Nanoporous Iron Oxide/Carbon Composites through In-Situ Deposition of Prussian Blue Nanoparticles on Graphene Oxide Nanosheets and Subsequent Thermal Treatment for Supercapacitor Applications
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Synthesis of GO Nanosheets
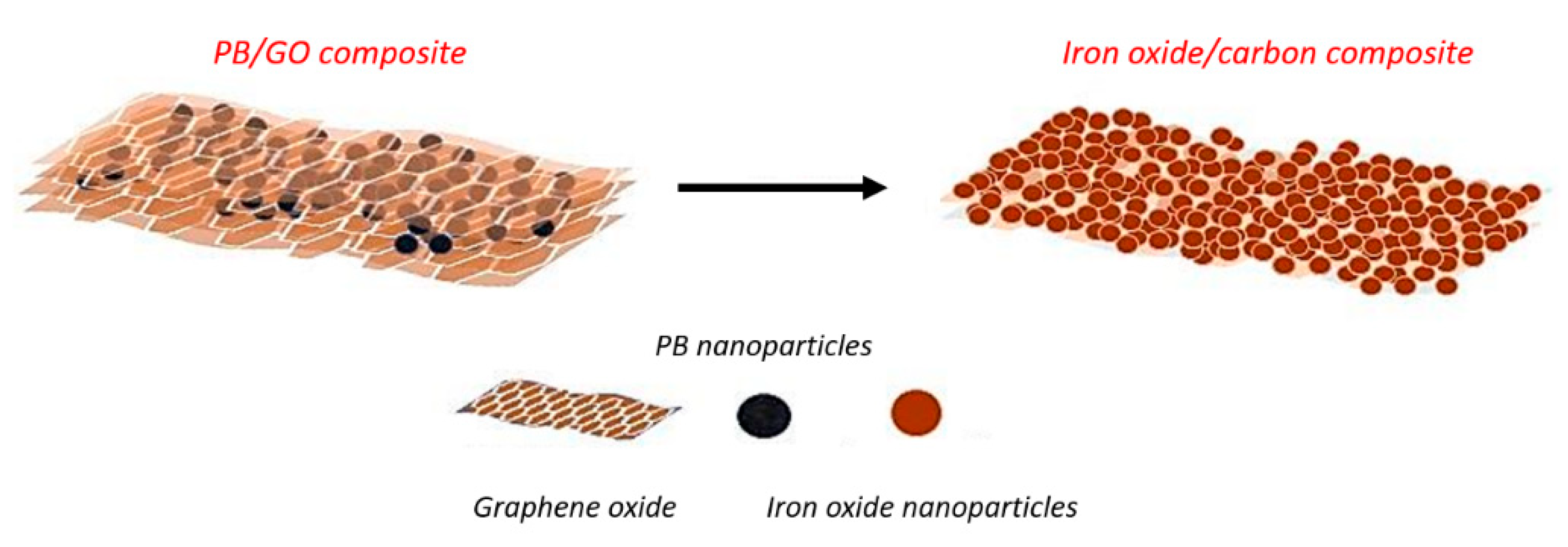
2.3. In-Situ Growth of PB Nanoparticles on the Surface of GO Nanosheets (PB/GO Composite) and the Subsequent Thermal Conversion to Nanoporous Iron Oxide/Carbon Composite
2.4. Characterization
2.5. Electrochemical Measurements
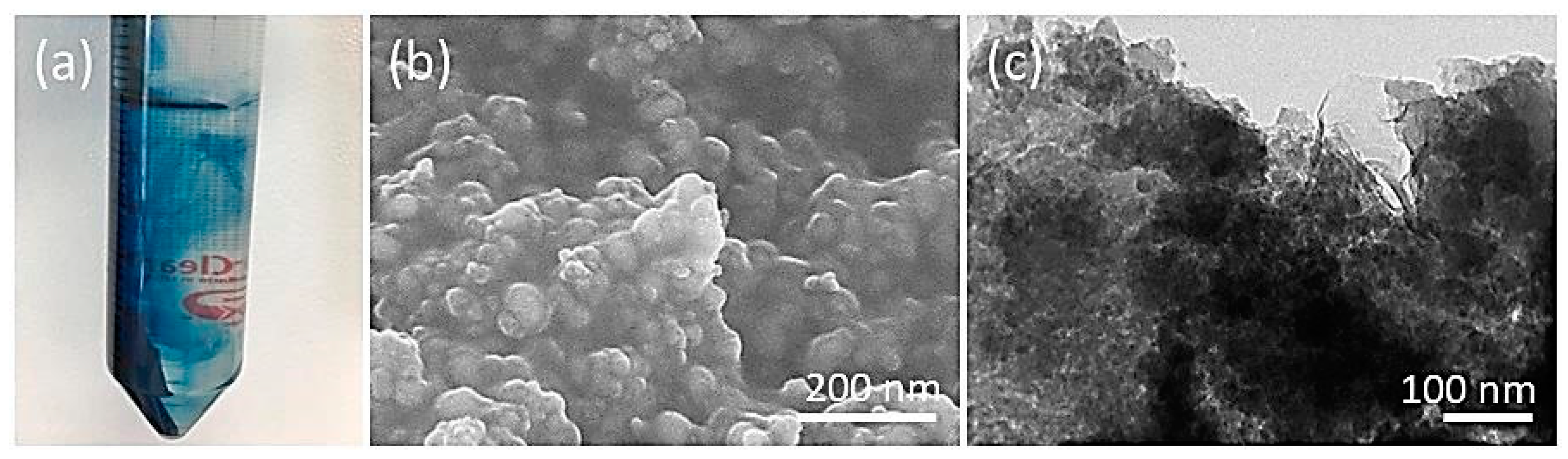
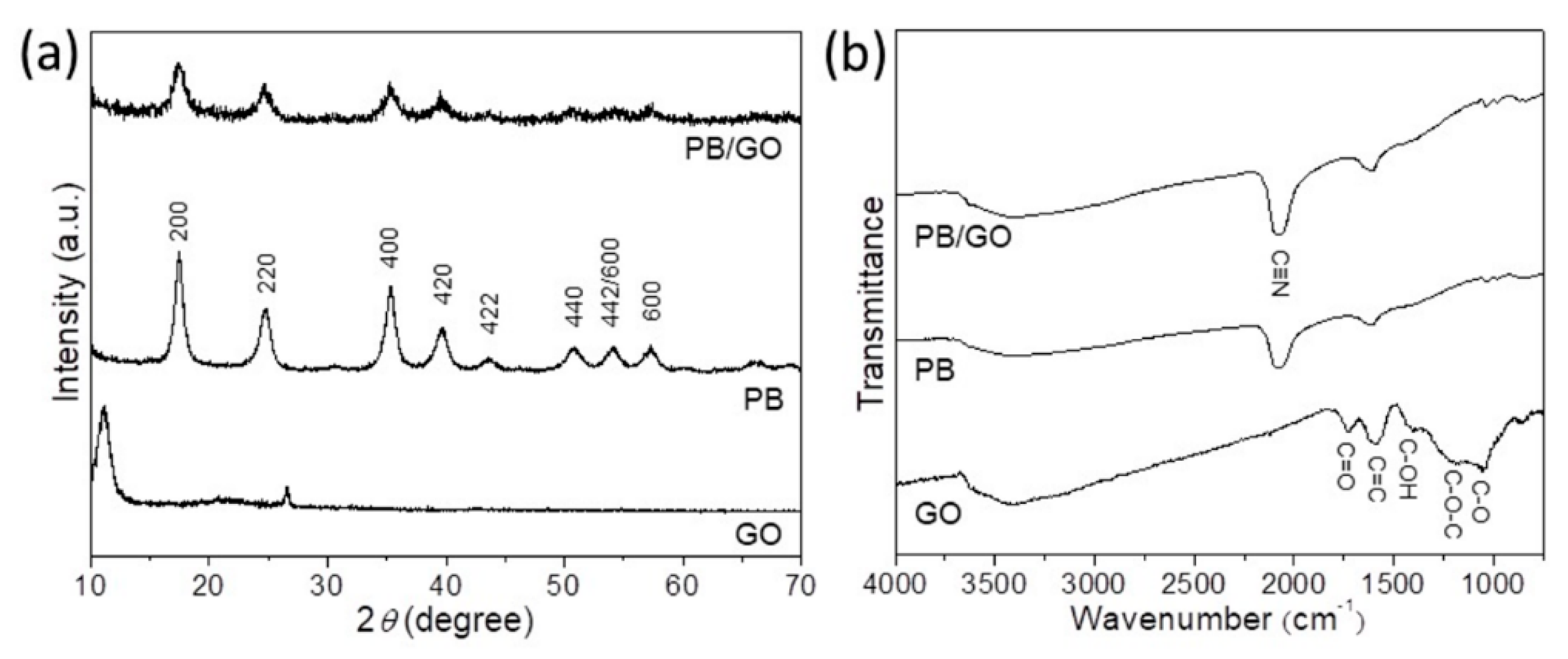
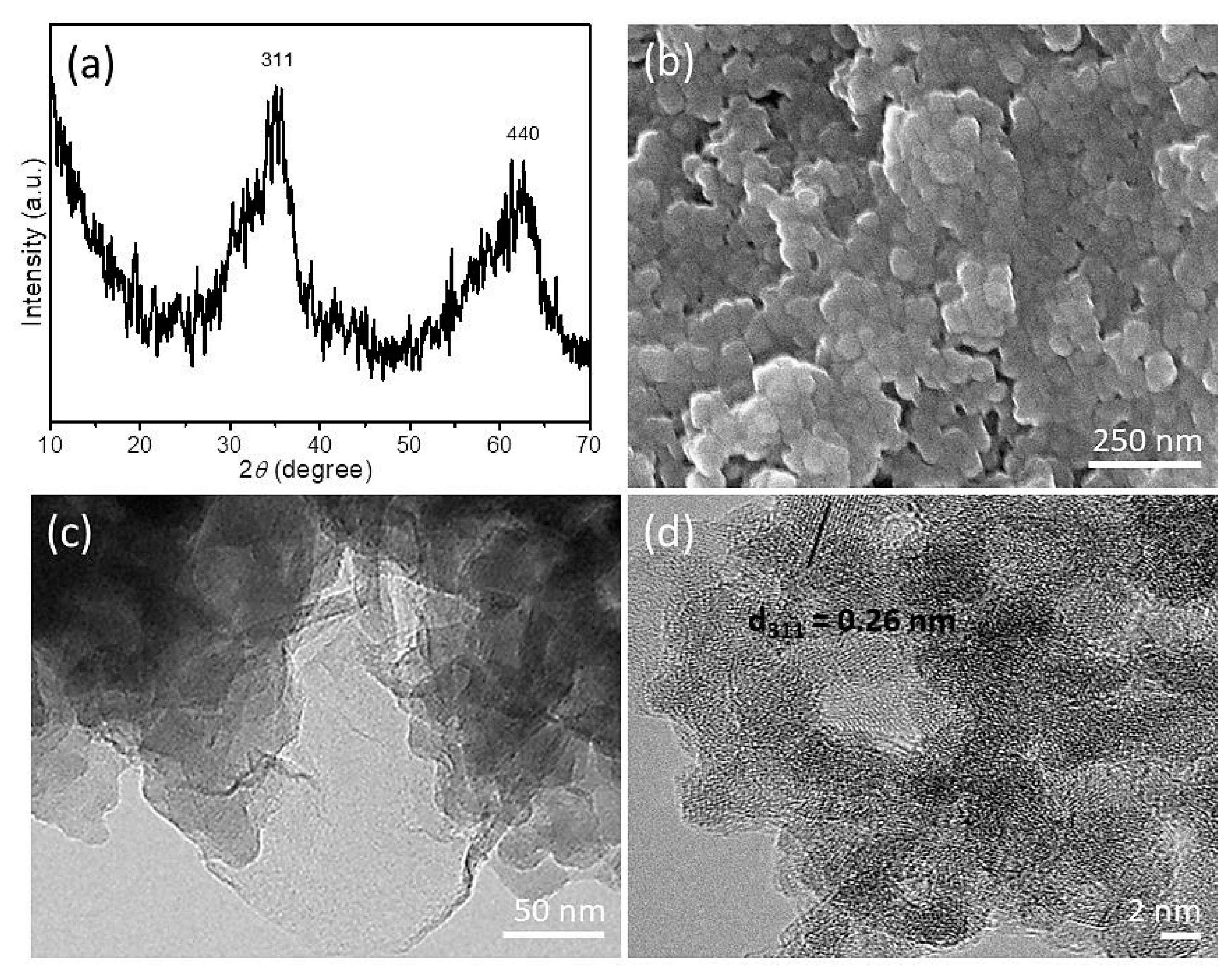
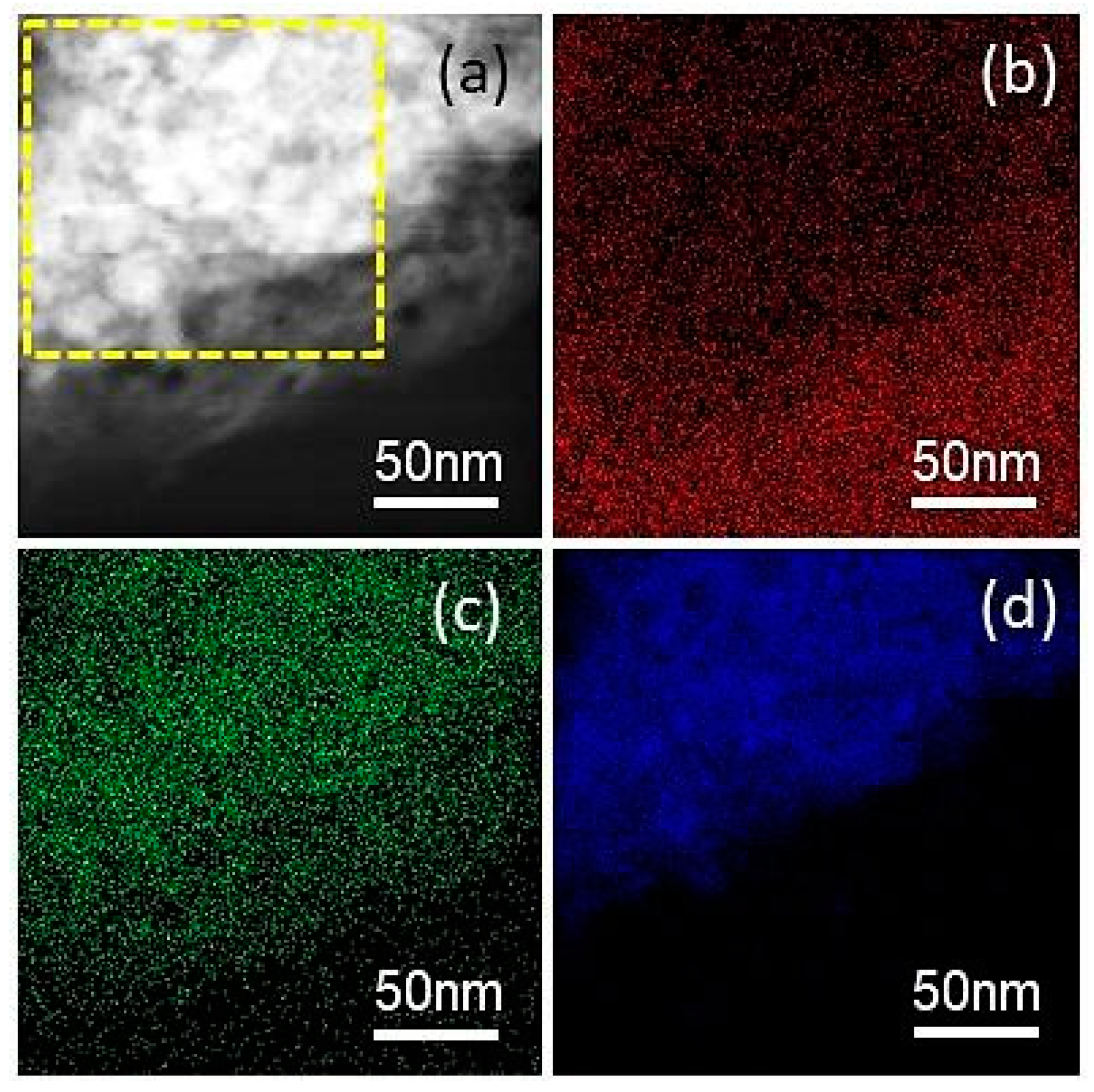
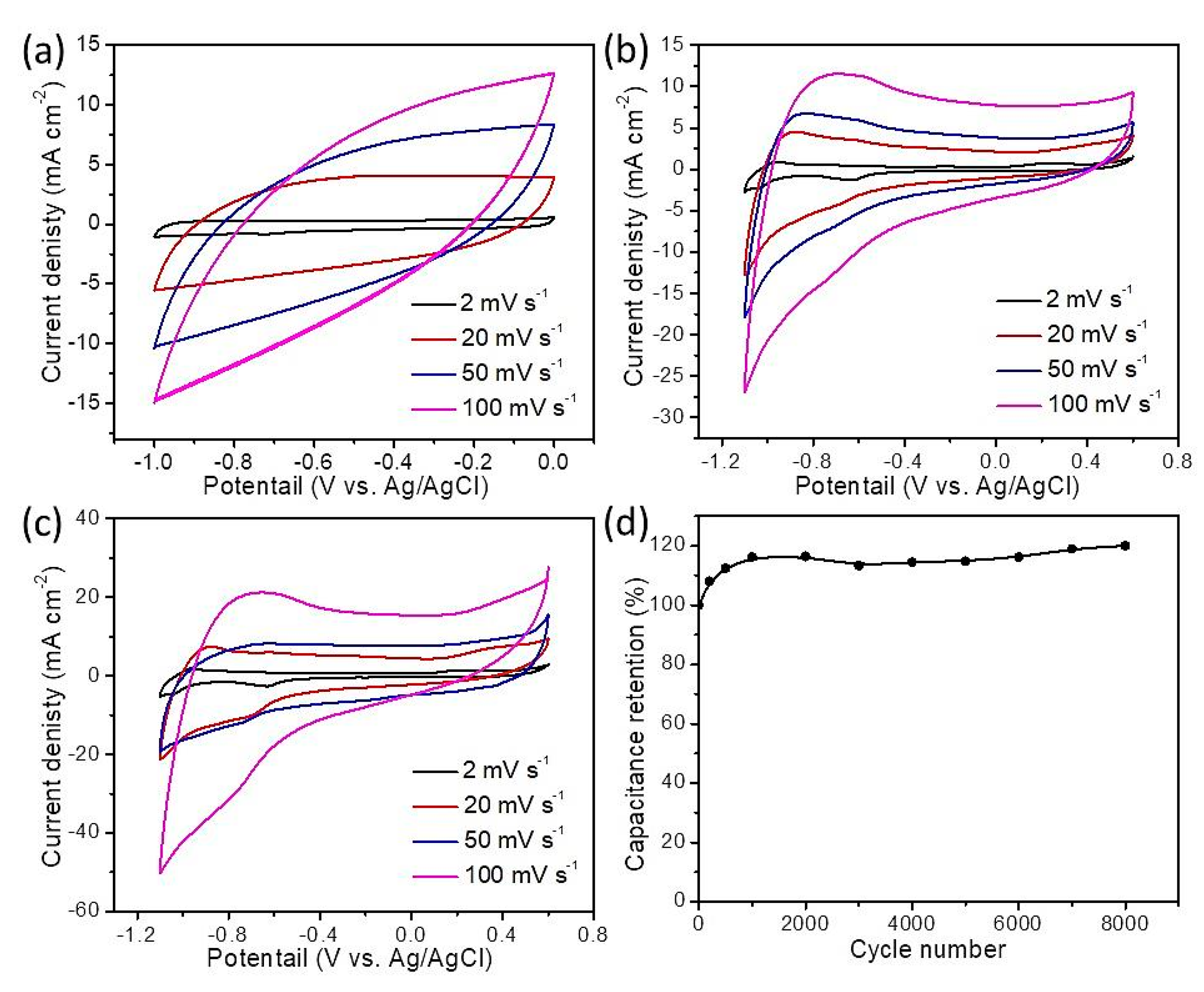
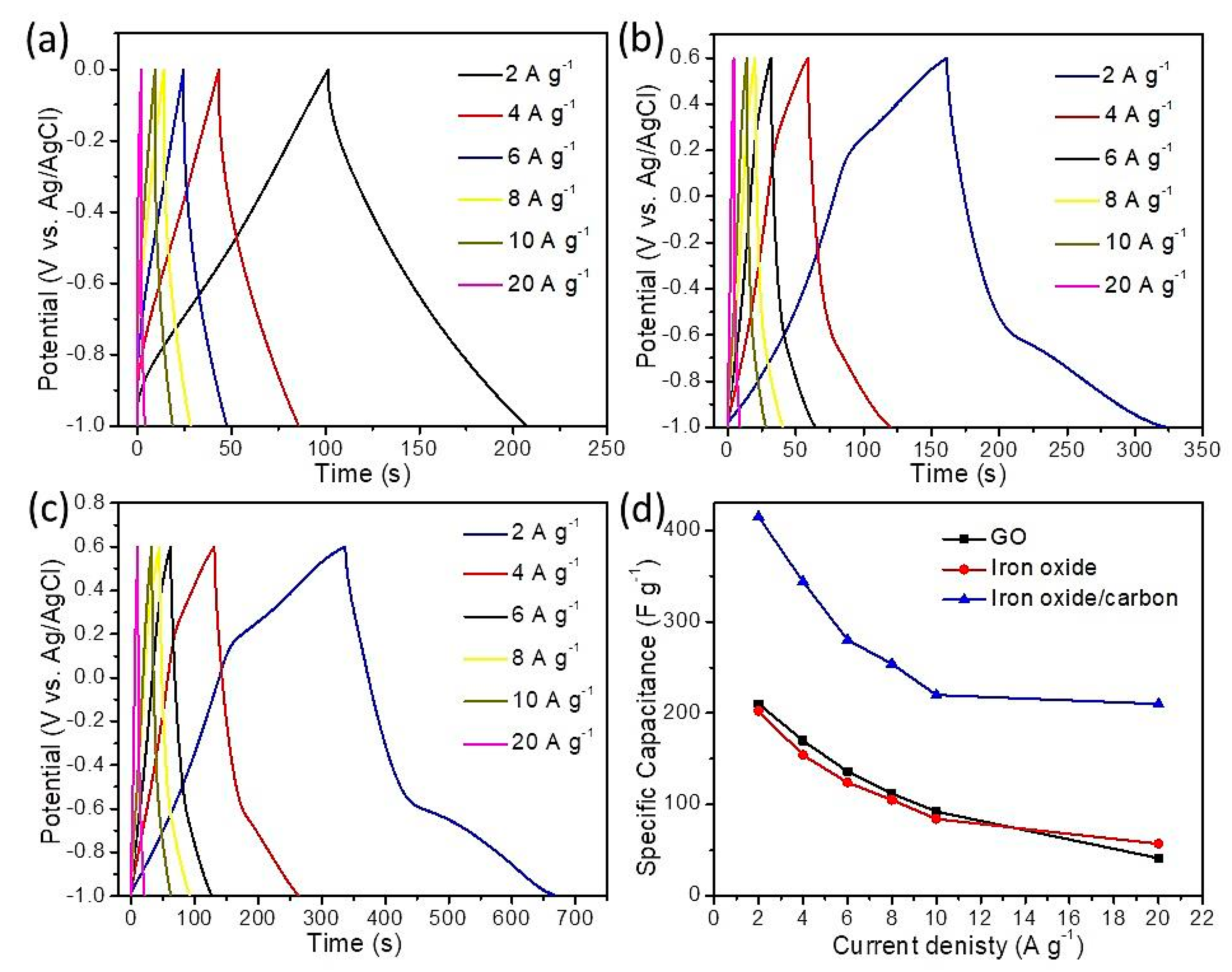
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tanaka, S.; Salunkhe, R.R.; Kaneti, Y.V.; Malgras, V.; Alshehri, S.M.; Ahamad, T.; Zakaria, M.B.; Dou, S.X.; Yamauchi, Y.; Hossain MS, A. Prussian blue derived iron oxide nanoparticles wrapped in graphene oxide sheets for electrochemical supercapacitors. RSC Adv. 2017, 7, 33994–33999. [Google Scholar] [CrossRef]
- Wang, Z.L.; Sun, K.; Henzie, J.; Hao, X.; Ide, Y.; Takei, T.; Bando, Y.; Yamauchi, Y. Electrochemically in situ controllable assembly of hierarchically-ordered and integrated inorganic-carbon hybrids for efficient hydrogen evolution. Mater. Horiz. 2018, 5, 1194–1203. [Google Scholar] [CrossRef]
- Sun, D.; Wang, H.; Deng, B.; Zhang, H.; Wang, L.; Wan, Q.; Yan, X.; Qu, M. A Mn Fe based Prussian blue Analogue@Reduced graphene oxide composite as high capacity and superior rate capability anode for lithium-ion batteries. Carbon 2019, 143, 706–713. [Google Scholar] [CrossRef]
- Cao, L.; Liu, Y.; Zhang, B.; Lu, L. In situ controllable growth of prussian blue nanocubes on reduced graphene oxide: Facile synthesis and their application as enhanced nanoelectrocatalyst for H2O2 reduction. ACS Appl. Mater. Interfaces 2010, 2, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Leeb, P.S.; Li, C. 3D carbon based nanostructures for advanced supercapacitors. Energy Environ. Sci. 2013, 6, 41–53. [Google Scholar] [CrossRef]
- Wong, M.H.; Zhang, Z.; Yang, X.; Chen, X.; Ying, J.Y. One-pot in situ redox synthesis of hexacyanoferrate/conductive polymer hybrids as lithium-ion battery cathodes. Chem. Commun. 2015, 51, 13674–13677. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Krumeich, F.; Nesper, R. Nanocomposite of manganese ferrocyanide and graphene: A promising cathode material for rechargeable lithium ion batteries. Electrochem. Commun. 2013, 34, 246–249. [Google Scholar] [CrossRef]
- Daneshvar, F.; Aziz, A.; Abdelkader, A.M.; Zhang, T.; Sue, H.-J.; Welland, M.E. Porous SnO2-CuxO nanocomposite thin film on carbon nanotubes as electrodes for high performance supercapacitors. Nanotechnology 2019, 30, 015401. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-L.; Wang, J.-Z.; Chew, S.-Y.; Liu, H.-K.; Dou, S.-X. Electrodeposition of MnO2 nanowires on carbon nanotube paper as free-standing, flexible electrode for supercapacitors. Electrochem Commun. 2008, 10, 1724–1727. [Google Scholar] [CrossRef]
- Chen, Z.; Augustyn, V.; Wen, J.; Zhang, Y.; Shen, M.; Dunn, B.; Lu, Y. High-Performance Supercapacitors Based on Intertwined CNT/V2O5 Nanowire Nanocomposites. Adv. Mater. 2011, 23, 791–795. [Google Scholar] [CrossRef]
- Zakaria, M.B.; Tan, H.; Kim, J.; Badjah, A.Y.; Naushad, M.; Habila, M.; Wabaidur, S.; Alothman, Z.A.; Yamauchi, Y.; Lin, J. Structurally controlled layered Ni3C/graphene hybrids using cyano-bridged coordination polymers. Electrochem. Commun. 2019, 100, 74–8075. [Google Scholar] [CrossRef]
- Cai, Z.X.; Wang, Z.L.; Kim, J.; Yamauchi, Y. Hollow Functional Materials Derived from Metal-Organic Frameworks: Synthetic Strategies, Conversion Mechanisms, and Electrochemical Applications. Adv. Mater. 2019, 31, 1804903. [Google Scholar] [CrossRef]
- Tanaka, S.; Zakaria, M.B.; Kaneti, Y.V.; Jikihara, Y.; Nakayama, T.; Zaman, M.; Bando, Y.; Hossain, M.S.A. Gold-loaded nanoporous iron oxide cubes derived from Prussian blue as carbon monoxide oxidation catalyst at room temperature. ChemistrySelect 2018, 3, 13464–13469. [Google Scholar] [CrossRef]
- Liu, H.D.; Zhang, J.L.; Xu, D.D.; Huang, L.H.; Tan, S.Z.; Mai, W.J. Easy one-step hydrothermal synthesis of nitrogen-doped reduced graphene oxide/iron oxide hybrid as efficient supercapacitor material. J. Solid State Electrochem. 2014, 19, 135–144. [Google Scholar] [CrossRef]
- Zakaria, M.B.; Li, C.; Ji, Q.; Jiang, B.; Tominaka, S.; Ide, Y.; Hill, J.P.; Ariga, K.; Yamauchi, Y. Self-construction from 2D to 3D: One-Pot layer-by-layer assembly of graphene oxide sheets held together by coordination polymers. Angew. Chem. Int. Ed. 2016, 55, 8426–8430. [Google Scholar] [CrossRef] [PubMed]
- Azhar, A.; Zakaria, M.B.; Lin, J.; Chikyow, T.; Martin, D.J.; Alghamdi, Y.G.; Alshehri, A.A.; Bando, Y.; Hossain, M.S.A.; Wu, K.C.W.; et al. Graphene-wrapped nanoporous nickel-cobalt oxide flakes for electrochemical supercapacitors. ChemistrySelect 2018, 3, 8505–8510. [Google Scholar] [CrossRef]
- Chen, Y.L.; Hu, Z.A.; Chang, Y.Q.; Wang, H.W.; Zhang, Z.Y.; Yang, Y.Y.; Wu, H.Y. Zinc oxide/reduced graphene oxide composites and electrochemical capacitance enhanced by homogeneous incorporation of reduced graphene oxide sheets in zinc oxide matrix. J. Phys. Chem. C 2011, 115, 2563–2571. [Google Scholar] [CrossRef]
- Huang, X.; Zhi, C.; Jiang, P.; Golberg, D.; Bando, Y.; Tanaka, T. Temperature-dependent electrical property transition of graphene oxide paper. Nanotechnology 2012, 23, 455705. [Google Scholar] [CrossRef]
- Muthusamy, S.; Charles, J.; Renganathan, B.; Sastikumar, D. In situ growth of Prussian blue nanocubes on polypyrrole nanoparticles: Facile synthesis, characterization and their application as fiber optic gas sensor. J. Mater. Sci. 2018, 53, 15401–15417. [Google Scholar] [CrossRef]
- Vermisoglou, E.C.; Devlin, E.; Giannakopoulou, T.; Romanos, G.; Boukos, N.; Psycharis, V.; Lei, C.; Lekakou, C.; Petridis, D.; Trapalis, C. Reduced graphene oxide/iron carbide nanocomposites for magnetic and supercapacitor applications. J. Alloys Compd. 2014, 590, 102–109. [Google Scholar] [CrossRef]
- Liu, X.W.; Yao, Z.J.; Wang, Y.F.; Wei, X.W. Graphene oxide sheet-prussian blue nanocomposites: Green synthesis and their extraordinary electrochemical properties. Coll. Surf. B Biointerfaces 2010, 81, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.C.; Haldorai, Y.; Lee, G.W.; Hwang, S.K.; Han, Y.K.; Roh, C.; Huh, Y.S. Porous three-dimensional graphene foam/Prussian blue composite for efficient removal of radioactive137Cs. Sci. Rep. 2015, 5, 17510. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Gu, M.; Pan, L.; Xu, J.; Han, L.; Yi, F.Y. The interlocked: In situ fabrication of graphene@prussian blue nanocomposite as high-performance supercapacitor. Dalton Trans. 2018, 47, 13126–13134. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, H.; Zhai, J.; Sun, L.; Zhao, Y.; Yu, H. Magnetic prussian blue/graphene oxide nanocomposites caged in calcium alginate microbeads for elimination of cesium ions from water and soil. Chem. Eng. J. 2014, 246, 10–19. [Google Scholar] [CrossRef]
- Hu, M.; Belik, A.A.; Imura, M.; Mibu, K.; Tsujimoto, Y.; Yamauchi, Y. Synthesis of superparamagnetic nanoporous iron oxide particles with hollow interiors by using Prussian blue coordination polymers. Chem. Mater. 2012, 24, 2698–2707. [Google Scholar] [CrossRef]
- Zakaria, M.B.; Belik, A.A.; Liu, C.; Hsieh, H.; Liao, Y.; Malgras, V.; Yamauchi, Y.; Wu, K.C. Prussian blue derived nanoporous iron oxides as anticancer drug carriers for magnetic-guided chemotherapy. Chem. Asian J. 2015, 10, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Chen, S.; Yang, S.; Chen, W.; Weng, W.; Cheng, Y.; Zhu, M. Flexible all-solid-state asymmetric supercapacitor based on transition metal oxide nanorods/reduced graphene oxide hybrid fibers with high energy density. Carbon 2017, 113, 151–158. [Google Scholar] [CrossRef]
- Li, M.; Pan, F.; Shi, E.; Choo, G.; Lv, Y.; Chen, Y.; Xue, J. Designed construction of a graphene and iron oxide freestanding electrode with enhanced flexible energy-storage performance. ACS Appl. Mater. Interfaces 2016, 8, 6972–6981. [Google Scholar] [CrossRef]
- Mallick, S.; Jana, P.P.; Raj, C.R. Asymmetric supercapacitor based on chemically coupled hybrid material of Fe2O3-Fe3O4 heterostructure and nitrogen-doped reduced graphene oxide. ChemElectroChem 2018, 5, 2348–2356. [Google Scholar] [CrossRef]
- Liu, M.; Sun, J. In situ growth of monodisperse Fe3O4 nanoparticles on graphene as flexible paper for supercapacitor. J. Mater. Chem. A 2014, 2, 12068–12074. [Google Scholar] [CrossRef]
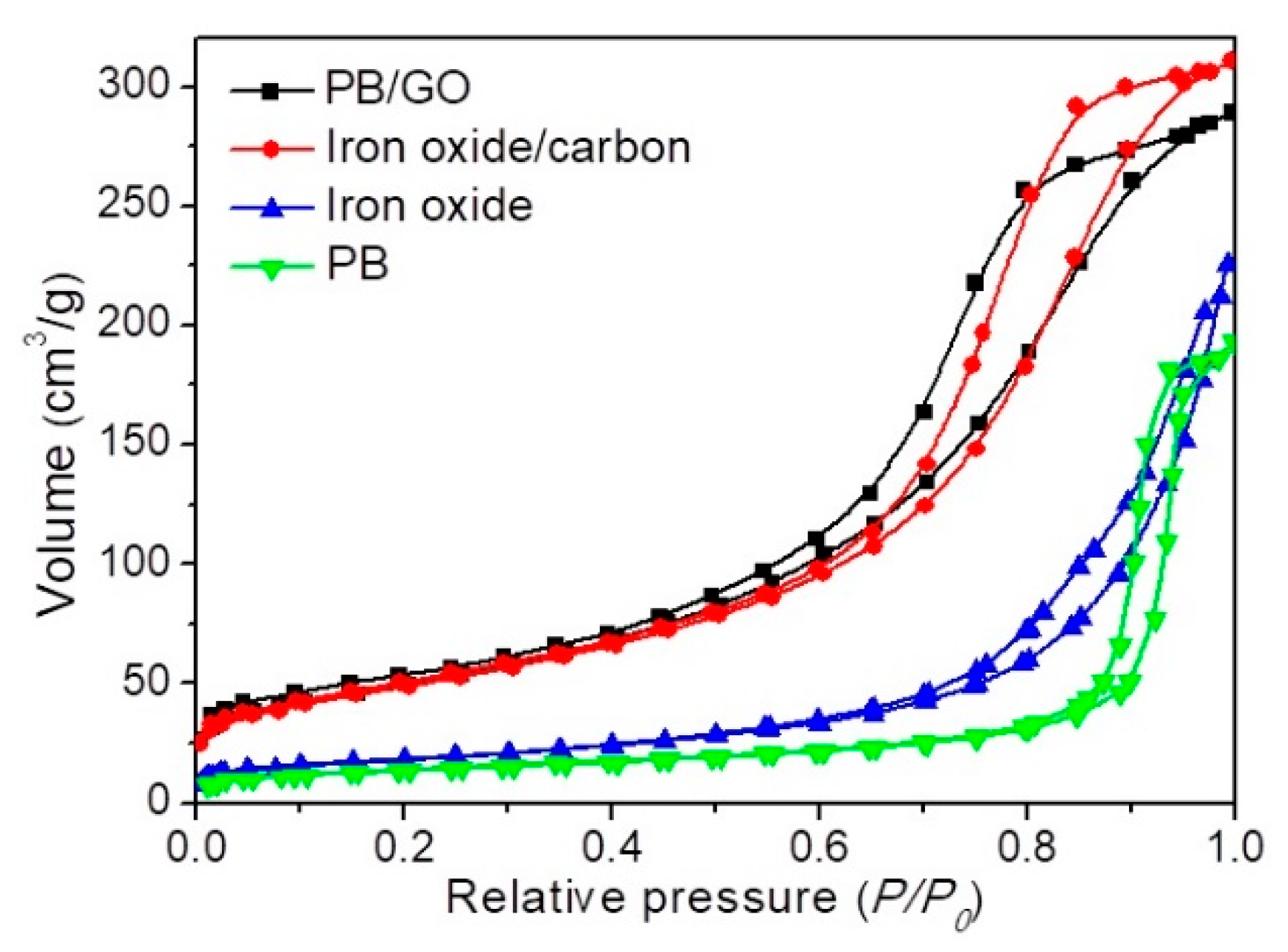
| Sample | Surface Area/m2 g−1 | Pore Volume/cm3 g−1 |
|---|---|---|
| PB | 36.1 | 0.291 |
| Iron oxide | 60.3 | 0.343 |
| PB/GO | 152.6 | 0.453 |
| Iron oxide/carbon | 145.5 | 0.487 |
| Scan Rate/mV s−1 | Specific Capacitance/F g−1 | ||
|---|---|---|---|
| GO | Iron Oxide | Iron Oxide/Carbon | |
| 2 | 220.0 | 264.0 | 551.5 |
| 20 | 205.3 | 145.5 | 450.0 |
| 50 | 110.0 | 91.70 | 340.0 |
| 100 | 97.00 | 82.00 | 275.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azhar, A.; Yamauchi, Y.; Allah, A.E.; Alothman, Z.A.; Badjah, A.Y.; Naushad, M.; Habila, M.; Wabaidur, S.; Wang, J.; Zakaria, M.B. Nanoporous Iron Oxide/Carbon Composites through In-Situ Deposition of Prussian Blue Nanoparticles on Graphene Oxide Nanosheets and Subsequent Thermal Treatment for Supercapacitor Applications. Nanomaterials 2019, 9, 776. https://doi.org/10.3390/nano9050776
Azhar A, Yamauchi Y, Allah AE, Alothman ZA, Badjah AY, Naushad M, Habila M, Wabaidur S, Wang J, Zakaria MB. Nanoporous Iron Oxide/Carbon Composites through In-Situ Deposition of Prussian Blue Nanoparticles on Graphene Oxide Nanosheets and Subsequent Thermal Treatment for Supercapacitor Applications. Nanomaterials. 2019; 9(5):776. https://doi.org/10.3390/nano9050776
Chicago/Turabian StyleAzhar, Alowasheeir, Yusuke Yamauchi, Abeer Enaiet Allah, Zeid A. Alothman, Ahmad Yacine Badjah, Mu. Naushad, Mohamed Habila, Saikh Wabaidur, Jie Wang, and Mohamed Barakat Zakaria. 2019. "Nanoporous Iron Oxide/Carbon Composites through In-Situ Deposition of Prussian Blue Nanoparticles on Graphene Oxide Nanosheets and Subsequent Thermal Treatment for Supercapacitor Applications" Nanomaterials 9, no. 5: 776. https://doi.org/10.3390/nano9050776
APA StyleAzhar, A., Yamauchi, Y., Allah, A. E., Alothman, Z. A., Badjah, A. Y., Naushad, M., Habila, M., Wabaidur, S., Wang, J., & Zakaria, M. B. (2019). Nanoporous Iron Oxide/Carbon Composites through In-Situ Deposition of Prussian Blue Nanoparticles on Graphene Oxide Nanosheets and Subsequent Thermal Treatment for Supercapacitor Applications. Nanomaterials, 9(5), 776. https://doi.org/10.3390/nano9050776









