An Electrospun Preparation of the NC/GAP/Nano-LLM-105 Nanofiber and Its Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
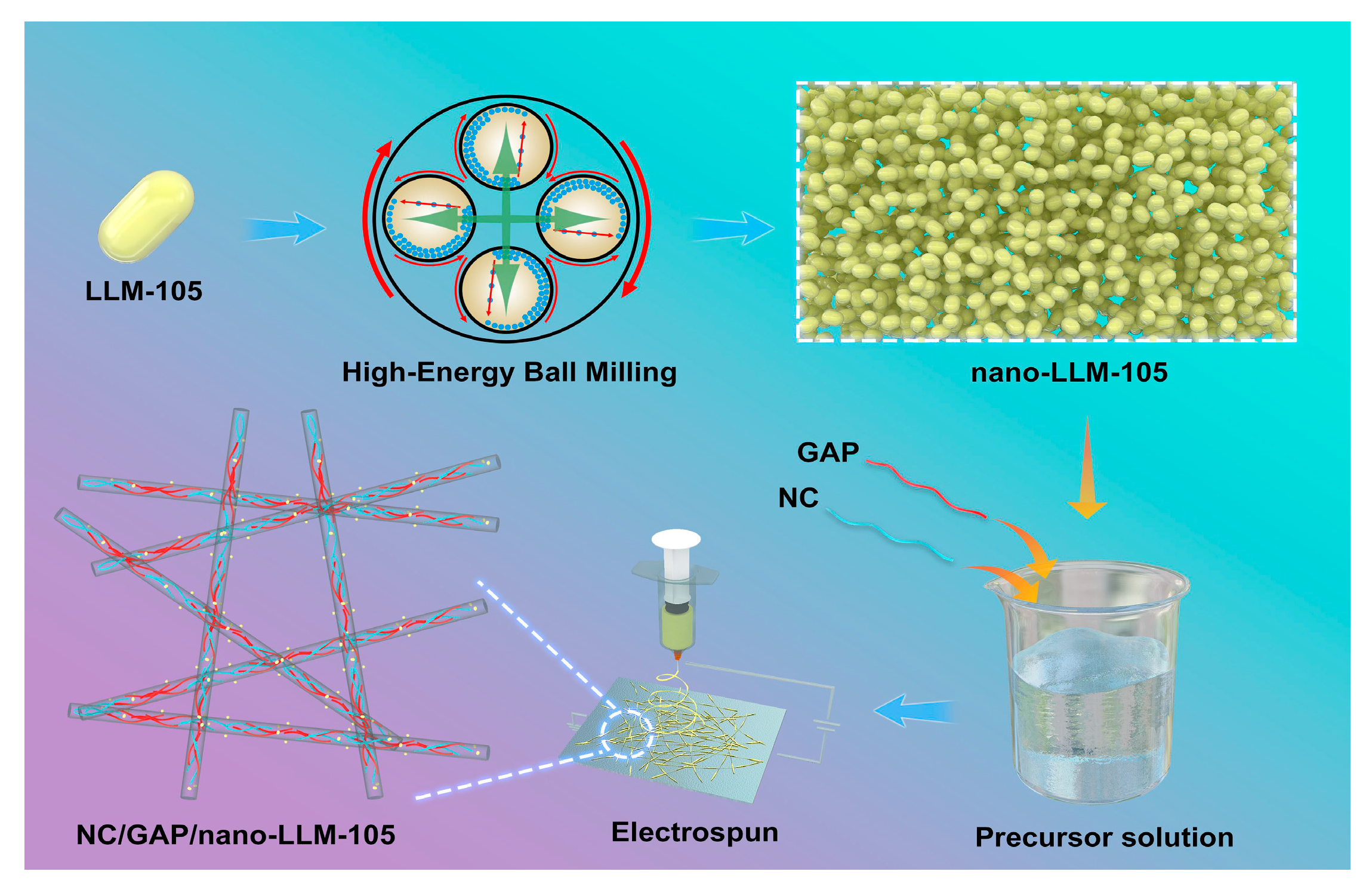
2.2. Fabrication of Nanofibers
2.3. Characterization
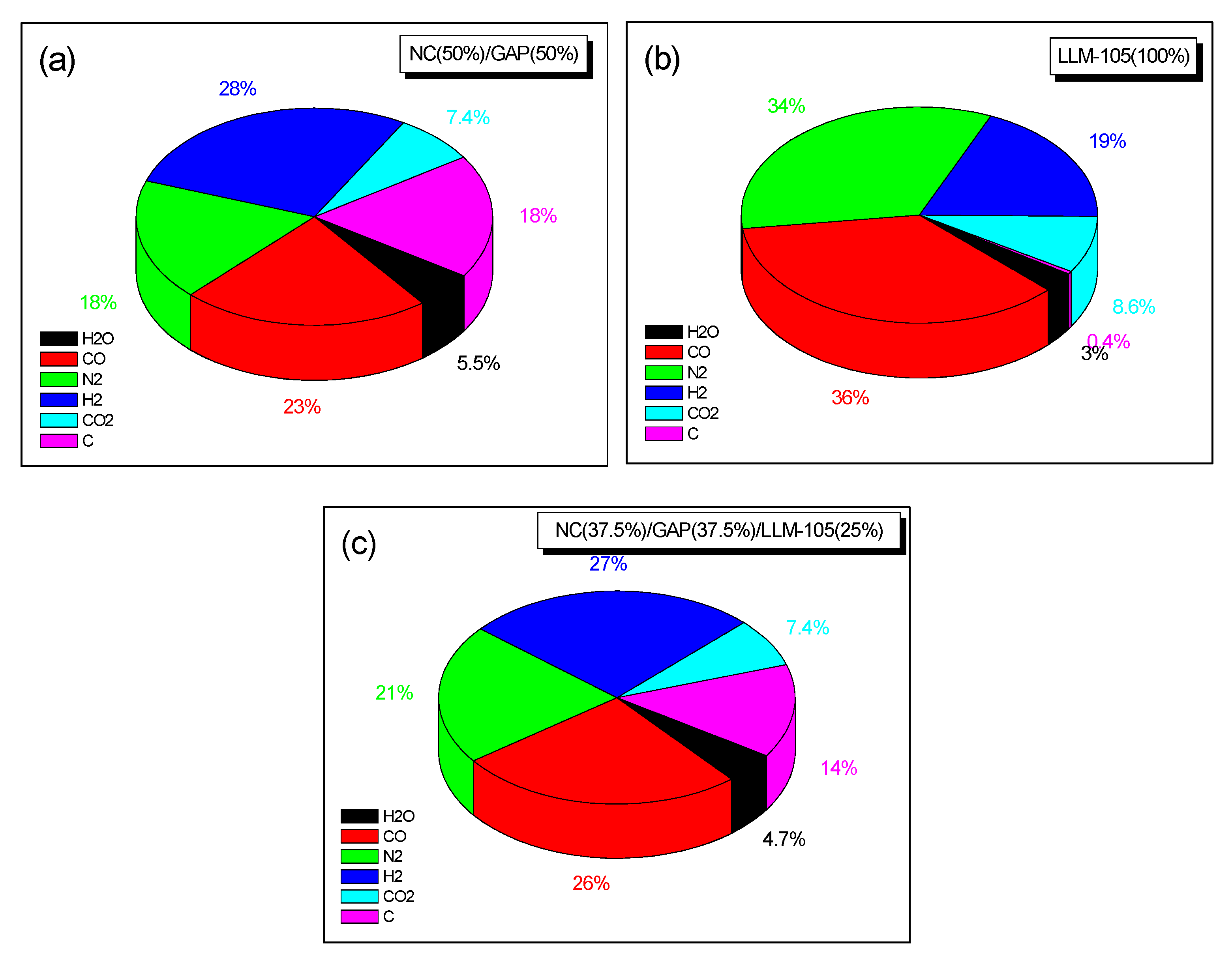
3. Results and Discussion
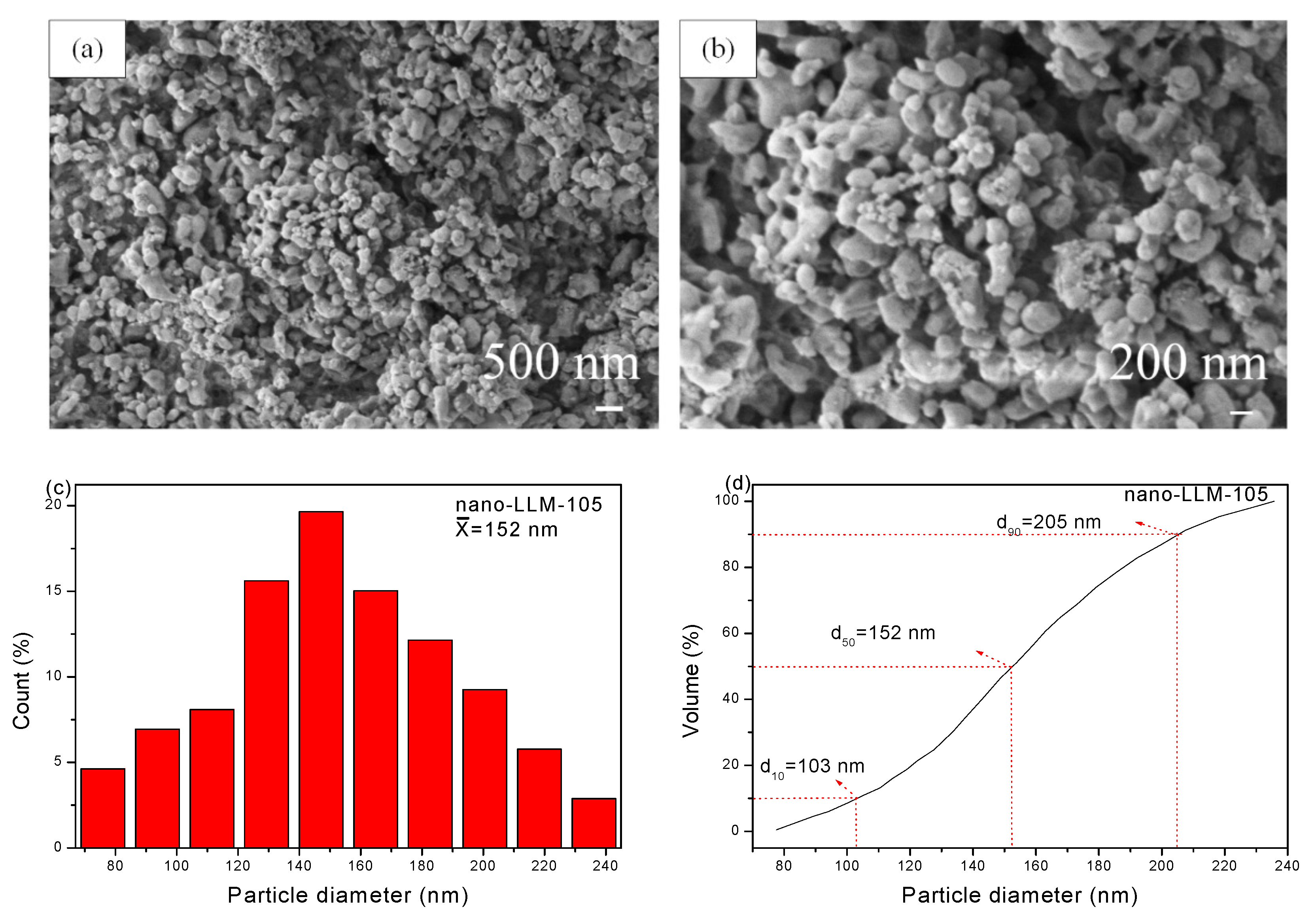
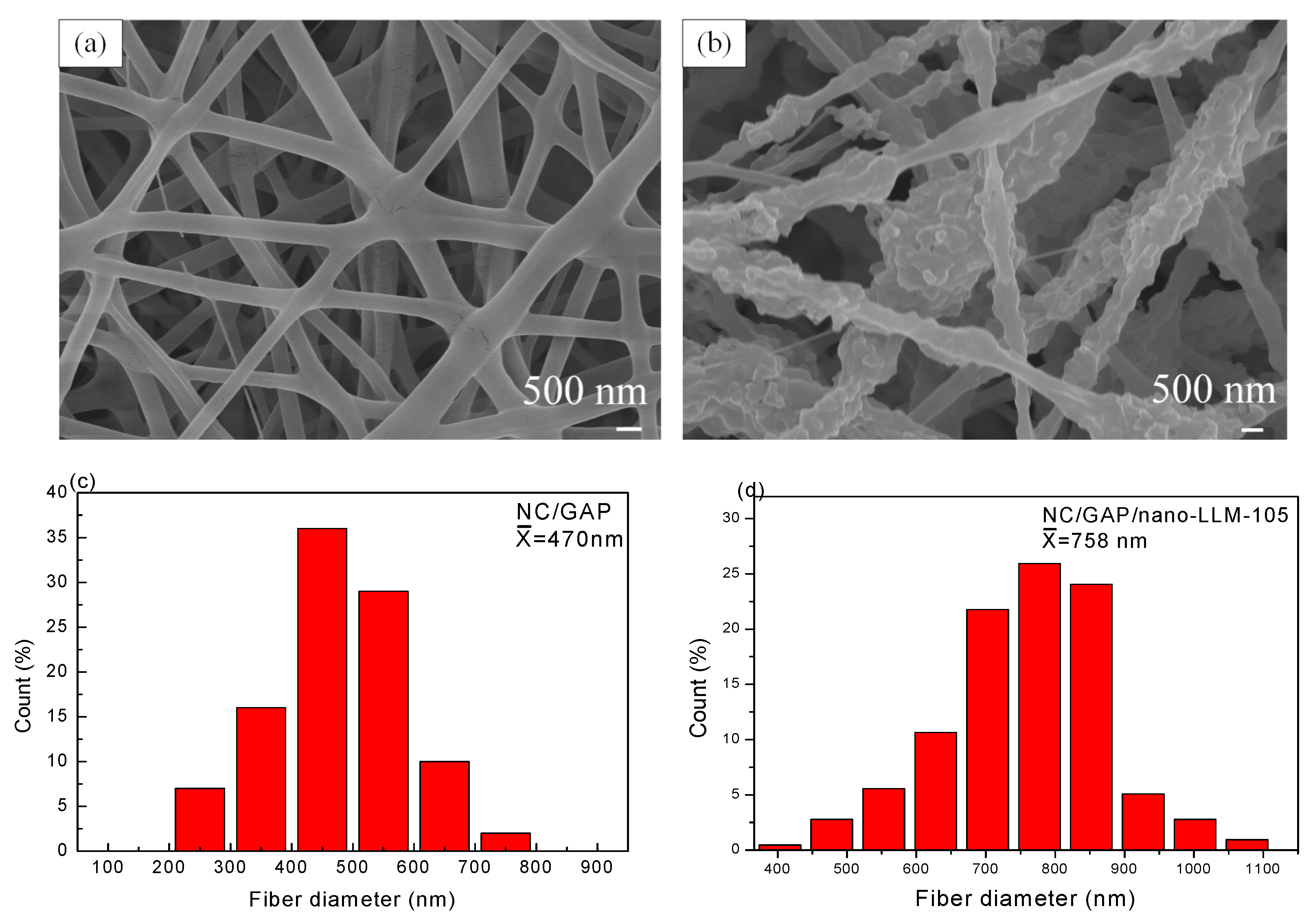
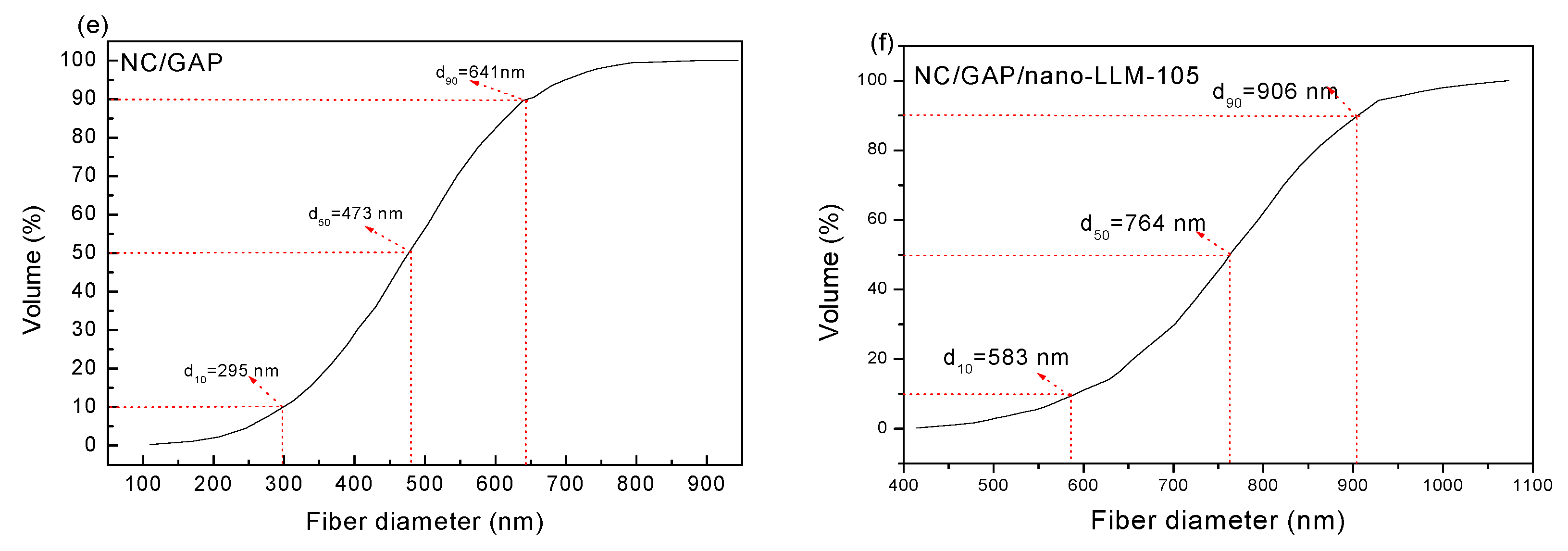
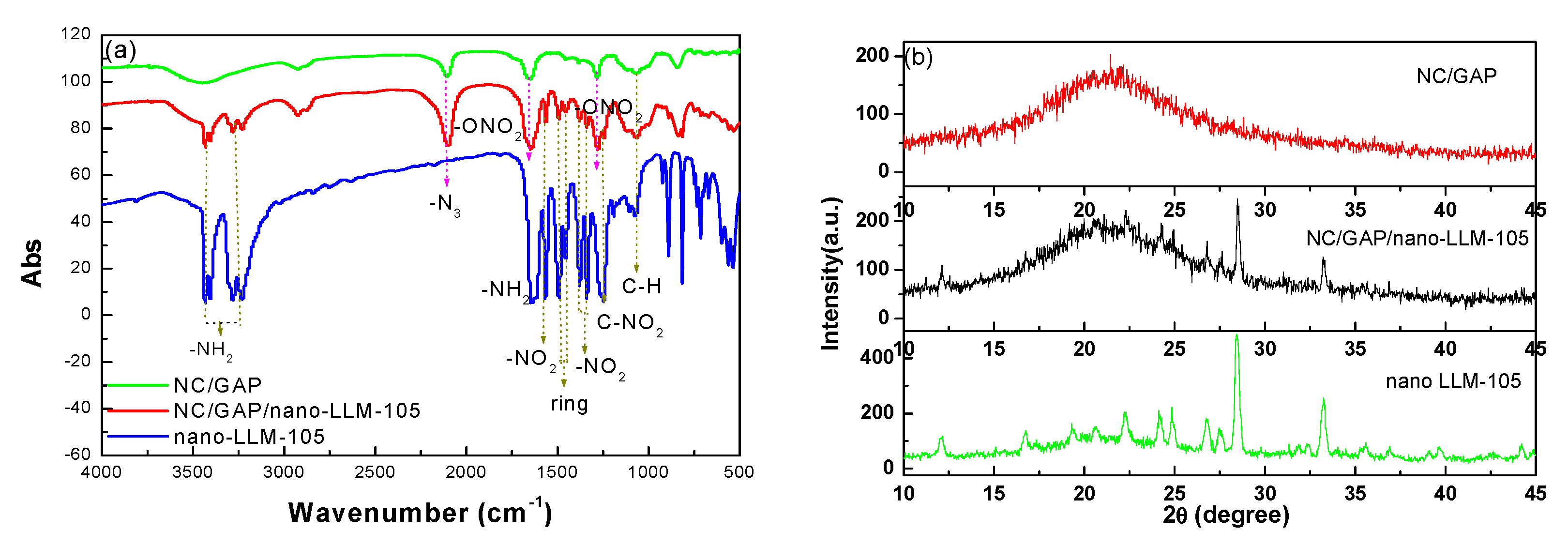
3.1. Morphology and Structure
3.2. Thermal Analysis
3.3. Energetic Performance and Sensitivities
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, W.Z.; An, C.W.; Wang, J.Y.; Dong, J.; Geng, X.H. Preparation and Properties of An Insensitive Booster Explosive Based on LLM-105. Propell. Explos. Pyrot. 2013, 38, 136–141. [Google Scholar] [CrossRef]
- Ma, H.X.; Song, J.R.; Zhao, F.Q.; Gao, H.X.; Hu, R.Z. Crystal Structure, Safety Performance and Density-Functional Theoretical Investigation of 2,6-Diamino-3, 5-dinitropyrazine-1-oxide (LLM-105). Chin. J. Chem. 2008, 26, 1997–2002. [Google Scholar] [CrossRef]
- Pagoria, P.; Zhang, M.X.; Zuckerman, N.; Lee, G.; Mitchell, A.; DeHope, A.; Clifford, G.; Patrick, C.; Gallagher, P. Synthetic Studies of 2,6-Diamino-3, 5-Dinitropyrazine-1-Oxide (LLM-105) from Discovery to Multi-Kilogram Scale. Propell. Explos. Pyrot. 2017, 42, 1–14. [Google Scholar] [CrossRef]
- Tarver, C.M.; Urtiew, P.A.; Tran, T.D. Sensitivity of 2,6-Diamino-3, 5- Dinitropyrazine-1-Oxide. J. Energ. Mater. 2005, 23, 183–203. [Google Scholar] [CrossRef]
- Manaa, M.R.; Kuo, I.F.W.; Fried, L.E. First-principles high-pressure unreacted equation of state and heat of formation of crystal 2,6-diamino-3, 5-dinitropyrazine-1-oxide (LLM-105). J. Chem. Phys. 2014, 141, 064702. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.W.; Sue, G.; Taylor, N.E.; Walley, S.M.; Jardine, A.P.; Glauser, A.; French, S.; Wortley, S. Characterisation of the impact response of energetic materials: observation of a lowlevel reaction in 2,6-diamino-3,5-dinitropyrazine-1-oxide (LLM-105). RSC Adv. 2016, 10, 27896–27900. [Google Scholar] [CrossRef]
- Huang, C.; Liu, J.; Ding, L.; Wang, D.J.; Yang, Z.J.; Nie, F. Facile Fabrication of Nanoparticles Stacked 2,6-diamino-3,5-dinitropyrazine-1-oxide(LLM-105) Sub-microspheres via Electrospray Deposition. Propell. Explos. Pyrot. 2017, 42, 1–7. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, P.; Yang, Z.J.; Gao, B.; Zhang, J.H.; Wang, P.; Nie, F.; Longyu Liao, L.H. Preparation and Properties of Submicrometer-Sized LLM-105 via Spray-Crystallization Method. Propell. Explos. Pyrot. 2014, 39, 653–657. [Google Scholar] [CrossRef]
- Deng, P.; Liu, Y.; Luo, P.; Wang, J.X.; Liu, Y.; Wang, D.J.; He, Y. Two-steps synthesis of sandwich-like graphene oxide/LLM-105 nanoenergetic composites using functionalized graphene. Mater. Lett. 2017, 194, 156–159. [Google Scholar] [CrossRef]
- Zhuang, X.B.; Huang, B.; Gao, B.; Qiao, Z.Q. Preparation of rectangular micro-rods by nano-LLM-105 self-assembly. Chin. J. Energ. Mater. 2016, 24, 433–438. [Google Scholar] [CrossRef]
- Kucheyev, S.O.; Gash, A.E.; Lorenz, T. Deformation and fracture of LLM-105 molecular crystals studied by nanoindentation. Mater. Res. Express. 2014, 1, 025036. [Google Scholar] [CrossRef]
- Zuckerman, N.B.; Shusteff, M.; Pagoria, P.F.; Gash, A.E. Microreactor flow synthesis of the secondary high explosive 2, 6-diamino-3, 5-dinitropyrazine-1- oxide (LLM-105). J. Flow Chem. 2015, 5, 178–182. [Google Scholar] [CrossRef]
- Babu, V.J.; Nair, A.S.; Zhu, P.N.; Ramakrishna, S. Synthesis and characterization of rice grains like Nitrogen-doped TiO2 nanostructures by electrospinning photocatalysis. Mater. Lett. 2011, 65, 3064–3068. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, B.H.; Yang, K.S. TiO2 nanoparticles loaded on graphene/carbon composite nanofibers by electrospinning for increased photocatalysis. Carbon 2012, 50, 2472–2481. [Google Scholar] [CrossRef]
- He, T.S.; Zhou, Z.F.; Xu, W.B.; Ren, F.M.; Ma, H.H.; Wang, J. Preparation and photocatalysis of TiO2–fluoropolymer electrospun fiber nanocomposites. Polymer 2009, 50, 3031–3036. [Google Scholar] [CrossRef]
- An, S.; Joshi, B.N.; Lee, M.W.; Kim, N.Y.; Yoon, S.S. Electrospun graphene-ZnO nanofiber mats for photocatalysis applications. Appl. Surf. Sci. 2014, 294, 24–28. [Google Scholar] [CrossRef]
- Sorayani Bafqi, M.S.; Bagherzadeh, R.; Latifi, M. Fabrication of composite PVDF-ZnO nanofiber mats by electrospinning for energy scavenging application with enhanced efficiency. J. Polym. Res. 2015, 22, 130. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotech. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrost. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Kohsari, I.; Zandavar, H.; Koudehi, M.F.; Mirsadeghi, S. Electrospinning and thermal characterization of nitrocellulose nanofibers containing a composite of diaminofurazan, aluminum nano-powder and iron oxide nanoparticles. Cellulose 2019, 26, 4405–4415. [Google Scholar] [CrossRef]
- Yan, S.; Jian, G.; Zachariah, M.R. Electrospun Nanofiber-Based Thermite Textiles and their Reactive Properties. ACS Appl. Mater. Inter. 2012, 4, 6432–6435. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Huang, R.H.; Yan, S. Preparation of NC/CL-20 composite fibers by electrospinning. In Proceedings of the 2016 International Workshop on Material Science and Environmental Engineering (IWMSEE2016), Wuhan, China, 22–24 January 2016; pp. 165–172. [Google Scholar] [CrossRef]
- Li, Y.C.; Yang, H.T.; Hong, Y.; Yang, Y.; Cheng, Y.; Chen, H.H. Electrospun nanofiber-based nanoboron/nitrocellulose composite and their reactive properties. J. Therm. Anal. Calorim. 2017, 130, 1063–1068. [Google Scholar] [CrossRef]
- Selim, K.; Özkar, S.; Yilmaz, L. Thermal characterization of glycidyl azide polymer (GAP) and GAP-based binders for composite propellants. J. Appl. Polym. Sci. 2000, 77, 538–546. [Google Scholar] [CrossRef]
- You, J.S.; Kweon, J.O.; Kang, S.C.; Noh, S.T. A kinetic study of thermal decomposition of glycidyl azide polymer (GAP)-based energetic thermoplastic polyurethanes. Macromol. Res. 2010, 18, 1226–1232. [Google Scholar] [CrossRef]
- Nazare, A.N.; Asthana, S.N.; Singh, H. Glycidyl Azide Polymer (GAP)-An Energetic Component of Advanced Solid Rocket Propellants-A Review. J. Energ. Mater. 1992, 10, 43–63. [Google Scholar] [CrossRef]
- Li, J.Y.; Zhang, H.B.; Xu, J.J.; Sun, J. IR Absorption Peaks Assignments of LLM-105 by Temperature Dependent FT-IR Spectroscopy. Chin. J. Energ. Mater. 2015, 23, 507–510. [Google Scholar] [CrossRef]
- Shin, W.G.; Han, D.; Park, Y.; Hyun, H.S.; Sung, H.G.; Sohn, Y.K. Combustion of boron particles coated with an energetic polymer material. Korean J. Chem. Eng. 2016, 33, 3016–3020. [Google Scholar] [CrossRef]
- Wang, Y.; Song, X.L.; Song, D.; Liang, L.; An, C.W.; Wang, J.Y. Synthesis, thermolysis, and sensitivities of HMX/NC energetic nanocomposites. J. Hazard. Mater. 2016, 312, 73–83. [Google Scholar] [CrossRef]
- Song, X.L.; Wang, Y.; An, C.W.; Guo, X.D.; Li, F.S. Dependence of particle morphology and size on the mechanical sensitivity and thermal stability of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine. J. Hazard. Mater. 2008, 159, 222–229. [Google Scholar] [CrossRef]
- Lin, C.M.; Gong, F.Y.; Yang, Z.J.; Pan, L.P.; Liu, S.J.; Li, J.; Guo, S.Y. Bio-inspired fabrication of core@shell structured TATB/polydopamine microparticles via in situ polymerization with tunable mechanical properties. Polym. Test. 2018, 68, 126–134. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Waller, G.H.; Liu, Y.; Liu, M.L.; Wong, C.P. 3D Nitrogen-doped graphene prepared by pyrolysis of graphene oxide with polypyrrole for electrocatalysis of oxygen reduction reaction. Nano Energy 2013, 2, 241–248. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Song, X.L.; Huang, H.; Li, F.S. Characteristics and properties of nitrocellulose/glycidyl azide polymer/2, 4, 6, 8, 10, 12-hexanitro-2, 4, 6, 8, 10, 12-hexaazaisowurtzitane nanocomposites synthesized using a sol–gel supercritical method. Nanomater. Nanotechno. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Ye, B.Y.; An, C.W.; Zhang, Y.R.; Song, C.K.; Geng, X.H.; Wang, J.Y. One-Step Ball Milling Preparation of Nanoscale CL-20/Graphene Oxide for Significantly Reduced Particle Size and Sensitivity. Nanoscale Res. Lett. 2018, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, X.L.; Li, F. Thermal Behavior and Decomposition Mechanism of Ammonium Perchlorate and Ammonium Nitrate in the Presence of Nanometer Triaminoguanidine Nitrate. ACS Omega 2019, 4, 214–225. [Google Scholar] [CrossRef]
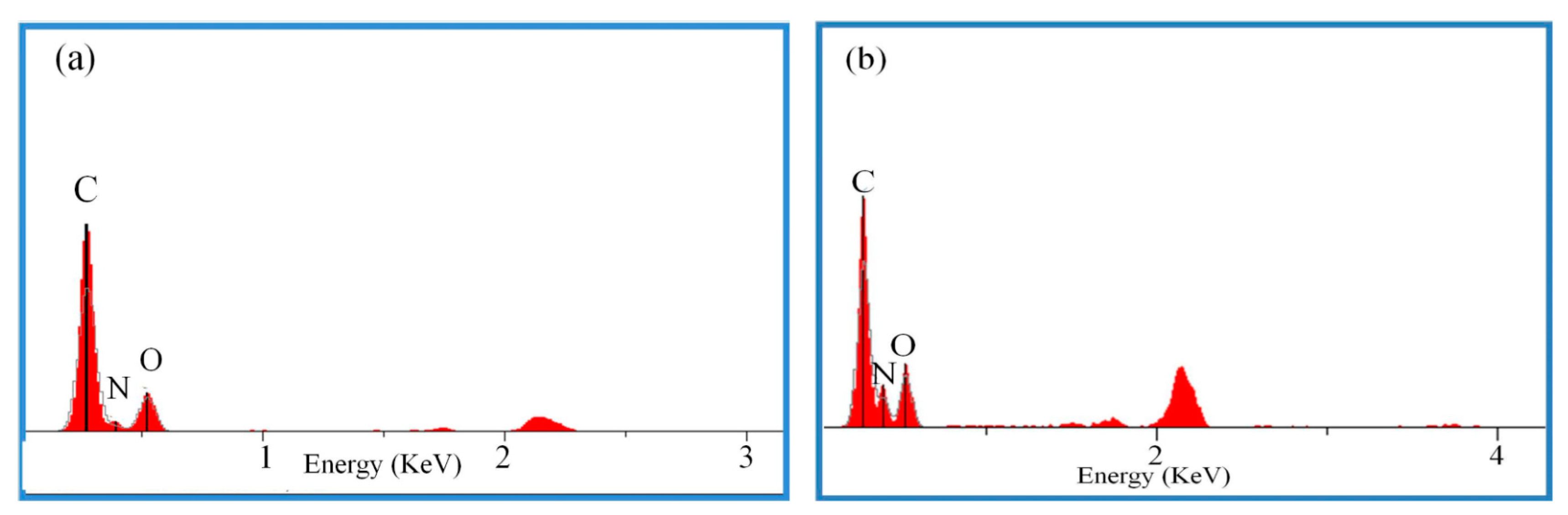
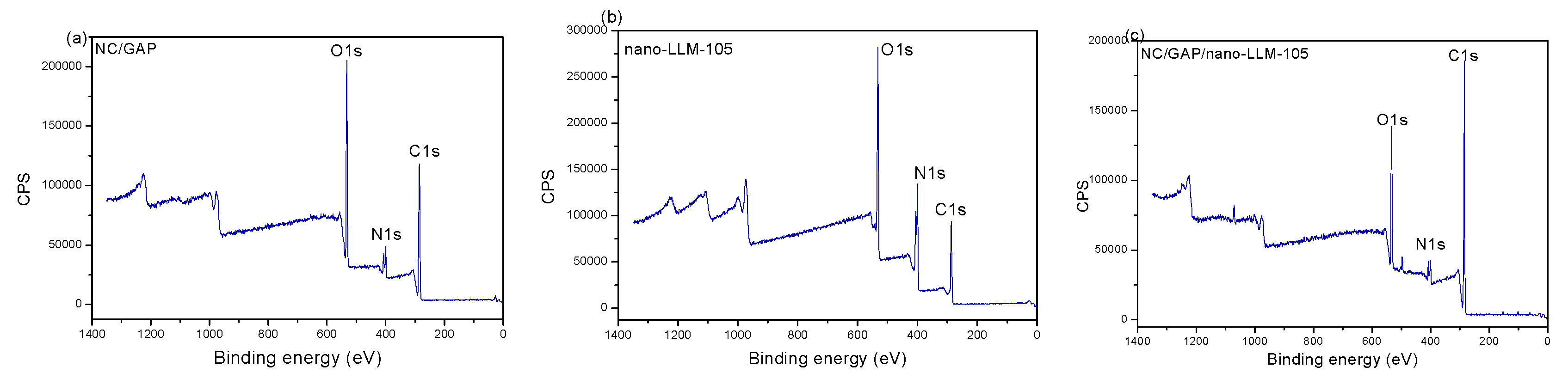
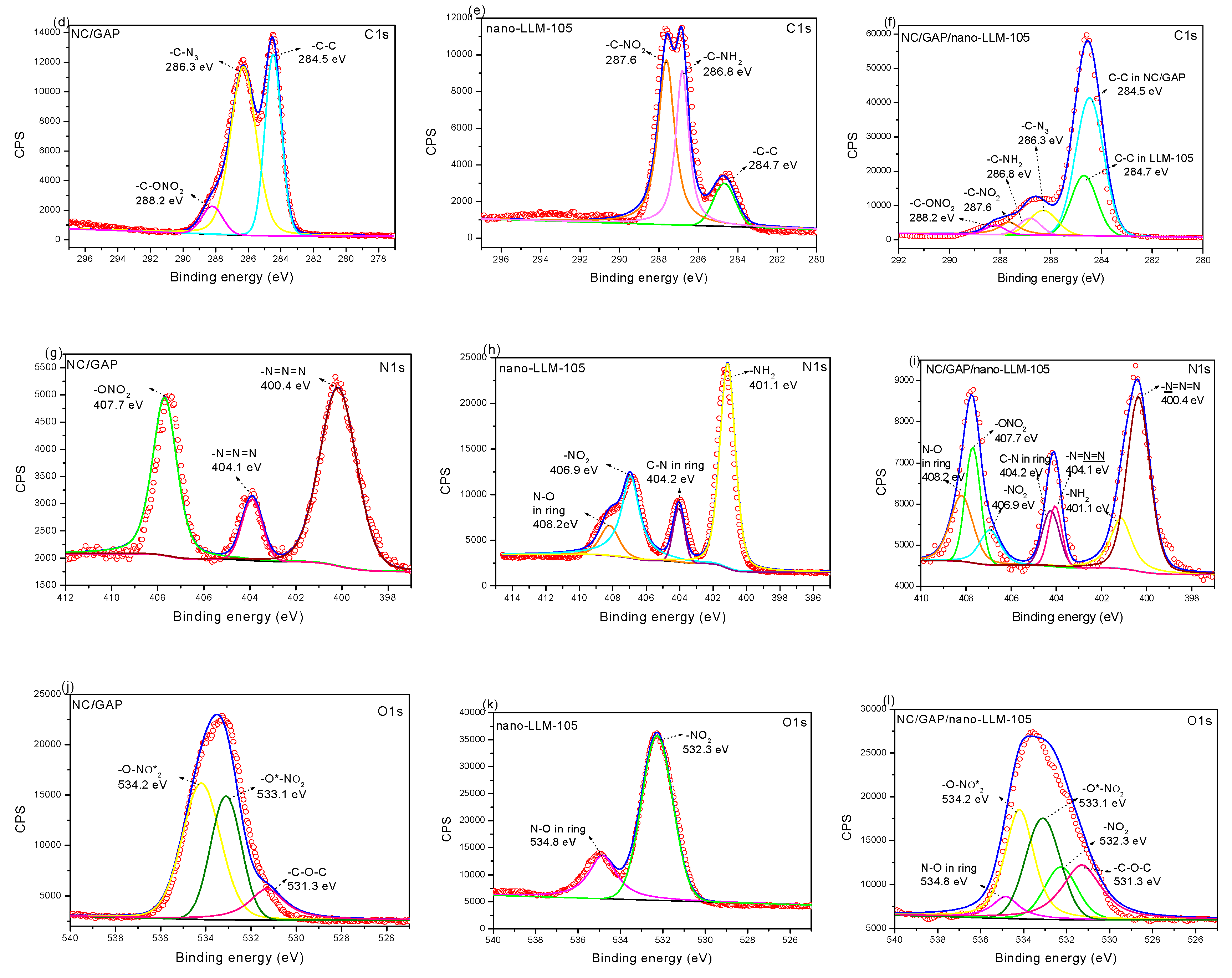
| Sample | C Content (%) | N Content (%) | O Content (%) |
|---|---|---|---|
| NC/GAP | 31.45 | 27.5 | 37.2 |
| NC/GAP/nano-LLM-105 | 29.14 | 30.20 | 37.16 |
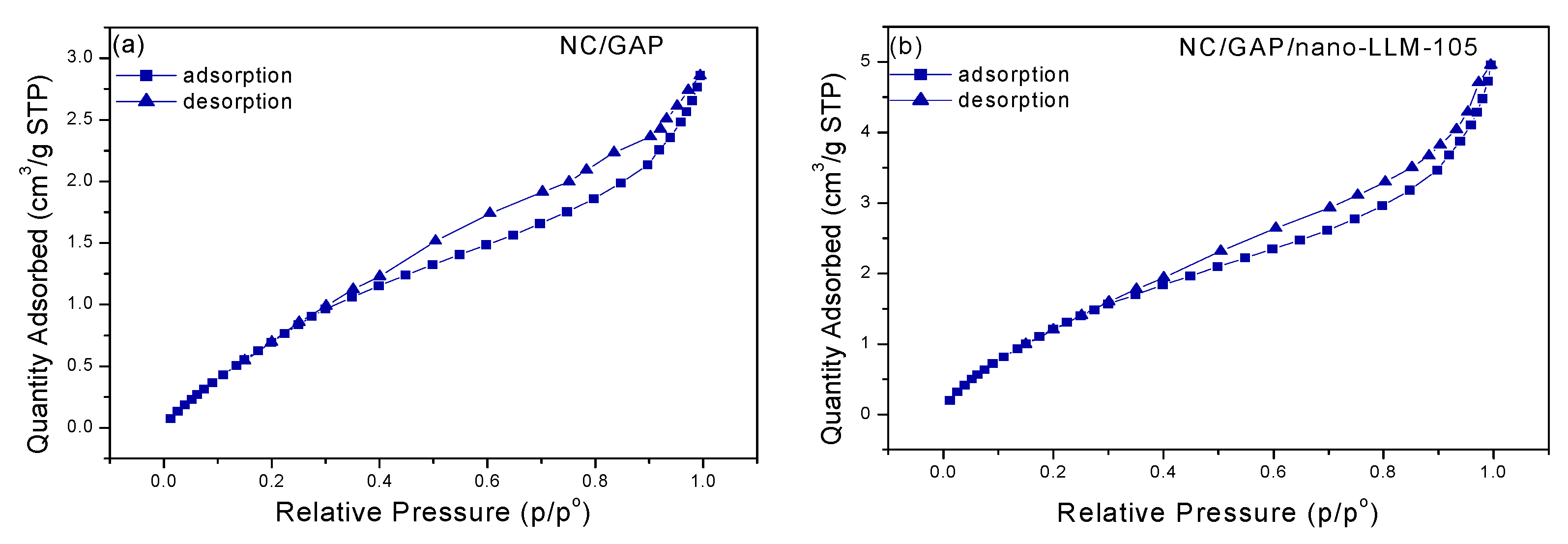
| Samples | BET Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Pore Size (nm) |
|---|---|---|---|
| NC/GAP | 4.3573 | 0.004422 | 4.05911 |
| NC/GAP/nano-LLM-105 | 6.0545 | 0.007664 | 5.06352 |
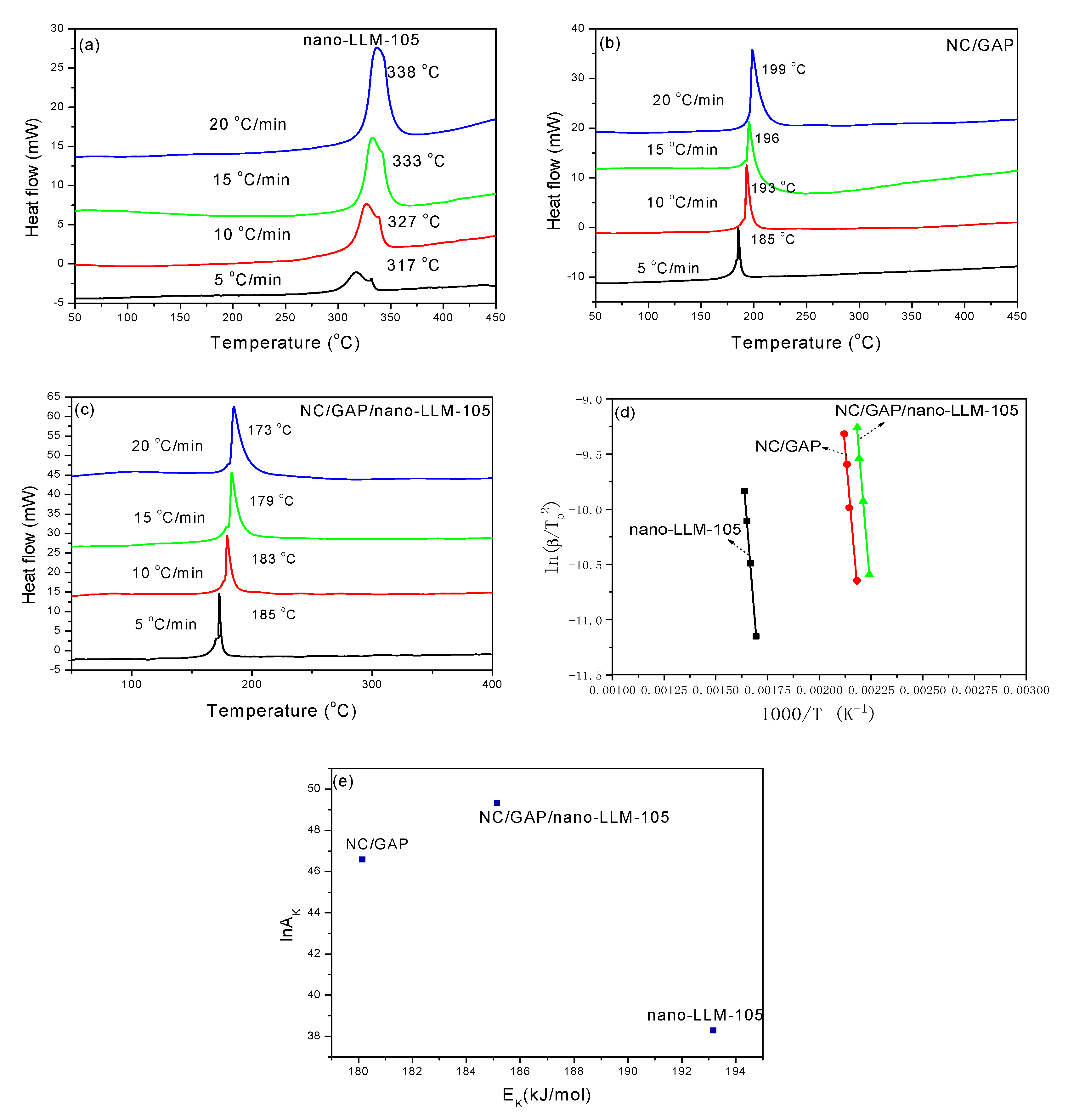
| Samples | Tp (K) | Thermodynamics | Kinetics | ||||
|---|---|---|---|---|---|---|---|
| ΔH≠ (kJ·mol−1) | ΔG≠ (kJ·mol−1) | ΔS≠ (J·mol−1·K−1) | EK (kJ·mol−1) | lnAK | k (s−1) | ||
| Nano-LLM-105 | 606 | 188 | 152 | 59 | 193 | 38 | 1.0 |
| NC/GAP | 468 | 176 | 115 | 130 | 180 | 47 | 1.4 |
| NC/GAP/Nano-LLM-105 | 456 | 181 | 111 | 153 | 185 | 49 | 1.7 |
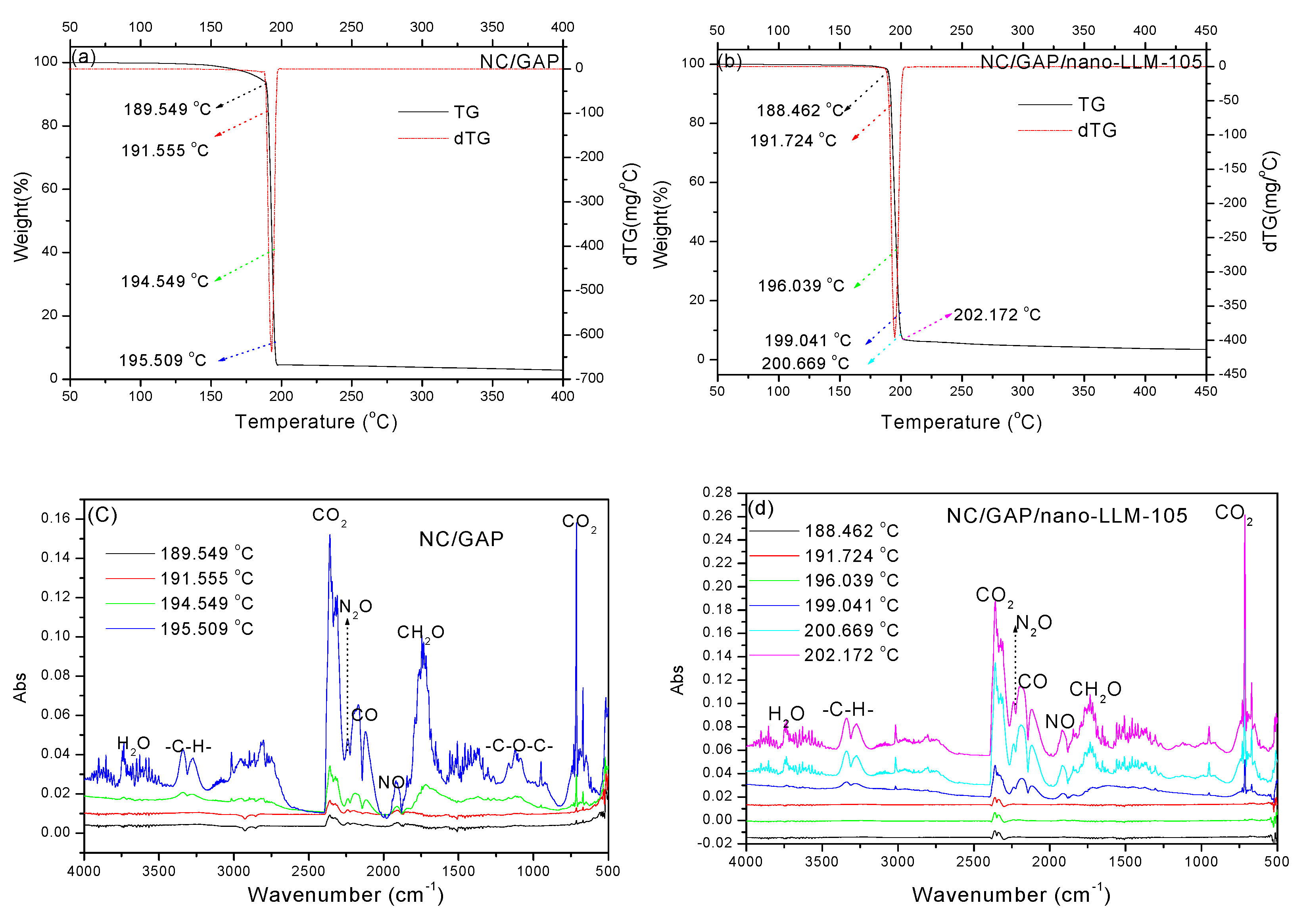
| Samples | Peaks and Attribution (cm−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| 3739–3560 | 2309–2360 | 2339 | 2113–2199 | 1901–1924 | 3271–3379 | 1691–1788 | 1077–1130 | |
| NC/GAP | H2O | CO2 | N2O | CO | NO | -C-H | -CH2O | C-O-C |
| NC/GAP/nano-LLM-105 | H2O | CO2 | N2O | CO | NO | -C-H | -CH2O | no |
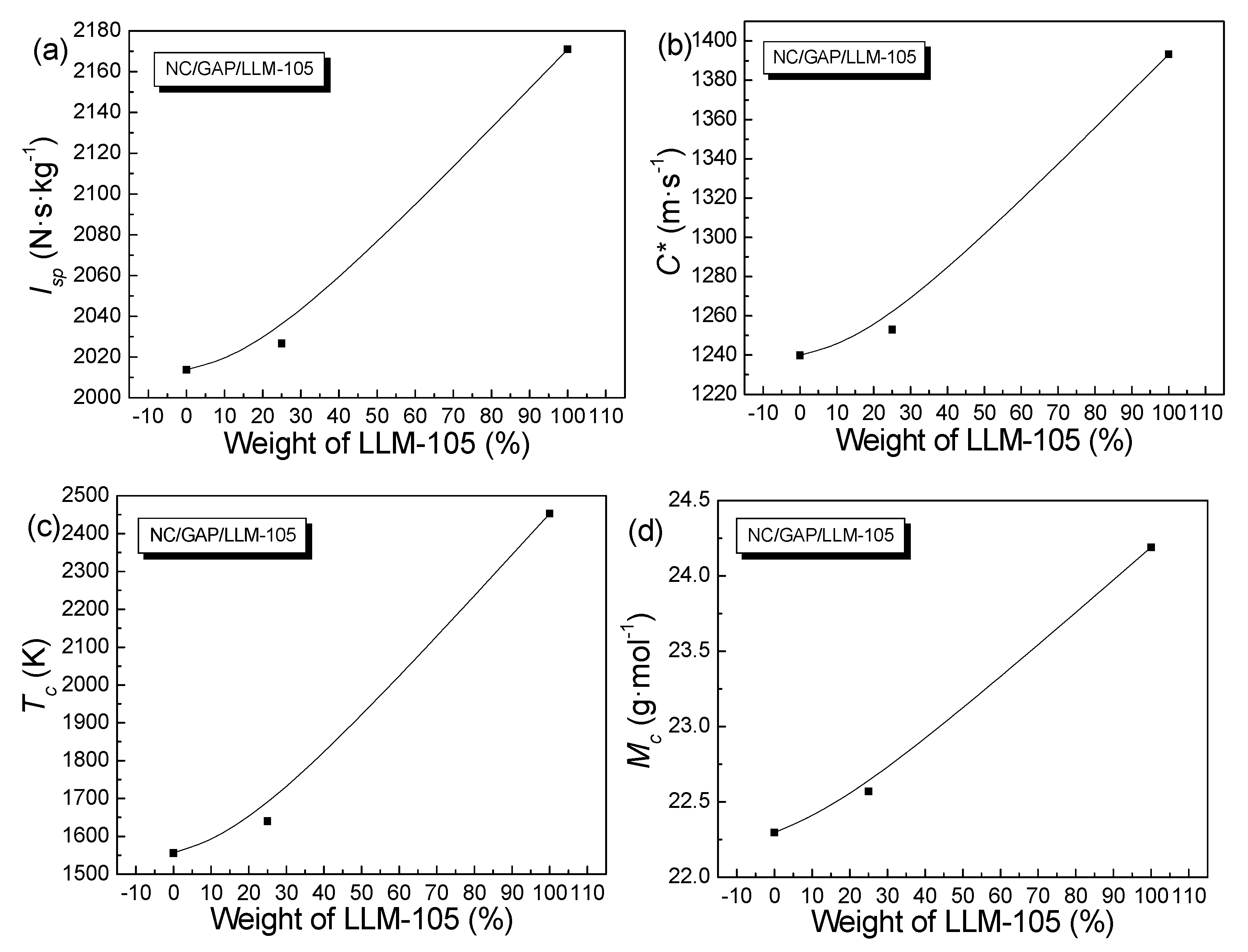
| Samples | Impact Sensitivity | Energy Performance | |||
|---|---|---|---|---|---|
| H50 (cm) | Isp (N·s·kg−1) | C* (m·s−1) | Tc (K) | Mc (g·mol−1) | |
| NC(50%)/GAP(50%) | 60 | 2014 | 1240 | 1556 | 22 |
| LLM-105(100%) | 113 | 2171 | 1393 | 2453 | 24 |
| NC(37.5%)/GAP(37.5%)/LLM-105(25%) | 78 | 2027 | 1253 | 1640 | 23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, T.; Wang, Y.; Huang, H.; Shang, F.; Song, X. An Electrospun Preparation of the NC/GAP/Nano-LLM-105 Nanofiber and Its Properties. Nanomaterials 2019, 9, 854. https://doi.org/10.3390/nano9060854
Luo T, Wang Y, Huang H, Shang F, Song X. An Electrospun Preparation of the NC/GAP/Nano-LLM-105 Nanofiber and Its Properties. Nanomaterials. 2019; 9(6):854. https://doi.org/10.3390/nano9060854
Chicago/Turabian StyleLuo, Tingting, Yi Wang, Hao Huang, Feifei Shang, and Xiaolan Song. 2019. "An Electrospun Preparation of the NC/GAP/Nano-LLM-105 Nanofiber and Its Properties" Nanomaterials 9, no. 6: 854. https://doi.org/10.3390/nano9060854
APA StyleLuo, T., Wang, Y., Huang, H., Shang, F., & Song, X. (2019). An Electrospun Preparation of the NC/GAP/Nano-LLM-105 Nanofiber and Its Properties. Nanomaterials, 9(6), 854. https://doi.org/10.3390/nano9060854





