Design and Regulation of Novel MnFe2O4@C Nanowires as High Performance Electrode for Supercapacitor
Abstract
:1. Introduction
2. Materials and Methods
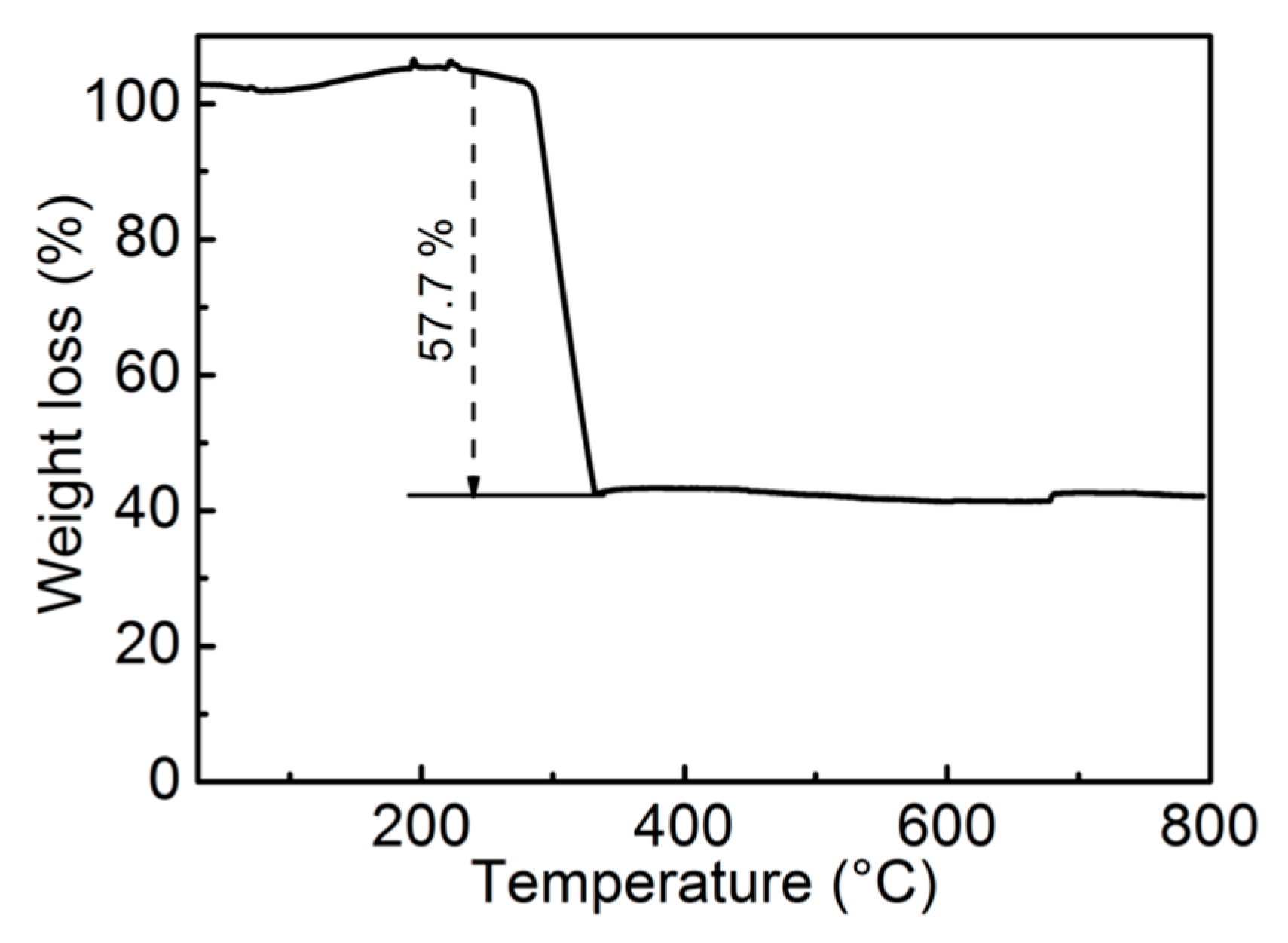
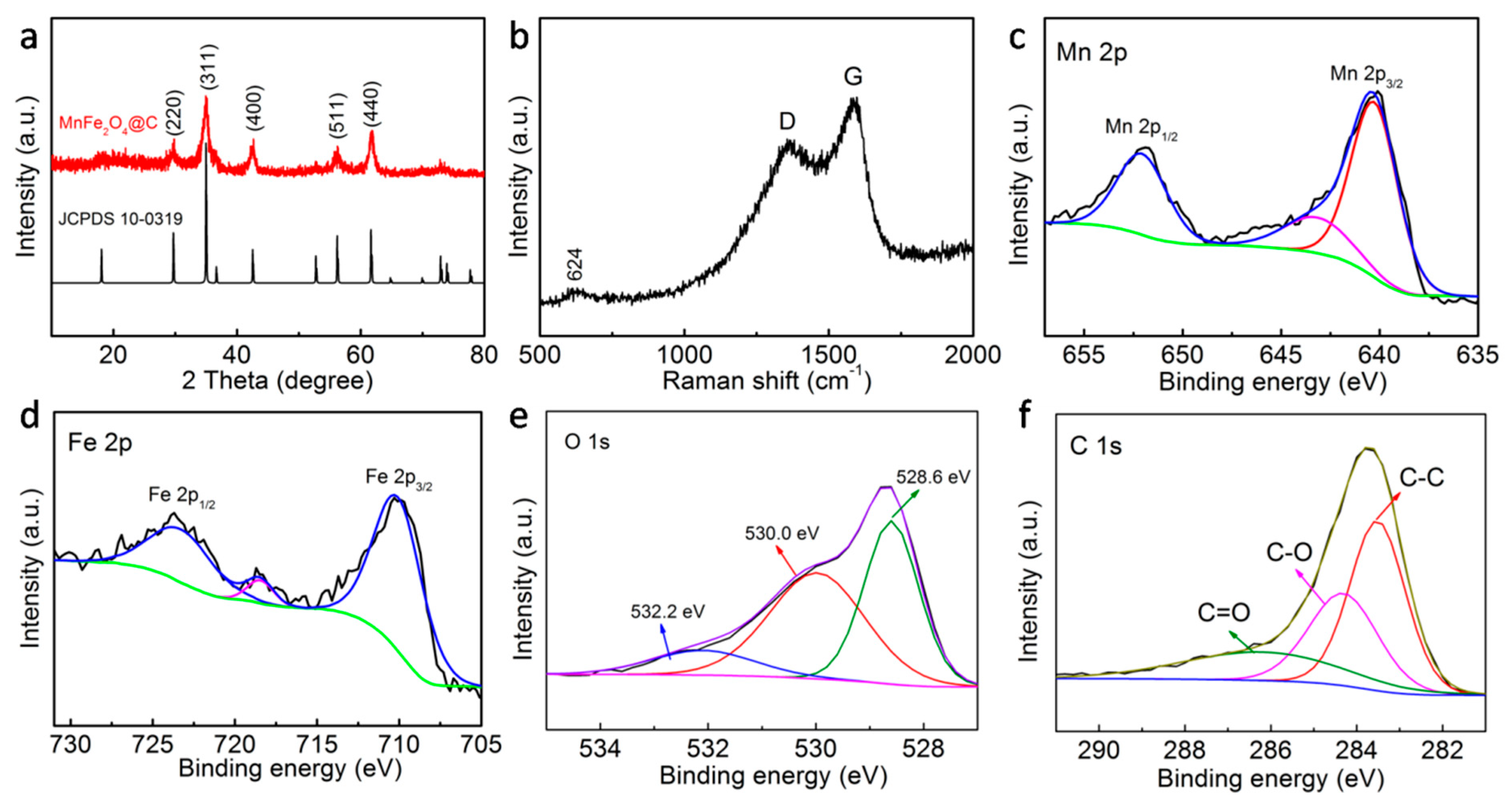
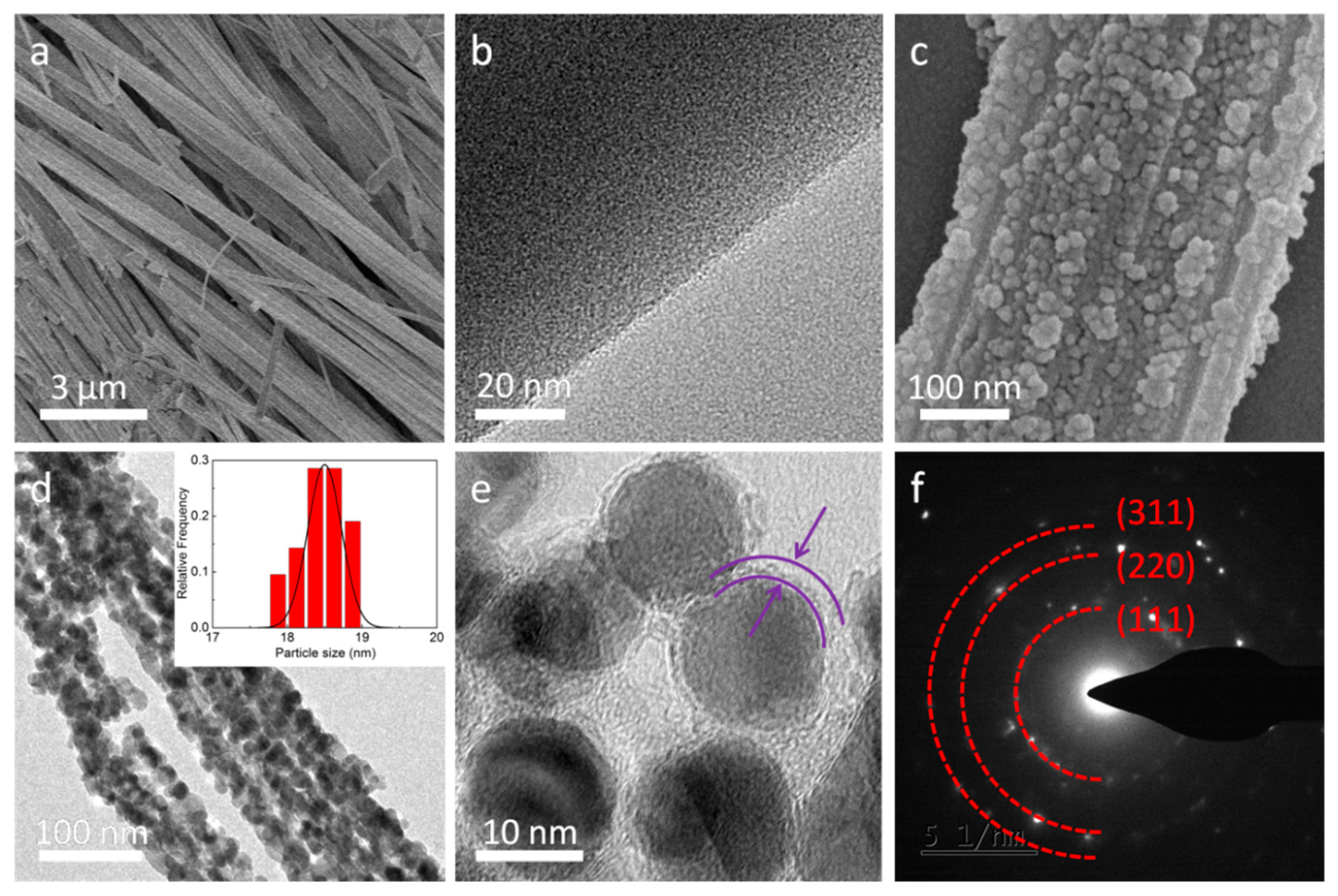
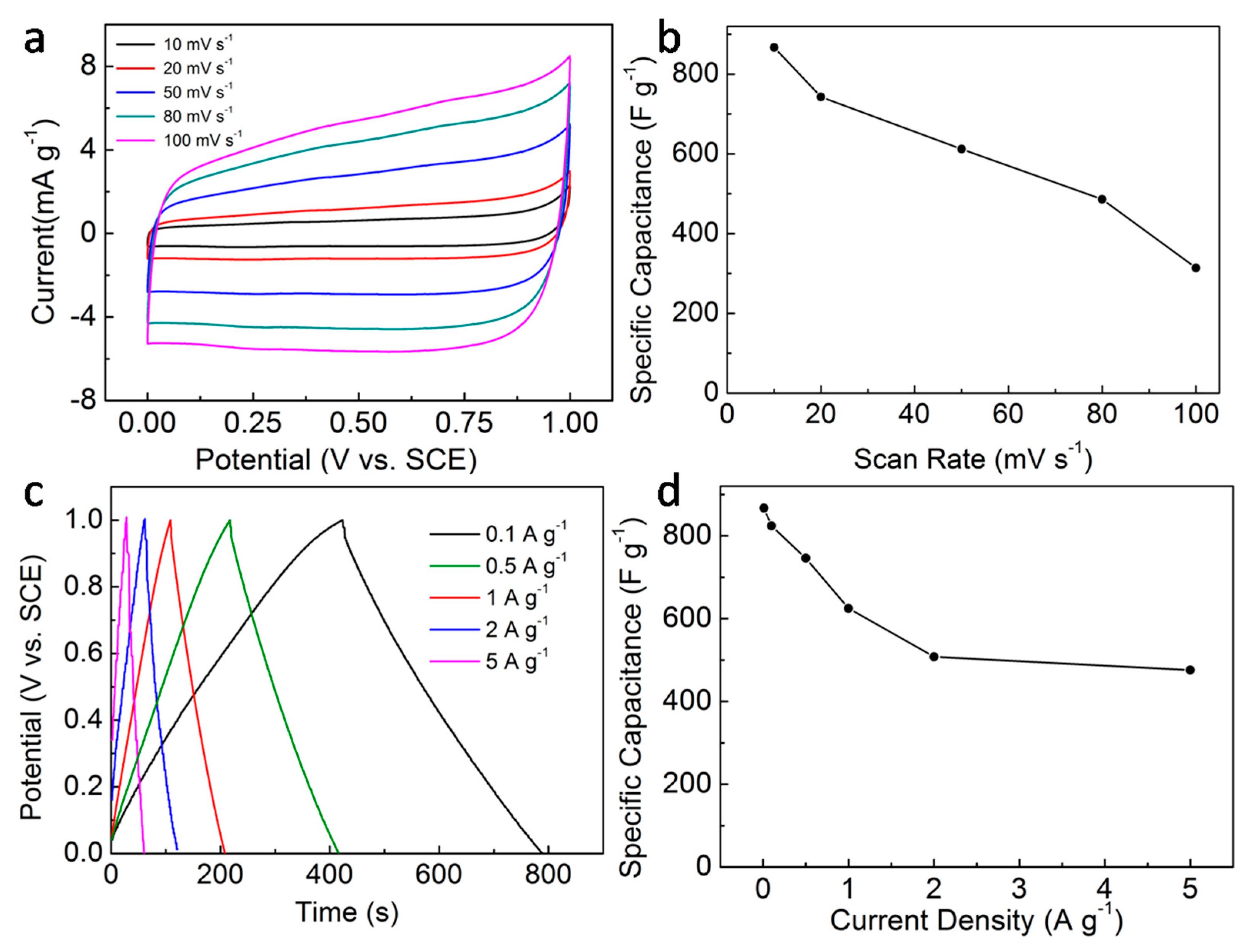
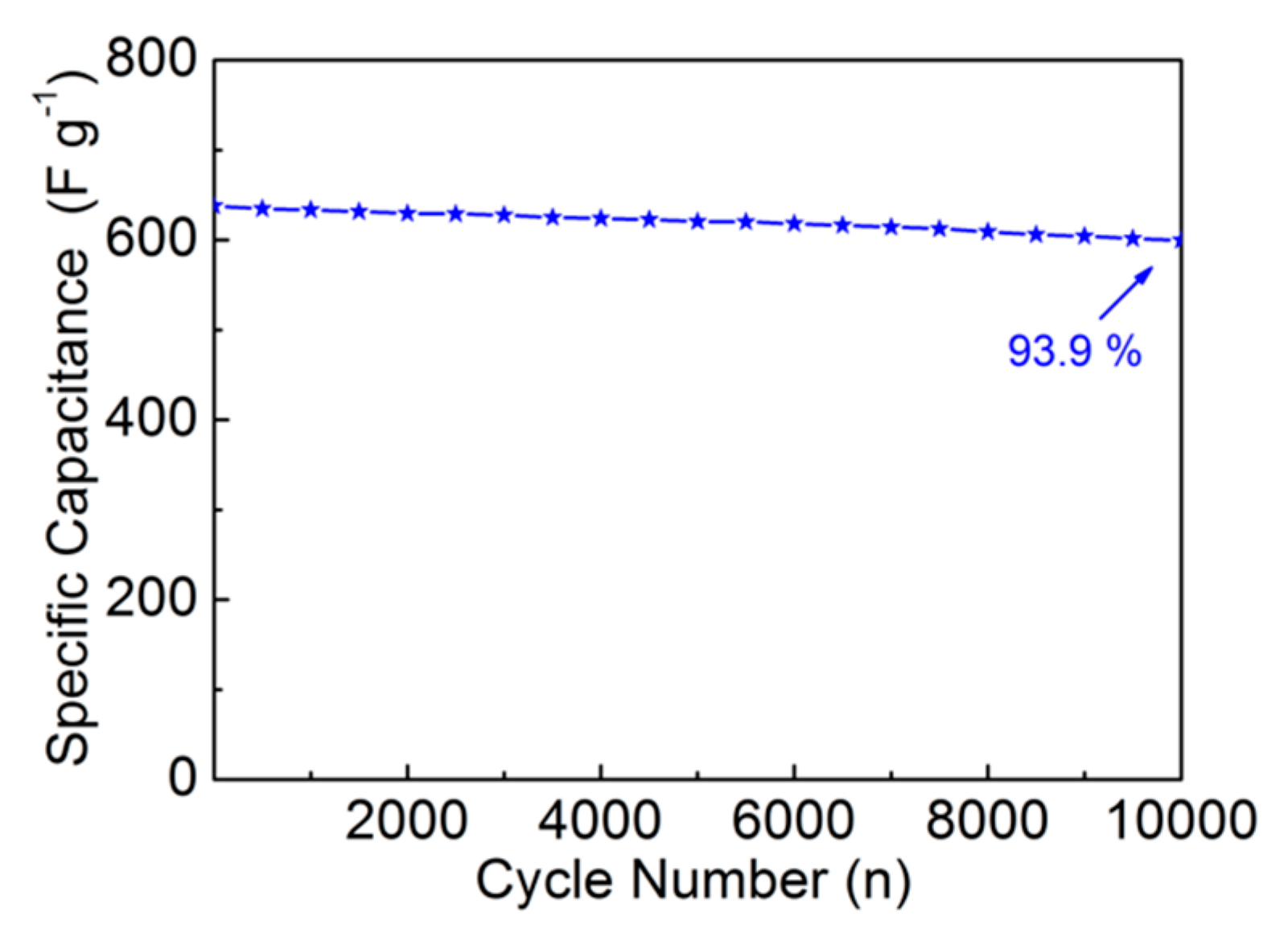
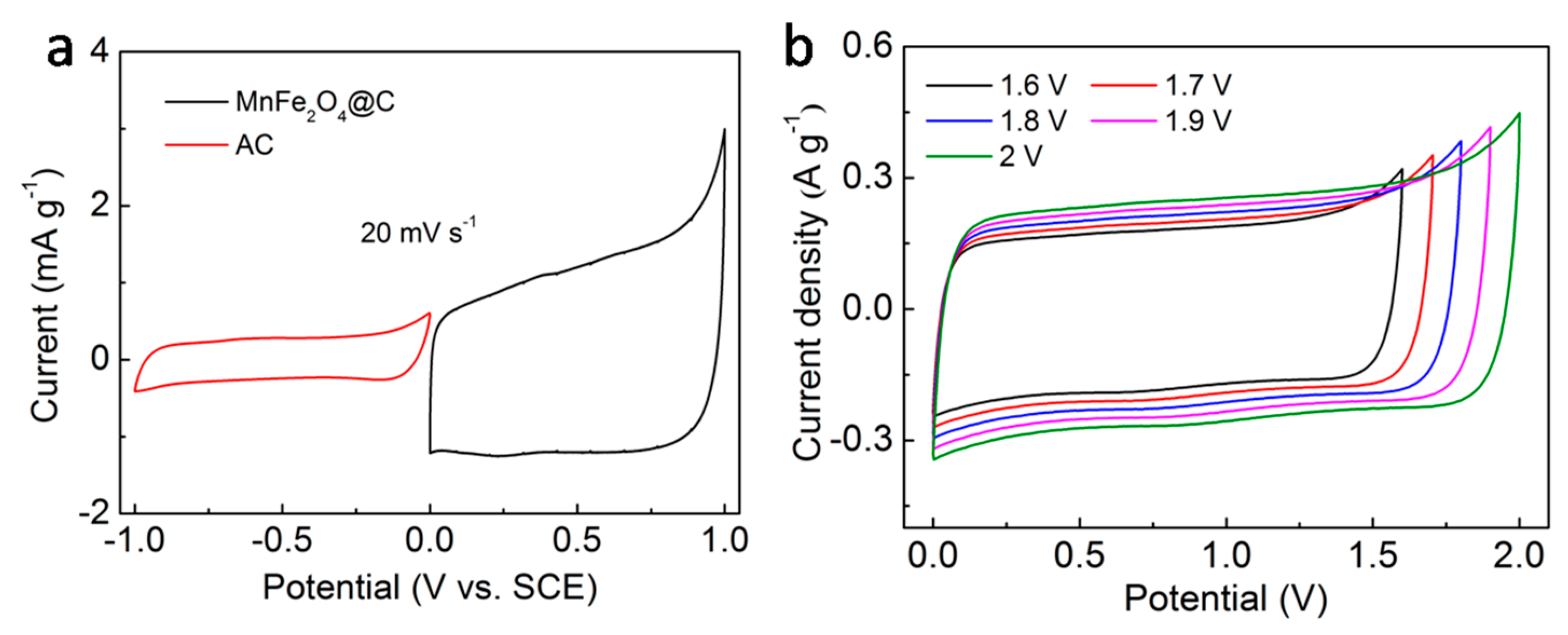
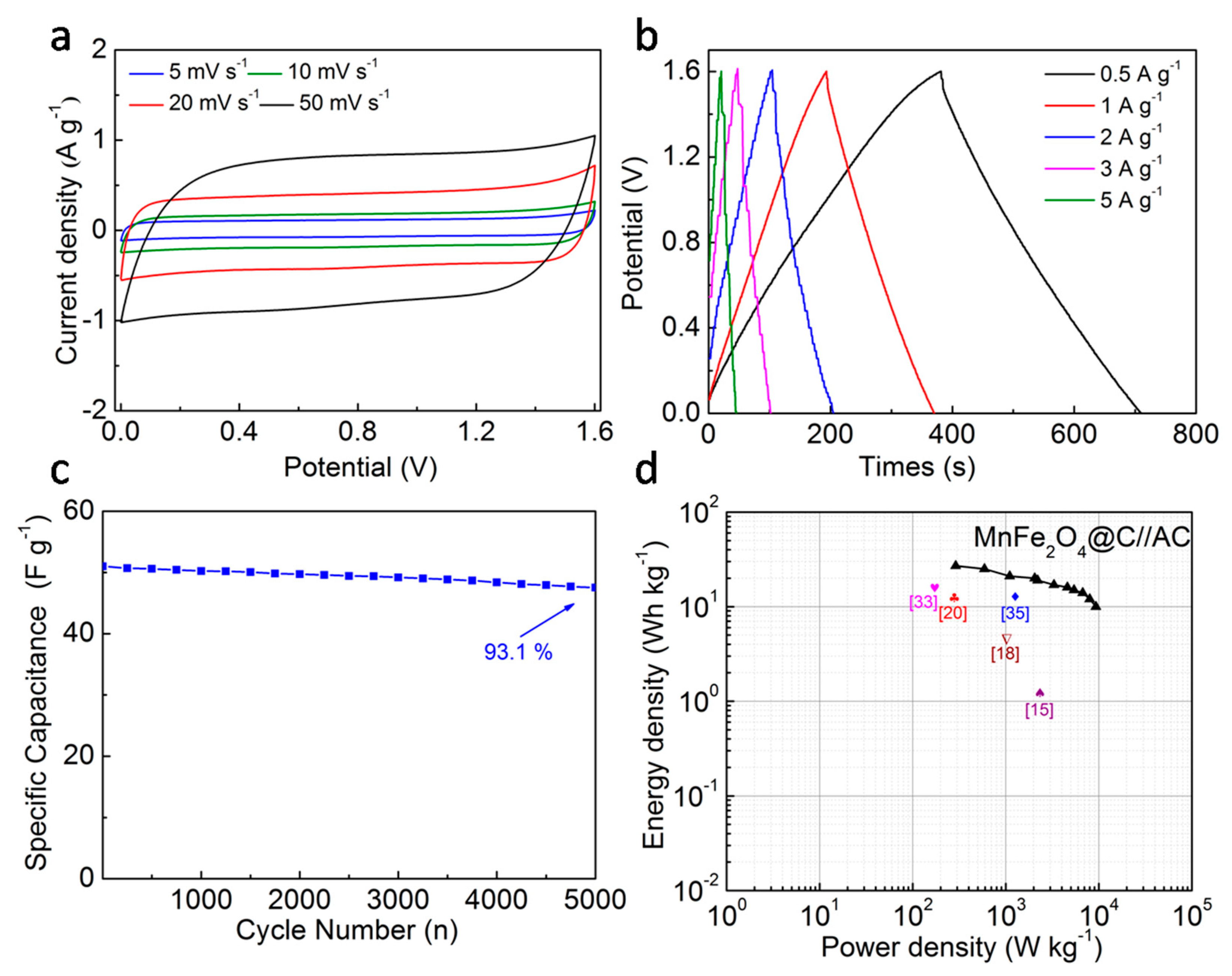
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lu, Q.; Chen, J.; Xiao, J. Nanostructured electrodes for high-performance pseudocapacitors. Angew. Chem. Inter. Edit. 2013, 52, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Li, W.; Wang, M.; Deng, Q.; Wang, Y. High energy density aqueous asymmetric supercapacitors based on MnO2@C branch dendrite nanoarchitectures. Electrochim. Acta 2018, 283, 603–610. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest advances in supercapacitors: from new electrode materials to novel device designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef] [PubMed]
- ZCendrowski, K.; Kukulka, W.; Kedzierski, T.; Zhang, S.; Mijowska, E. Poly (vinylidene fluoride) and carbon derivative structures from eco-friendly MOF-5 for supercapacitor electrode preparation with improved electrochemical performance. Nanomaterials 2018, 8, 890. [Google Scholar] [CrossRef]
- Yan, A.; Wang, X.; Cheng, J. Research progress of NiMn layered double hydroxides for supercapacitors: a review. Nanomaterials 2018, 8, 747. [Google Scholar] [CrossRef]
- Raj, S.; Kar, P.; Roy, P. Facile synthesis of flower-like morphology Cu0.27Co2.73O4 for a high-performance supercapattery with extraordinary cycling stability. Chem. Comm. 2018, 54, 12400–12403. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X.; An, C.; Wang, Y.; Jiao, L.; Yuan, H. Facile synthesis route of porous MnCo2O4 and CoMn2O4 nanowires and their excellent electrochemical properties in supercapacitors. J. Mater. Chem. A 2014, 2, 16480–16488. [Google Scholar] [CrossRef]
- Ramesh, S.; Vikraman, D.; Karuppasamy, K.; Yadav, H.; Sivasamy, A.; Kim, H.; Kim, J.; Kim, H. Controlled synthesis of SnO2@NiCo2O4/nitrogen doped multiwalled carbon nanotube hybrids as an active electrode material for supercapacitors. J. Alloy. Compd. 2019, 794, 186–194. [Google Scholar] [CrossRef]
- Vikraman, D.; Karuppasamy, K.; Hussain, S.; Kathalingam, A.; Sanmugam, A.; Jung, J.; Kim, H. One-pot facile methodology to synthesize MoS2-graphene hybrid nanocomposites for supercapacitors with improved electrochemical capacitance. Compos. Part B Eng. 2019, 161, 555–563. [Google Scholar] [CrossRef]
- Kate, R.; Khalate, S.; Deokate, R. Overview of nanostructured metal oxides and pure nickel oxide (NiO) electrodes for supercapacitors: A review. J. Alloy. Compd. 2018, 734, 89–111. [Google Scholar] [CrossRef]
- Chen, N.; Younis, A.; Huang, S.; Chu, D.; Li, S. Advanced three-dimensional hierarchical Pr6O11@Ni-Co oxides-based core-shell electrodes for supercapacitance application. J. Alloy. Compd. 2018, 783, 772–778. [Google Scholar] [CrossRef]
- Zhu, F.; Yan, M.; Liu, Y.; Shen, H.; Lei, Y.; Shi, W. Hexagonal prism-like hierarchical Co9S8@Ni(OH)2 core-shell nanotubes on carbon fibers for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 22782–22789. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, M.; Meng, Y.; Fang, P.; Lu, X.; Tong, Y. Iron-based supercapacitor electrodes: advances and challenges. Adv. Energy Mater. 2016, 6, 1601053. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, H.; Wang, J.; Liu, J.; Guo, J.; Zhang, X.; Weeks, B.; Shen, T.; Wei, S.; Guo, Z. Electropolymerized polyaniline/manganese iron oxide hybrids with an enhanced color switching response and electrochemical energy storage. J. Mater. Chem. A 2015, 3, 20778–20790. [Google Scholar] [CrossRef]
- Xiong, P.; Hu, C.; Fan, Y.; Zhang, W.; Zhu, J.; Wang, X. Ternary manganese ferrite/graphene/polyaniline nanostructure with enhanced electrochemical capacitance performance. J. Power Sources 2014, 266, 384–392. [Google Scholar] [CrossRef]
- Sankar, K.; Selvan, R. The preparation of MnFe2O4 decorated flexible graphene wrapped with PANI and its electrochemical performance for hybrid supercapacitors. RSC adv. 2014, 4, 17555–17566. [Google Scholar] [CrossRef]
- Li, B.; Fu, Y.; Xia, H.; Wang, X. High-performance asymmetric supercapacitor based on MnFe2O4/graphene nanocomposite as anode material. Mater. Lett. 2014, 122, 193–196. [Google Scholar] [CrossRef]
- Cai, W.; Lai, T.; Dai, W.; Ye, S. A facile approach to fabricate flexible all-solid-state supercapacitors based on MnFe2O4/graphene hybrids. J. Power Sources 2014, 255, 170–178. [Google Scholar] [CrossRef]
- Kuo, S.; Wu, N. Electrochemical characterization of MnFe2O4/carbon black composite aqueous supercapacitors. J. Power Sources 2006, 162, 1437–1443. [Google Scholar] [CrossRef]
- Ghadimi, L.; Arsalani, N.; Tabrizi, A.; Mohammadi, A.; Ahadzadeh, I. Novel nanocomposite of MnFe2O4 and nitrogen-doped carbon from polyaniline carbonization as electrode material for symmetric ultra-stable supercapacitor. Electrchim. Acta 2018, 282, 116–127. [Google Scholar] [CrossRef]
- Ganguli, A.; Ganguly, A.; Vaidya, S. Microemulsion-based synthesis of nanocrystalline materials. Chem. Soc. Rev. 2010, 39, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, W.; Lu, J.; Hou, D.; Ke, Y.; Li, G.; Tang, Z.; Kang, X.; Chen, S. Hierarchical spheres constructed by defect-rich MoS2/carbon nanosheets for efficient electrocatalytic hydrogen evolution. Nano Energy 2016, 22, 490–498. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, G.; Li, H.; Hu, D.; Dou, S. Electrode materials based on micro-emulsion polymerized polyaniline and their capacitive property. Int. J. Electrochem. Sci. 2019, 14, 238–249. [Google Scholar] [CrossRef]
- Vestal, C.; Zhang, Z. Effects of surface coordination chemistry on the magnetic properties of MnFe2O4 spinel ferrite nanoparticles. J. Am. Chem. Soc. 2003, 125, 9828–9833. [Google Scholar] [CrossRef]
- Zeng, H.; Rice, P.; Wang, S.; Sun, S. Shape-controlled synthesis and shape-induced texture of MnFe2O4 nanoparticles. J. Am. Chem. Soc. 2004, 126, 11458–11459. [Google Scholar] [CrossRef] [PubMed]
- Kahmel, R.; Borah, J. Clustering of MnFe2O4 nanoparticles and the effect of field intensity in the generation of heat for hyperthermia application. Nanotechnology 2019, 30, 035706. [Google Scholar]
- Mishra, A.; Aharma, V.; Mohanty, T.; Kuanr, B. Microstructural and magnetic properties of rGO/MnFe2O4 nanocomposites; relaxation dynamics. J. Alloy. Compd. 2019, 790, 983–991. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, N. Characterization of MnFe2O4/LiMn2O4 aqueous asymmetric supercapacitor. J. Power Sources 2011, 196, 851–854. [Google Scholar] [CrossRef]
- Kogularasu, S.; Akilarasan, M.; Chen, S.; Elaiyappillai, E.; Johnson, P.; Chen, T.; Al-Hemaid, F.; Ali, M.; Elshikh, M. A comparative study on conventionally prepared MnFe2O4 nanospheres and template-synthesized novel MnFe2O4 nano-agglomerates as the electrodes for biosensing of mercury contaminations and supercapacitor applications. Electrochim. Acta 2018, 290, 533–543. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, N.; Yu, C.; Jiao, L.; Chen, J. MnFe2O4@C nanofibers as high-performance anode for sodium-ion batteries. Nano Lett. 2016, 16, 3321–3328. [Google Scholar] [CrossRef]
- Fu, H.; Ma, S.; Zhao, P.; Xu, S.; Zhan, S. Activation of peroxymonosulfate by graphitized hierarchical porous biochar and MnFe2O4 magnetic nanoarchitecture for organic pollutants degradation: structure dependence and mechanism. Chem. Eng. J. 2019, 360, 157–170. [Google Scholar] [CrossRef]
- Younis, A.; Chu, D.; Li, S. Ethanol-directed morphological evolution of hierarchical CeOx architectures as advanced elctrochemical capacitor. J. Mater. Chem. A 2015, 3, 13970–13977. [Google Scholar] [CrossRef]
- Sankar, K.; Selvan, R. The ternary MnFe2O4/graphene/polyaniline hybrid composite as negative electrode for supercapacitors. J. Power Sources 2015, 275, 399–407. [Google Scholar] [CrossRef]
- Su, L.; Lei, S.; Liu, L.; Liu, L.; Zhang, Y.; Shi, S.; Yan, X. Sprinkling MnFe2O4 quantum dots on nitrogen-doped graphene sheets: the formation mechanism and application for high-performance supercapacitor electrodes. J. Mater. Chem. A 2018, 6, 9997–10007. [Google Scholar] [CrossRef]
- Vignesh, V.; Subramani, K.; Sathish, M.; Navamathavan, R. Electrochemical investigation of manganese ferrites prepared via a facile synthesis route for supercapacitor applications. Colloid. Surface A 2018, 538, 668–677. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, L.; Yan, F.; Dong, C.; An, C. Design and Regulation of Novel MnFe2O4@C Nanowires as High Performance Electrode for Supercapacitor. Nanomaterials 2019, 9, 777. https://doi.org/10.3390/nano9050777
Geng L, Yan F, Dong C, An C. Design and Regulation of Novel MnFe2O4@C Nanowires as High Performance Electrode for Supercapacitor. Nanomaterials. 2019; 9(5):777. https://doi.org/10.3390/nano9050777
Chicago/Turabian StyleGeng, Lei, Fengfeng Yan, Chenhao Dong, and Cuihua An. 2019. "Design and Regulation of Novel MnFe2O4@C Nanowires as High Performance Electrode for Supercapacitor" Nanomaterials 9, no. 5: 777. https://doi.org/10.3390/nano9050777
APA StyleGeng, L., Yan, F., Dong, C., & An, C. (2019). Design and Regulation of Novel MnFe2O4@C Nanowires as High Performance Electrode for Supercapacitor. Nanomaterials, 9(5), 777. https://doi.org/10.3390/nano9050777




