SPIONs Prepared in Air through Improved Synthesis Methodology: The Influence of γ-Fe2O3/Fe3O4 Ratio and Coating Composition on Magnetic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methodology
2.2.1. SPIONs Synthesis
2.2.2. SPIONs Coating
2.2.3. Characterization Techniques
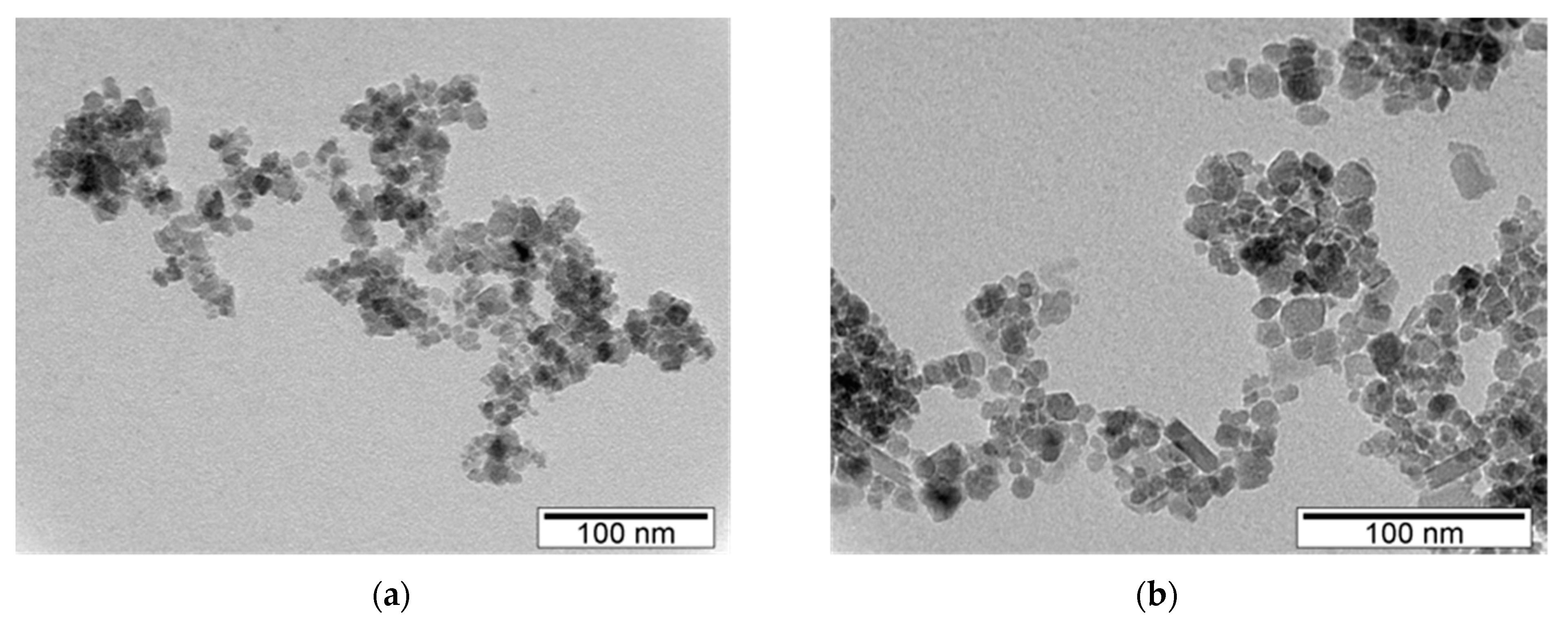
Transmission Electron Microscopy (TEM)
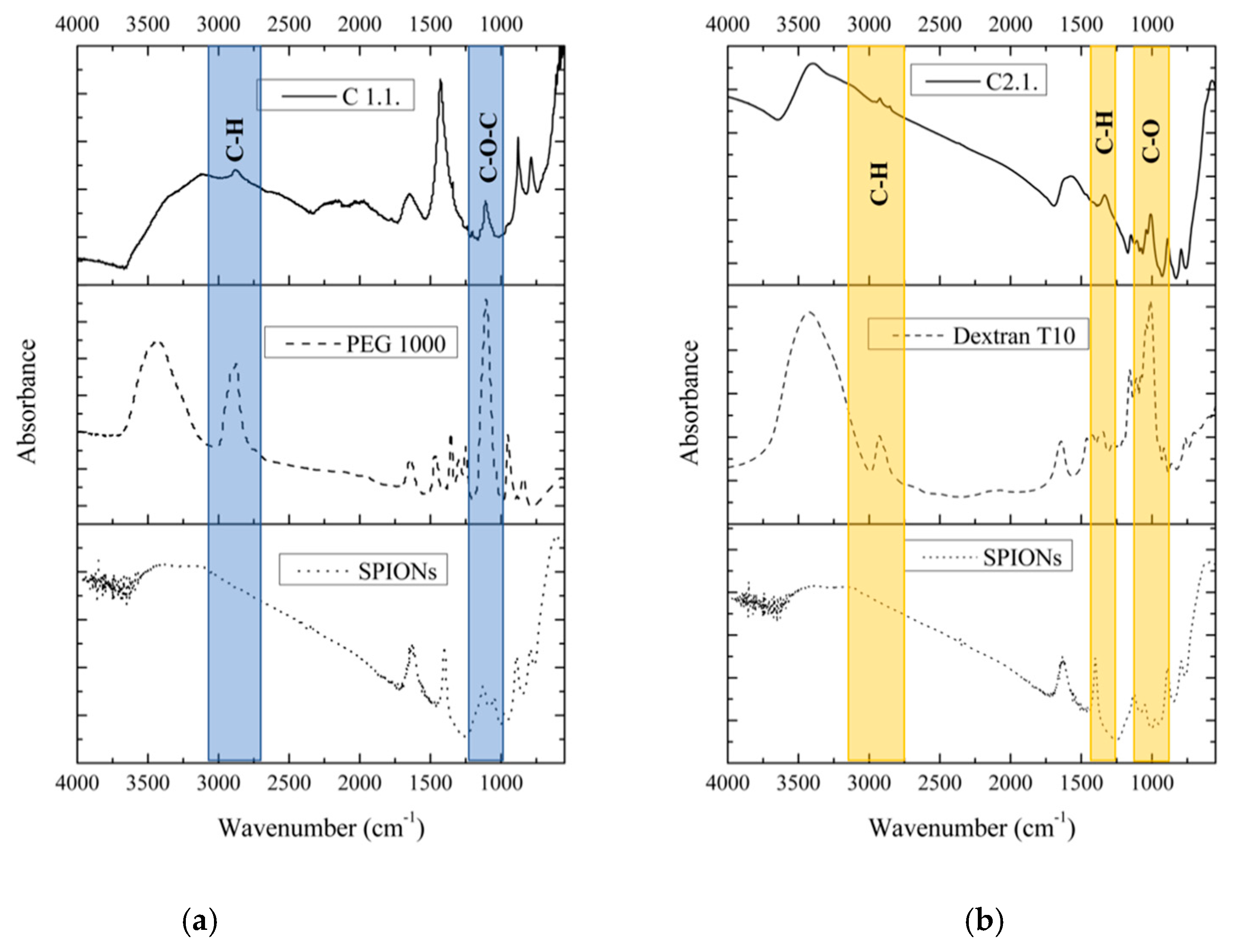
Fourier Transform Infrared (FTIR) Spectroscopy
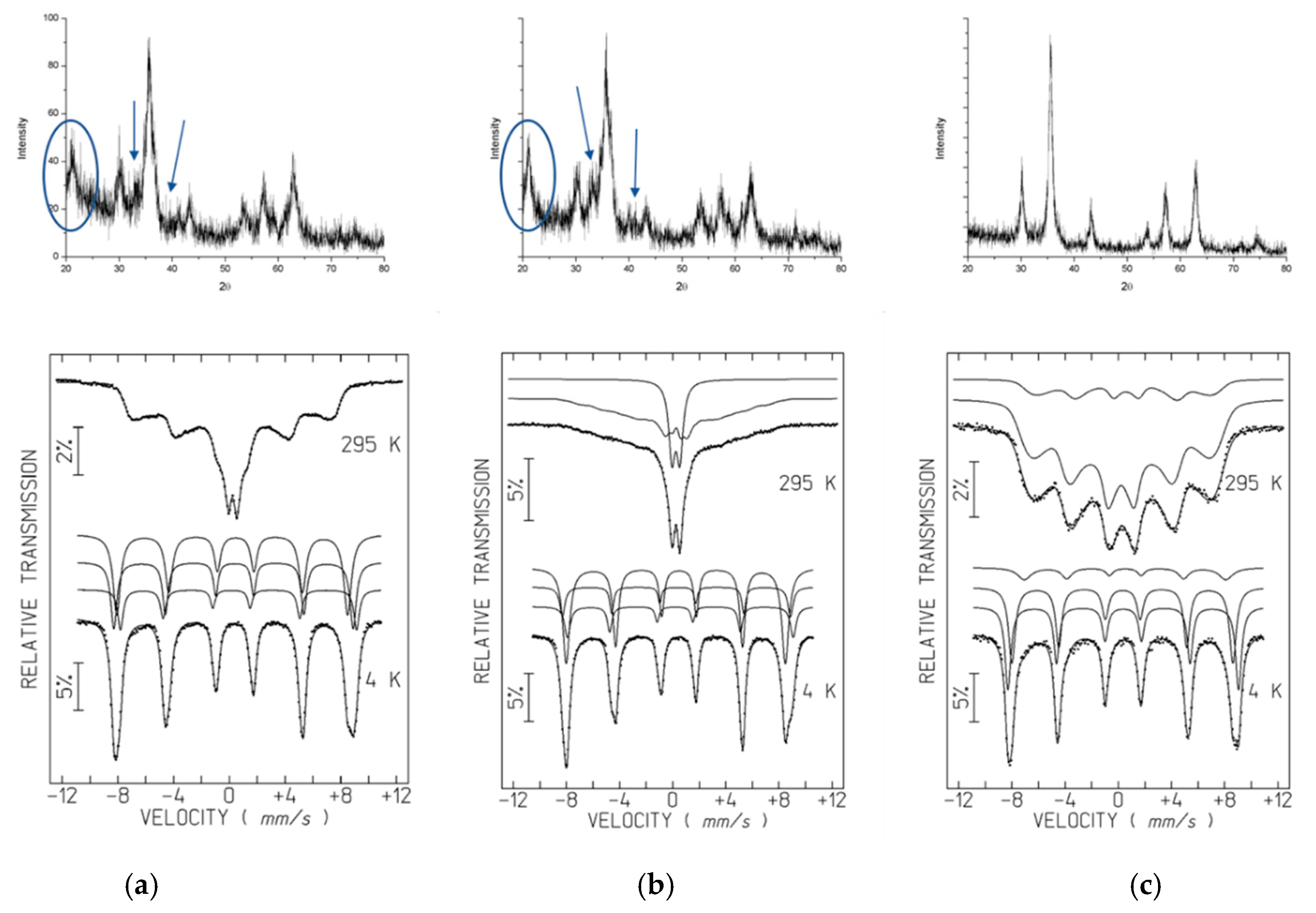
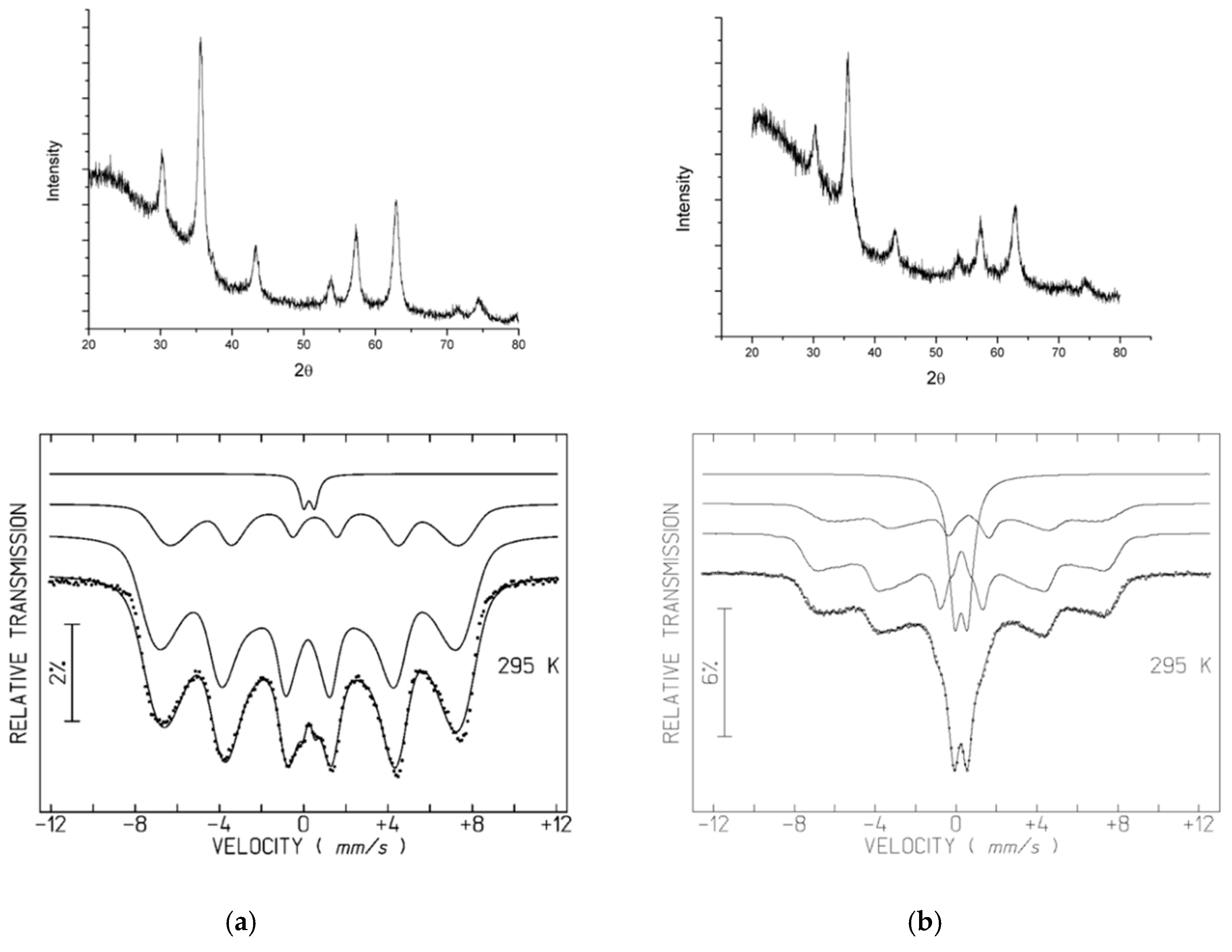
Powder X-ray Diffraction (XRD)
Mössbauer Spectroscopy
Magnetization Measurements
3. Results
3.1. Size, Morphology and Structural Analysis
3.2. XRD and Mössbauer Characterization
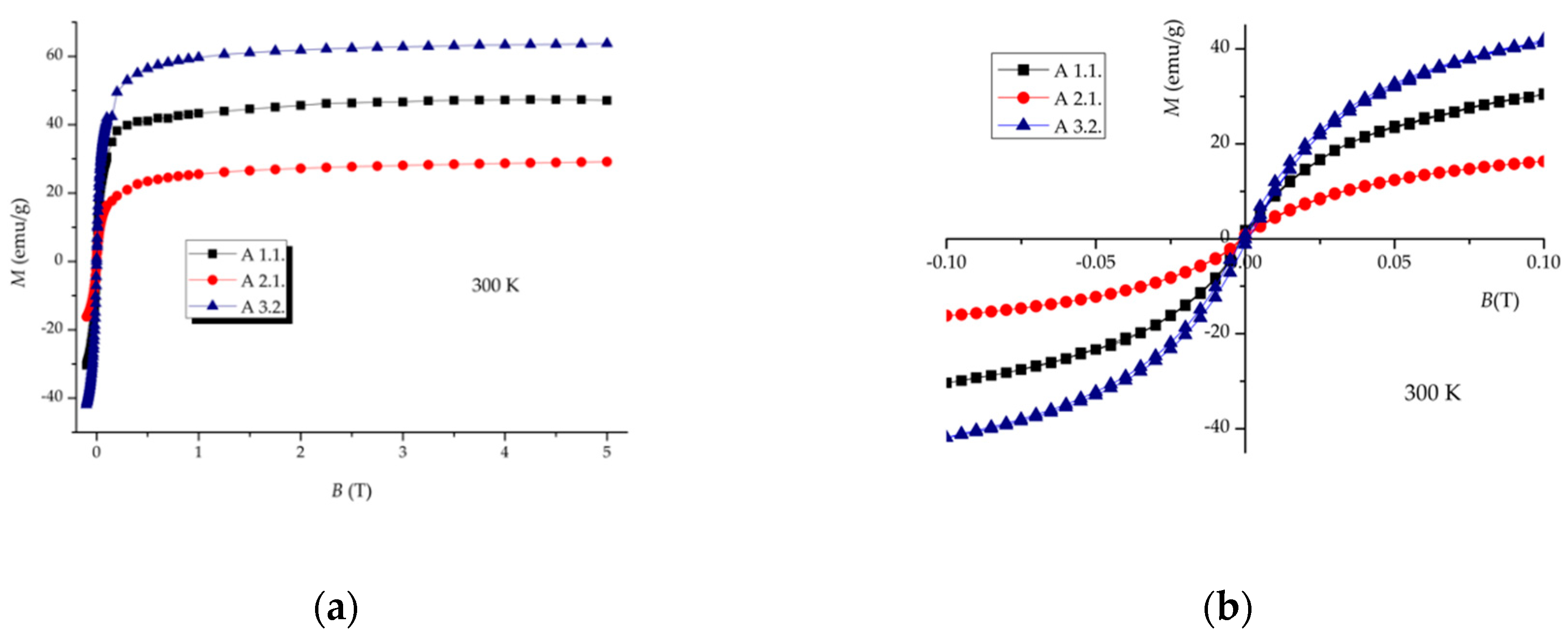
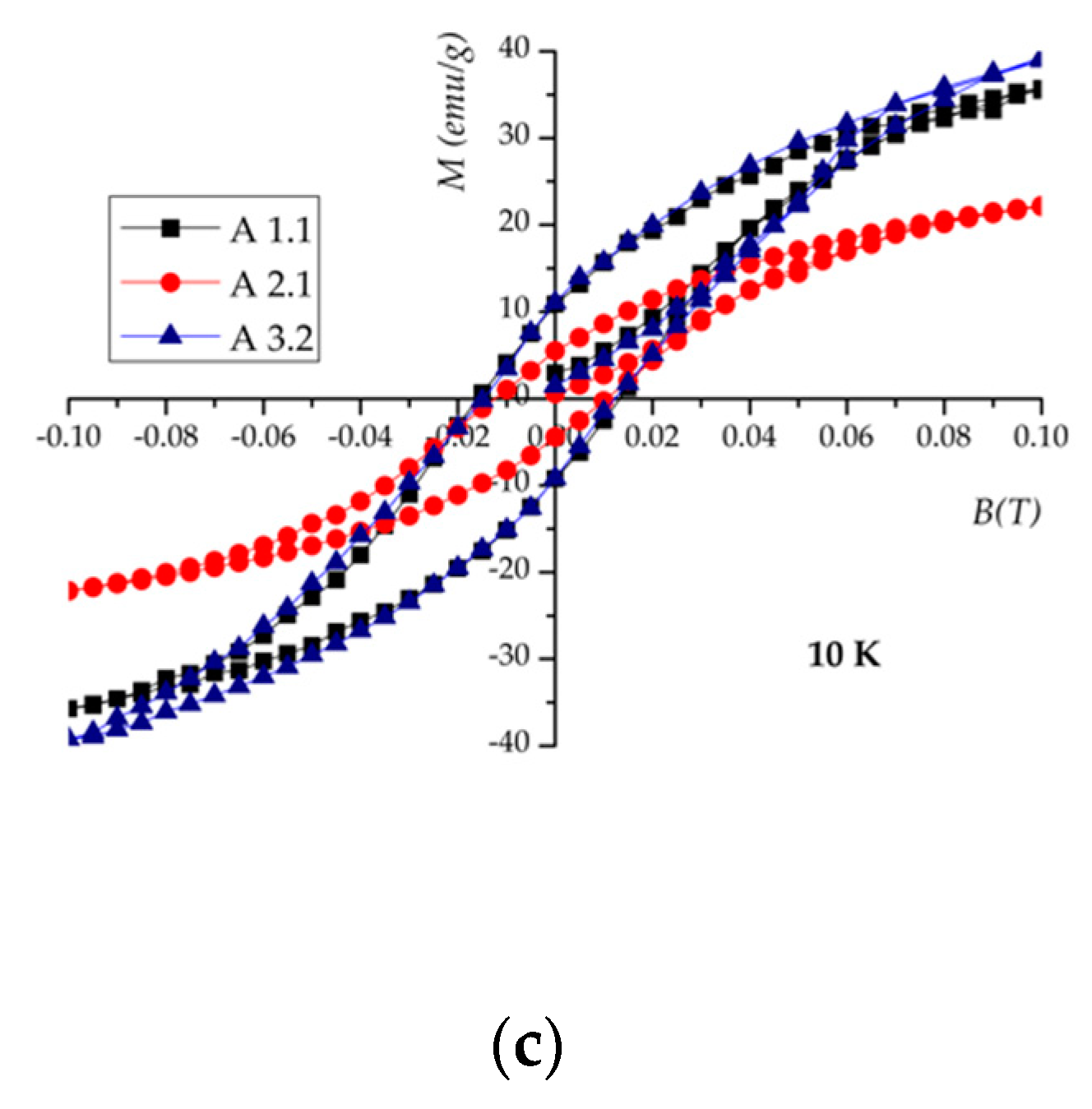
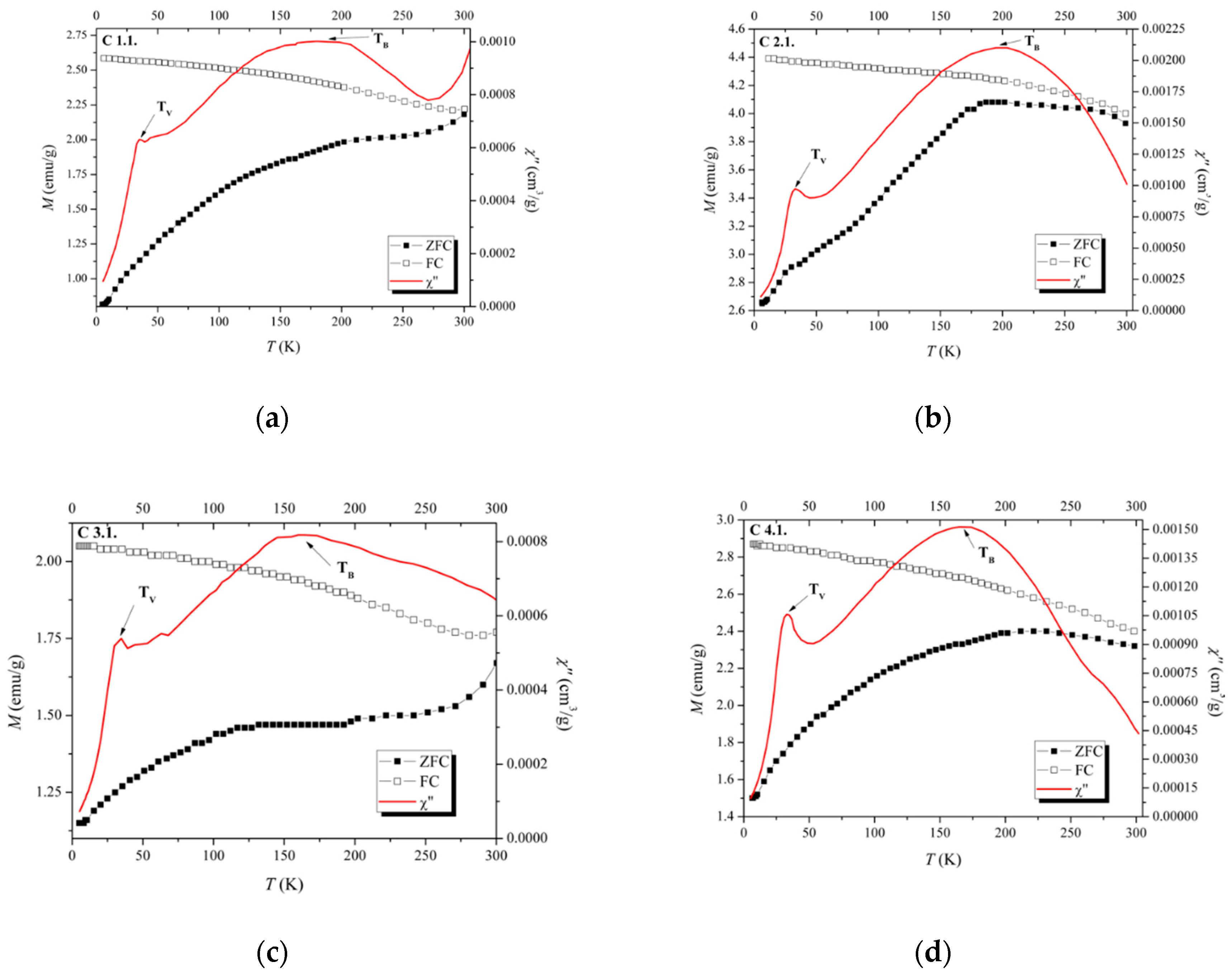
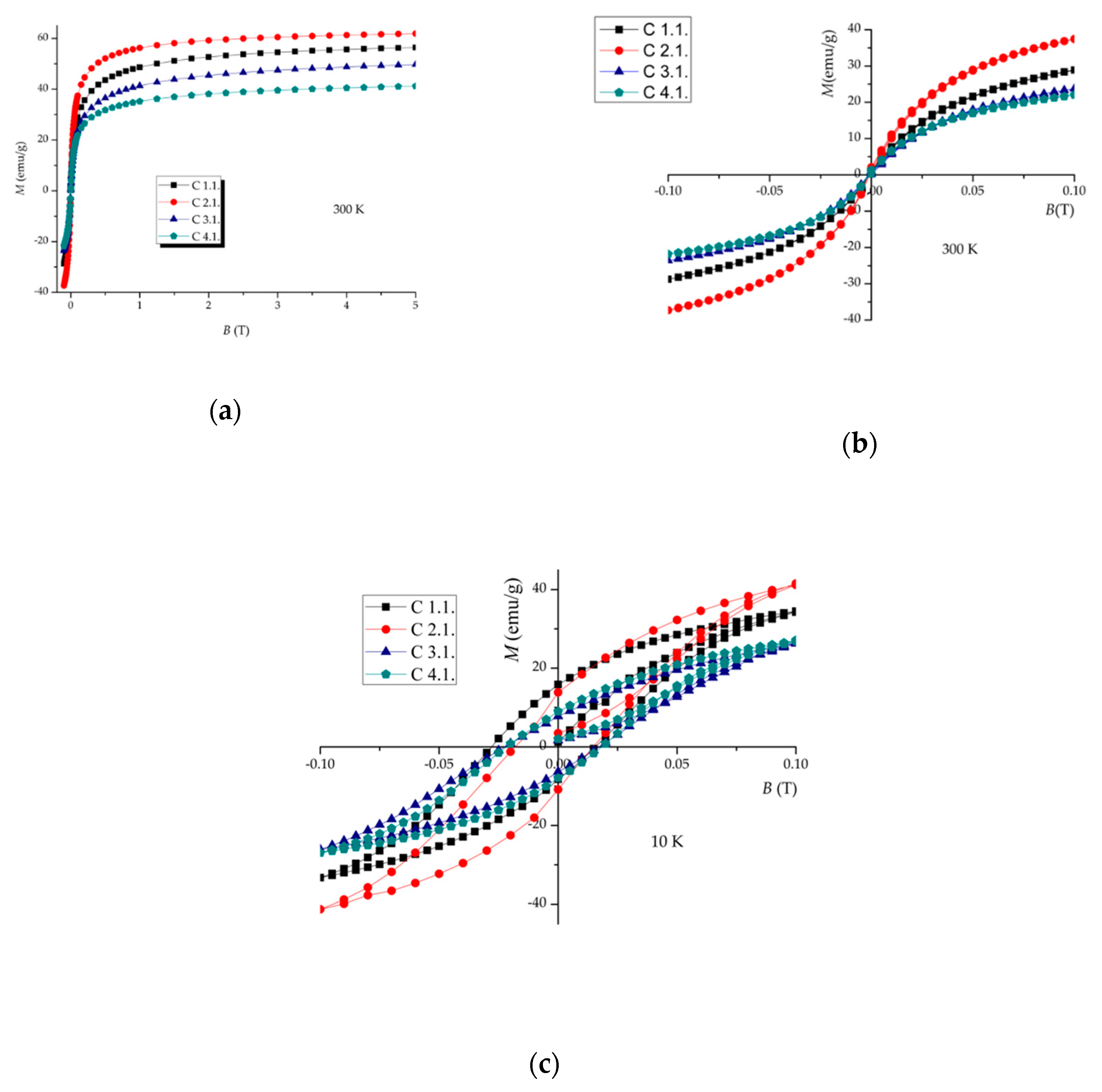
3.3. Magnetic Properties Evaluation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kang, T.; Li, F.; Baik, S.; Shao, W.; Ling, D. Surface design of magnetic nanoparticles for stimuli-responsive cancer imaging and therapy. Biomaterials 2017, 136, 98–114. [Google Scholar] [CrossRef]
- Li, R.; Liu, B.; Gao, J. The application of nanoparticles in diagnosis and theranostics of gastric cancer. Cancer Lett. 2017, 386, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, P.; Artursson, P. Oral absorption of peptides and nanoparticles across the human intestine: Opportunities, limitations and studies in human tissues. Adv. Drug Deliv. Rev. 2016, 106, 256–276. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Mirando, A.C.; Popel, A.S.; Green, J.J. Gene Delivery nanoparticles to modulate angiogenesis. Adv. Drug Deliv. Rev. 2017, 119, 20–43. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.-M.; Lee, K. Nanomaterials for Theranostics: Recent Advances and future challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. 2014, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Thakor, A.S.; Gambhir, S.S. Nanooncology: The future of cancer diagnosis and therapy. CA Cancer J. Clin. 2013, 63, 395–418. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.; Mitragotri, S.; Tong, S. Multifunctional nanoparticles for drug delivery and molecular imaging. Annu. Rev. Biomed. Eng. 2013, 15, 253–282. [Google Scholar] [CrossRef]
- Mura, S.; Couvreur, P. Nanotheranostics for personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1394–1416. [Google Scholar] [CrossRef]
- Prijic, S.; Sersa, G. Magnetic nanoparticles as targeted delivery systems in oncology. Radiol. Oncol. 2011, 45, 1–16. [Google Scholar] [CrossRef]
- Veiseh, O.; Gunn, J.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef] [PubMed]
- Majidi, S.; Sehrig, F.Z.; Samiei, M.; Milani, M.; Abbasi, E.; Dadashzadeh, K.; Akbarzadeh, A. Magnetic nanoparticles: Applications in gene delivery and gene therapy. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Domingues, I.; Gonçalves, M.C. Core-shell superparamagnetic nanoparticles with interesting properties as contrast agents for MRI. Mater. Chem. Phys. 2015, 168, 42–49. [Google Scholar] [CrossRef]
- Shen, Z.; Wu, A.; Chen, X. Iron oxide nanoparticles based contrast agents for magnetic resonance imaging. Mol. Pharm. 2017, 14, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Usov, N.A.; Nesmeyanov, M.S.; Tarasov, V.P. Magnetic vortices as efficient nano heaters in magnetic nanoparticle hyperthermia. Sci. Rep. 2018, 8, 1224. [Google Scholar] [CrossRef] [PubMed]
- Castellani, S.; Orlando, C.; Carbone, A.; Gioia, S.D.; Conese, M. Magnetofection enhances lentiviral-mediated transduction of airway epithelial cells through extracellular and cellular barriers. Genes 2016, 7, 103. [Google Scholar] [CrossRef]
- Zhuang, J.; Fan, K.; Gao, L.; Lu, D.; Feng, J.; Yang, D.; Gu, N.; Zhang, Y.; Liang, M.; Yan, X. Ex vivo detection of iron oxide magnetic nanoparticles in mice using their intrinsic peroxidase-mimicking activity. Mol. Pharm. 2012, 9, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, B.S.; Chen, B.-H. In vitro removal of toxic heavy metals by poly (γ-glutamic acid)-coated superparamagnetic nanoparticles. Int. J. Nanomed. 2012, 7, 4419–4432. [Google Scholar] [CrossRef][Green Version]
- Gao, Y.; Lim, J.; Teoh, S.-H.; Xu, C. Emerging translational research on magnetic nanoparticles for regenerative medicine. Chem. Soc. Rev. 2015, 44, 6306–6329. [Google Scholar] [CrossRef] [PubMed]
- Sneider, A.; VanDyke, D.; Paliwal, S.; Rai, P. Remotely triggered nano-theranostics for cancer applications. Nanotheranostics 2017, 1, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Wadajkar, A.S.; Menon, J.U.; Kadapure, T.; Tran, R.T.; Yang, J.; Nguyen, K.T. Design and application of magnetic-based theranostic nanoparticle systems. Recent Pat. Biomed. Eng. 2013, 6, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.; Fries, L. The role of complement in inflammation and phagocytosis. Immunol. Today 1991, 12, 322–326. [Google Scholar] [CrossRef]
- Gonçalves, M.C. Sol-Gel Silica Nanoparticles in Medicine: A natural choice. Design, synthesis and products. Molecules 2018, 23, 2021. [Google Scholar] [CrossRef]
- Gref, R.; Minamitake, Y.; Peracchia, M.T.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable long circulating polymeric nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.N. Innate and adaptive immunity: The immune response to foreign materials. In Biomaterials Science: An. Introduction to Materials in Medicine; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2004; pp. 304–318. [Google Scholar]
- Berry, C.C.; Wells, S.; Charles, S.; Curtis, A.S.G. Dextran and Albumin derivatised iron oxide nanoparticles: Influence on fibroblasts in vitro. Biomaterials 2003, 24, 4551–4557. [Google Scholar] [CrossRef]
- Chouly, C.; Pouliquen, D.; Lucet, I.; Jeune, J.J.; Jallet, P. Development of superparamagnetic nanoparticles for MRI: Effect of particle size, charge and surface nature on biodistribution. J. Microencapsul. 1996, 3, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Gaur, U.; Sahoo, S.K.; De, T.K.; Ghosh, P.C.; Maitra, A.; Ghosh, P.K. Biodistribution of fluoreceinated dextran using novel nanoparticles evading reticuloendothelial system. Int. J. Pharm. 2002, 202, 1–10. [Google Scholar] [CrossRef]
- Khiabani, S.S.; Farshbaf, M.; Akbarzadeh, A.; Davaran, S. Magnetic nanoparticles: Preparation methods, applications in cancer diagnosis and cancer therapy. Artif. Cells Nanomed. Biotechnol. 2017, 45, 6–17. [Google Scholar] [CrossRef]
- García-Jimeno, S.; Estelrich, J. Ferrofluid based on polyethylene glycol-coated iron oxide nanoparticles: Characterization and properties. Colloids Surf. A 2013, 420, 74–81. [Google Scholar] [CrossRef]
- Illés, E.; Szekeres, M.; Tóth, I.Y.; Farkas, K.; Földesi, I.; Szabó, A.; Iván, B.; Tombácz, E. PEGylation of superparamagnetic iron oxide nanoparticles with self-organizing polyacrylate-PEG brushes for contrast enhancement in MRI diagnosis. Nanomaterials 2018, 8, 776. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory and practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar] [PubMed]
- Duget, E.; Vasseur, S.; Mornet, S.; Devoisselle, J.M. Magnetic nanoparticles and their applications in medicine. Nanomedicine 2006, 1, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.C.; Fortes, L.M.; Pimenta, A.R.; Pereira, J.C.G.; Almeida, R.M.; Carvalho, M.D.; Ferreira, L.P.; Cruz, M.M.; Godinho, M. Silica/ormosil SPIONs for biomedical applications. Curr. Nanosci. 2013, 9, 599–608. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodriguez-Carvajal, J. WinPLOTR: A Windows tool for powder diffraction patterns analysis. Mater. Sci. Forum 2000, 378–381, 118–123. [Google Scholar] [CrossRef]
- Waerenborgh, J.C.; Rojas, D.P.; Shaula, A.L.; Mather, G.C.; Patrakeev, M.V.; Kharton, V.V.; Frade, J.R. Phase formation and iron oxidation state in SrFe(Al)O3-δ perovskites. Mater. Lett. 2005, 59, 1644–1648. [Google Scholar] [CrossRef]
- Hesse, J.; Rübartsch, A. Model independent evaluation of overlapped Mössbauer spectra. J. Phys. E 1974, 7, 526–532. [Google Scholar] [CrossRef]
- Asadi, H.; Khoee, S.; Deckers, R. Polymer-grafted superparamagnetic iron oxide nanoparticles as a potential stable system for magnetic resonance imaging and doxorubicin delivery. RSC Adv. 2016, 6, 83963. [Google Scholar] [CrossRef]
- Khairuddin; Pramono, E.; Utomo, S.B.; Wulandari, V.; Zahrotul, W.; Clegg, F. FTIR studies on the effect of concentration of polyethylene glycol on polimerization of Shellac. J. Phys. Conf. Ser. 2016, 776. [Google Scholar] [CrossRef]
- Unterwegen, H.; Subatzus, D.; Tietze, R.; Janko, C.; Poettler, M.; Stiegelschmitt, A.; Schuster, M.; Maake, C.; Boccaccini, A.R.; Alexiou, C. Hypericin-bearing magnetic iron oxide nanoparticles for selective drug delivery on photodynamic therapy. Int. J. Nanomed. 2015, 10, 6985–6996. [Google Scholar] [CrossRef]
- Mørup, S. Mössbauer effect studies of microcrystalline materials. In Mössbauer Spectroscopy Applied to Inorganic Chemistry; Long, G.J., Ed.; Plenum Press: New York, NY, USA, 1987. [Google Scholar]
- Murad, E. Clays and clay minerals: What can Mössbauer spectroscopy do to help understand them? Hyperfine Interact. 1998, 117, 39–70. [Google Scholar] [CrossRef]
- Roca, G.; Marco, J.F.; del Puerto Morales, M.; Serna, C.J. Effect of nature and particle size on properties of uniform magnetite and maghemite nanoparticles. J. Phys. Chem. C 2007, 111, 18577–18584. [Google Scholar] [CrossRef]
- Predoi, D.; Kuncser, V.; Tronc, E.; Nogues, M.; Russo, U.; Principi, G.; Filoti, G. Magnetic relaxation phenomena and inter-particle interactions in nanosized γ-Fe2O3 systems. J. Phys. Condens. Matter. 2003, 15, 1797–1811. [Google Scholar] [CrossRef]
- Murad, E.; Johnston, J.H. Iron oxides and oxyhydroxides. In Mossbauer Spectroscopy Applied to Inorganic Chemistry; Long, G.J., Ed.; Plenum Press: New York, NY, USA, 1987. [Google Scholar]
- Goya, G.F.; Berquó, T.S.; Fonseca, F.C.; Morales, M.P. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Appl. Phys. 2003, 94, 3520–3528. [Google Scholar] [CrossRef]
- Vandenberghe, R.E.; Barrero, C.A.; da Costa, G.M.; Van San, E.; De Grave, E. Mössbauer characterization of iron oxides and (oxy)hydroxides: The present state of the art. Hyperfine Interact. 2000, 126, 247–259. [Google Scholar] [CrossRef]
- Hong, J.; Xu, D.; Yu, J.; Gong, P.; Ma, H.; Yao, S. Facile synthesis of polymer-enveloped ultrasmall superparamagnetic iron oxide for magnetic resonance imaging. Nanotechnology 2007, 18, 135608. [Google Scholar] [CrossRef]


| Sample | Washing | Drying |
|---|---|---|
| A 1.1 | Centrifuged | Air |
| A 2.1 | Sedimented | Air |
| A 3.2 | Centrifuged | Vacuum |
| Sample | Sample Type | Synthesis |
|---|---|---|
| B 1.1 | SPIONs + PEG1000 | Method 1 |
| B 2.2 | SPIONs + Dextran T10 | Method 1 |
| C 1.1 | SPIONs + PEG1000 | Method 2 |
| C 2.1 | SPIONs + Dextran T10 | Method 2 |
| C 3.1 | SPIONs + PEG6000 | Method 2 |
| C 4.1 | SPIONs + Dextran T70 | Method 2 |
| Sample | TEMD (nm) | Phases Identified by XRD | Phases Identified by Mossbauer |
|---|---|---|---|
| A 1.1 | 10.5 ± 3.1 | goethite spinel | goethite maghemite |
| A 2.1 | 8.1 ± 1.9 | goethite spinel | goethite maghemite |
| A 3.2 | 7.5 ± 1.5 | goethite spinel | goethite maghemite magnetite |
| B 1.1 | 4.4 ± 1.1 | Goethite Ferrihydrite | Goethite Ferrihydrite |
| B 2.2 | 1.1 ± 0.4 | Ferrihydrite | Ferrihydrite |
| C 1.1 | 7.9 ± 2.3 | Spinel | Maghemite Magnetite |
| C 2.1 | 8.5 ± 1.9 | Spinel | Maghemite Magnetite |
| C 3.1 | 7.6 ± 2.1 | Spinel | Maghemite Magnetite |
| C 4.1 | 7.7 ± 2.0 | Spinel | Maghemite Magnetite |
| Sample | T | IS | QS | Bhf or <Bhf> | I | Fe Species |
|---|---|---|---|---|---|---|
| A1.1 | 295 K | 0.35 | −0.03 | 25.4 | 100% | * |
| A1.1 | 4 K | 0.50 0.45 0.45 | 0.52 −0.07 −0.25 | 52.5 53.3 51.4 | 16% 34% 50% | Fe3+ CN = 6 γ Fe2O3 Fe3+ CN = 4 γ Fe2O3 Fe3+ CN = 6 α FeOOH |
| A2.1 | 295 K | 0.35 0.32 | 0.60 −0.11 | −23.5 | 32% 68% | * |
| A2.1 | 4 K | 0.49 0.44 0.47 | 0.40 −0.10 −0.25 | 52.7 53.3 51.2 | 18% 15% 67% | Fe3+ CN = 6 γ Fe2O3 Fe3+ CN = 4 γ Fe2O3 Fe3+ CN = 6 α FeOOH |
| A3.2 | 295 K | 0.33 0.65 | 0.03 −0.18 | 30.6 33.3 | 84% 16% | Fe3+ CN = 4, 6 γ Fe2O3, Fe3O4 Fe2.5+ CN = 6 Fe3O4 |
| A3.2 | 4 K | 0.49 0.43 0.93 | 0.02 −0.03 0.12 | 53.7 51.5 46.2 | 39% 51% 10% | Fe3+ CN = 6 γ Fe2O3 Fe3+ CN = 4, 6 γ Fe2O3, Fe3O4 Fe2+ Fe3O4 |
| B1.1 | 295 K | 0.37 | 0.76 | − | 100% | Fe3+ CN = 6 Ferrihydrite, αFeOOH |
| B1.1 | 4 K | 0.50 0.49 0.48 | 0.35 −0.10 −0.25 | 50.3 46.3 50.5 | 9% 69% 22% | Fe3+ CN = 6 Ferrihydrite Fe3+ CN = 6 Ferrihydrite Fe3+ CN = 6 α FeOOH |
| B2.2 | 295 K | 0.37 | 0.75 | - | 100% | Fe3+ CN = 6 Ferrihydrite |
| B2.2 | 4 K | 0.50 0.49 | −0.04 −0.11 | 49.5 45.8 | 36% 64% | Fe3+ CN = 6 Ferrihydrite Fe3+ CN = 6 Ferrihydrite |
| C1.1 | 295 K | 0.35 0.64 0.35 | 0.01 −0.12 0.60 | 34.7 33.8 − | 91% 7% 2% | Fe3+ CN=4, 6 γ Fe2O3, Fe3O4 Fe2.5+ CN = 6 Fe3O4 Fe3+ CN = 4,6 γ Fe2O3 |
| C2.1 | 295 K | 0.33 0.65 0.36 | −0.02 0.02 0.47 | 34.3 49.1 − | 84% 14% 2% | Fe3+ CN=4, 6 γ Fe2O3, Fe3O4 Fe2.5+ CN = 6 Fe3O4 Fe3+ CN = 4,6 γ Fe2O3 |
| C3.1 | 295 K | 0.37 0.65 0.30 | −0.03 0.29 0.66 | 33.4 33.4 − | 90% 7% 3% | Fe3+ CN=4, 6 γ Fe2O3, Fe3O4 Fe2.5+ CN = 6 Fe3O4 Fe3+ CN = 4,6 γ Fe2O3 |
| C4.1 | 295 K | 0.29 0.64 0.37 | 0.11 −0.19 0.64 | 31.8 32.3 − | 42% 31% 27% | Fe3+ CN=4, 6 γ Fe2O3, Fe3O4 Fe2.5+ CN = 6 Fe3O4 Fe3+ CN = 4, 6 γ Fe2O3 |
| Sample | Ms * (emu/g) (300 K) | Ms * (emu/g) (10 K) | TB ** (K) | Tv ** (K) |
|---|---|---|---|---|
| A 1.1 | 47 | 56.5 | 126 | - |
| A 2.1 | 29 | 36.5 | 78.5 | - |
| A 3.2 | 64 | 71 | 150 | 37.7 |
| B 1.1 | ~8 | 13 | 112 | - |
| B 2.2 | ~1.4 | 4.5 | 20 | - |
| C 1.1 | ~56 | 65.5 | ~180 | 35.0 |
| C 2.1 | ~62 | 69.0 | ~194 | 32.8 |
| C 3.1 | ~50 | 58.0 | ~165 | 34.8 |
| C 4.1 | ~41 | 48.5 | ~160 | 32.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, J.C.; Gonçalves, M.C.; Pereira, L.C.J.; Vieira, B.J.C.; Waerenborgh, J.C. SPIONs Prepared in Air through Improved Synthesis Methodology: The Influence of γ-Fe2O3/Fe3O4 Ratio and Coating Composition on Magnetic Properties. Nanomaterials 2019, 9, 943. https://doi.org/10.3390/nano9070943
Matos JC, Gonçalves MC, Pereira LCJ, Vieira BJC, Waerenborgh JC. SPIONs Prepared in Air through Improved Synthesis Methodology: The Influence of γ-Fe2O3/Fe3O4 Ratio and Coating Composition on Magnetic Properties. Nanomaterials. 2019; 9(7):943. https://doi.org/10.3390/nano9070943
Chicago/Turabian StyleMatos, Joana C., M. Clara Gonçalves, Laura C. J. Pereira, Bruno J. C. Vieira, and João Carlos Waerenborgh. 2019. "SPIONs Prepared in Air through Improved Synthesis Methodology: The Influence of γ-Fe2O3/Fe3O4 Ratio and Coating Composition on Magnetic Properties" Nanomaterials 9, no. 7: 943. https://doi.org/10.3390/nano9070943
APA StyleMatos, J. C., Gonçalves, M. C., Pereira, L. C. J., Vieira, B. J. C., & Waerenborgh, J. C. (2019). SPIONs Prepared in Air through Improved Synthesis Methodology: The Influence of γ-Fe2O3/Fe3O4 Ratio and Coating Composition on Magnetic Properties. Nanomaterials, 9(7), 943. https://doi.org/10.3390/nano9070943






