Short- and Long-Range Mechanical and Chemical Interphases Caused by Interaction of Boehmite (γ-AlOOH) with Anhydride-Cured Epoxy Resins
Abstract
1. Introduction
2. Experimental
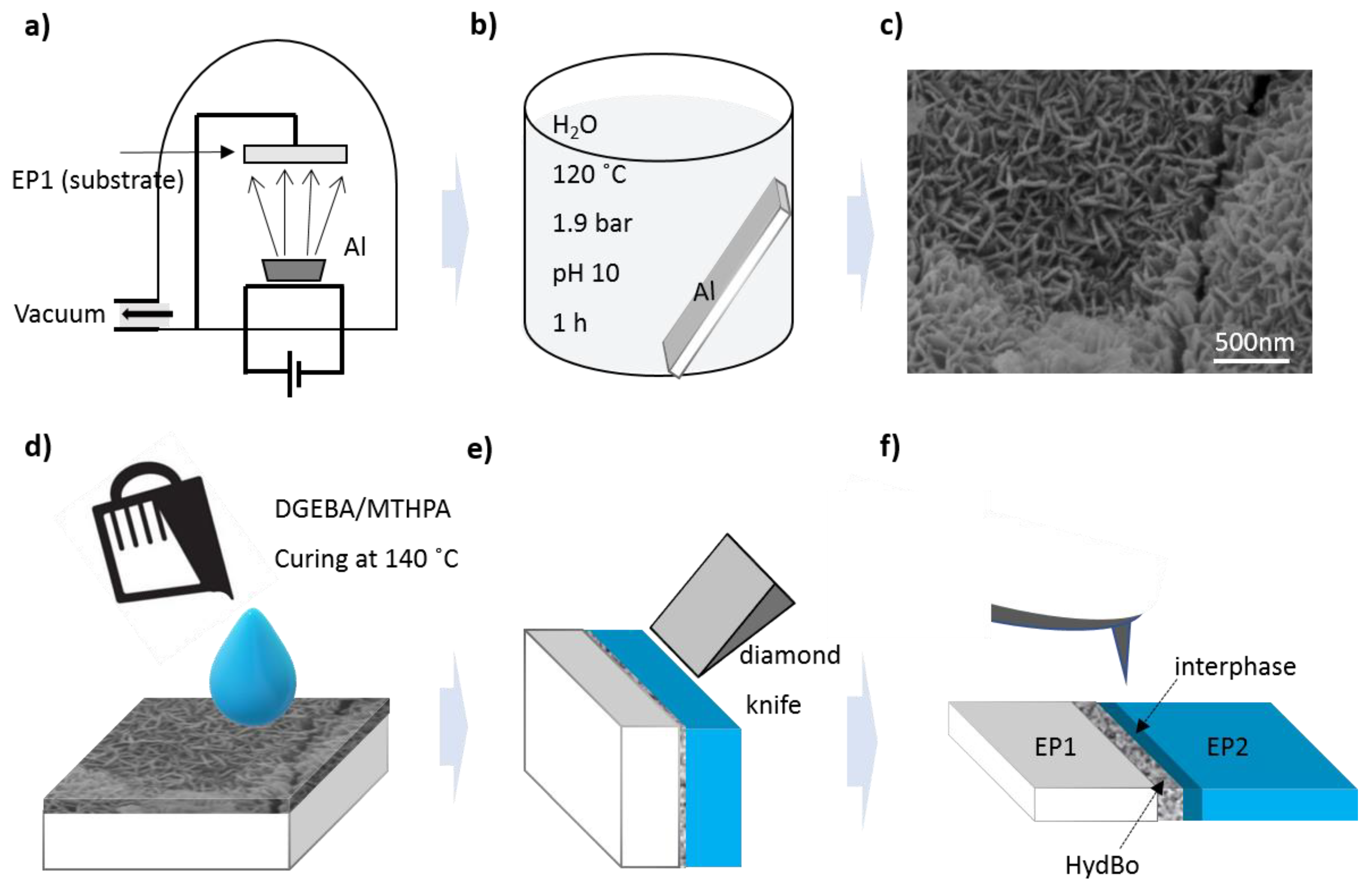
2.1. Materials and Sample Preparation
2.2. Characterization Methods for Short- and Long-Range Mechanical and Chemical Interphases
3. Results and Discussion
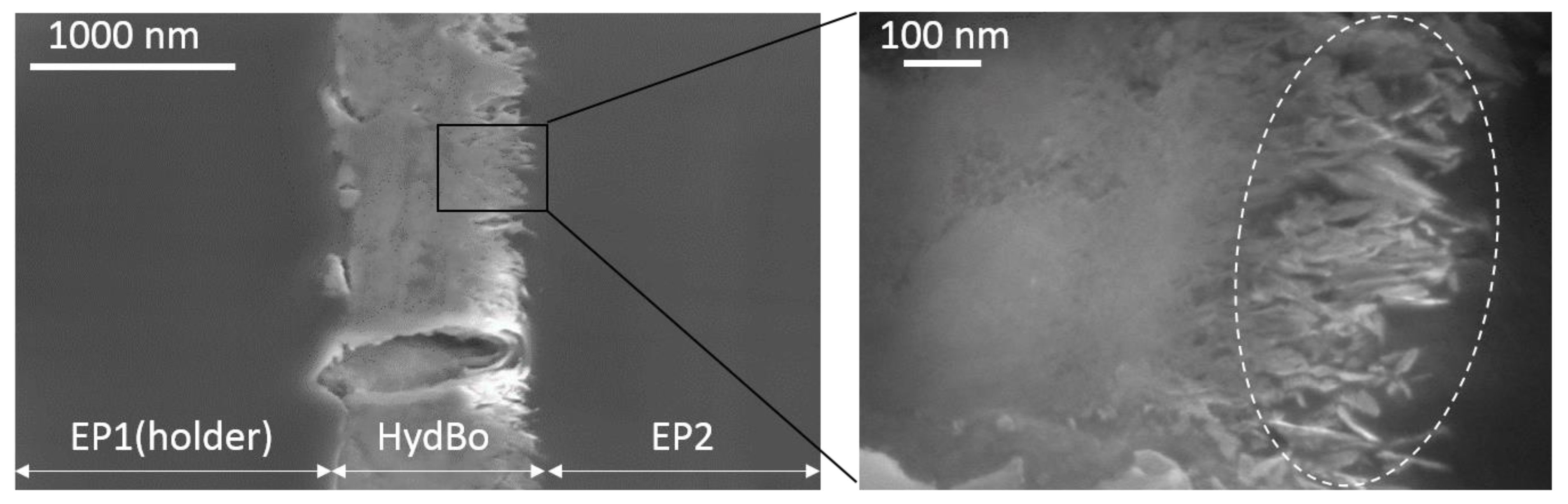
3.1. Morphology of the Interfacial Region
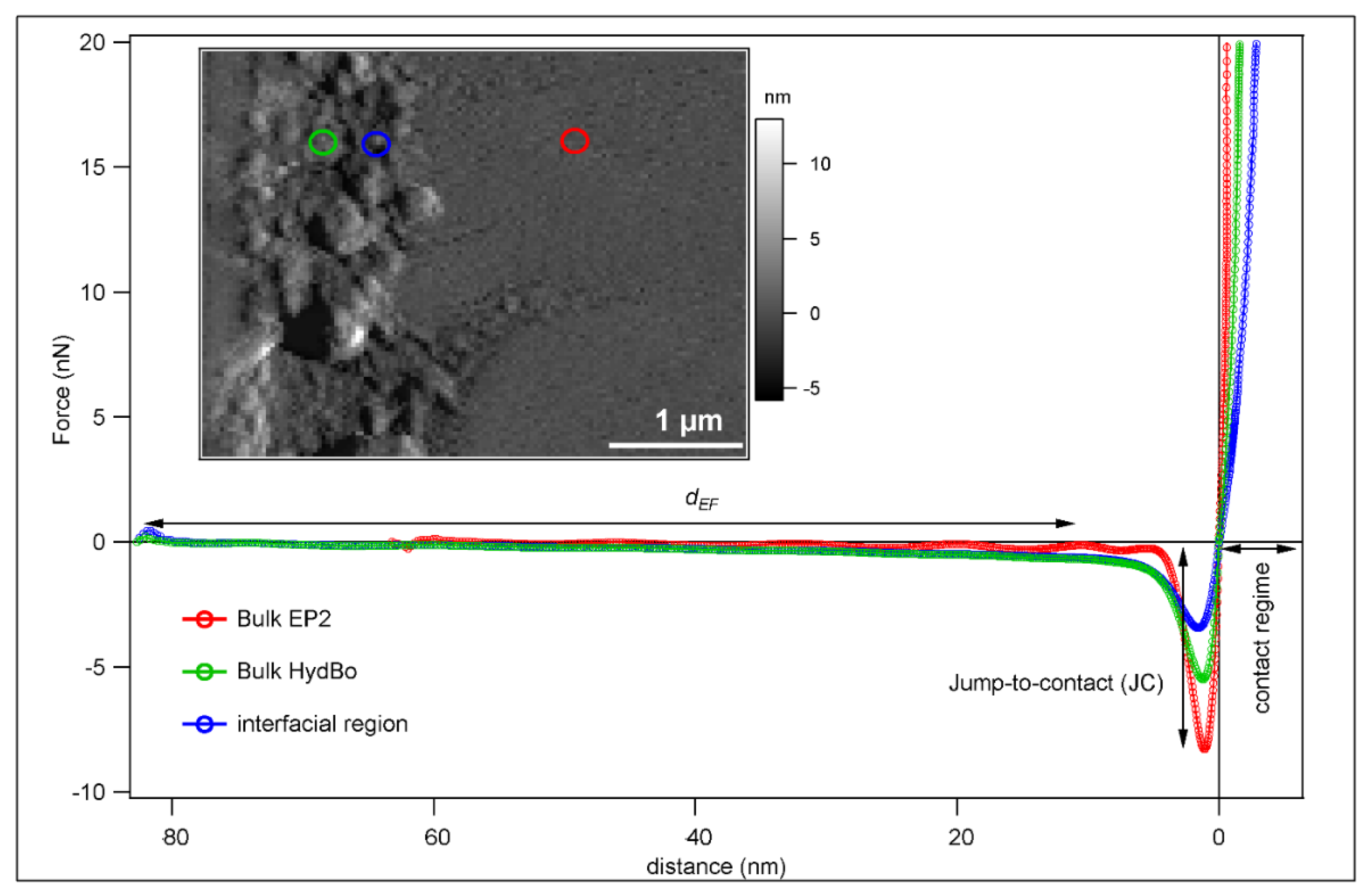
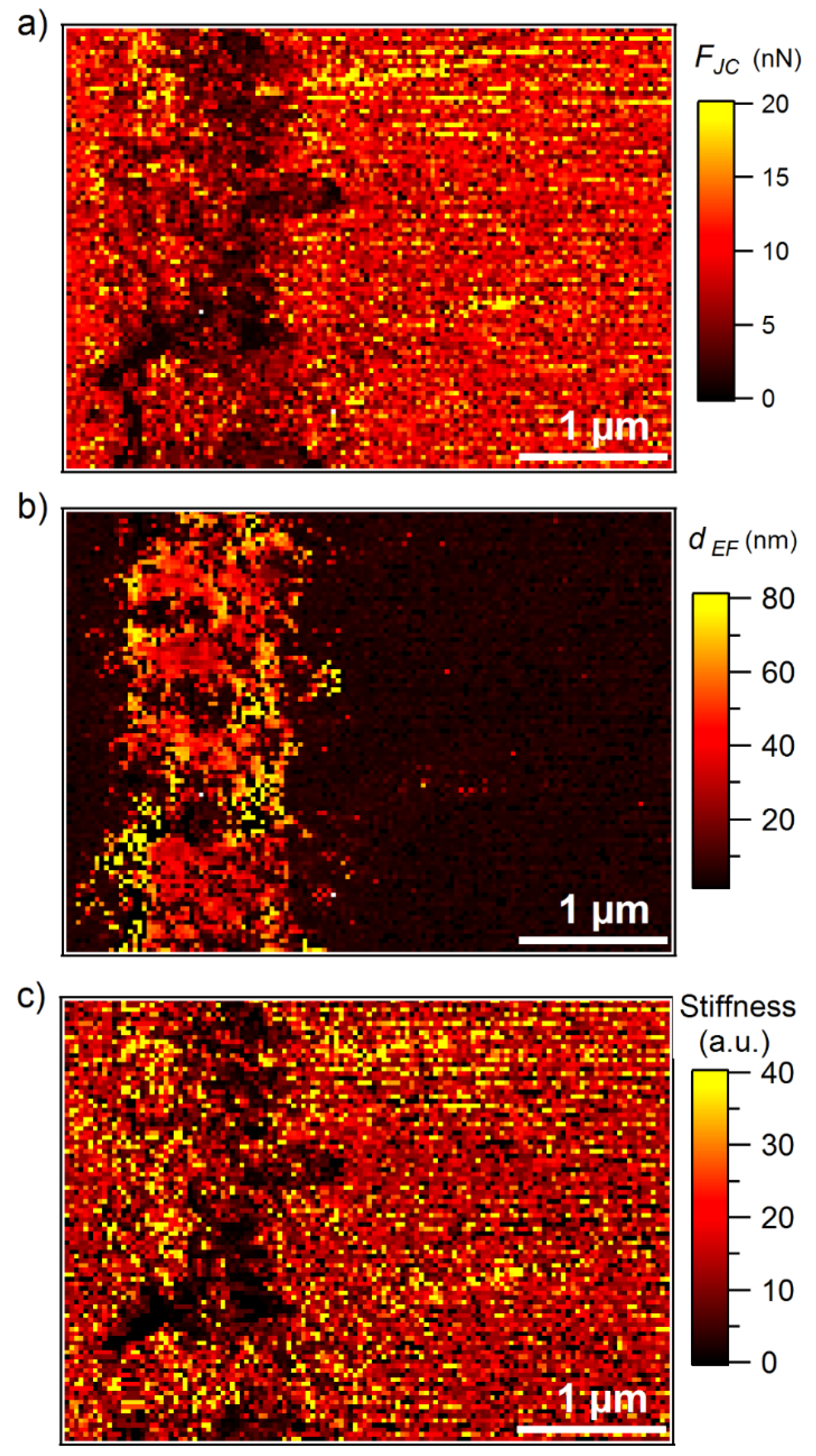
3.2. ImAFM Analysis
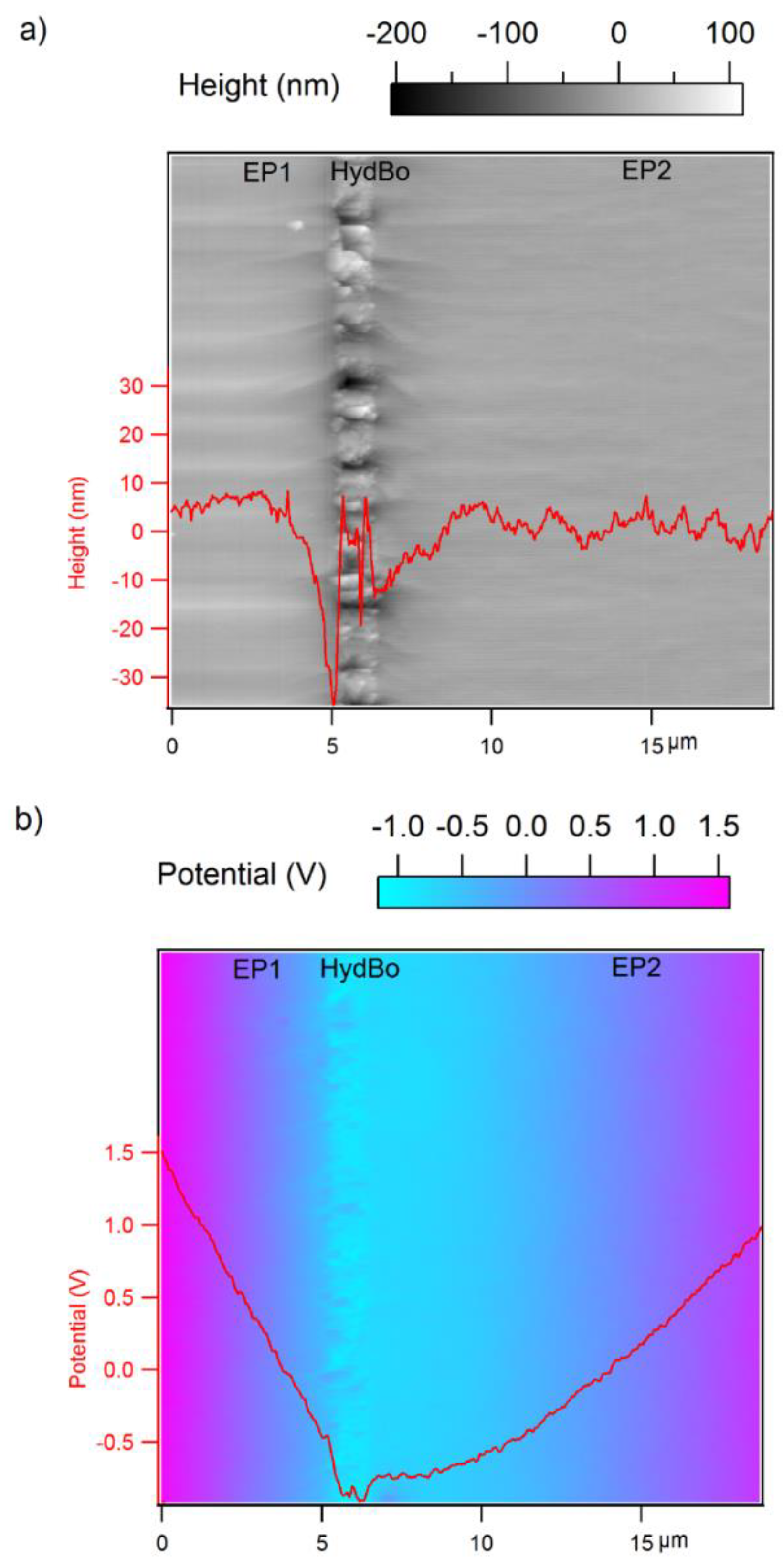
3.3. Surface Potential Measurements
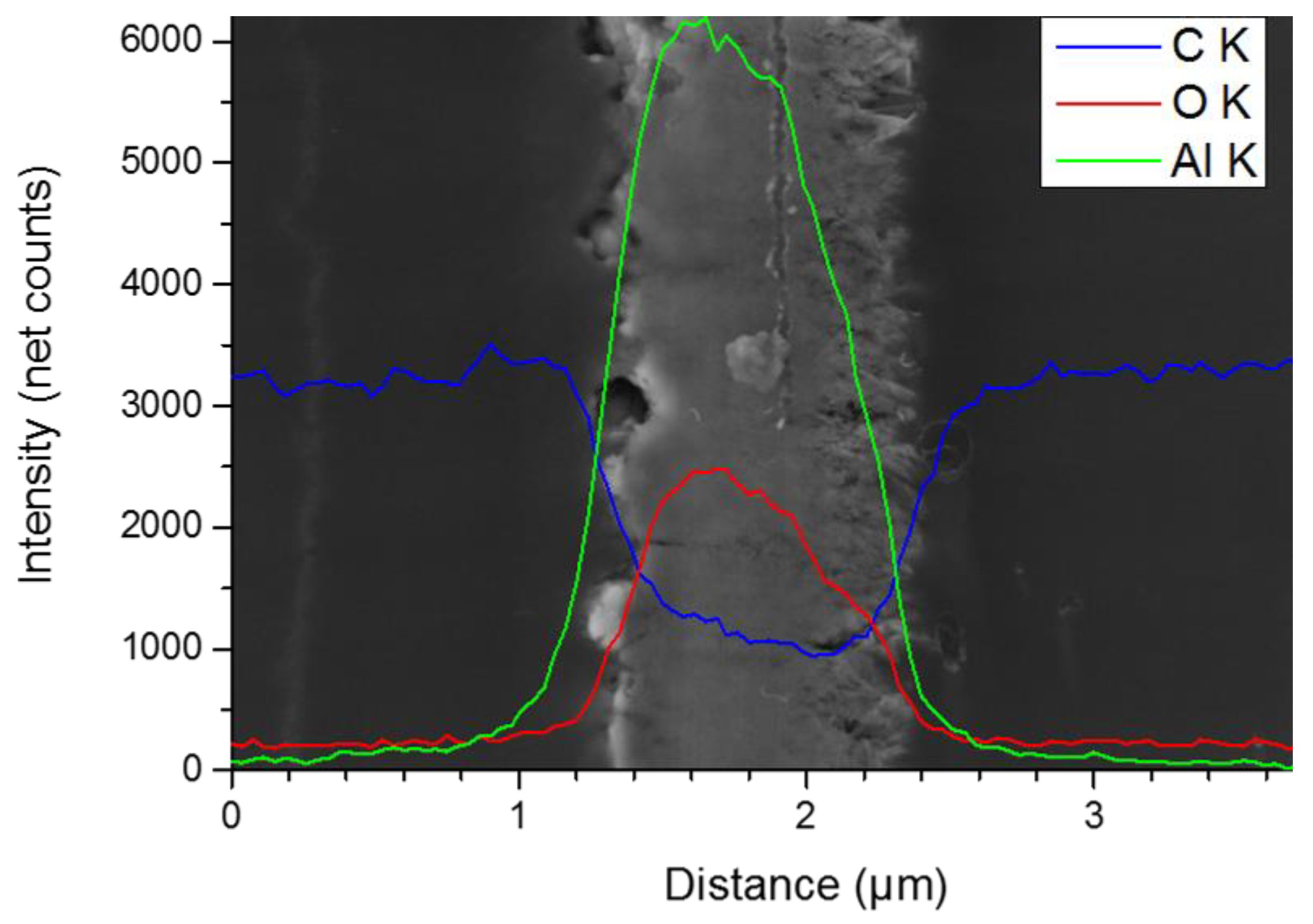
3.4. Elemental Analysis via EDS (Energy Dispersive X-Ray Spectroscopy)
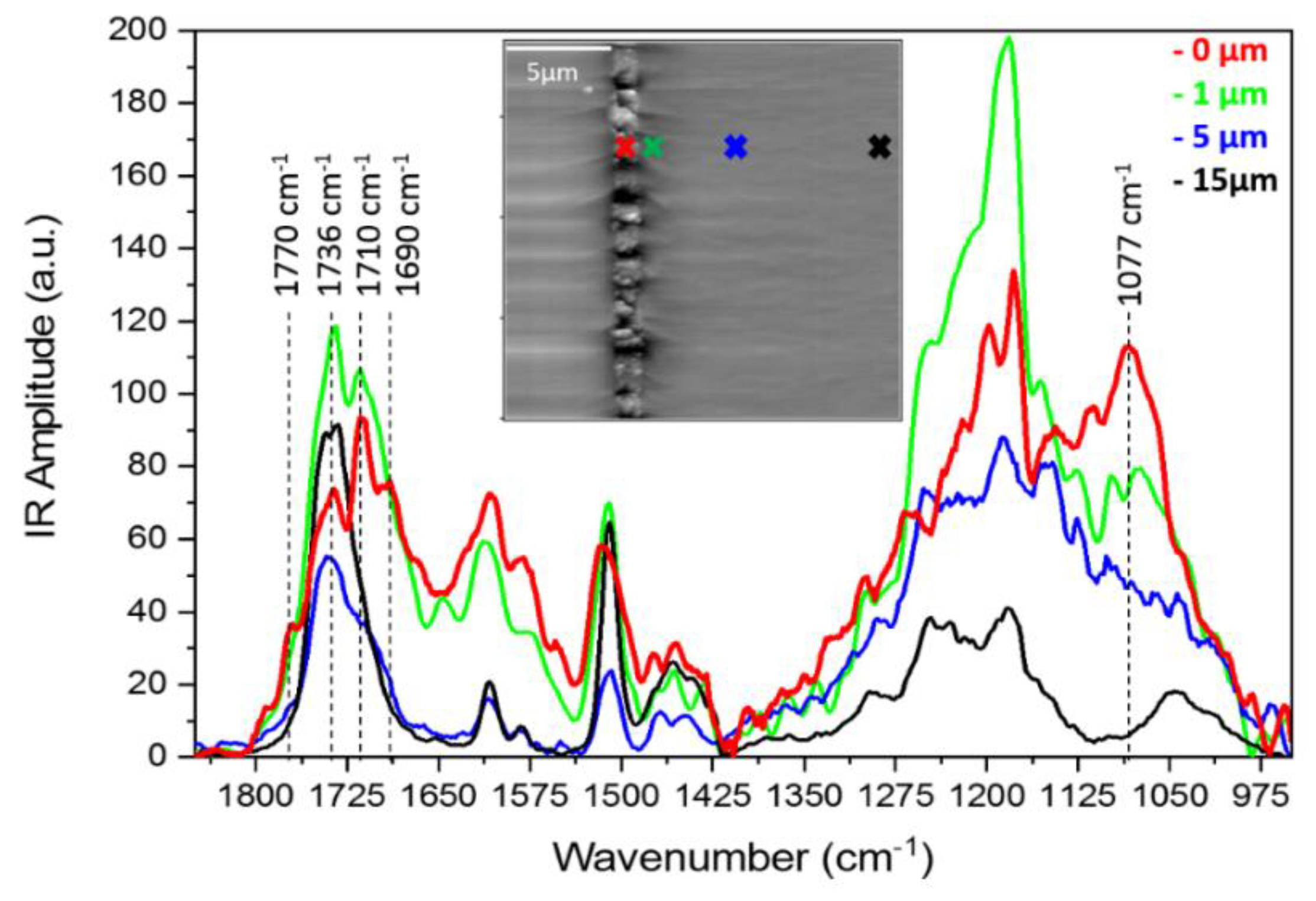
3.5. AFM-IR Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
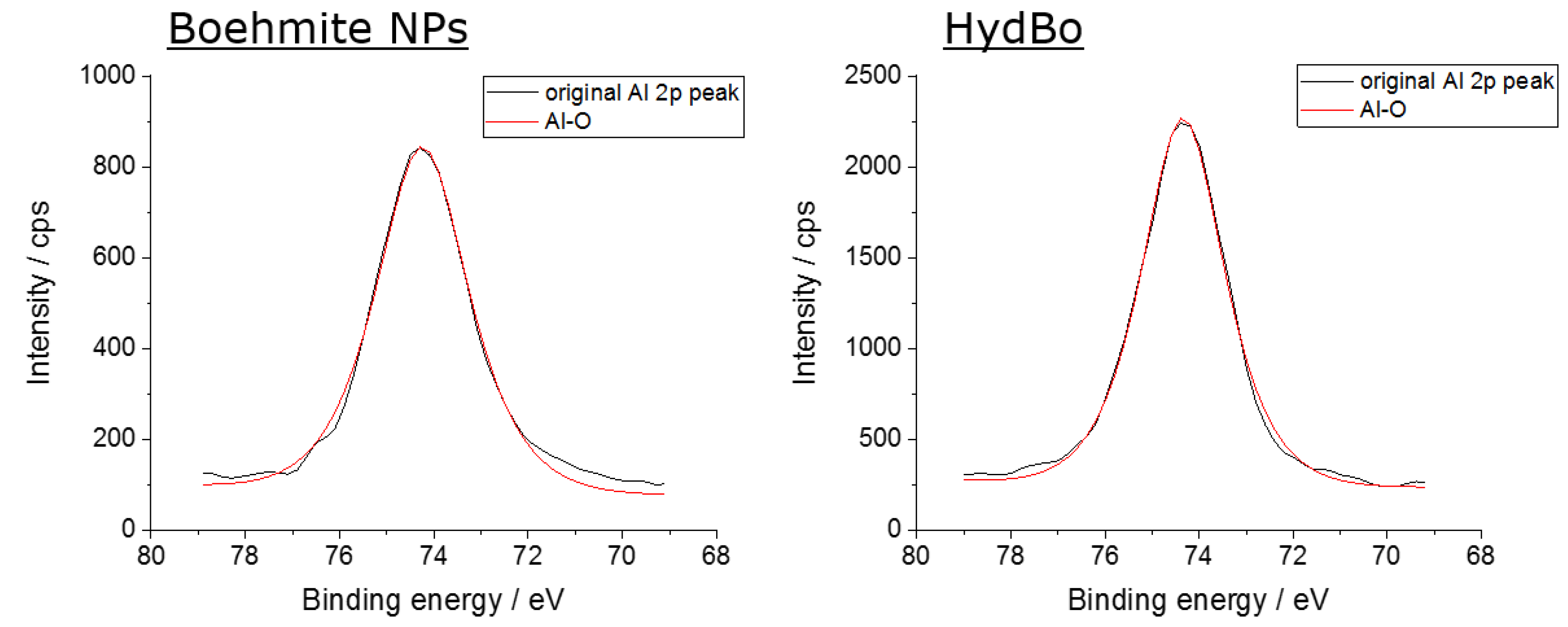
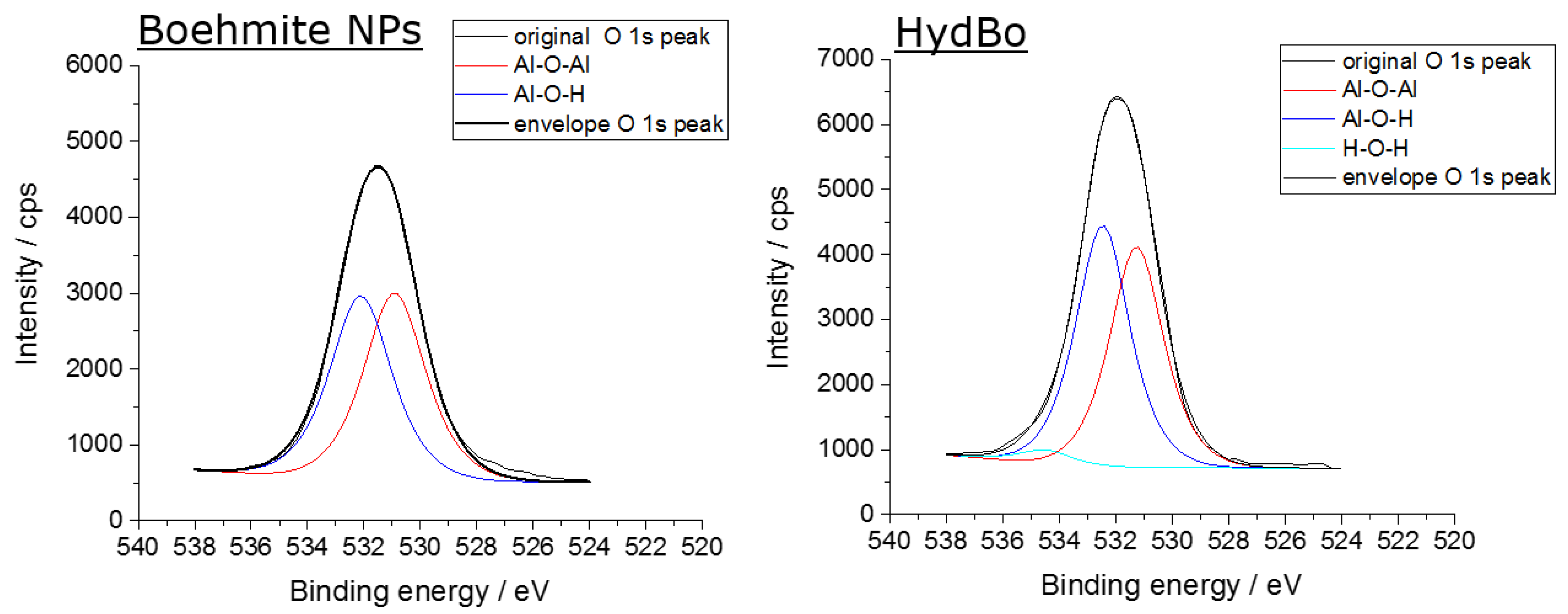
| Sample | Al-O-Al in % | Al-O-H in % | H2O |
|---|---|---|---|
| Commercially available boehmite (BNP) | 49.7 | 50.3 | - |
| HydBo | 48.2 | 49.7 | 2.1 |
Appendix B
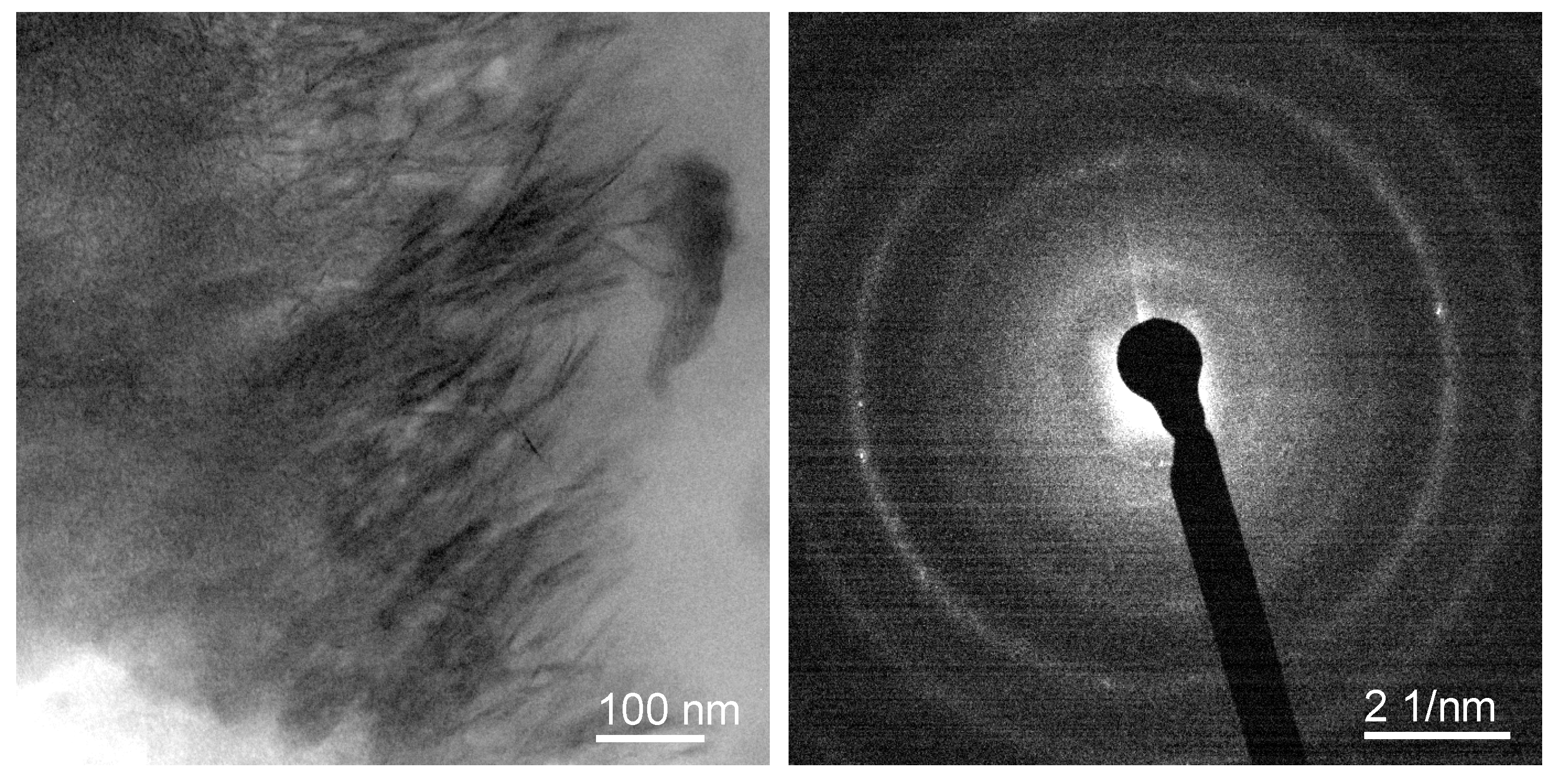
Appendix C
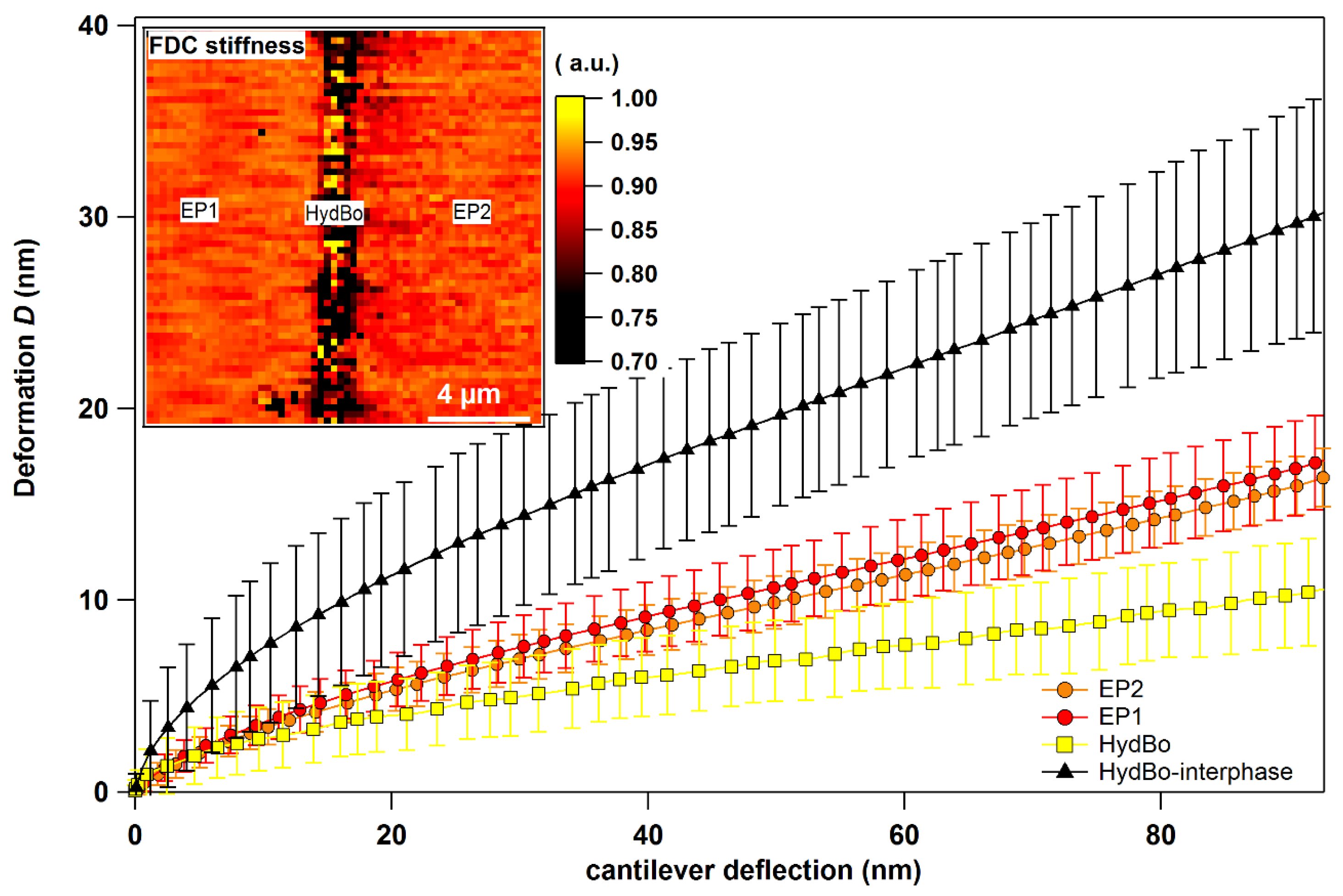
Appendix D
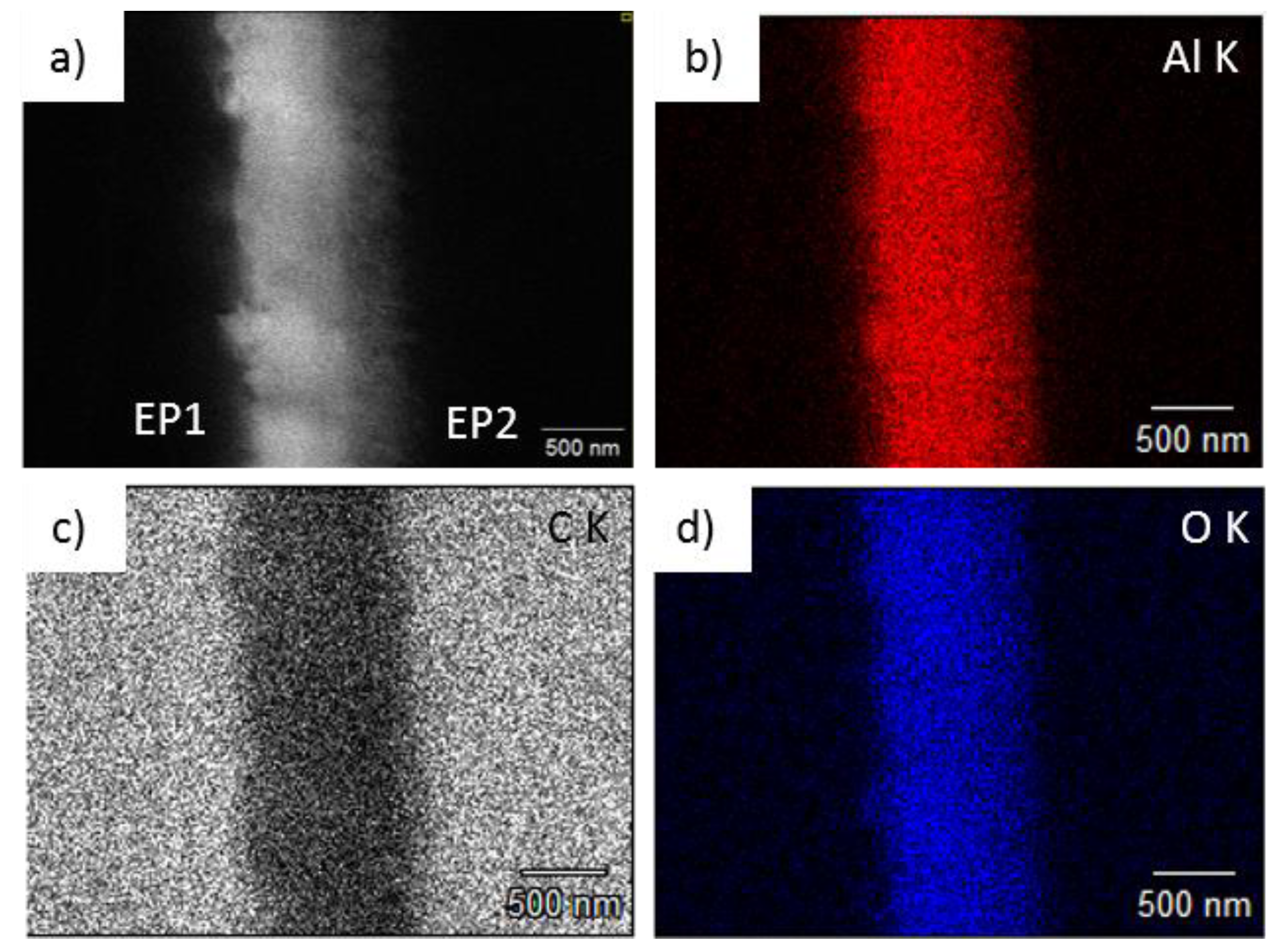
Appendix E
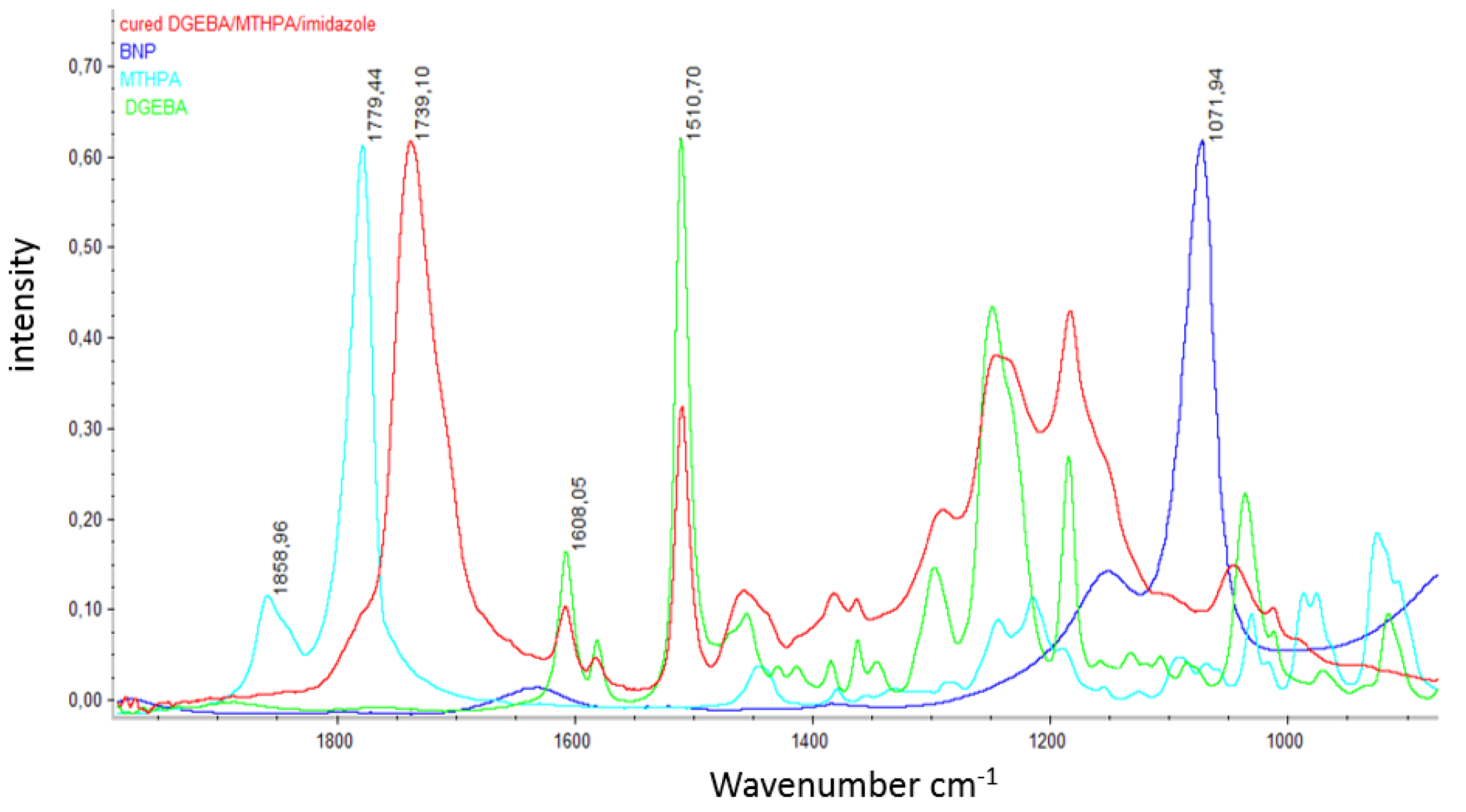
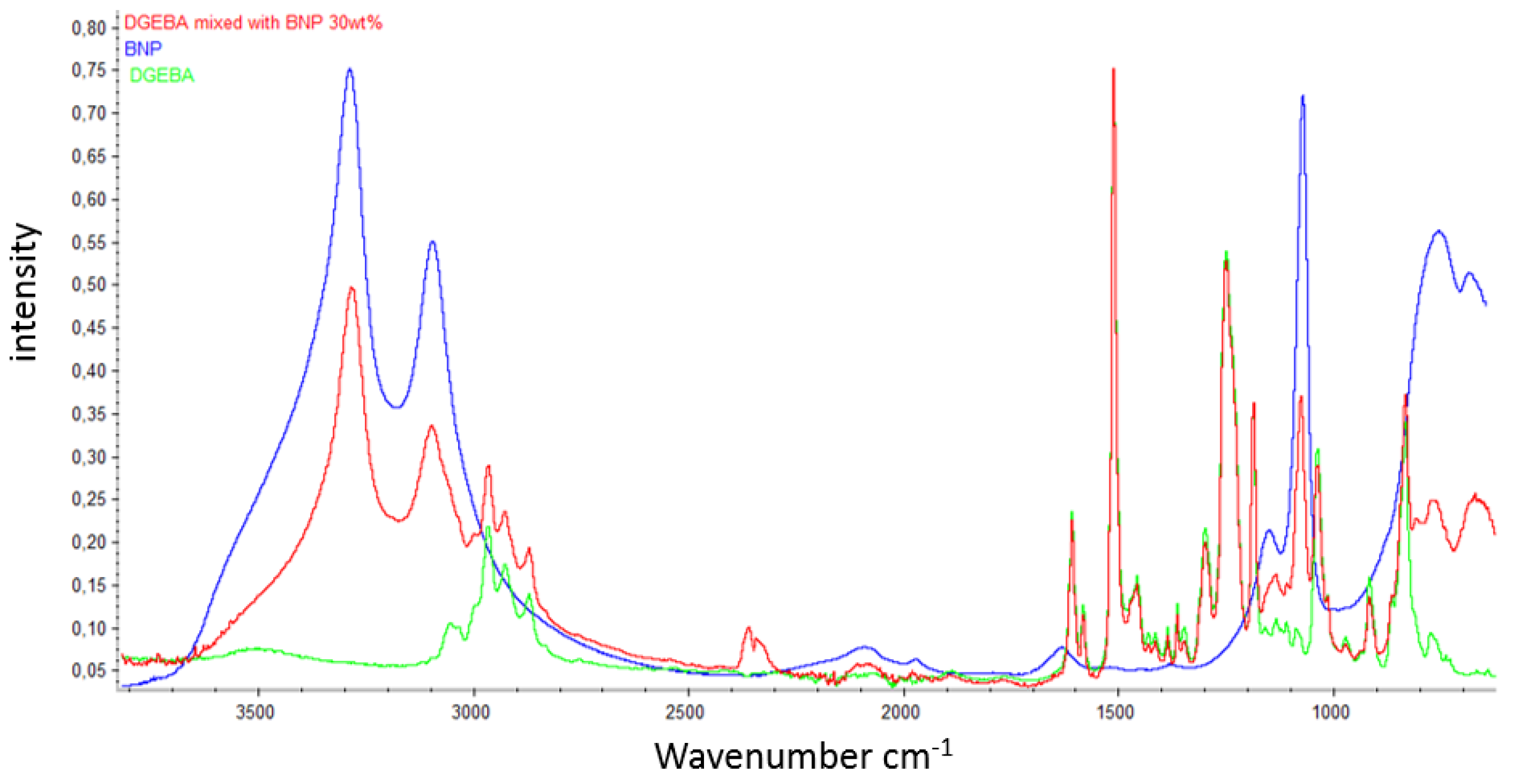
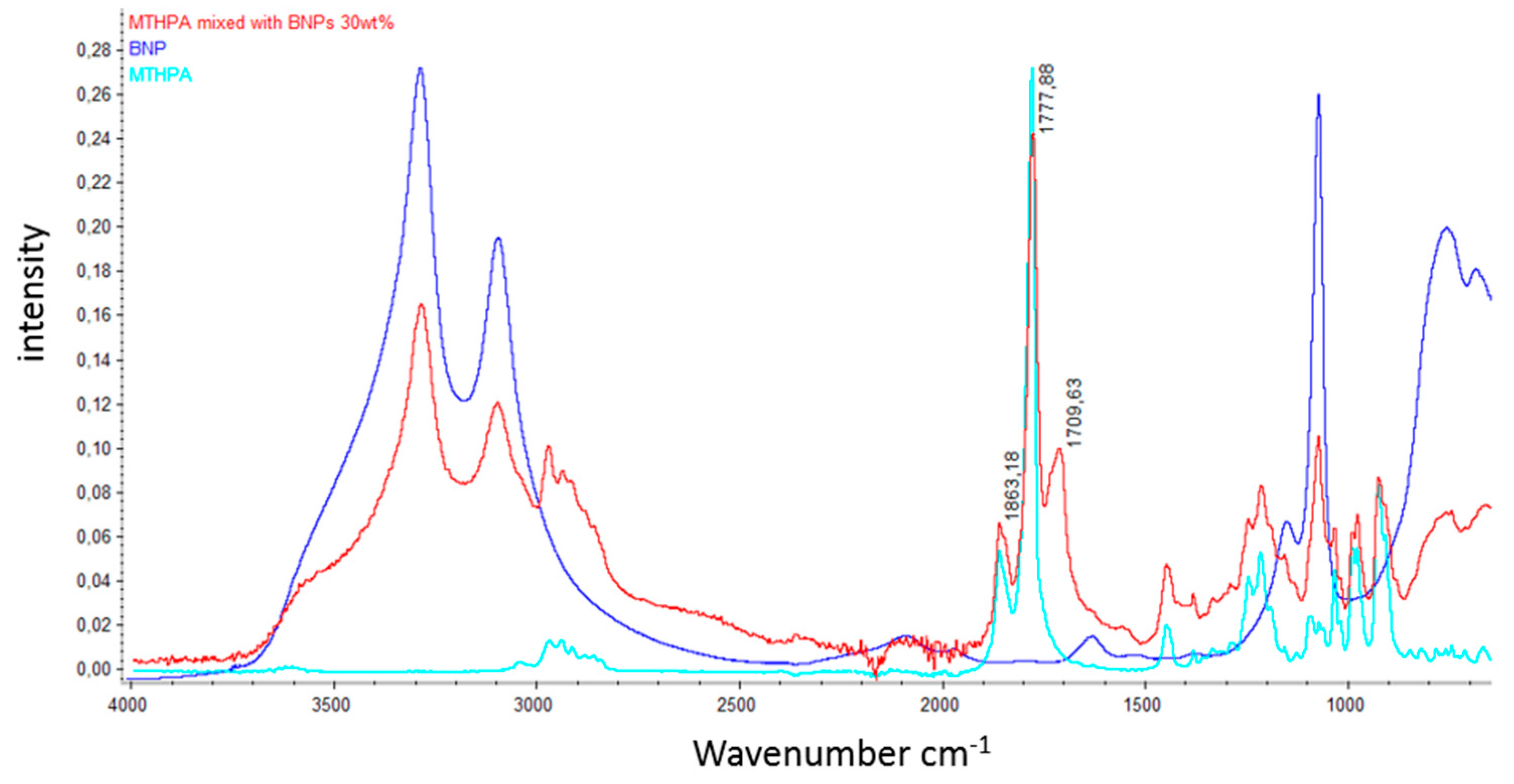
References
- Karger-Kocsis, J.; Lendvai, L. Polymer/boehmite nanocomposites: A review. J. Appl. Polym. Sci. 2017, 135, 24. [Google Scholar] [CrossRef]
- Blaszczak, P.; Brostow, W.; Datashvili, T.; Lobland, H.E.H. Rheology of low-density polyethylene+ Boehmite composites. Polym. Compos. 2010, 31, 1909–1913. [Google Scholar] [CrossRef]
- Streller, R.C.; Thomann, R.; Torno, O.; Mülhaupt, R. Morphology, crystallization behavior, and mechanical properties of isotactic poly (propylene) nanocomposites based on organophilic boehmites. Macromol. Mater. Eng. 2009, 294, 380–388. [Google Scholar] [CrossRef]
- Camino, G.; Maffezzoli, A.; Braglia, M.; De Lazzaro, M.; Zammarano, M. Effect of hydroxides and hydroxycarbonate structure on fire retardant effectiveness and mechanical properties in ethylene-vinyl acetate copolymer. Polym. Degrad. 2001, 74, 457–464. [Google Scholar] [CrossRef]
- Wawrzyn, E.; Schartel, B.; Karrasch, A.; Jäger, C. Flame-retarded bisphenol A polycarbonate/silicon rubber/bisphenol A bis (diphenyl phosphate): Adding inorganic additives. Polym. Degrad. 2014, 106, 74–87. [Google Scholar] [CrossRef]
- Laachachi, A.; Ferriol, M.; Cochez, M.; Cuesta, J.-M.L.; Ruch, D. A comparison of the role of boehmite (AlOOH) and alumina (Al2O3) in the thermal stability and flammability of poly (methyl methacrylate). Polym. Degrad. 2009, 94, 1373–1378. [Google Scholar] [CrossRef]
- Arlt, C. Wirkungsweisen nanoskaliger Böhmite in einem Polymer und seinem Kohlenstofffaserverbund unter Druckbelastung. Ph.D Thesis, Otto-von-Guericke Universität Magdeburg, Magdeburg, Germany, 5 August 2011. [Google Scholar]
- Exner, W.; Arlt, C.; Mahrholz, T.; Riedel, U.; Sinapius, M. Nanoparticles with various surface modifications as functionalized cross-linking agents for composite resin materials. Compos. Sci. Technol. 2012, 72, 1153–1159. [Google Scholar] [CrossRef]
- Jux, M.; Fankhänel, J.; Daum, B.; Mahrholz, T.; Sinapius, M.; Rolfes, R. Mechanical properties of epoxy/boehmite nanocomposites in dependency of mass fraction and surface modification-An experimental and numerical approach. Polymer 2018, 141, 34–35. [Google Scholar] [CrossRef]
- Duwe, S.; Arlt, C.; Aranda, S.; Riedel, U.; Ziegmann, G.J.C.S. A detailed thermal analysis of nanocomposites filled with SiO2, AlN or boehmite at varied contents and a review of selected rules of mixture. Compos. Sci. Technol. 2012, 72, 1324–1330. [Google Scholar] [CrossRef]
- Kyritsis, A.; Vikelis, G.; Maroulas, P.; Pissis, P.; Milosheva, B.; Kotsilkova, R.; Toplijska, A.; Silvestre, C.; Duraccio, D. Polymer dynamics in epoxy/alumina nanocomposites studied by various techniques. J. Appl. Polym. Sci. 2011, 121, 3613–3627. [Google Scholar]
- Vogelson, C.T.; Koide, Y.; Alemany, L.B.; Barron, A.R. Inorganic− organic hybrid and composite resin materials using carboxylate-alumoxanes as functionalized cross-linking agents. Chem. Mater. 2000, 12, 795–804. [Google Scholar] [CrossRef]
- Wu, Z.; Zhuo, Q.; Sun, T.; Wang, Z. Mechanical properties of epoxy resins reinforced with synthetic boehmite (AlOOH) nanosheets. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Sun, T.; Wu, Z.; Zhuo, Q.; Liu, X.; Wang, Z.; Fan, H. Surface functionalized boehmite sheets filled epoxy composites with enhanced mechanical and thermal properties. Polym. Adv. Technol. 2014, 25, 1604–1609. [Google Scholar] [CrossRef]
- Drescher, P.; Thomas, M.; Borris, J.; Riedel, U.; Arlt, C. Strengthening fibre/matrix interphase by fibre surface modification and nanoparticle incorporation into the matrix. Compos. Sci. Technol. 2013, 74, 60–66. [Google Scholar] [CrossRef]
- Ghasem, M.; Khorasani, Z.; Silbernagl, D.; Platz, D.; Sturm, H. Insights into nano-scale physical and mechanical properties of epoxy/boehmite nanocomposite using different AFM modes. Polymers 2019, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, M.G.Z.; Silbernagl, D.; Szymoniak, P.; Hodoroaba, V.-D.; Sturm, H. The effect of boehmite (AlOOH) on nanomechanical and thermomechanical properties correlated to crosslinking density of epoxy in epoxy/boehmite nanocomposites. Polymer 2019, 164, 174–182. [Google Scholar] [CrossRef]
- Holt, A.P.; Griffin, P.J.; Bocharova, V.; Agapov, A.L.; Imel, A.E.; Dadmun, M.D.; Sangoro, J.R.; Sokolov, A.P. Dynamics at the polymer/nanoparticle interface in poly (2-vinylpyridine)/silica nanocomposites. Macromolecules 2014, 47, 1837–1843. [Google Scholar] [CrossRef]
- Qiao, R.; Brinson, L.C. Simulation of interphase percolation and gradients in polymer nanocomposites. Compos. Sci. Technol. 2009, 69, 491–499. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Yu, H.; Zhao, L.; Qu, J. Controlled network structure and its correlations with physical properties of polycarboxyl octaphenylsilsesquioxanes-based inorganic–organic polymer nanocomposites. RSC Adv. 2012, 2, 2759–2767. [Google Scholar] [CrossRef]
- Ndoro, T.V.; Böhm, M.C.; Müller-Plathe, F. Interface and interphase dynamics of polystyrene chains near grafted and ungrafted silica nanoparticles. Macromolecules 2011, 45, 171–179. [Google Scholar] [CrossRef]
- Chung, J.; Munz, M.; Sturm, H. Stiffness variation in the interphase of amine-cured epoxy adjacent to copper microstructures. Surf. Interface Anal. 2007, 39, 624–633. [Google Scholar] [CrossRef]
- Munz, M.; Cappella, B.; Sturm, H.; Geuss, M.; Schulz, E. Materials contrasts and nanolithography techniques in scanning force microscopy (SFM) and their application to polymers and polymer composites. In Filler-Reinforced Elastomers/Sanning Force Microscopy; Springer: Berlin/Heidelberg, Germany, 2003; pp. 87–210. [Google Scholar]
- Fankhänel, J.; Kempe, A.; Rolfes, R. Simulating atomic force microscopy for the determination of the elastic properties of nanoparticle reinforced epoxy resin. In Proceedings of the 7th European Congress on Computational Methods in Applied Sciences and Engineering (ECCOMAS Congress’ 16), Crete, Greece, 5–10 June 2016. [Google Scholar]
- Silbernagl, E.; Cappella, B. Reconstruction of a hidden topography by single AFM force–distance curves. Surf. Sci. 2009, 603, 2363–2369. [Google Scholar] [CrossRef]
- Mäder, E.; Mai, K.; Pisanova, E. Interphase characterization in polymer composites-monitoring of interphasial behavior in dependence on the mode of loading. Compos. Interfaces 2000, 7, 133–147. [Google Scholar] [CrossRef]
- Munz, M.; Sturm, H.; Schulz, E.; Hinrichsen, G. The scanning force microscope as a tool for the detection of local mechanical properties within the interphase of fibre reinforced polymers. Composites Part A 1998, 29, 1251–1259. [Google Scholar] [CrossRef]
- Platz, D.; Forchheimer, D.; Tholén, E.A.; Haviland, D.B. Interaction imaging with amplitude-dependence force spectroscopy. Nat. Commun. 2013, 4, 1360. [Google Scholar] [CrossRef] [PubMed]
- Hart, R. A study of boehmite formation on aluminium surfaces by electron diffraction. Trans. Faraday Soc. 1954, 50, 269–273. [Google Scholar] [CrossRef]
- Harrington, R.; Nelson, H. An electron diffraction study of anodic films. Trans. Am. Inst. Min. Metall. Pet. Engvol. 1940, 137, 62. [Google Scholar]
- Bryan, J. The action of boiling distilled water on aluminium. J. Soc. Chem. Ind. London 1950, 69, 169–173. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, W.; Page, K.L.; Pearce, C.I.; Bowden, M.E.; Graham, T.R.; Shen, Z.; Li, P.; Wang, Z.; Kerisit, S.; et al. Size and Morphology Controlled synthesis of boehmite nanoplates and crystal growth mechanisms. Cryst. Growth Des. 2018, 18, 3596–3606. [Google Scholar] [CrossRef]
- Okada, K.; Nagashima, T.; Kameshima, Y.; Yasumori, A.; Tsukada, T. Relationship between formation conditions, properties, and crystallite size of boehmite. J. Colloid Interface Sci. 2002, 253, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Kocjan, A.; Dakskobler, A.; Kosmac, T. Superhydrophobic nanostructured boehmite coatings prepared by AlN powder hydrolysis. Int. J. Appl. Ceram. Technol. 2011, 8, 848–853. [Google Scholar] [CrossRef]
- Platz, D.; Tholén, E.A.; Pesen, D.; Haviland, D.B. Intermodulation atomic force microscopy. Appl. Phys. Lett. 2008, 92, 153106. [Google Scholar] [CrossRef]
- Nonnenmacher, M.; O’Boyle, M.P.; Wickramasinghe, H.K. Kelvin probe force microscopy. Appl. Phys. Lett. 1991, 58, 2921–2923. [Google Scholar] [CrossRef]
- Dazzi, A.; Prazeres, R.; Glotin, F.; Ortega, J. Local infrared microspectroscopy with subwavelength spatial resolution with an atomic force microscope tip used as a photothermal sensor. Opt. Lett. 2005, 30, 2388–2390. [Google Scholar] [CrossRef]
- Dazzi, A. Sub-100-nanometer infrared spectroscopy and imaging based on a near-field photothermal technique (PTIR). Biomed. Vib. Spectro. 2008, 291–312. [Google Scholar]
- Dazzi, A.; Prater, C.B.; Hu, Q.; Chase, D.B.; Rabolt, J.F.; Marcott, C. AFM–IR: combining atomic force microscopy and infrared spectroscopy for nanoscale chemical characterization. Appl. Spectrosc. 2012, 66, 1365–1384. [Google Scholar] [CrossRef]
- Dazzi, A.; Prazeres, R.; Glotin, F.; Ortega, J.M. Subwavelength infrared spectromicroscopy using an AFM as a local absorption sensor. Infrared Phys. Technol. 2006, 49, 113–121. [Google Scholar] [CrossRef]
- Hodoroaba, V.D.; Motzkus, C.; Macé, T.; Vaslin-Reimann, S. Performance of high-resolution SEM/EDX systems equipped with transmission mode (TSEM) for imaging and measurement of size and size distribution of spherical nanoparticles. Microsc. Microanal. 2014, 20, 602–612. [Google Scholar] [CrossRef]
- Butt, H.J. Measuring electrostatic, van der Waals, and hydration forces in electrolyte solutions with an atomic force microscope. Biophys. J. 1991, 60, 1438–1444. [Google Scholar] [CrossRef]
- Rosa-Zeiser, A.; Weilandt, E.; Hild, S.; Marti, O. The simultaneous measurement of elastic, electrostatic and adhesive properties by scanning force microscopy: pulsed-force mode operation. Meas. Sci. Technol. 1997, 8, 1333. [Google Scholar] [CrossRef]
- Butt, H.-J.; Cappella, B.; Kappl, M. Force measurements with the atomic force microscope: Technique, interpretation and applications. Surf. Sci. Rep. 2005, 59, 1–152. [Google Scholar] [CrossRef]
- Fernàndez-Francos, X.; Kazarian, S.G.; Ramis, X.; Serra, A. Simultaneous monitoring of curing shrinkage and degree of cure of thermosets by attenuated total reflection Fourier transform infrared (ATR FT-IR) spectroscopy. Appl. Spectrosc. 2013, 67, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Fripiat, J.J.; Bosmans, H.J.; Rouxhet, P.G. Proton mobility in solids. I. Hydrogenic vibration modes and proton delocalization in boehmite. J. Phys. Chem. 1967, 71, 1097–1111. [Google Scholar] [CrossRef]
- Fernàndez-Francos, X.; Ramis, X.; Serra, A. From curing kinetics to network structure: A novel approach to the modeling of the network buildup of epoxy–anhydride thermosets. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 61–75. [Google Scholar] [CrossRef]
- Borgani, R.; Pallon, L.K.; Hedenqvist, M.S.; Gedde, U.W.; Haviland, D.B. Local charge injection and extraction on surface-modified Al2O3 nanoparticles in LDPE. Nano Lett. 2016, 16, 5934–5937. [Google Scholar] [CrossRef] [PubMed]
- Giesa, T.; Arslan, M.; Pugno, N.M.; Buehler, M.J. Nanoconfinement of spider silk fibrils begets superior strength, extensibility, and toughness. Nano Lett. 2011, 11, 5038–5046. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A.; Chen, P.-Y.; Lin, A.Y.-M.; Seki, Y. Biological materials: structure and mechanical properties. Prog. Mater Sci. 2008, 53, 1–206. [Google Scholar] [CrossRef]
- Fratzl, P.; Gupta, H.S.L.; Fischer, F.D.; Kolednik, O. Hindered crack propagation in materials with periodically varying Young’s modulus—lessons from biological materials. Adv. Mater. 2007, 19, 2657–2661. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Duong, L.V.; Wood, B.J.; Frost, R.L. XPS study of the major minerals in bauxite: gibbsite, bayerite and (pseudo-) boehmite. J. Colloid Interface Sci. 2006, 296, 572–576. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasem Zadeh Khorasani, M.; Elert, A.-M.; Hodoroaba, V.-D.; Agudo Jácome, L.; Altmann, K.; Silbernagl, D.; Sturm, H. Short- and Long-Range Mechanical and Chemical Interphases Caused by Interaction of Boehmite (γ-AlOOH) with Anhydride-Cured Epoxy Resins. Nanomaterials 2019, 9, 853. https://doi.org/10.3390/nano9060853
Ghasem Zadeh Khorasani M, Elert A-M, Hodoroaba V-D, Agudo Jácome L, Altmann K, Silbernagl D, Sturm H. Short- and Long-Range Mechanical and Chemical Interphases Caused by Interaction of Boehmite (γ-AlOOH) with Anhydride-Cured Epoxy Resins. Nanomaterials. 2019; 9(6):853. https://doi.org/10.3390/nano9060853
Chicago/Turabian StyleGhasem Zadeh Khorasani, Media, Anna-Maria Elert, Vasile-Dan Hodoroaba, Leonardo Agudo Jácome, Korinna Altmann, Dorothee Silbernagl, and Heinz Sturm. 2019. "Short- and Long-Range Mechanical and Chemical Interphases Caused by Interaction of Boehmite (γ-AlOOH) with Anhydride-Cured Epoxy Resins" Nanomaterials 9, no. 6: 853. https://doi.org/10.3390/nano9060853
APA StyleGhasem Zadeh Khorasani, M., Elert, A.-M., Hodoroaba, V.-D., Agudo Jácome, L., Altmann, K., Silbernagl, D., & Sturm, H. (2019). Short- and Long-Range Mechanical and Chemical Interphases Caused by Interaction of Boehmite (γ-AlOOH) with Anhydride-Cured Epoxy Resins. Nanomaterials, 9(6), 853. https://doi.org/10.3390/nano9060853






