F, Wang Q, Yao W, Sun K, Zhang R, Zhao J, Lou Z, Li J. Significantly Enhanced Electrical Performances of Eco-Friendly Dielectric Liquids for Harsh Conditions with Fullerene. Nanomaterials. 2019; 9(7):989. https://doi.org/10.3390/nano9070989
Chicago/Turabian StyleHuang, Zhengyong, Feipeng Wang, Qiang Wang, Wei Yao, Kai Sun, Ruiqi Zhang, Jianying Zhao, Ziyi Lou, and Jian Li. 2019. "Significantly Enhanced Electrical Performances of Eco-Friendly Dielectric Liquids for Harsh Conditions with Fullerene" Nanomaterials 9, no. 7: 989. https://doi.org/10.3390/nano9070989
APA StyleHuang, Z., Wang, F., Wang, Q., Yao, W., Sun, K., Zhang, R., Zhao, J., Lou, Z., & Li, J. (2019). Significantly Enhanced Electrical Performances of Eco-Friendly Dielectric Liquids for Harsh Conditions with Fullerene. Nanomaterials, 9(7), 989. https://doi.org/10.3390/nano9070989







