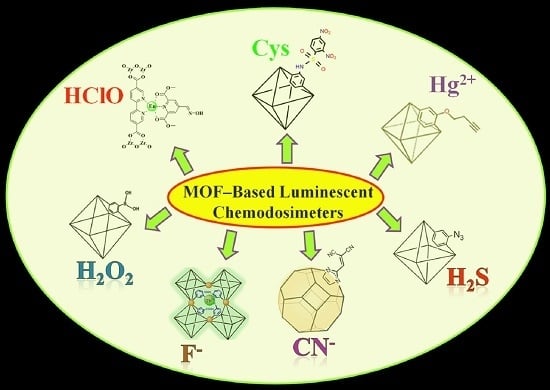
Recent Progress in Metal–Organic Framework (MOF) Based Luminescent Chemodosimeters
Abstract
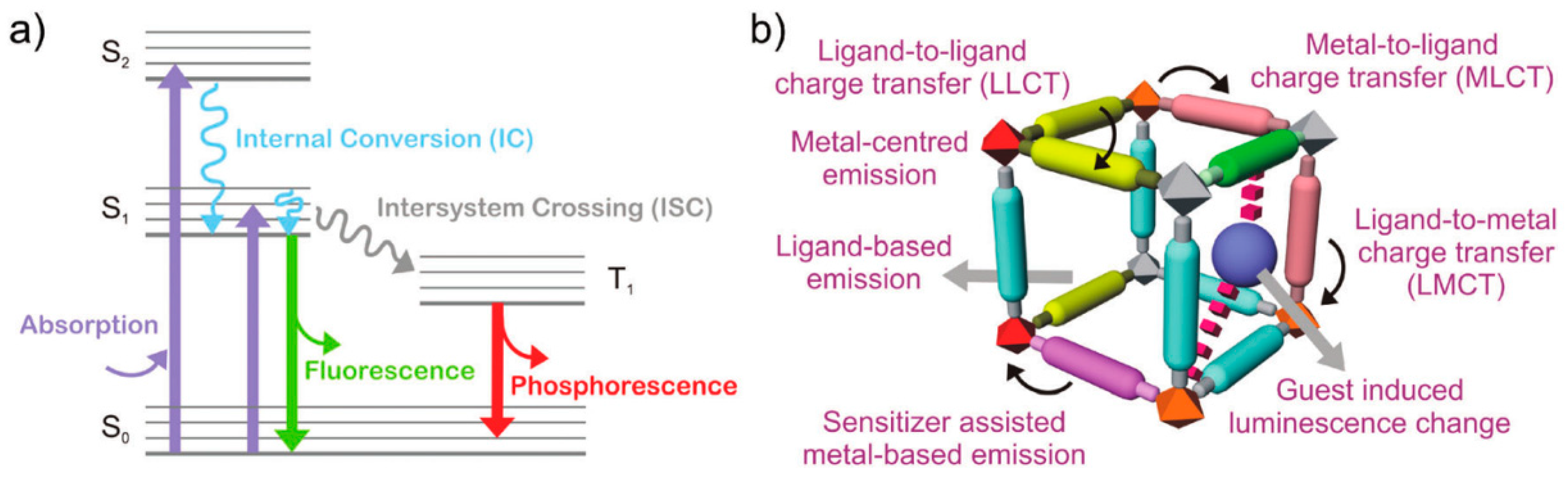
1. Introduction
2. MOF-Based Luminescent Chemodosimeters for Sulfur Compounds
2.1. MOF-Based Chemodosimeters for H2S
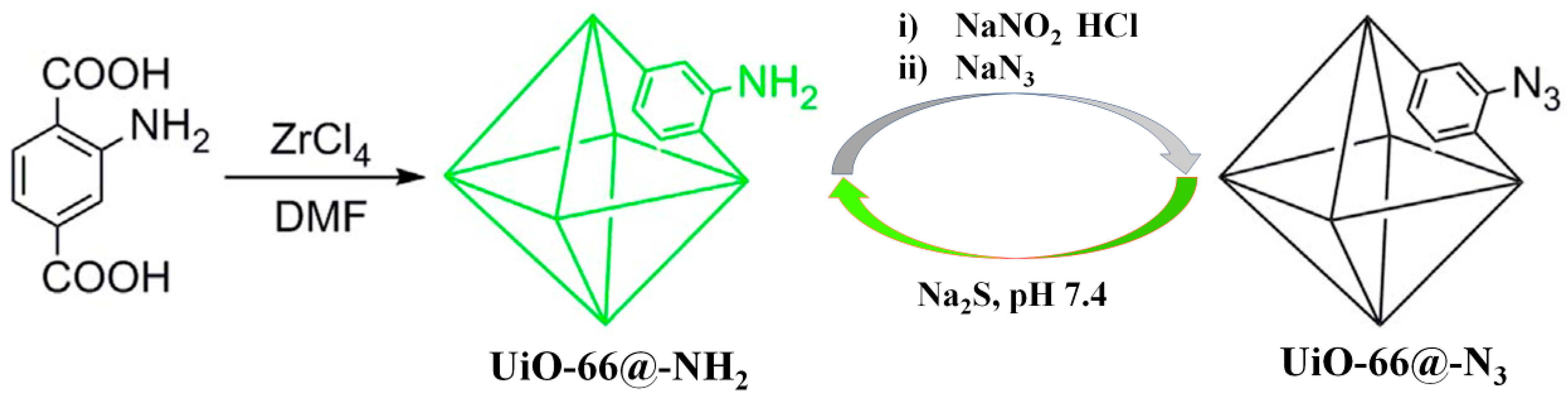
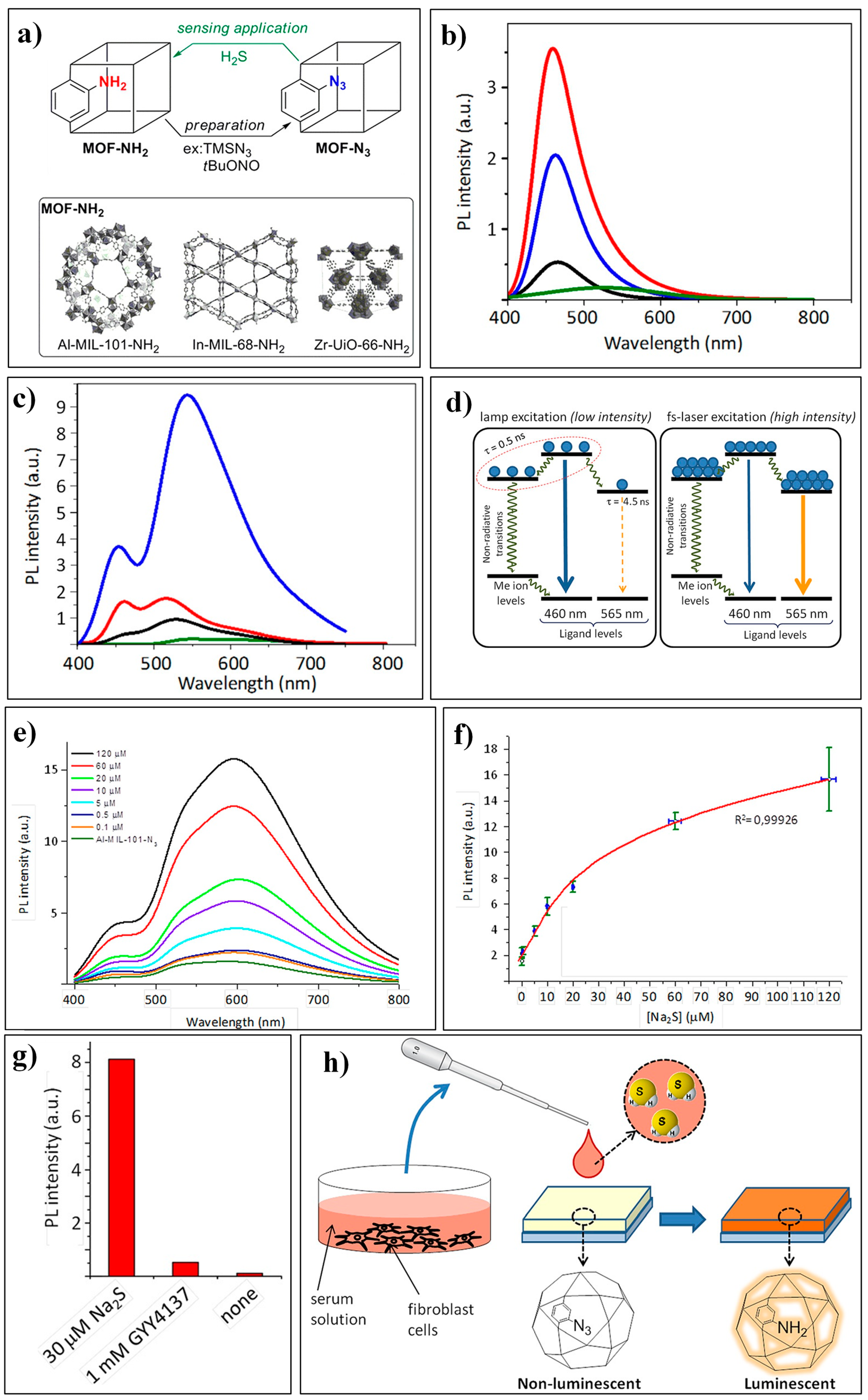
2.1.1. Based on the H2S-Mediated Reduction of Azide/Nitro Group to Amine
2.1.2. Based on the Binding Reaction between S2− and Cu2+
2.1.3. Other MOF-Based Chemodosimeters for H2S
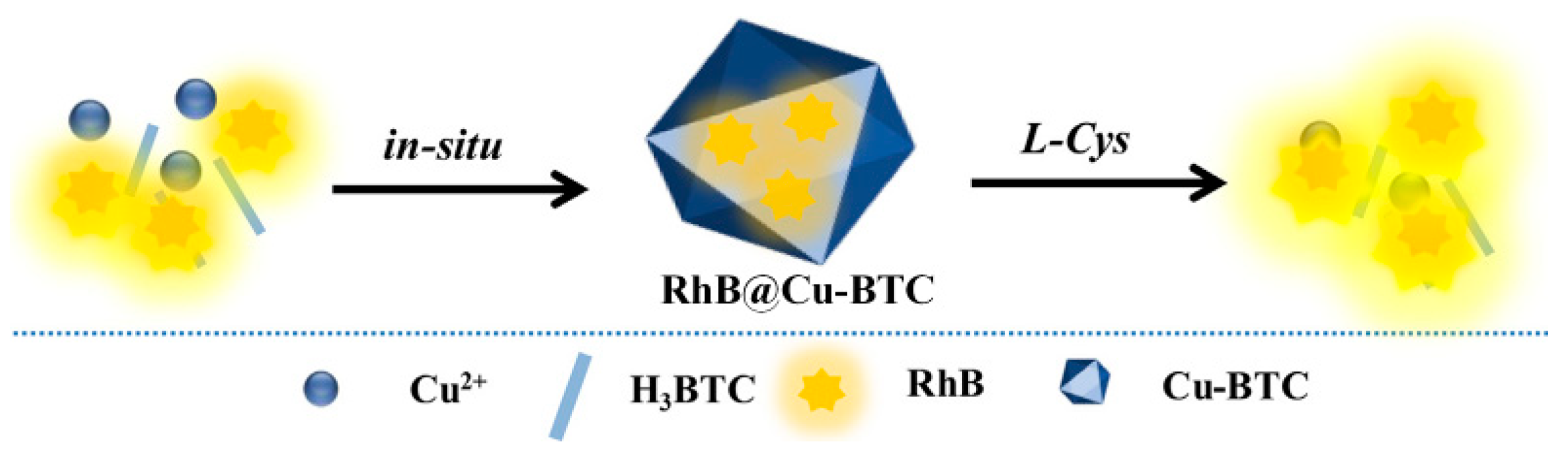
2.2. MOF-Based Chemodosimeters for Biothiols
3. MOF-Based Chemodosimeters for Other Redox-Active Biomolecules
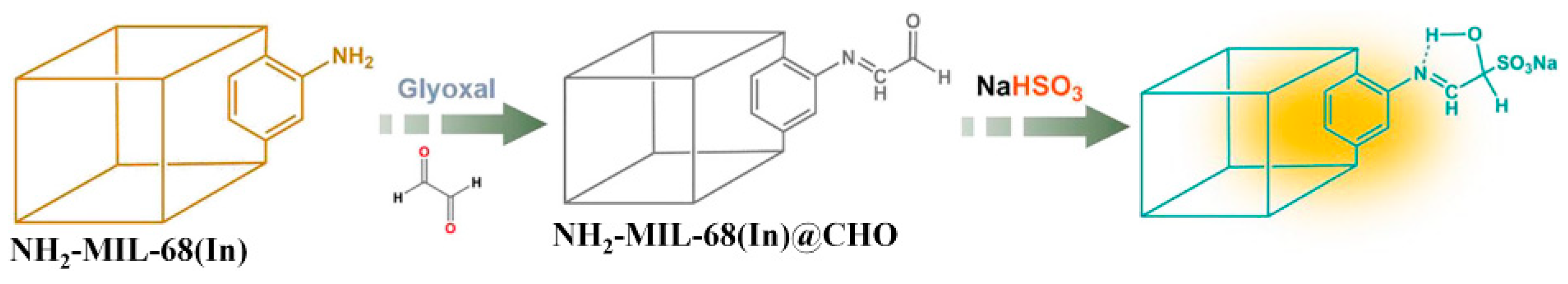
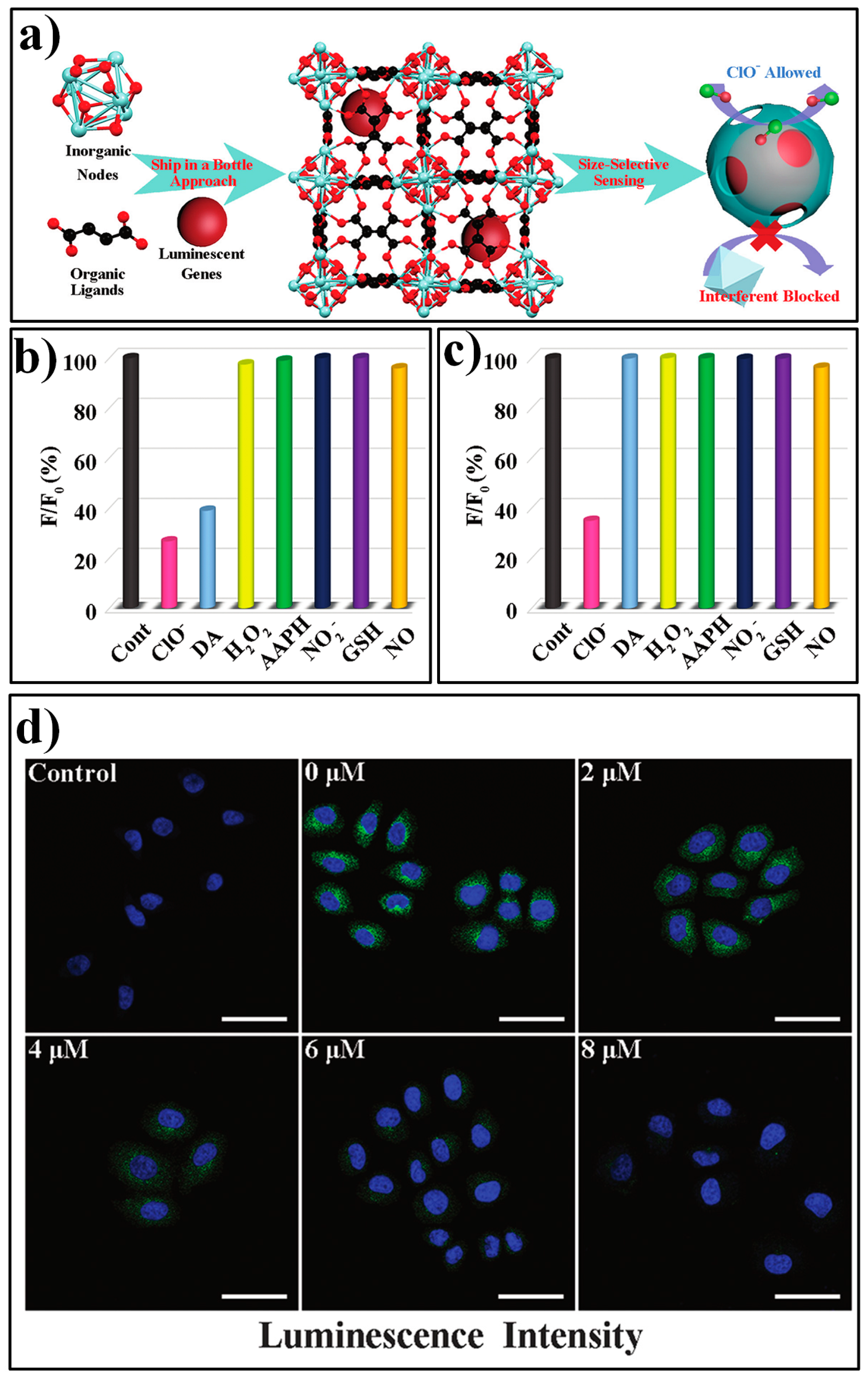
3.1. MOF-Based Chemodosimeters for HClO
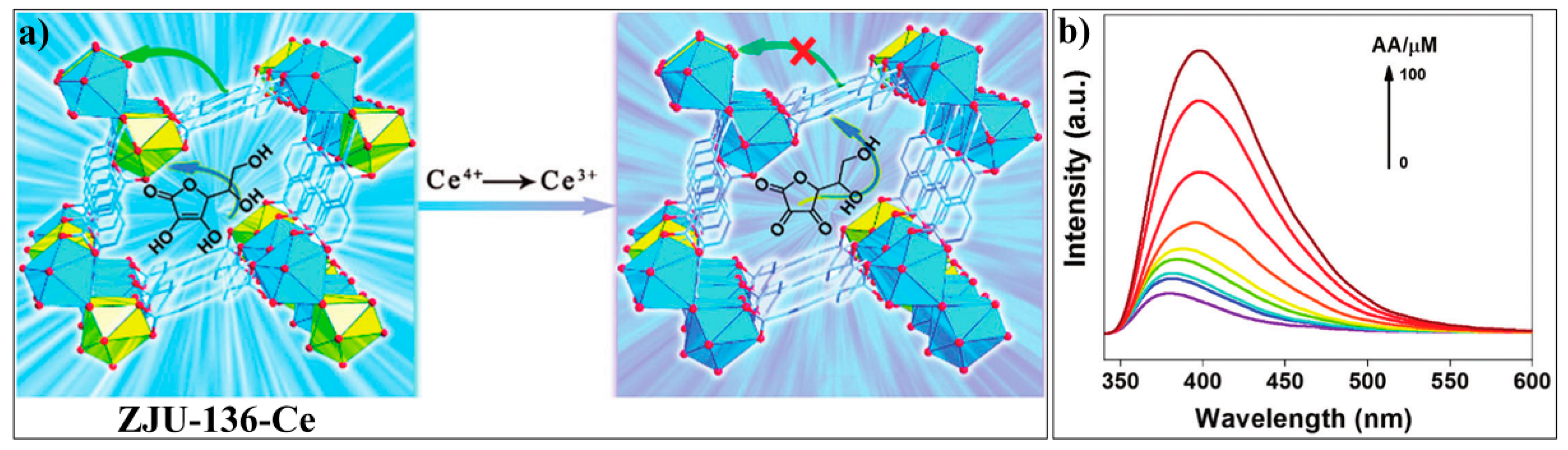
3.2. MOF-Based Chemodosimeters for Ascorbic Acid
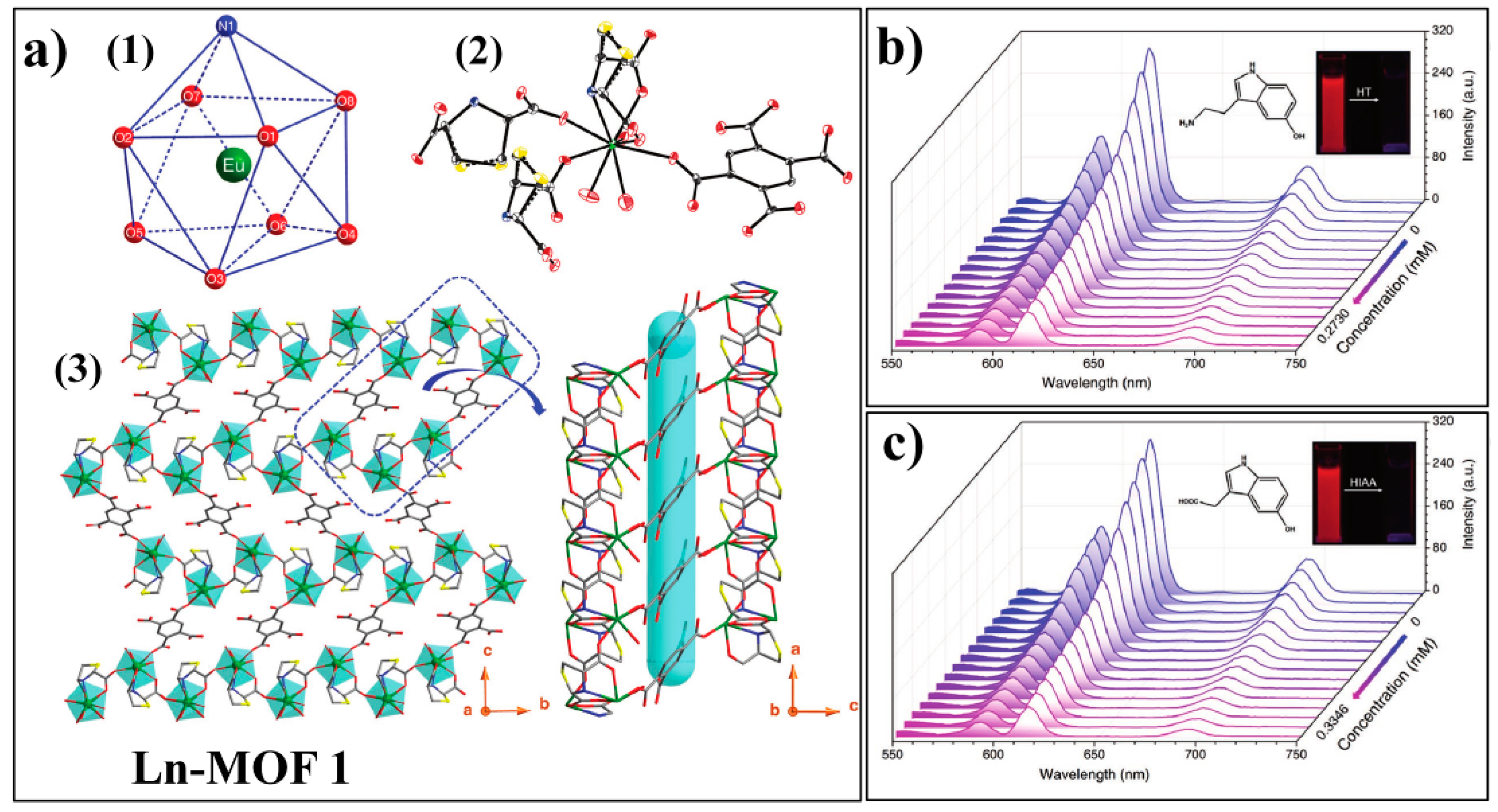
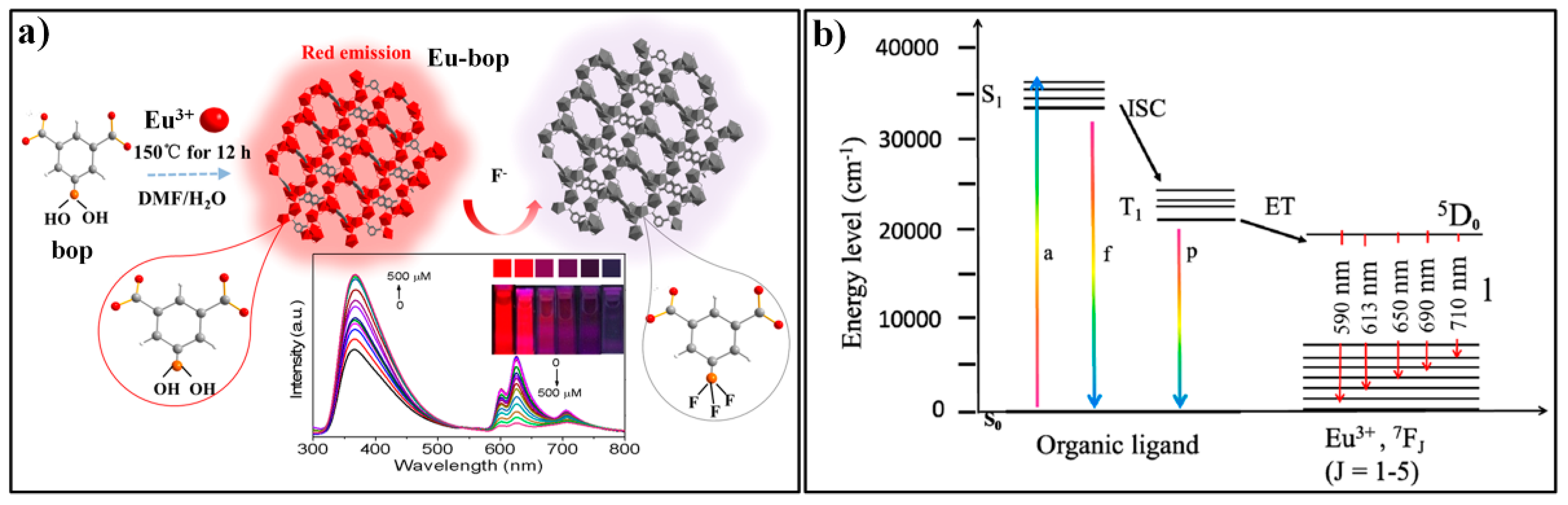
3.3. MOF-Based Chemodosimeter for 5-Hydroxytryptamine
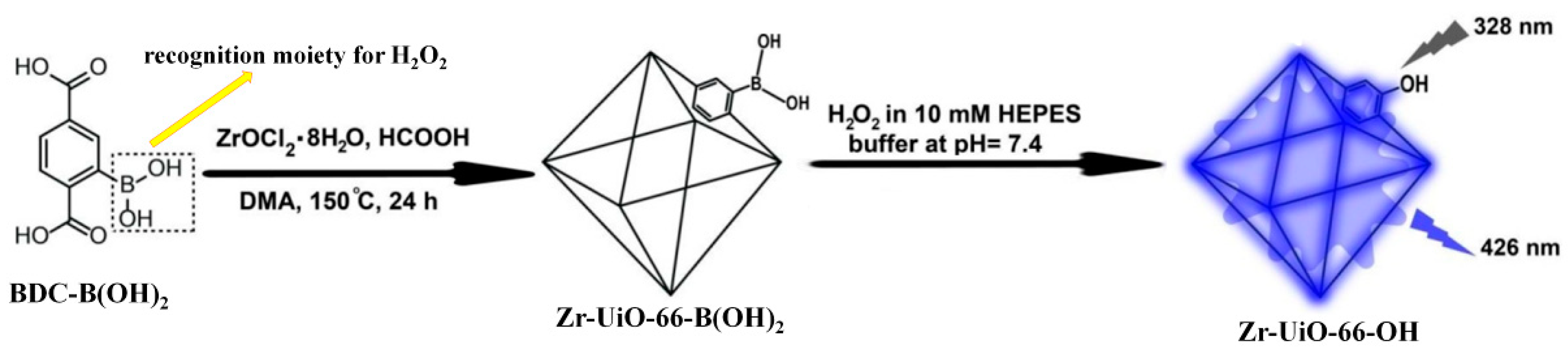
3.4. MOF-Based Chemodosimeter for H2O2
4. MOF-Based Chemodosimeters for Ions
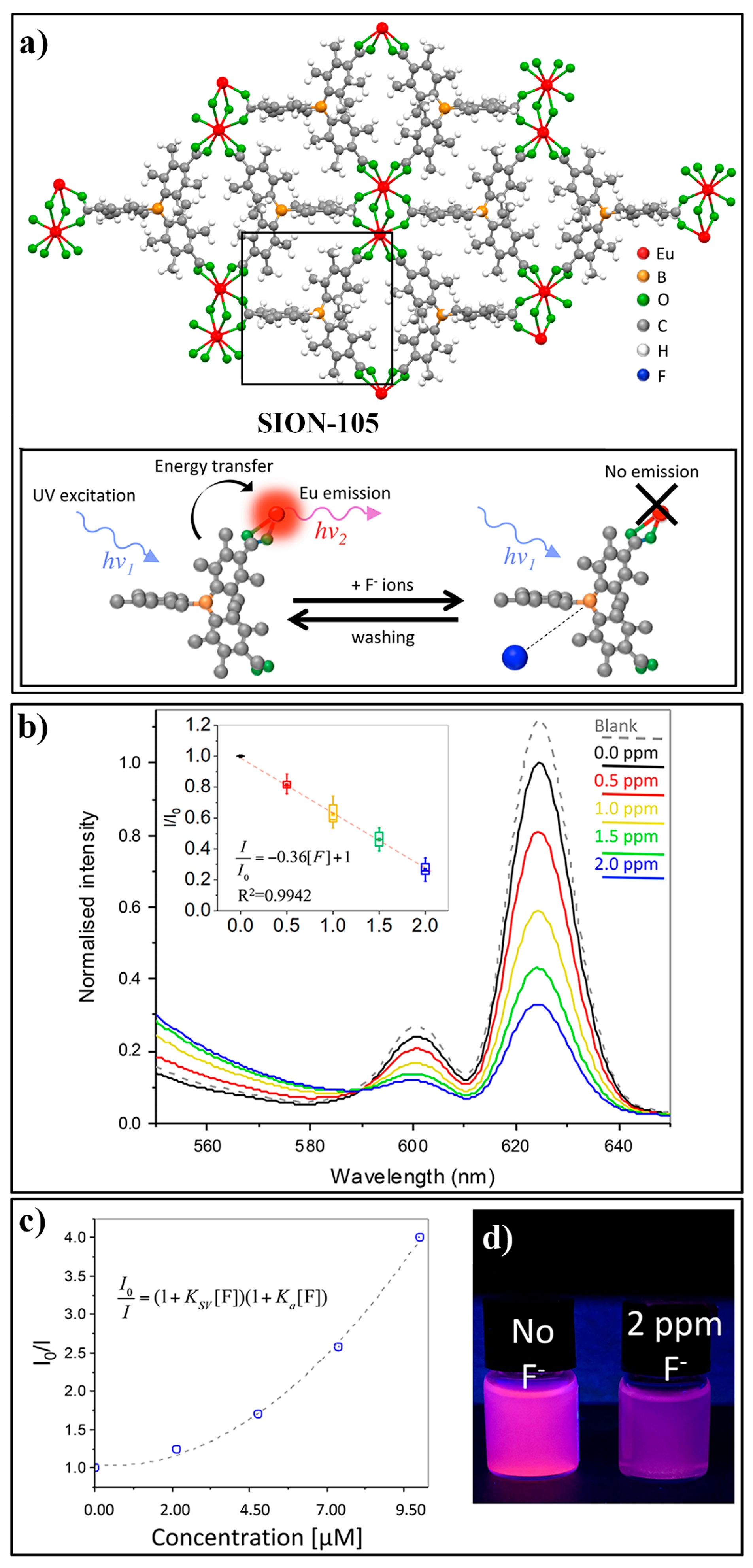
4.1. MOF-Based Chemodosimeters for Fluoride Ions
4.2. MOF-Based Chemodosimeter for Hg2+
4.3. MOF-Based Chemodosimeter for CN−
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IPA | isophthalic acid |
| NDC | napthalene-2,6-dicarboxylic acid |
| TCPP | meso-tetrakis (4-carboxylphenyl) porphyrin |
| HBSS | Hanks’ balanced salt solutions |
| BBS | boricacid−borax buffer |
| SNP | S-nitroso-N-acetyl-dl-penicillamine |
| PPG | dl-propargylglycine |
| TO | thiazole orange |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| FCA | flow cytometry analysis |
| CLSM | confocal laser scanning microscopy |
| Cys | cysteine |
| Hcy | homocysteine |
| GSH | glutathione |
| DNP | dinitrophenyl |
| PET | photoinduced electron transfer |
| H3tca | 4,4′,4″-tricarboxyltriphenylamine |
| Ppca | 1H-pyrrolo-[2,3-b]pyridine-2-carbaldehyde |
| PLQY | photoluminescence quantum yield |
| RhB | rhodamine B |
| AA | ascorbic acid |
| Isp | isophthalic acid |
| 5-bop | 5-boronoisophthalic acid |
| H3tctb | tris (p-carboxylic acid) tridurylborane |
| H2TDA | thiazolidine 2,4-dicaboxylic acid |
| BDC-B(OH)2 | 2-boronobenzene-1,4-dicarboxylic acid |
| ZIF | Zeolitic imidazolate frameworks |
References
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- James, S.L. Metal-organic frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Rowsell, J.L.C.; Yaghi, O.M. Metal-organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Cook, T.R.; Zheng, Y.R.; Stang, P.J. Metal-Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal-Organic Materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Li, J.R.; Ma, Y.G.; McCarthy, M.C.; Sculley, J.; Yu, J.M.; Jeong, H.K.; Balbuena, P.B.; Zhou, H.C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, X.; Zheng, B.; Huang, L.; Hang, C.; Jiao, Y.; Cao, X.; Zeng, W.; Yun, R. Highly Selective Carbon Dioxide Capture and Cooperative Catalysis of a Water-Stable Acylamide-Functionalized Metal–Organic Framework. Eur. J. Inorg. Chem. 2018, 2018, 1309–1314. [Google Scholar] [CrossRef]
- Zheng, B.; Luo, X.; Wang, Z.; Zhang, S.; Yun, R.; Huang, L.; Zeng, W.; Liu, W. An unprecedented water stable acylamide-functionalized metal–organic framework for highly efficient CH4/CO2 gas storage/separation and acid–base cooperative catalytic activity. Inorg. Chem. Front. 2018, 5, 2355–2363. [Google Scholar] [CrossRef]
- Zheng, B.; Huang, L.; Cao, X.; Shen, S.; Cao, H.; Hang, C.; Zeng, W.; Wang, Z. A highly porous acylamide decorated MOF-505 analogue exhibiting high and selective CO2 gas uptake capability. CrystEngComm 2018, 20, 1874–1881. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, H.; Wang, Z.; Ozaki, N.; Hang, C.; Luo, X.; Huang, L.; Zeng, W.; Yang, M.; Duan, J. A highly porous rht-type acylamide-functionalized metal–organic framework exhibiting large CO2 uptake capabilities. Chem. Commun. 2016, 52, 12988–12991. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Chen, L.F.; Cui, H.; Zhang, J.Y.; Zhang, L.; Su, C.Y. Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Garcia, H.; Xamena, F. Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ke, F.; Zhu, J. MOF-Derived Ultrathin Cobalt Phosphide Nanosheets as Efficient Bifunctional Hydrogen Evolution Reaction and Oxygen Evolution Reaction Electrocatalysts. Nanomaterials 2018, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Ferey, G.; Morris, R.E.; Serre, C. Metal-Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]
- Illes, B.; Wuttke, S.; Engelke, H. Liposome-Coated Iron Fumarate Metal-Organic Framework Nanoparticles for Combination Therapy. Nanomaterials 2017, 7, 351. [Google Scholar] [CrossRef] [PubMed]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Hu, Z.C.; Deibert, B.J.; Li, J. Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef]
- Cui, Y.J.; Yue, Y.F.; Qian, G.D.; Chen, B.L. Luminescent Functional Metal-Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Yuan, S.; Day, G.; Wang, X.; Yang, X.Y.; Zhou, H.C. Luminescent sensors based on metal-organic frameworks. Coord. Chem. Rev. 2018, 354, 28–45. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, S.; Luo, J.; Huang, R.; Cai, H.; Yan, W.; Yang, H. A Novel Tb@Sr-MOF as Self-Calibrating Luminescent Sensor for Nutritional Antioxidant. Nanomaterials 2018, 8, 796. [Google Scholar] [CrossRef] [PubMed]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lustig, W.P.; Li, J. Sensing and capture of toxic and hazardous gases and vapors by metal-organic frameworks. Chem. Soc. Rev. 2018, 47, 4729–4756. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.G.; Wang, H.; Chen, B.; Zou, Y.; Gu, Z.G.; Zhao, Z.J.; Shen, L. A luminescent metal-organic framework constructed using a tetraphenylethene-based ligand for sensing volatile organic compounds. Chem. Commun. 2015, 51, 1677–1680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Feng, G.; Song, Z.; Zhou, Y.-P.; Chao, H.-Y.; Yuan, D.; Tan, T.T.Y.; Guo, Z.; Hu, Z.; Tang, B.Z.; et al. Two-Dimensional Metal–Organic Framework with Wide Channels and Responsive Turn-On Fluorescence for the Chemical Sensing of Volatile Organic Compounds. J. Am. Chem. Soc. 2014, 136, 7241–7244. [Google Scholar] [CrossRef]
- Hao, J.N.; Yan, B. Highly sensitive and selective fluorescent probe for Ag+ based on a Eu3+ post-functionalized metal-organic framework in aqueous media. J. Mater. Chem. A 2014, 2, 18018–18025. [Google Scholar] [CrossRef]
- Yang, C.X.; Ren, H.B.; Yan, X.P. Fluorescent Metal Organic Framework MIL-53(Al) for Highly Selective and Sensitive Detection of Fe3+ in Aqueous Solution. Anal. Chem. 2013, 85, 7441–7446. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, B. A ratiometric fluorescent sensor with dual response of Fe3+/Cu2+ based on europium post-modified sulfone-metal-organic frameworks and its logical application. Talanta 2019, 197, 291–298. [Google Scholar] [CrossRef]
- Helal, A.; Nguyen, H.L.; Al-Ahmed, A.; Cordova, K.E.; Yamani, Z.H. An Ultrasensitive and Selective Metal–Organic Framework Chemosensor for Palladium Detection in Water. Inorg. Chem. 2019, 58, 1738–1741. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.Y.; Wang, J.; He, C.; Zhang, X.L.; Wang, Y.T.; Liu, T.; Duan, C.Y. Luminescent Metal-Organic Frameworks for Selectively Sensing Nitric Oxide in an Aqueous Solution and in Living Cells. Adv. Funct. Mater. 2012, 22, 1698–1703. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Qi, X.-L.; Lin, R.-B.; Cheng, X.-N.; Liao, P.-Q.; Zhang, J.-P.; Chen, X.-M. Porous Cu(I) Triazolate Framework and Derived Hybrid Membrane with Exceptionally High Sensing Efficiency for Gaseous Oxygen. Adv. Funct. Mater. 2014, 24, 5866–5872. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, S.-Y.; Liu, J.; Zhang, L.; Chen, D.; Chen, J.; Ma, Y.; Zhang, J.-P.; Dai, Z.; Zou, X. In Situ Enzyme Immobilization with Oxygen-Sensitive Luminescent Metal–Organic Frameworks to Realize “All-in-One” Multifunctions. Chem. Eur. J. 2019, 25, 5463–5471. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-B.; Liu, S.-Y.; Ye, J.-W.; Li, X.-Y.; Zhang, J.-P. Photoluminescent Metal–Organic Frameworks for Gas Sensing. Adv. Sci. 2016, 3, 1500434. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yan, B. A ratiometric fluorescent pH sensor based on nanoscale metal–organic frameworks (MOFs) modified by europium(III) complexes. Chem. Commun. 2014, 50, 13323–13326. [Google Scholar] [CrossRef] [PubMed]
- Harbuzaru, B.V.; Corma, A.; Rey, F.; Jordá, J.L.; Ananias, D.; Carlos, L.D.; Rocha, J. A Miniaturized Linear pH Sensor Based on a Highly Photoluminescent Self-Assembled Europium(III) Metal–Organic Framework. Angew. Chem. Int. Ed. 2009, 48, 6476–6479. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, K.; He, H.; Yue, D.; Zhao, D.; Cui, Y.; Yang, Y.; Qian, G. A stable lanthanide-functionalized nanoscale metal-organic framework as a fluorescent probe for pH. Sens. Actuators B Chem. 2018, 254, 1069–1077. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Shan, D.; Chen, J.; Zhang, S.; Lu, X. Dual-Emitting Fluorescent Metal–Organic Framework Nanocomposites as a Broad-Range pH Sensor for Fluorescence Imaging. Anal. Chem. 2018, 90, 7056–7063. [Google Scholar] [CrossRef]
- He, C.B.; Lu, K.D.; Lin, W.B. Nanoscale Metal-Organic Frameworks for Real-Time Intracellular pH Sensing in Live Cells. J. Am. Chem. Soc. 2014, 136, 12253–12256. [Google Scholar] [CrossRef]
- Jiang, H.L.; Feng, D.W.; Wang, K.C.; Gu, Z.Y.; Wei, Z.W.; Chen, Y.P.; Zhou, H.C. An Exceptionally Stable, Porphyrinic Zr Metal-Organic Framework Exhibiting pH-Dependent Fluorescence. J. Am. Chem. Soc. 2013, 135, 13934–13938. [Google Scholar] [CrossRef] [PubMed]
- Holler, C.J.; Muller-Buschbaum, K. The First Dinitrile Frameworks of the Rare Earth Elements: (3)(infinity) LnCl(3)(1,4-Ph(CN)(2)) and (3)(infinity) Ln(2)Cl(6)(1,4-Ph(CN)(2)), Ln = Sm, Gd, Tb, Y; Access to Novel Metal-Organic Frameworks by Solvent Free Synthesis in Molten 1,4-Benzodinitrile. Inorg. Chem. 2008, 47, 10141–10149. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.J.; Kalinovskyy, Y.; Griffin, S.L.; Wilson, C.; Blight, B.A.; Forgan, R.S. Functional Versatility of a Series of Zr Metal–Organic Frameworks Probed by Solid-State Photoluminescence Spectroscopy. J. Am. Chem. Soc. 2017, 139, 6253–6260. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.L.; Stavila, V.; Zeitler, T.R.; White, M.I.; Thornberg, S.M.; Greathouse, J.A.; Allendorf, M.D. Ultrasensitive Humidity Detection Using Metal–Organic Framework-Coated Microsensors. Anal. Chem. 2012, 84, 7043–7051. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jing, P.T.; Yan, N.; Hilbers, M.; Zhang, H.; Rothenberg, G.; Tanase, S. Dual-mode humidity detection using a lanthanide-based metal-organic framework: Towards multifunctional humidity sensors. Chem. Commun. 2017, 53, 4465–4468. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yue, D.; Jiang, K.; Zhang, L.; Li, C.X.; Qian, G.D. Isostructural Tb3+/Eu3+ Co-Doped Metal Organic Framework Based on Pyridine-Containing Dicarboxylate Ligands for Ratiometric Luminescence Temperature Sensing. Inorg. Chem. 2019, 58, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, W.; Lv, R.; Zhai, A.; Li, X.L.; Gu, W.; Liu, X. Dual-Function Mixed-Lanthanide Metal-Organic Framework for Ratiometric Water Detection in Bioethanol and Temperature Sensing. Anal. Chem. 2019, 91, 2148–2154. [Google Scholar] [CrossRef]
- Wieme, J.; Lejaeghere, K.; Kresse, G.; Van Speybroeck, V. Tuning the balance between dispersion and entropy to design temperature-responsive flexible metal-organic frameworks. Nat. Commun. 2018, 9, 10. [Google Scholar] [CrossRef]
- Kimura, H.; Nagai, Y.; Umemura, K.; Kimura, Y. Physiological Roles of Hydrogen Sulfide: Synaptic Modulation, Neuroprotection, and Smooth Muscle Relaxation. Antioxid. Redox Signal. 2005, 7, 795–803. [Google Scholar] [CrossRef]
- Li, L.; Rose, P.; Moore, P.K. Hydrogen Sulfide and Cell Signaling. Annu. Rev. Pharmacool. Toxicol. 2011, 51, 169–187. [Google Scholar] [CrossRef]
- Watanabe, M.; Osada, J.; Aratani, Y.; Kluckman, K.; Reddick, R.; Malinow, M.R.; Maeda, N. Mice deficient in cystathionine beta-synthase: Animal models for mild and severe homocyst(e)inemia. Proc. Natl. Acad. Sci. USA 1995, 92, 1585–1589. [Google Scholar] [CrossRef] [PubMed]
- Dorman, D.C.; Moulin, F.J.-M.; McManus, B.E.; Mahle, K.C.; James, R.A.; Struve, M.F. Cytochrome Oxidase Inhibition Induced by Acute Hydrogen Sulfide Inhalation: Correlation with Tissue Sulfide Concentrations in the Rat Brain, Liver, Lung, and Nasal Epithelium. Toxicol. Sci. 2002, 65, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.L.; Scafa, N.; Xu, L.P.; Zhou, S.F.; Al-Ghanem, K.A.; Mahboob, S.; Fugetsu, B.; Zhang, X.J. Electrochemical hydrogen sulfide biosensors. Analyst 2016, 141, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Llobet, E.; Brunet, J.; Pauly, A.; Ndiaye, A.; Varenne, C. Nanomaterials for the Selective Detection of Hydrogen Sulfide in Air. Sensors 2017, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.N.; Zhu, C.Z.; Du, D.; Lin, Y.H. A review of optical probes based on nanomaterials for the detection of hydrogen sulfide in biosystems. Anal. Chim. Acta 2019, 1061, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.S.; Chen, W.; Xian, M.; Chang, C.J. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem. Soc. Rev. 2015, 44, 4596–4618. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhang, Y.; Ruan, K.; Meng, F.; Li, T.; Guan, J.; Du, L.; Qu, P.; Xu, M. A highly selective long-wavelength fluorescent probe for hydrazine and its application in living cell imaging. Spectrochim. Acta A 2017, 184, 355–360. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, Y.; Ruan, K.; Chen, W.; Zhou, B.; Tan, X.; Wang, Y.; Zhao, L.; Zhang, G.; Qu, P.; et al. A naphthalimide-based chemodosimetric probe for ratiometric detection of hydrazine. Sens. Actuators B Chem. 2017, 244, 417–424. [Google Scholar] [CrossRef]
- Yu, F.; Han, X.; Chen, L. Fluorescent probes for hydrogen sulfide detection and bioimaging. Chem. Commun. 2014, 50, 12234–12249. [Google Scholar] [CrossRef]
- Peng, H.; Cheng, Y.; Dai, C.; King, A.L.; Predmore, B.L.; Lefer, D.J.; Wang, B. A Fluorescent Probe for Fast and Quantitative Detection of Hydrogen Sulfide in Blood. Angew. Chem. Int. Ed. 2011, 50, 9672–9675. [Google Scholar] [CrossRef]
- Lippert, A.R.; New, E.J.; Chang, C.J. Reaction-Based Fluorescent Probes for Selective Imaging of Hydrogen Sulfide in Living Cells. J. Am. Chem. Soc. 2011, 133, 10078–10080. [Google Scholar] [CrossRef] [PubMed]
- Hammers, M.D.; Taormina, M.J.; Cerda, M.M.; Montoya, L.A.; Seidenkranz, D.T.; Parthasarathy, R.; Pluth, M.D. A Bright Fluorescent Probe for H2S Enables Analyte-Responsive, 3D Imaging in Live Zebrafish Using Light Sheet Fluorescence Microscopy. J. Am. Chem. Soc. 2015, 137, 10216–10223. [Google Scholar] [CrossRef] [PubMed]
- Ke, B.; Wu, W.; Liu, W.; Liang, H.; Gong, D.; Hu, X.; Li, M. Bioluminescence Probe for Detecting Hydrogen Sulfide in Vivo. Anal. Chem. 2016, 88, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, C.; Yang, Z.; Chen, J.; He, Y.; Jiao, Y.; He, W.; Qiu, L.; Cen, J.; Guo, Z. A Ratiometric Fluorescent Probe for Rapid Detection of Hydrogen Sulfide in Mitochondria. Angew. Chem. Int. Ed. 2013, 52, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, J.; Zhang, W.; Ma, X.; Lv, J.; Tang, B. A near-infrared ratiometric fluorescent probe for rapid and highly sensitive imaging of endogenous hydrogen sulfide in living cells. Chem. Sci. 2013, 4, 2551–2556. [Google Scholar] [CrossRef]
- Huang, Z.; Ding, S.; Yu, D.; Huang, F.; Feng, G. Aldehyde group assisted thiolysis of dinitrophenyl ether: A new promising approach for efficient hydrogen sulfide probes. Chem. Commun. 2014, 50, 9185–9187. [Google Scholar] [CrossRef] [PubMed]
- Men, J.; Yang, X.; Zhang, H.; Zhou, J. A near-infrared fluorescent probe based on nucleophilic substitution–cyclization for selective detection of hydrogen sulfide and bioimaging. Dyes Pigments 2018, 153, 206–212. [Google Scholar] [CrossRef]
- Sasakura, K.; Hanaoka, K.; Shibuya, N.; Mikami, Y.; Kimura, Y.; Komatsu, T.; Ueno, T.; Terai, T.; Kimura, H.; Nagano, T. Development of a Highly Selective Fluorescence Probe for Hydrogen Sulfide. J. Am. Chem. Soc. 2011, 133, 18003–18005. [Google Scholar] [CrossRef]
- Liang, Z.H.; Tsoi, T.H.; Chan, C.F.; Dai, L.X.; Wu, Y.D.; Du, G.Y.; Zhu, L.Z.; Lee, C.S.; Wong, W.T.; Law, G.L.; et al. A smart “off-on” gate for the in situ detection of hydrogen sulphide with Cu(II)-assisted europium emission. Chem. Sci. 2016, 7, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Vikrant, K.; Kumar, V.; Ok, Y.S.; Kim, K.-H.; Deep, A. Metal-organic framework (MOF)-based advanced sensing platforms for the detection of hydrogen sulfide. TrAC Trends Anal. Chem. 2018, 105, 263–281. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Saha, T.; Desai, A.V.; Talukdar, P.; Ghosh, S.K. Metal-organic framework based highly selective fluorescence turn-on probe for hydrogen sulphide. Sci. Rep. 2014, 4, 7053. [Google Scholar] [CrossRef] [PubMed]
- Nagarkar, S.S.; Desai, A.V.; Ghosh, S.K. A Nitro-Functionalized Metal–Organic Framework as a Reaction-Based Fluorescence Turn-On Probe for Rapid and Selective H2S Detection. Chem. Eur. J. 2015, 21, 9994–9997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.; Hu, Q.; Cui, Y.; Yang, Y.; Qian, G. Postsynthetic modification of metal–organic framework for hydrogen sulfide detection. Appl. Surf. Sci. 2015, 355, 814–819. [Google Scholar] [CrossRef]
- Buragohain, A.; Biswas, S. Cerium-based azide- and nitro-functionalized UiO-66 frameworks as turn-on fluorescent probes for the sensing of hydrogen sulphide. CrystEngComm 2016, 18, 4374–4381. [Google Scholar] [CrossRef]
- Nandi, S.; Reinsch, H.; Banesh, S.; Stock, N.; Trivedi, V.; Biswas, S. Rapid and highly sensitive detection of extracellular and intracellular H2S by an azide-functionalized Al(III)-based metal–organic framework. Dalton Trans. 2017, 46, 12856–12864. [Google Scholar] [CrossRef] [PubMed]
- Dalapati, R.; Balaji, S.N.; Trivedi, V.; Khamari, L.; Biswas, S. A dinitro-functionalized Zr(IV)-based metal-organic framework as colorimetric and fluorogenic probe for highly selective detection of hydrogen sulphide. Sens. Actuators B Chem. 2017, 245, 1039–1049. [Google Scholar] [CrossRef]
- Das, A.; Banesh, S.; Trivedi, V.; Biswas, S. Extraordinary sensitivity for H2S and Fe(III) sensing in aqueous medium by Al-MIL-53-N3 metal–organic framework: In vitro and in vivo applications of H2S sensing. Dalton Trans. 2018, 47, 2690–2700. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Banesh, S.; Trivedi, V.; Biswas, S. A dinitro-functionalized metal–organic framework featuring visual and fluorogenic sensing of H2S in living cells, human blood plasma and environmental samples. Analyst 2018, 143, 1482–1491. [Google Scholar] [CrossRef]
- Legrand, A.; Pastushenko, A.; Lysenko, V.; Geloen, A.; Quadrelli, E.A.; Canivet, J.; Farrusseng, D. Enhanced Ligand-Based Luminescence in Metal–Organic Framework Sensor. ChemNanoMat 2016, 2, 866–872. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Yue, D.; Zhang, J.; Wang, J.; Li, B.; Yang, Y.; Cui, Y.; Qian, G. Flexible Metal–Organic Framework-Based Mixed-Matrix Membranes: A New Platform for H2S Sensors. Small 2018, 14, 1801563. [Google Scholar] [CrossRef]
- Ma, Y.; Su, H.; Kuang, X.; Li, X.; Zhang, T.; Tang, B. Heterogeneous Nano Metal–Organic Framework Fluorescence Probe for Highly Selective and Sensitive Detection of Hydrogen Sulfide in Living Cells. Anal. Chem. 2014, 86, 11459–11463. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, C.; Yang, P.; Li, X.; Tong, L.; Huang, F.; Yue, J.; Tang, B. A CuO-functionalized NMOF probe with a tunable excitation wavelength for selective detection and imaging of H2S in living cells. Nanoscale 2018, 10, 15793–15798. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Q.; Xia, T.; Zhang, J.; Yang, Y.; Cui, Y.; Chen, B.; Qian, G. Turn-on and Ratiometric Luminescent Sensing of Hydrogen Sulfide Based on Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2016, 8, 32259–32265. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, L.; Jiang, K.; He, H.; Yang, Y.; Cui, Y.; Li, B.; Qian, G. Nanoscale fluorescent metal–organic framework composites as a logic platform for potential diagnosis of asthma. Biosens. Bioelectron. 2019, 130, 65–72. [Google Scholar] [CrossRef]
- Zheng, X.; Fan, R.; Song, Y.; Wang, A.; Xing, K.; Du, X.; Wang, P.; Yang, Y. A highly sensitive turn-on ratiometric luminescent probe based on postsynthetic modification of Tb3+@Cu-MOF for H2S detection. J. Mater. Chem. C 2017, 5, 9943–9951. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhang, L.; Jiang, K.; Cui, Y.; Yang, Y.; Qian, G. A nanoscale Zr-based fluorescent metal-organic framework for selective and sensitive detection of hydrogen sulfide. J. Solid State Chem. 2017, 255, 97–101. [Google Scholar] [CrossRef]
- Cao, Y.-Y.; Guo, X.-F.; Wang, H. High sensitive luminescence metal-organic framework sensor for hydrogen sulfide in aqueous solution: A trial of novel turn-on mechanism. Sens. Actuators B Chem. 2017, 243, 8–13. [Google Scholar] [CrossRef]
- Reddie, K.G.; Carroll, K.S. Expanding the functional diversity of proteins through cysteine oxidation. Curr. Opin. Chem. Biol. 2008, 12, 746–754. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. Metal Binding Affinity and Selectivity in Metalloproteins: Insights from Computational Studies. Annu. Rev. Biophys. 2008, 37, 97–116. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Carroll, K.S. Cysteine-Mediated Redox Signaling: Chemistry, Biology, and Tools for Discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef]
- Lieberman, M.W.; Wiseman, A.L.; Shi, Z.Z.; Carter, B.Z.; Barrios, R.; Ou, C.N.; Chévez-Barrios, P.; Wang, Y.; Habib, G.M.; Goodman, J.C.; et al. Growth retardation and cysteine deficiency in gamma-glutamyl transpeptidase-deficient mice. Proc. Natl. Acad. Sci. USA 1996, 93, 7923–7926. [Google Scholar] [CrossRef]
- Wang, S.Q.; Shen, S.L.; Zhang, Y.R.; Dai, X.; Zhao, B.X. Recent Progress in Fluorescent Probes for the Detection of Biothiols. Chin. J. Org. Chem. 2014, 34, 1717–1729. [Google Scholar] [CrossRef]
- Niu, L.-Y.; Chen, Y.-Z.; Zheng, H.-R.; Wu, L.-Z.; Tung, C.-H.; Yang, Q.-Z. Design strategies of fluorescent probes for selective detection among biothiols. Chem. Soc. Rev. 2015, 44, 6143–6160. [Google Scholar] [CrossRef]
- Ding, S.; Liu, M.; Hong, Y. Biothiol-specific fluorescent probes with aggregation-induced emission characteristics. Sci. China Chem. 2018, 61, 882–891. [Google Scholar] [CrossRef]
- Yin, C.-X.; Xiong, K.-M.; Huo, F.-J.; Salamanca, J.C.; Strongin, R.M. Fluorescent Probes with Multiple Binding Sites for the Discrimination of Cys, Hcy, and GSH. Angew. Chem. Int. Ed. 2017, 56, 13188–13198. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Peng, X.; Yoon, J. Fluorescent and colorimetric probes for detection of thiols. Chem. Soc. Rev. 2010, 39, 2120–2135. [Google Scholar] [CrossRef]
- Ren, X.; Tian, H.; Yang, L.; He, L.; Geng, Y.; Liu, X.; Song, X. Fluorescent probe for simultaneous discrimination of Cys/Hcy and GSH in pure aqueous media with a fast response under a single-wavelength excitation. Sens. Actuators B Chem. 2018, 273, 1170–1178. [Google Scholar] [CrossRef]
- Yang, L.; Xiong, H.; Su, Y.; Tian, H.; Liu, X.; Song, X. A red-emitting water-soluble fluorescent probe for biothiol detection with a large Stokes shift. Chin. Chem. Lett. 2019, 30, 563–565. [Google Scholar] [CrossRef]
- Sharma, S.; Ghosh, S.K. Metal–Organic Framework-Based Selective Sensing of Biothiols via Chemidosimetric Approach in Water. ACS Omega 2018, 3, 254–258. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Jiang, M.; Li, Y.; Xia, L.; Wu, P. Aldehyde-functionalized metal–organic frameworks for selective sensing of homocysteine over Cys, GSH and other natural amino acids. Chem. Commun. 2018, 54, 1004–1007. [Google Scholar] [CrossRef]
- Gui, B.; Meng, Y.; Xie, Y.; Tian, J.; Yu, G.; Zeng, W.; Zhang, G.; Gong, S.; Yang, C.; Zhang, D.; et al. Tuning the Photoinduced Electron Transfer in a Zr-MOF: Toward Solid-State Fluorescent Molecular Switch and Turn-On Sensor. Adv. Mater. 2018, 30, 1802329. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Han, J.; Jing, H.; Gao, Z.; Huang, H.; Wang, Y.; Zhong, C. Design of “turn-on” fluorescence sensor for L-Cysteine based on the instability of metal-organic frameworks. Microporous Mesoporous Mater. 2018, 268, 88–92. [Google Scholar] [CrossRef]
- Sen, A.; Desai, A.V.; Samanta, P.; Dutta, S.; Let, S.; Ghosh, S.K. Post-synthetically modified metal–organic framework as a scaffold for selective bisulphite recognition in water. Polyhedron 2018, 156, 1–5. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, T.; Zhao, D.; Cui, Y.; Yang, Y.; Qian, G. In situ secondary growth of Eu(III)-organic framework film for fluorescence sensing of sulfur dioxide. Sens. Actuators B Chem. 2018, 260, 63–69. [Google Scholar] [CrossRef]
- Fang, F.C. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat. Rev. Microbiol. 2004, 2, 820–832. [Google Scholar] [CrossRef]
- Li, H.; Cao, Z.; Moore, D.R.; Jackson, P.L.; Barnes, S.; Lambeth, J.D.; Thannickal, V.J.; Cheng, G. Microbicidal Activity of Vascular Peroxidase 1 in Human Plasma via Generation of Hypochlorous Acid. Infect. Immun. 2012, 80, 2528–2537. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Soto, N.O.; Horstkotte, B.; March, J.G.; de Alba, P.L.L.; Martínez, L.L.; Martín, V.C. An environmental friendly method for the automatic determination of hypochlorite in commercial products using multisyringe flow injection analysis. Anal. Chim. Acta 2008, 611, 182–186. [Google Scholar] [CrossRef]
- Ordeig, O.; Mas, R.; Gonzalo, J.; Del Campo, F.J.; Muñoz, F.J.; de Haro, C. Continuous Detection of Hypochlorous Acid/Hypochlorite for Water Quality Monitoring and Control. Electroanalysis 2005, 17, 1641–1648. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Liu, Y.; Feng, X.; Zhao, B.-X. Recent progress in the development of fluorescent probes for the detection of hypochlorous acid. Sens. Actuators B Chem. 2017, 240, 18–36. [Google Scholar] [CrossRef]
- Duan, Q.; Jia, P.; Zhuang, Z.; Liu, C.; Zhang, X.; Wang, Z.; Sheng, W.; Li, Z.; Zhu, H.; Zhu, B.; et al. Rational Design of a Hepatoma-Specific Fluorescent Probe for HOCl and Its Bioimaging Applications in Living HepG2 Cells. Anal. Chem. 2019, 91, 2163–2168. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wu, I.C.; DuFort, C.C.; Carlson, M.A.; Wu, X.; Chen, L.; Kuo, C.-T.; Qin, Y.; Yu, J.; Hingorani, S.R.; et al. Photostable Ratiometric Pdot Probe for in Vitro and in Vivo Imaging of Hypochlorous Acid. J. Am. Chem. Soc. 2017, 139, 6911–6918. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.H.; Hao, Y.; Zeng, K.; Fan, S.; Li, F.; Yuan, S.; Ding, X.; Xu, M.; Liu, Y.-N. A benzothiazole-based fluorescent probe for hypochlorous acid detection and imaging in living cells. Spectrochim. Acta A 2018, 199, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Kuang, Y.; Ma, F.; Chen, S.; Long, Y. A sensitive spectrofluorometric method for detection of berberine hydrochloride using Ag nanoclusters directed by natural fish sperm DNA. Biosens. Bioelectron. 2016, 85, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, H.; Hong, Y.; Yu, M.; Zeng, R.; Long, Y.; Chen, J. Selective visualization of endogenous hypochlorous acid in zebrafish during lipopolysaccharide-induced acute liver injury using a polymer micelles-based ratiometric fluorescent probe. Biosens. Bioelectron. 2018, 99, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, H.; Zhang, D.; Zeng, X.; Zeng, R.; Xiao, L.; Tao, H.; Long, Y.; Yi, P.; Chen, J. Two-photon fluorescent probe for lysosome-targetable hypochlorous acid detection within living cells. Sens. Actuators B Chem. 2018, 255, 2223–2231. [Google Scholar] [CrossRef]
- Xue, M.; Wang, H.; Chen, J.; Ren, J.; Chen, S.; Yang, H.; Zeng, R.; Long, Y.; Zhang, P. Ratiometric fluorescent sensing of endogenous hypochlorous acid in lysosomes using AIE-based polymeric nanoprobe. Sens. Actuators B Chem. 2019, 282, 1–8. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Tang, Y.; Zhang, C.C.; Fu, H.; Wu, N.; Ma, L.; Gao, J.; Wang, Q. Oxidative deoximation reaction induced recognition of hypochlorite based on a new fluorescent lanthanide-organic framework. Chem. Eng. J. 2018, 351, 364–370. [Google Scholar] [CrossRef]
- Ye, Y.; Zhao, L.; Hu, S.; Liang, A.; Li, Y.; Zhuang, Q.; Tao, G.; Gu, J. Specific detection of hypochlorite based on the size-selective effect of luminophore integrated MOF-801 synthesized by a one-pot strategy. Dalton Trans. 2019, 48, 2617–2625. [Google Scholar] [CrossRef]
- Cai, K.; Zeng, M.; Wang, L.; Song, Y.; Chen, L. Ratiometric Fluorescent Detection of ClO− Based on Dual-Emission F1-Rubpy@Nanoscale Metal-Organic Frameworks. ChemistrySelect 2019, 4, 2649–2655. [Google Scholar] [CrossRef]
- Yue, D.; Zhao, D.; Zhang, J.; Zhang, L.; Jiang, K.; Zhang, X.; Cui, Y.; Yang, Y.; Chen, B.; Qian, G. A luminescent cerium metal–organic framework for the turn-on sensing of ascorbic acid. Chem. Commun. 2017, 53, 11221–11224. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Huang, Y.; Zhang, L.; Jiang, K.; Zhang, X.; Cui, Y.; Yu, Y.; Qian, G. Ratiometric luminescence sensing based on a mixed Ce/Eu metal–organic framework. J. Mater. Chem. C 2018, 6, 2054–2059. [Google Scholar] [CrossRef]
- Yue, D.; Huang, Y.; Zhang, J.; Zhang, X.; Cui, Y.; Yang, Y.; Qian, G. A Two-Dimensional Metal–Organic Framework as a Fluorescent Probe for Ascorbic Acid Sensing. Eur. J. Inorg. Chem. 2018, 2018, 173–177. [Google Scholar] [CrossRef]
- Wu, S.; Lin, Y.; Liu, J.; Shi, W.; Yang, G.; Cheng, P. Rapid Detection of the Biomarkers for Carcinoid Tumors by a Water Stable Luminescent Lanthanide Metal–Organic Framework Sensor. Adv. Funct. Mater. 2018, 28, 1707169. [Google Scholar] [CrossRef]
- Chan, J.; Dodani, S.C.; Chang, C.J. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 2012, 4, 973. [Google Scholar] [CrossRef] [PubMed]
- Sk, M.; Banesh, S.; Trivedi, V.; Biswas, S. Selective and Sensitive Sensing of Hydrogen Peroxide by a Boronic Acid Functionalized Metal–Organic Framework and Its Application in Live-Cell Imaging. Inorg. Chem. 2018, 57, 14574–14581. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, P.V.; Patel, S.V.; Bhalani, K.D.; Shah, D.; Shah, V.S.; Mehta, K.G. Prevalence of dental fluorosis & dental caries in association with high levels of drinking water fluoride content in a district of Gujarat, India. Indian J. Med. Res. 2012, 135, 873–877. [Google Scholar]
- Chirani, R.A.; Foray, H. Dental fluorosis: Etiological diagnosis. Arch. Pediatr. 2005, 12, 284–287. [Google Scholar] [CrossRef]
- Souza, D.C.C.; Maltz, M.; Hashizume, L.N. Fluoride retention in saliva and in dental biofilm after different home-use fluoride treatments. Braz. Oral Res. 2014, 28, 255–259. [Google Scholar] [CrossRef]
- Dhillon, A.; Nair, M.; Kumar, D. Analytical methods for determination and sensing of fluoride in biotic and abiotic sources: A review. Anal. Methods 2016, 8, 5338–5352. [Google Scholar] [CrossRef]
- Wu, S.; Han, T.; Guo, J.; Cheng, Y. Poly(3-aminophenylboronic acid)-reduced graphene oxide nanocomposite modified electrode for ultrasensitive electrochemical detection of fluoride with a wide response range. Sens. Actuators B Chem. 2015, 220, 1305–1310. [Google Scholar] [CrossRef]
- Snowden, T.S.; Anslyn, E.V. Anion recognition: Synthetic receptors for anions and their application in sensors. Curr. Opin. Chem. Biol. 1999, 3, 740–746. [Google Scholar] [CrossRef]
- Michigami, Y.; Kuroda, Y.; Ueda, K.; Yamamoto, Y. Determination of urinary fluoride by ion chromatography. Anal. Chim. Acta 1993, 274, 299–302. [Google Scholar] [CrossRef]
- Xu, X.R.; Li, H.B.; Gu, J.-D.; Paeng, K.J. Determination of Fluoride in Water by Reversed-Phase High-Performance Liquid Chromatography using F−-La3+-Alizarin Complexone Ternary Complex. Chromatographia 2004, 59, 745–747. [Google Scholar] [CrossRef]
- Han, J.; Zhang, J.; Gao, M.; Hao, H.; Xu, X. Recent advances in chromo-fluorogenic probes for fluoride detection. Dyes Pigments 2019, 162, 412–439. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.F.; Yoon, J. Fluorescence and Colorimetric Chemosensors for Fluoride-Ion Detection. Chem. Rev. 2014, 114, 5511–5571. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wu, Y.C.; You, J.Y.; Cao, L.; Ding, S.; Jiang, K.; Wang, Z.Y. New Progress in the Design, Synthesis and Application of Fluorescent Probes for Fluoride Ion Detection. Chin. J. Org. Chem. 2016, 36, 2559–2582. [Google Scholar] [CrossRef]
- Wang, X.; Hu, R.; Li, S.Y. Design Strategy and Application of F− Luminescent Probes. Prog. Chem. 2018, 30, 1364–1379. [Google Scholar]
- Chen, B.; Wang, L.; Zapata, F.; Qian, G.; Lobkovsky, E.B. A Luminescent Microporous Metal–Organic Framework for the Recognition and Sensing of Anions. J. Am. Chem. Soc. 2008, 130, 6718–6719. [Google Scholar] [CrossRef]
- Zheng, H.-Y.; Lian, X.; Qin, S.-J.; Yan, B. Novel “Turn-On” Fluorescent Probe for Highly Selectively Sensing Fluoride in Aqueous Solution Based on Tb3+-Functionalized Metal–Organic Frameworks. ACS Omega 2018, 3, 12513–12519. [Google Scholar] [CrossRef]
- Yang, Z.-R.; Wang, M.-M.; Wang, X.-S.; Yin, X.-B. Boric-Acid-Functional Lanthanide Metal–Organic Frameworks for Selective Ratiometric Fluorescence Detection of Fluoride Ions. Anal. Chem. 2017, 89, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, F.M.; Nguyen, T.N.; Shyshkanov, S.; Gładysiak, A.; Favre, P.; Zacharia, A.; Itskos, G.; Dyson, P.J.; Stylianou, K.C. Selective, Fast-Response, and Regenerable Metal–Organic Framework for Sampling Excess Fluoride Levels in Drinking Water. J. Am. Chem. Soc. 2019, 141, 3052–3058. [Google Scholar] [CrossRef] [PubMed]
- Dorea, J.G.; Donangelo, C.M. Early (in uterus and infant) exposure to mercury and lead. Clin. Nutr. 2006, 25, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.H.; Pickering, I.J.; George, G.N. The Chemical Form of Mercury in Fish. Science 2003, 301, 1203. [Google Scholar] [CrossRef] [PubMed]
- Llobet, J.M.; Falcó, G.; Casas, C.; Teixidó, A.; Domingo, J.L. Concentrations of Arsenic, Cadmium, Mercury, and Lead in Common Foods and Estimated Daily Intake by Children, Adolescents, Adults, and Seniors of Catalonia, Spain. J. Agric. Food. Chem. 2003, 51, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Ayensu, W.K.; Ninashvili, N.; Sutton, D. Review: Environmental exposure to mercury and its toxicopathologic implications for public health. Environ. Technol. 2003, 18, 149–175. [Google Scholar] [CrossRef] [PubMed]
- Harada, M. Minamata Disease: Methylmercury Poisoning in Japan Caused by Environmental Pollution. Crit. Rev. Toxicol. 1995, 25, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kuang, Y.; Zhang, P.; Huang, Y.; Wen, A.; Zeng, X.; Feng, R.; Nie, H.; Jiang, X.; Long, Y. A dual-functional spectroscopic probe for simultaneous monitoring Cu2+ and Hg2+ ions by two different sensing nature based on novel fluorescent gold nanoclusters. Sens. Actuators B Chem. 2017, 253, 283–291. [Google Scholar] [CrossRef]
- Hao, Y.; Xiong, D.; Wang, L.; Chen, W.; Zhou, B.; Liu, Y.-N. A reversible competition colorimetric assay for the detection of biothiols based on ruthenium-containing complex. Talanta 2013, 115, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Hao, Y.; Zhang, Y.; Liu, B.; Zhu, X.; Qu, P.; Li, D.; Xu, M. A water-soluble and retrievable ruthenium-based probe for colorimetric recognition of Hg(II) and Cys. Spectrochim. Acta A 2016, 165, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Suherman, A.L.; Tanner, E.E.L.; Compton, R.G. Recent developments in inorganic Hg2+ detection by voltammetry. TrAC Trends Anal. Chem. 2017, 94, 161–172. [Google Scholar] [CrossRef]
- Mahato, P.; Saha, S.; Das, P.; Agarwalla, H.; Das, A. An overview of the recent developments on Hg2+ recognition. RSC Adv. 2014, 4, 36140–36174. [Google Scholar] [CrossRef]
- Chen, S.; Tang, J.; Kuang, Y.; Fu, L.; Ma, F.; Yang, Y.; Chen, G.; Long, Y. Selective deposition of HgS at the corner sites of triangular silver nanoprism and its tunable LSPR for colorimetric Hg2+ detection. Sens. Actuators B Chem. 2015, 221, 1182–1187. [Google Scholar] [CrossRef]
- Quang, D.T.; Kim, J.S. Fluoro- and Chromogenic Chemodosimeters for Heavy Metal Ion Detection in Solution and Biospecimens. Chem. Rev. 2010, 110, 6280–6301. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, Q.; Feng, W.; Li, F. Luminescent Chemodosimeters for Bioimaging. Chem. Rev. 2013, 113, 192–270. [Google Scholar] [CrossRef] [PubMed]
- Samanta, P.; Desai, A.V.; Sharma, S.; Chandra, P.; Ghosh, S.K. Selective Recognition of Hg2+ ion in Water by a Functionalized Metal–Organic Framework (MOF) Based Chemodosimeter. Inorg. Chem. 2018, 57, 2360–2364. [Google Scholar] [CrossRef] [PubMed]
- Panda, M.; Robinson, N.C. Kinetics and mechanism for the binding of HCN to cytochrome c oxidase. Biochemistry 1995, 34, 10009–10018. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, X.; Zhou, W.; Gao, H.; Wu, J. Extraction of gold from alkaline cyanide solution by the tetradecyldimethylbenzylammonium chloride/tri-n-butyl phosphate/n-heptane system based on a microemulsion mechanism. Phys. Chem. Chem. Phys. 2002, 4, 4489–4494. [Google Scholar] [CrossRef]
- Attar, A.; Cubillana-Aguilera, L.; Naranjo-Rodríguez, I.; de Cisneros, J.L.; Palacios-Santander, J.M.; Amine, A. Amperometric inhibition biosensors based on horseradish peroxidase and gold sononanoparticles immobilized onto different electrodes for cyanide measurements. Bioelectrochemistry 2015, 101, 84–91. [Google Scholar] [CrossRef]
- Kang, H.-I.; Shin, H.-S. Derivatization Method of Free Cyanide Including Cyanogen Chloride for the Sensitive Analysis of Cyanide in Chlorinated Drinking Water by Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chem. 2015, 87, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, X.; Kim, H.N.; Yoon, J. Sensors for the optical detection of cyanide ion. Chem. Soc. Rev. 2010, 39, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, L.; Chen, X.; Yoon, J. Recent progress in the development of fluorometric and colorimetric chemosensors for detection of cyanide ions. Chem. Soc. Rev. 2014, 43, 4312–4324. [Google Scholar] [CrossRef] [PubMed]
- Udhayakumari, D. Chromogenic and fluorogenic chemosensors for lethal cyanide ion. A comprehensive review of the year 2016. Sens. Actuators B Chem. 2018, 259, 1022–1057. [Google Scholar] [CrossRef]
- Hao, Y.; Nguyen, K.H.; Zhang, Y.; Zhang, G.; Fan, S.; Li, F.; Guo, C.; Lu, Y.; Song, X.; Qu, P.; et al. A highly selective and ratiometric fluorescent probe for cyanide by rationally altering the susceptible H-atom. Talanta 2018, 176, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Kumar, N.; Samanta, P.; Desai, A.V.; Ghosh, S.K. A Post-Synthetically Modified MOF for Selective and Sensitive Aqueous-Phase Detection of Highly Toxic Cyanide Ions. Chem. Eur. J. 2016, 22, 864–868. [Google Scholar] [CrossRef]
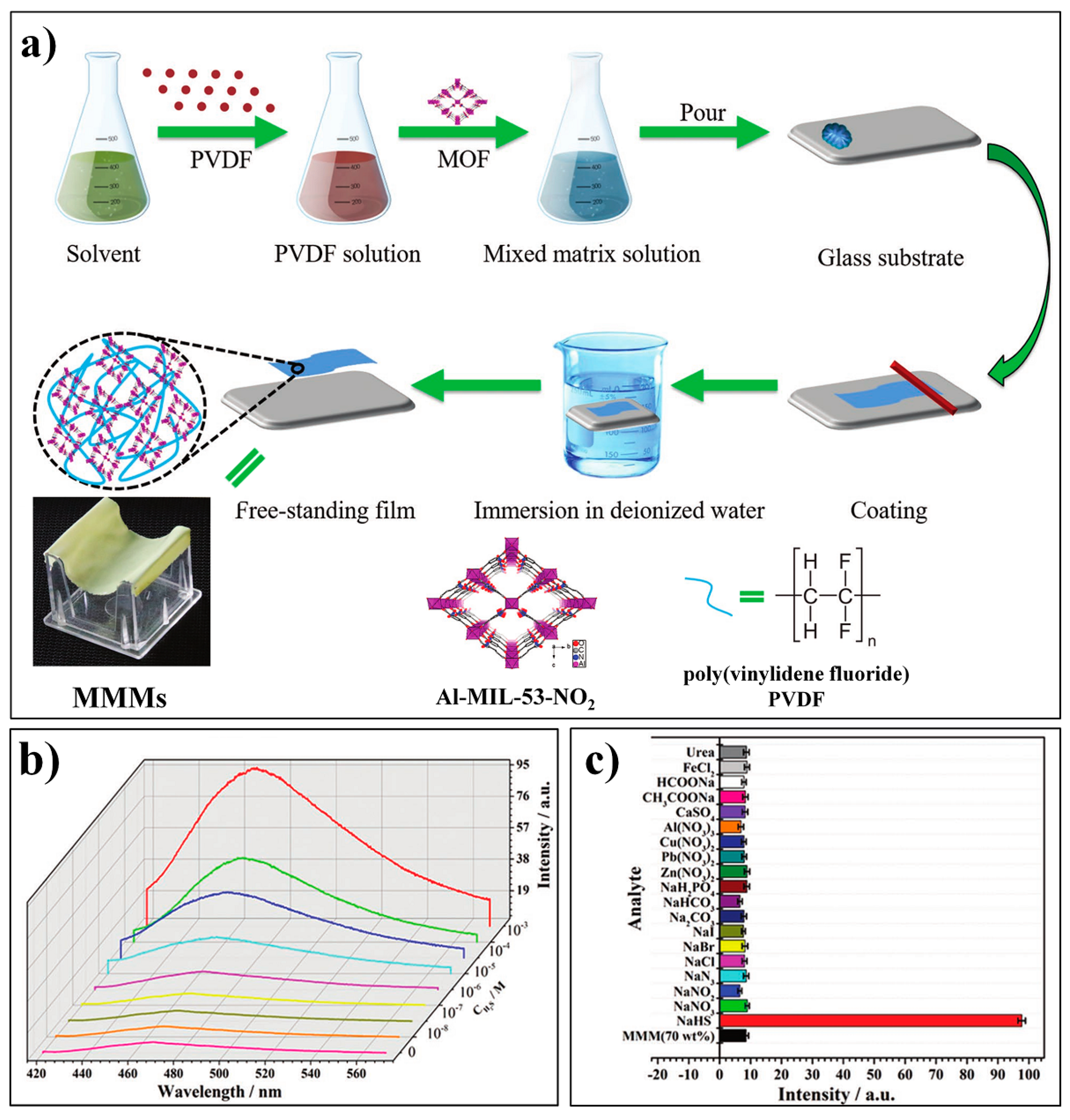
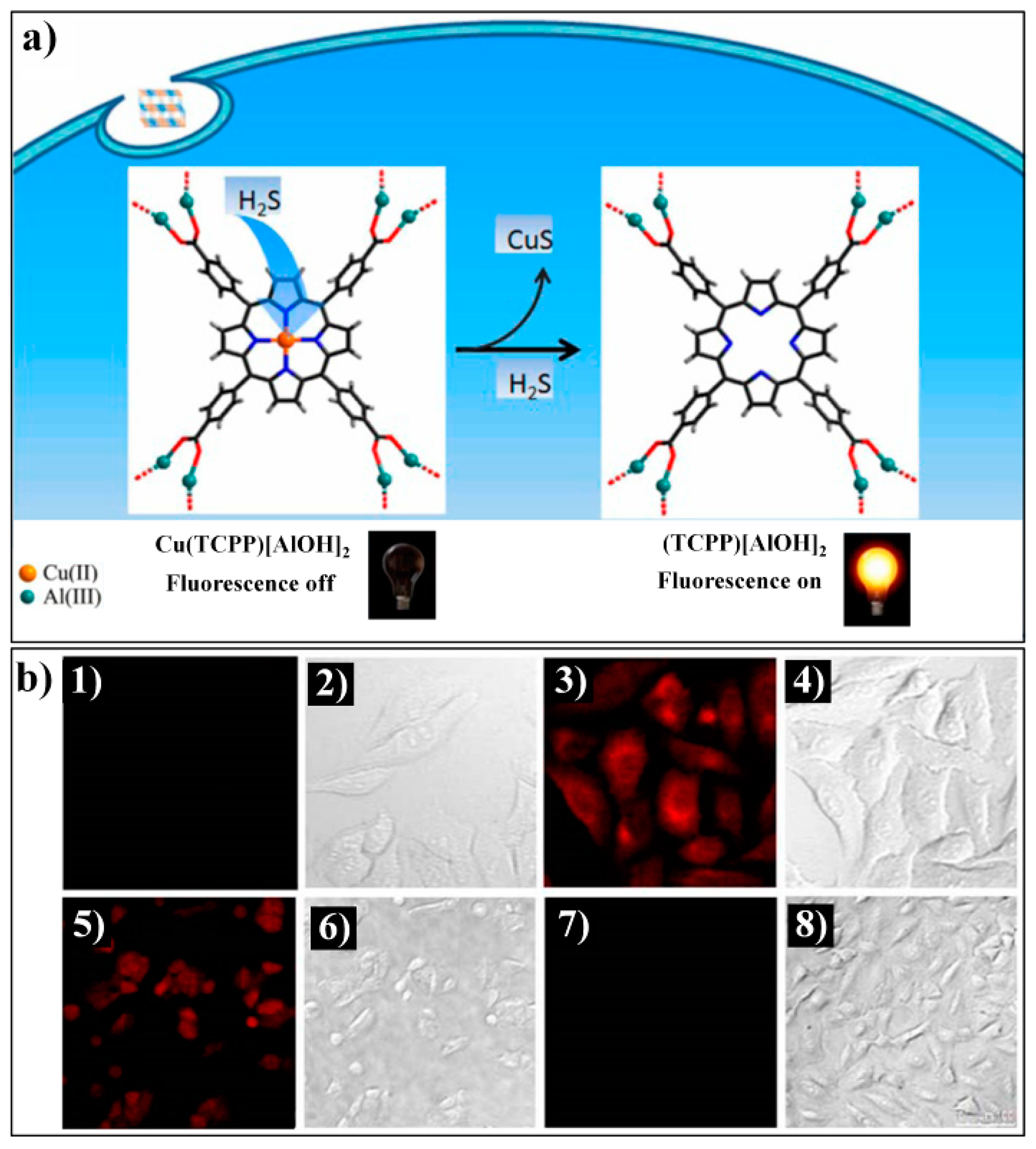
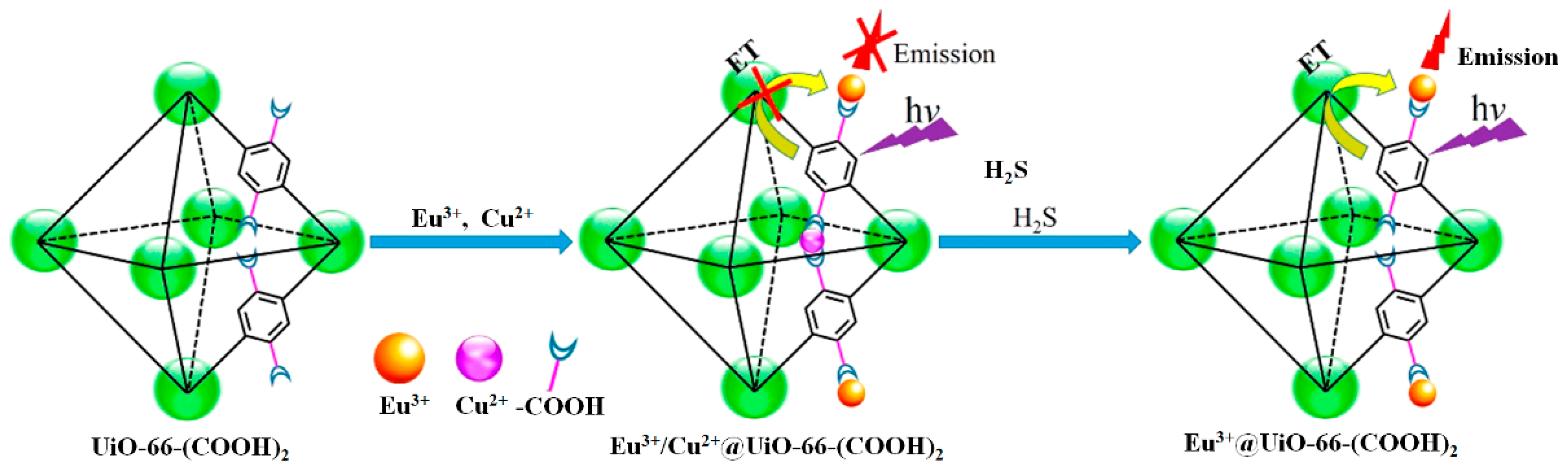
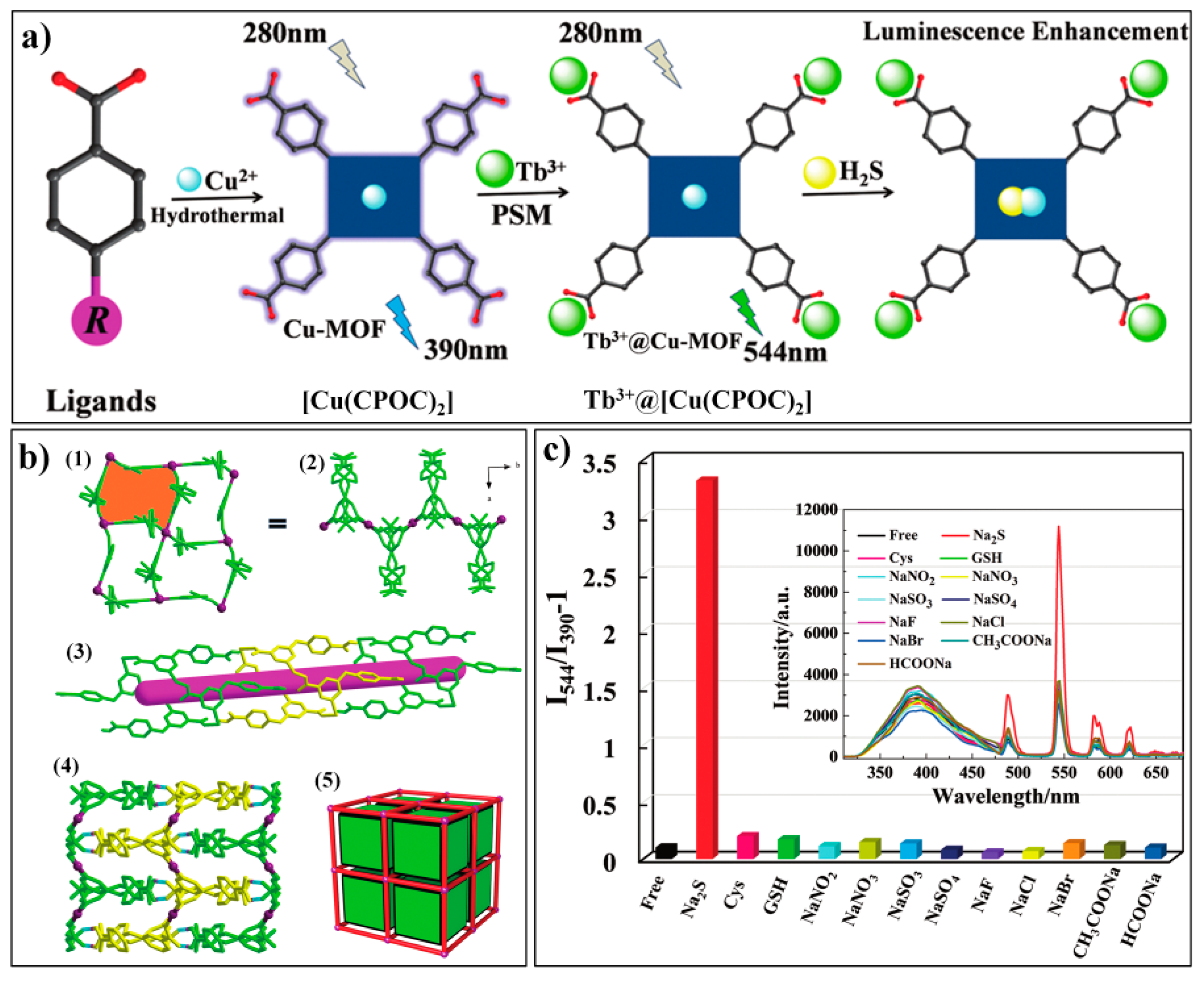
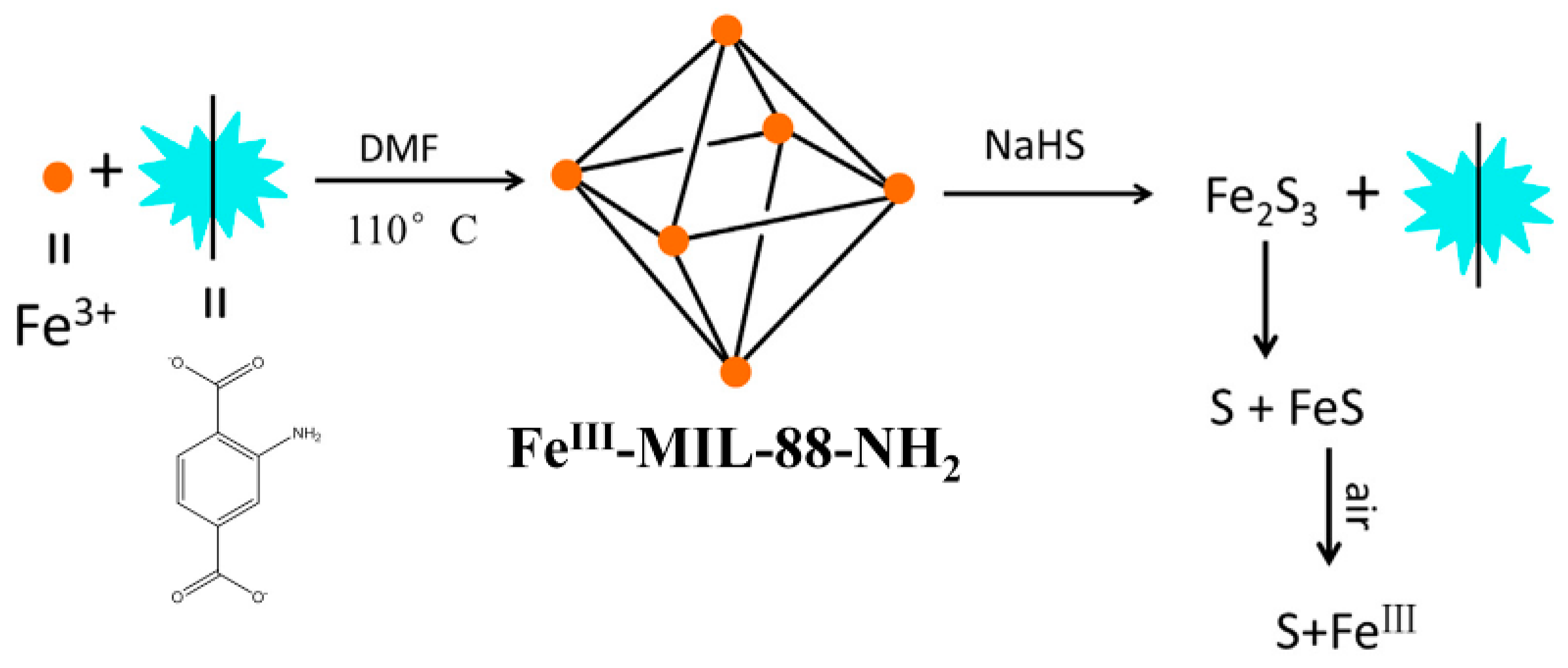
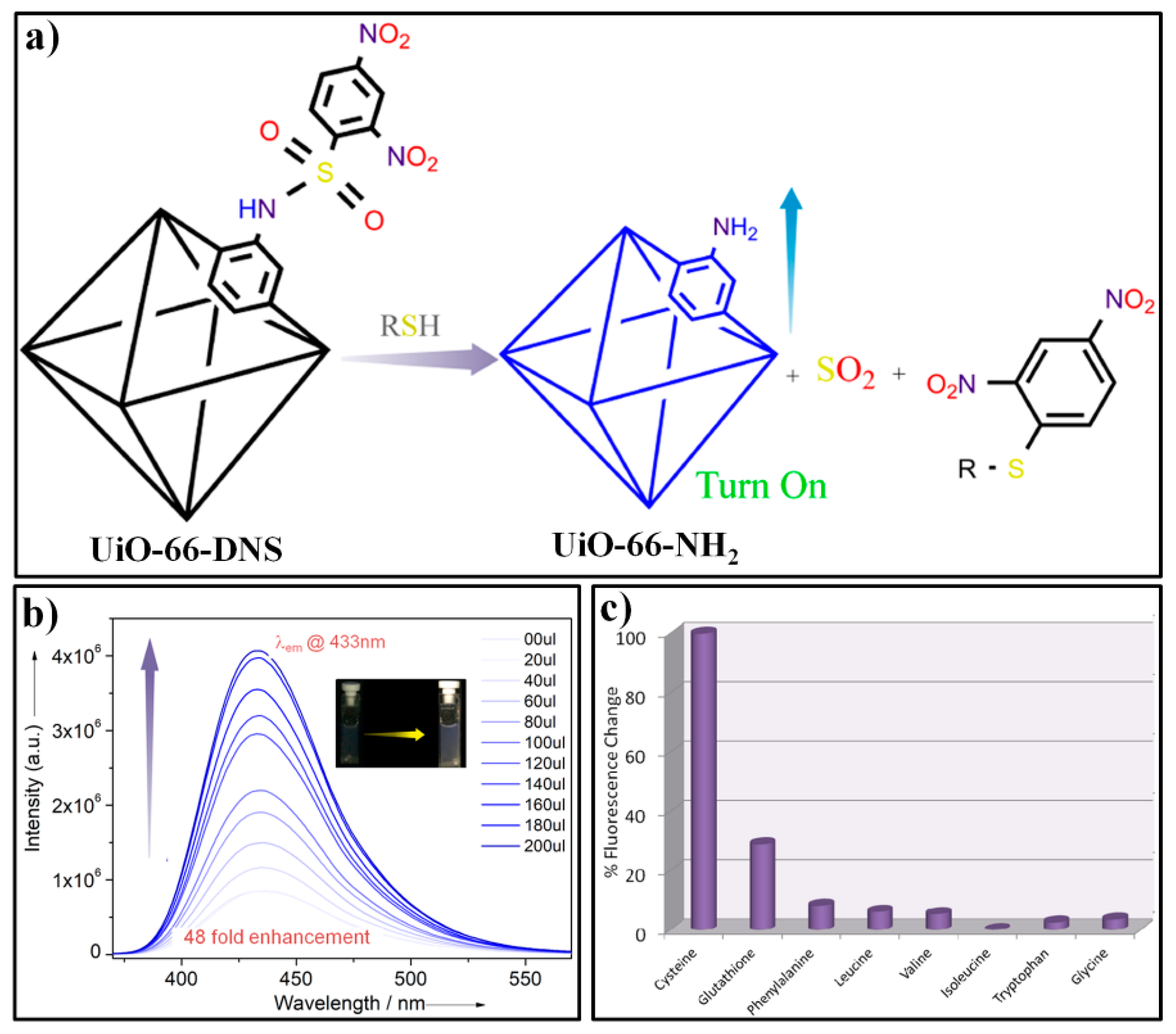
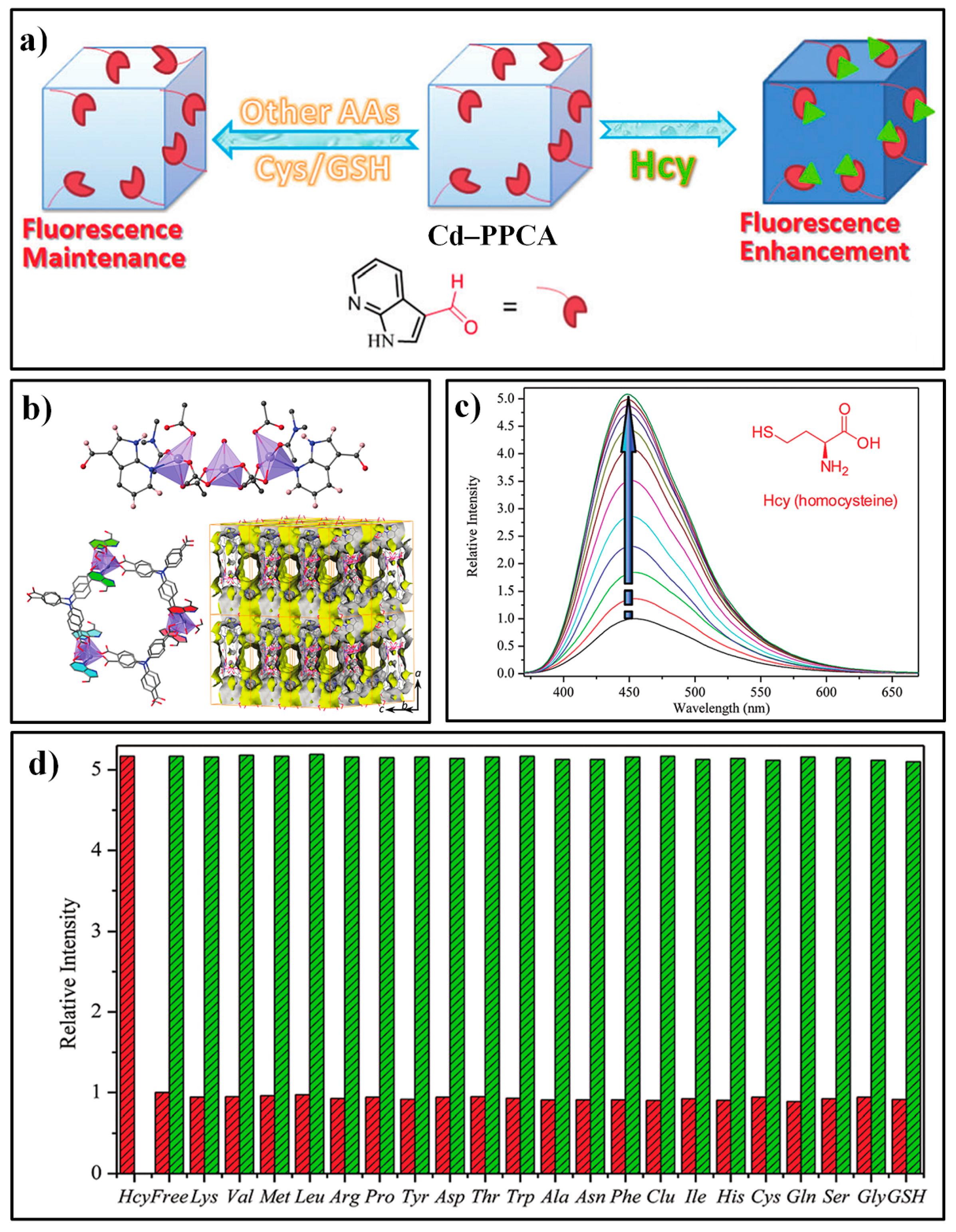
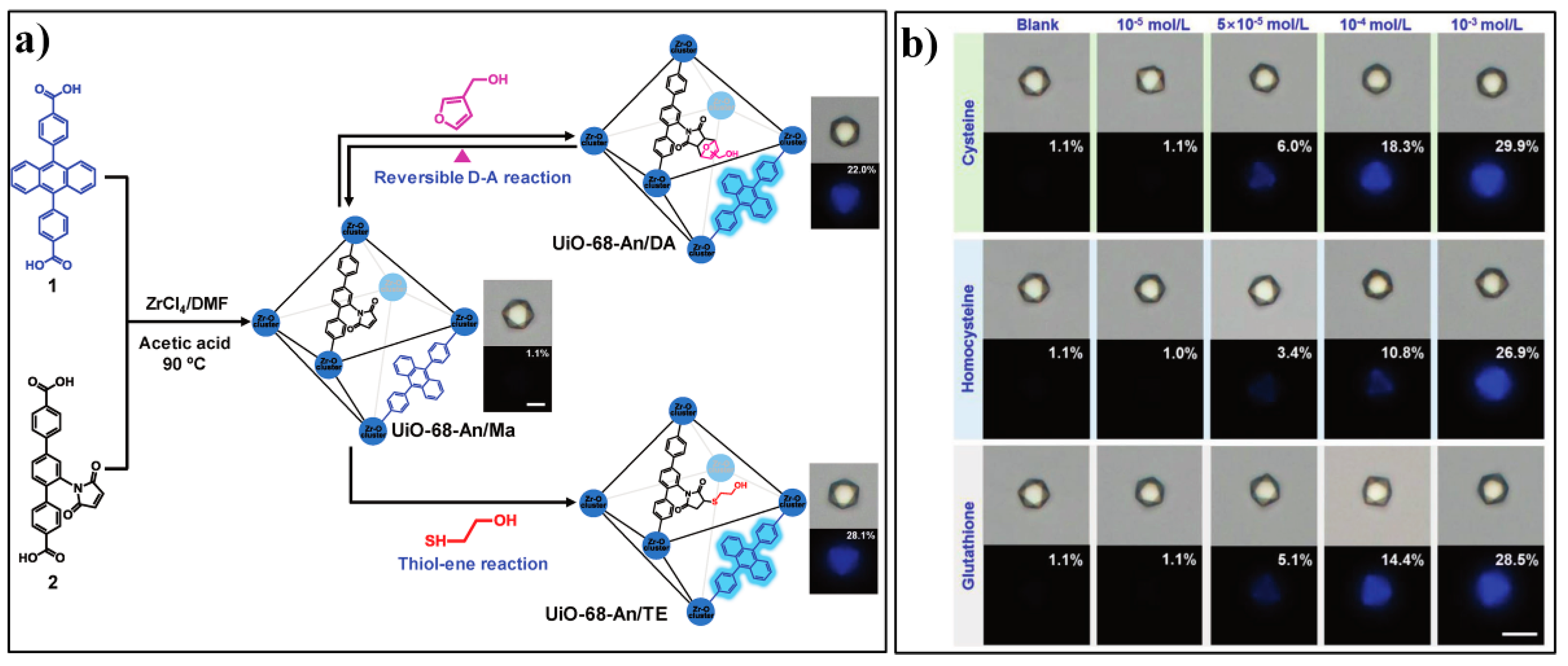

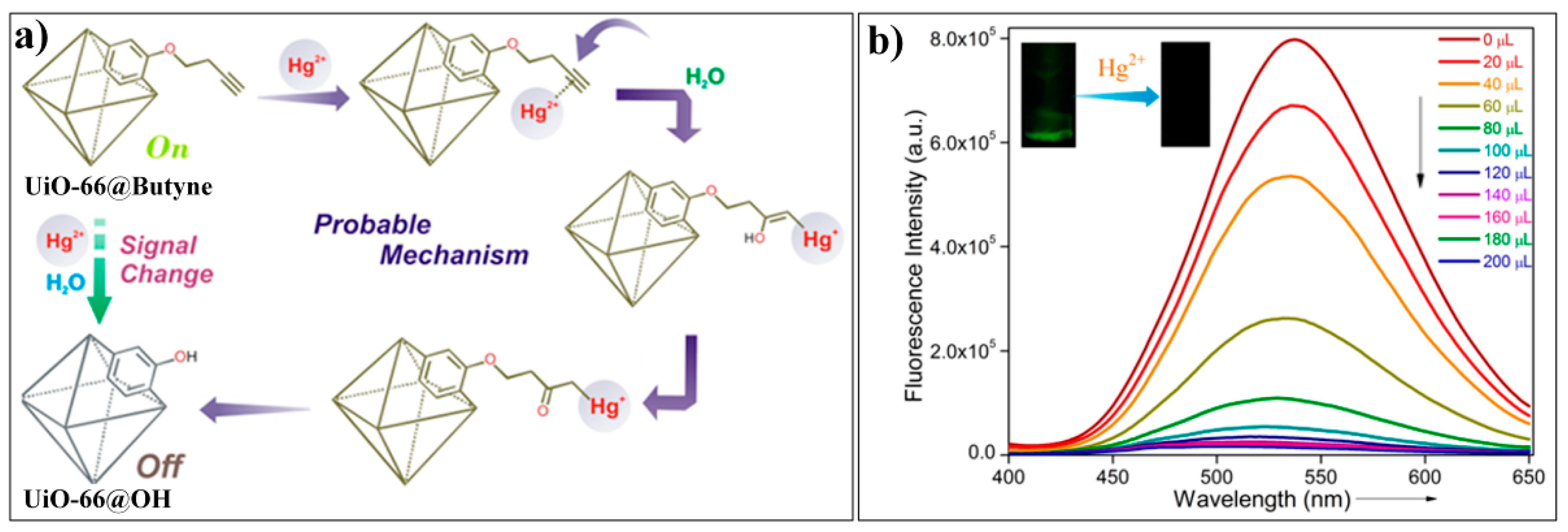
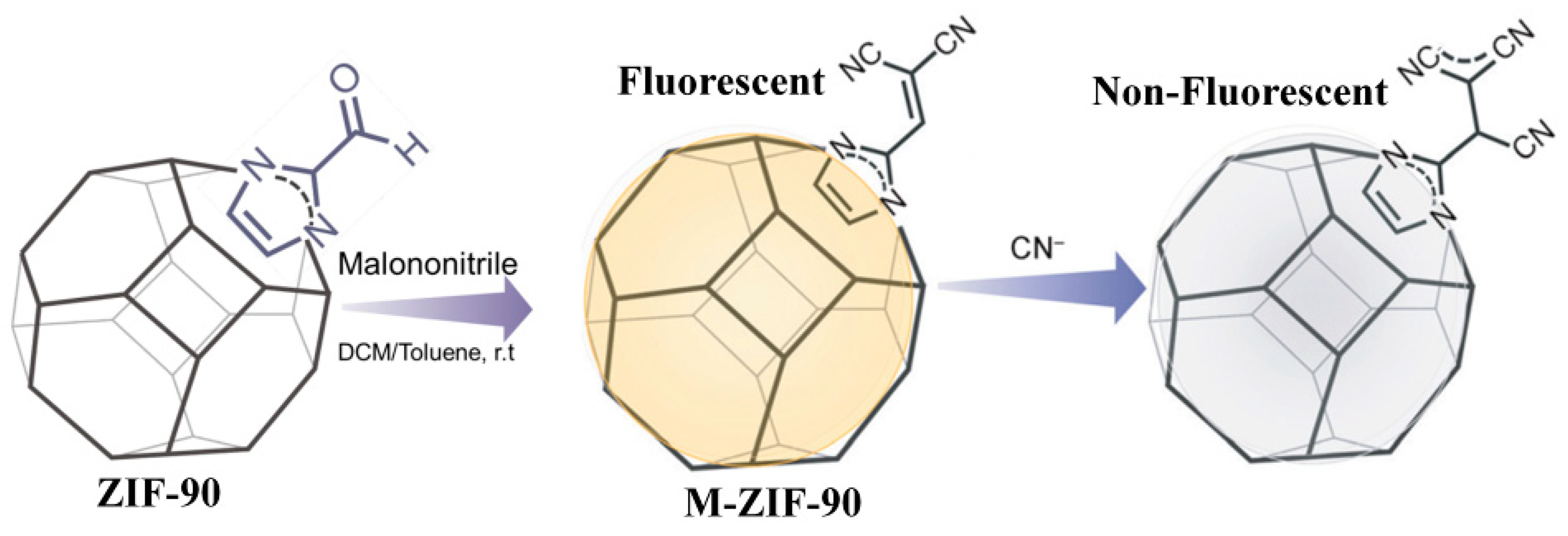
| MOF Formula | λex/λem (nm) | Dynamic Range | LOD 1 | RT 2 | Media | Real Sampe | Ref. |
|---|---|---|---|---|---|---|---|
| Zr6O4(OH)4(BDC-N3)6 | 334/436 | 0–4 mM | 117 μM | 180 s | HEPES buffer (10 mM, pH 7.4) | Live cells | [71] |
| Zr6O4(OH)4(BDC-NO2)6 | 334/436 | 0–4 mM | 188 μM | 460 s | HEPES buffer (10 mM, pH 7.4) | -- | [72] |
| Zn4O(OH)4(BDC-N3)3 | 395/455 | 0–0.5 mM | 28.3 μM | 90 s | HEPES ethanol buffer (10 mM, pH 7.4) | -- | [73] |
| Ce6O4(OH)4(BDC-N3)6 | 334/429 | 0–3.5 mM | 12.2 μM | 760 s | HEPES buffer (10 mM, pH 7.4) | -- | [74] |
| Ce6O4(OH)4(BDC-NO2)6 | 334/429 | 0–3.5 mM | 34.8 μM | 480 s | HEPES buffer (10 mM, pH 7.4) | -- | [74] |
| Al(OH)(IPA-N3) | 330/405 | 0–0.06 mM | 2.65 μM | 420 s | HEPES buffer (10 mM, pH 7.4) | Live cells | [75] |
| Zr6O4(OH)4((NDC-(NO2)2)6 | 390/474 | 0.1–0.7 mM | 20 μM | 50 min | HEPES buffer (10 mM, pH 7.4) | Live cells | [76] |
| Al(OH)(BDC-N3) | 315/425 | 0.2–1.6 μM | 90.47 nM | 60 s | HEPES buffer (10 mM, pH 7.4) | Live cells | [77] |
| Al3O4(OH)4(BDC-(NO2)2)6 | 345/527 | 0.1–0.6 mM | 14.14 μM | 40 min | HEPES buffer (10 mM, pH 7.4) | Live cells | [78] |
| Al3(O)(OH)(BDC-N3)3 | 343/460, 565 | 0.1–120 μM | 100 nM | -- | Hank’s balanced salt solution | Cell sample | [79] |
| Al(OH)(BDC-NO2)/poly(vinylidene fluoride) | 396/466 | 0–0.1 mM | 92.31 nM | -- | PVDF membrane | Lake water | [80] |
| Cu(TCPP)[AlOH]2 | 419/602, 650 | 0–10 μM | 16 nM | instant | BBS buffer (20 mM, pH 7.4) | Live cells | [81] |
| CuO@TO@UiO-66 | 510/~560 | 0–100 μM | 0.51 μM | instant | Tris-HCl butter (20 mM, pH 7.4) | Live cells | [82] |
| Eu3+/Cu2+@UiO-66-(COOH)2 | 305/393, 615 | 0–625 μM | 5.45 μM | 30 s | HEPES buffer (10 mM, pH 7.4) | -- | [83] |
| Eu3+/Ag+@UiO-66-(COOH)2 | 305/615 | 0–2.5 mM | 23.53 μM | 30 s | HEPES buffer (10 mM, pH 7.4) | Serum | [84] |
| Tb3+@[Cu(CPOC)2] | 280/390, 544 | 0–1.6 mM | 13.25 μM | 2 min | HEPES buffer (10 mM, pH 7.4) | -- | [85] |
| UiO-66-CH = CH2 Zr6O4(OH)4(BDC-CH = CH2)6 | 328/382 | 0–0.05 mM | 6.46 μM | 10 s | HEPES buffer (10 mM, pH 7.4) | Live cells | [86] |
| FeIII-MIL-88-NH2 | 333/~440 | 60–100 μM | 10 μM | 5 min | Aqueous solution | -- | [87] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Y.; Chen, S.; Zhou, Y.; Zhang, Y.; Xu, M. Recent Progress in Metal–Organic Framework (MOF) Based Luminescent Chemodosimeters. Nanomaterials 2019, 9, 974. https://doi.org/10.3390/nano9070974
Hao Y, Chen S, Zhou Y, Zhang Y, Xu M. Recent Progress in Metal–Organic Framework (MOF) Based Luminescent Chemodosimeters. Nanomaterials. 2019; 9(7):974. https://doi.org/10.3390/nano9070974
Chicago/Turabian StyleHao, Yuanqiang, Shu Chen, Yanli Zhou, Yintang Zhang, and Maotian Xu. 2019. "Recent Progress in Metal–Organic Framework (MOF) Based Luminescent Chemodosimeters" Nanomaterials 9, no. 7: 974. https://doi.org/10.3390/nano9070974
APA StyleHao, Y., Chen, S., Zhou, Y., Zhang, Y., & Xu, M. (2019). Recent Progress in Metal–Organic Framework (MOF) Based Luminescent Chemodosimeters. Nanomaterials, 9(7), 974. https://doi.org/10.3390/nano9070974