Grouping of Poorly Soluble Low (Cyto)Toxic Particles: Example with 15 Selected Nanoparticles and A549 Human Lung Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation and Characterization of Nanoparticle Suspensions
2.3. Cell Culture
2.4. MTT Assay
2.5. The Estimation of Phospholipid Rich Organelle Quantity
2.6. The Estimation of the Cellular Load of Acid Organelles
2.7. Statistical Analysis
3. Results
3.1. Particle Characteristics
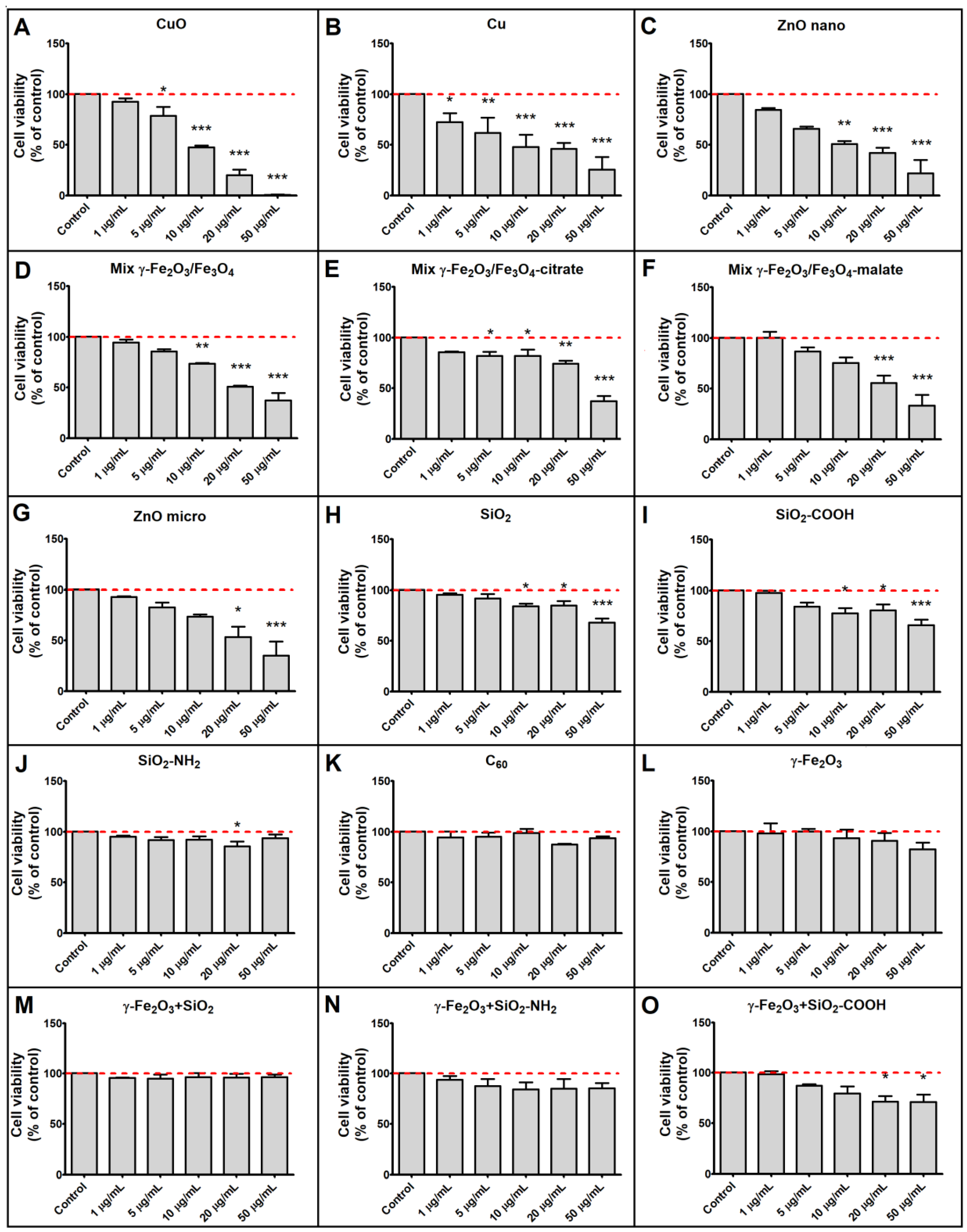
3.2. Cytotoxicity of Tested Particles
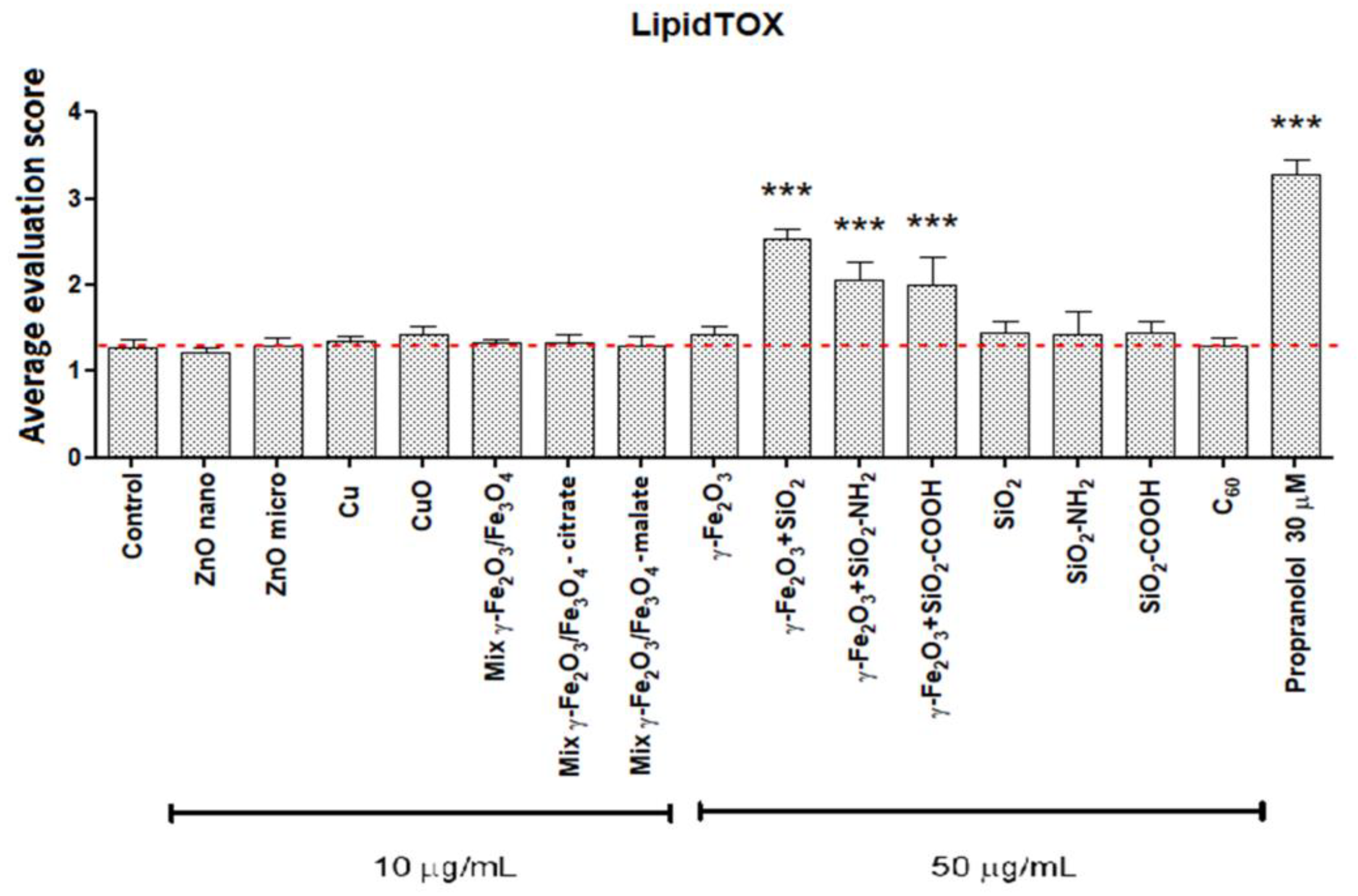
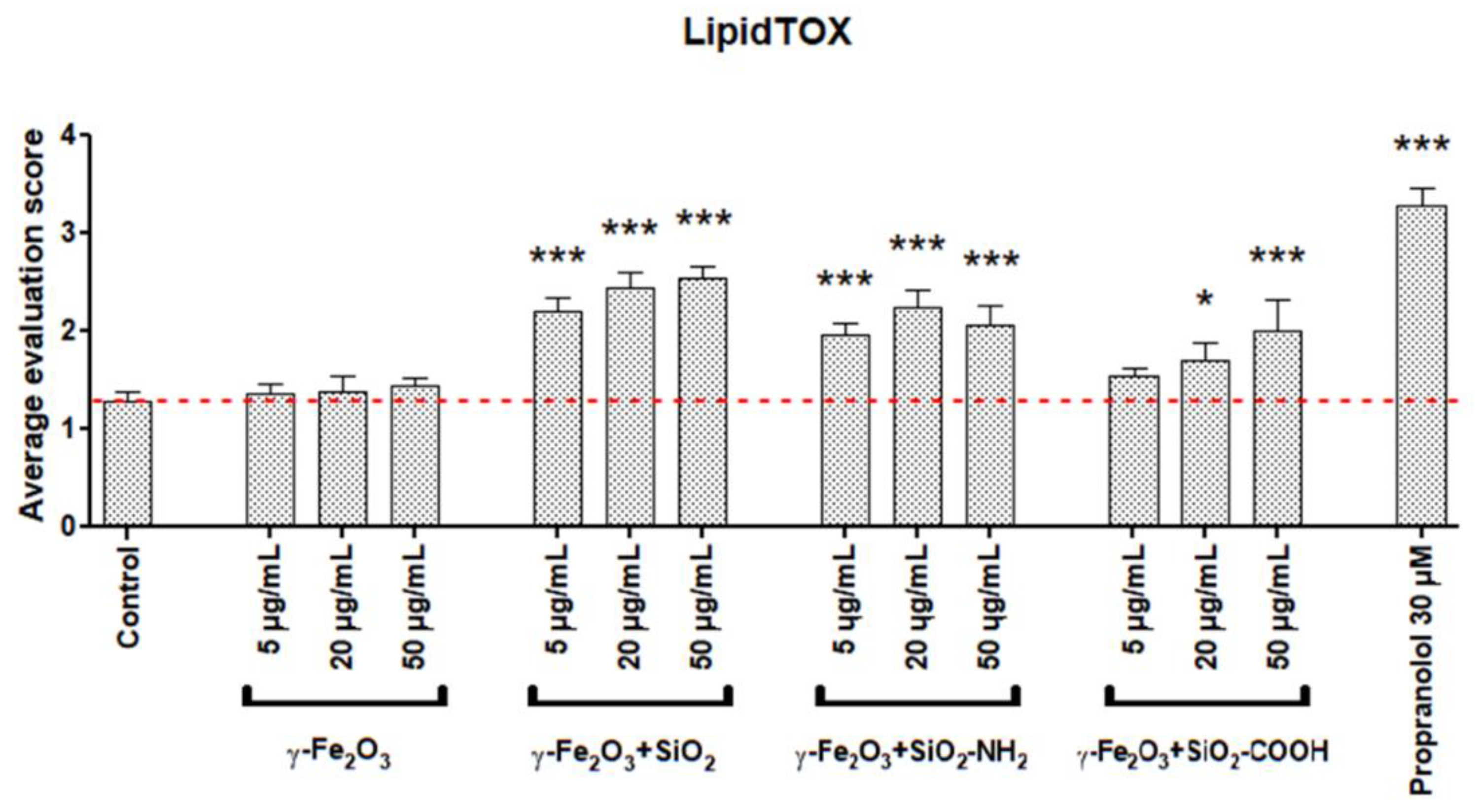
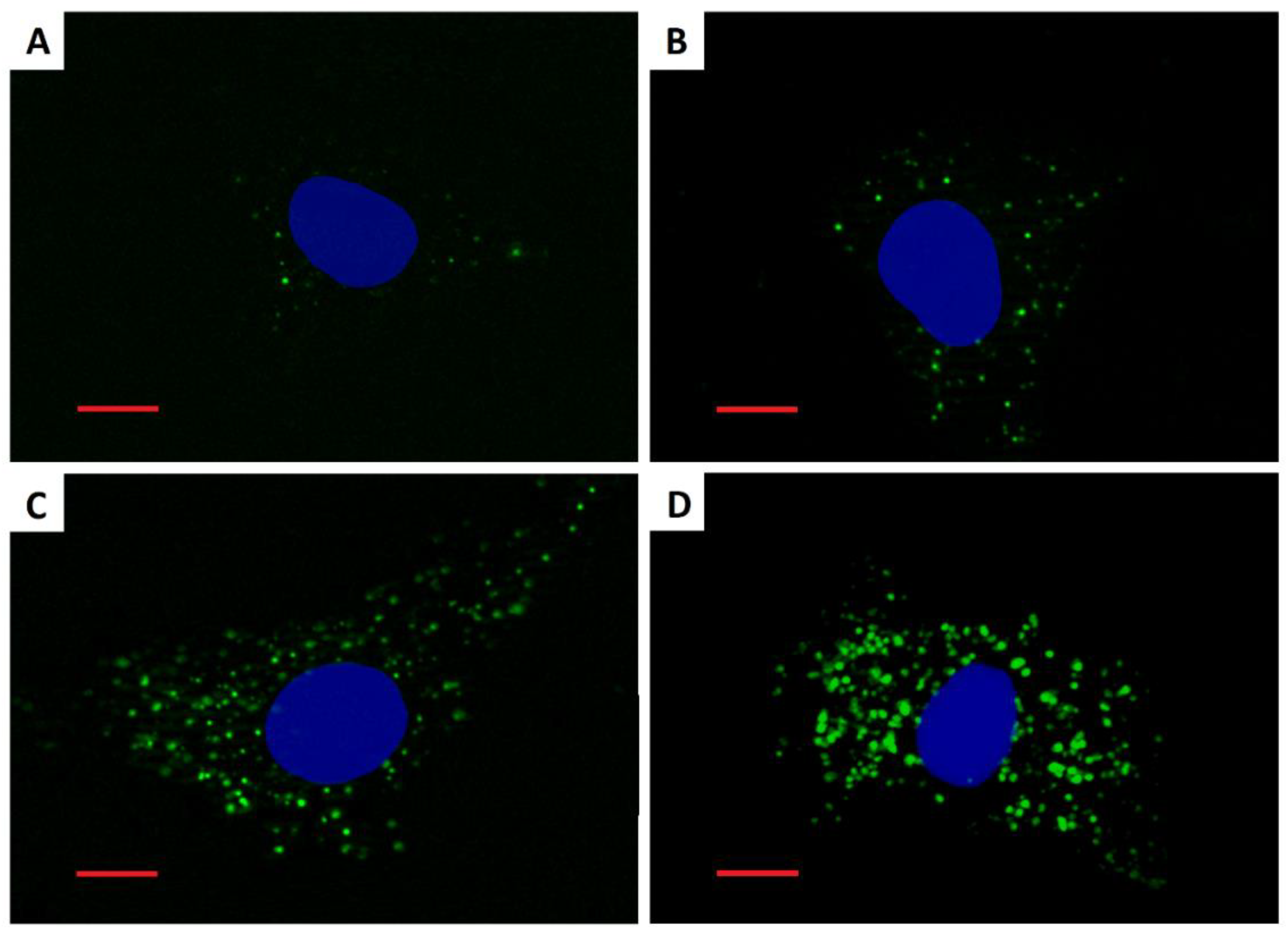
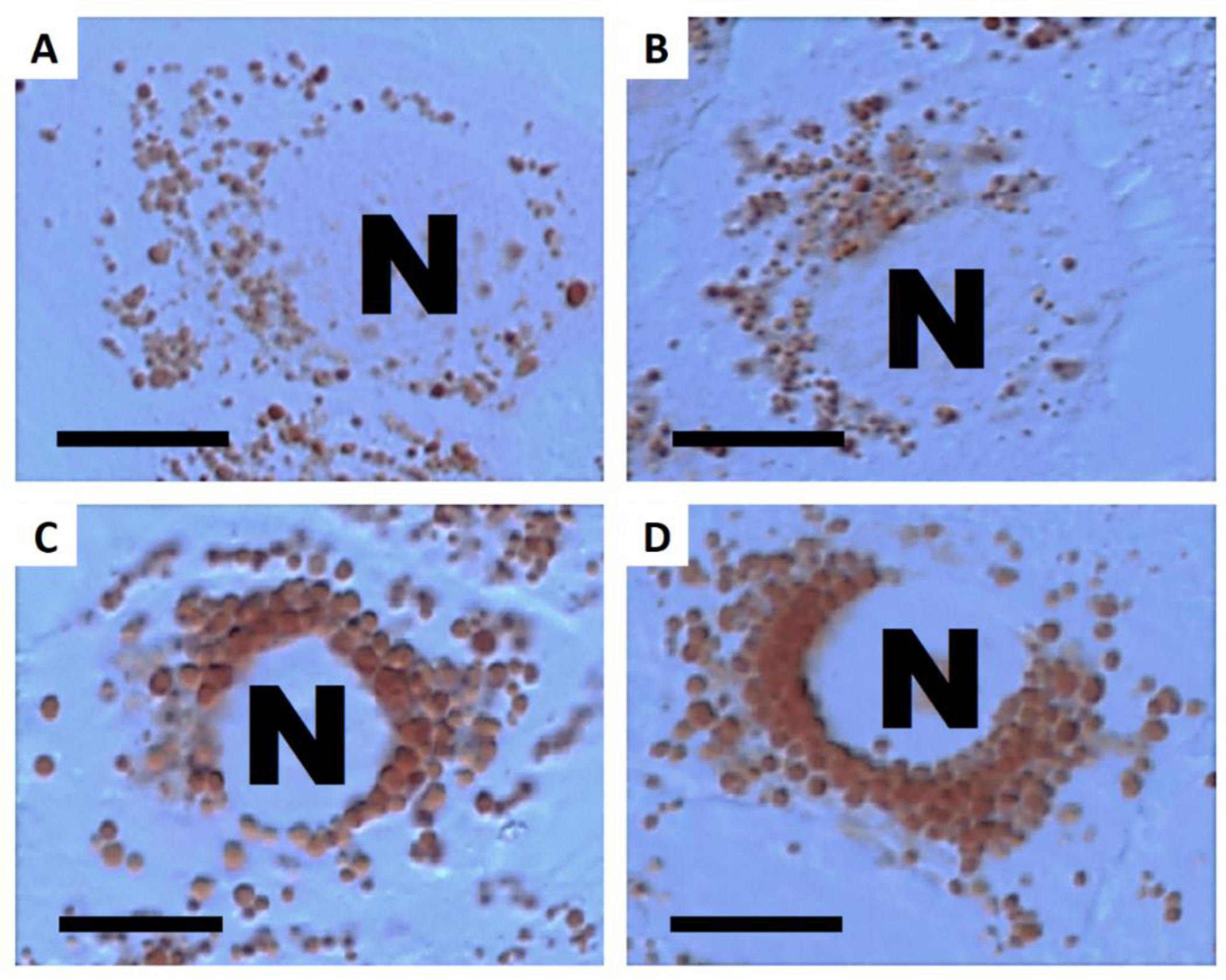
3.3. Cellular Load of Phospholipid Rich Organelles
3.4. Cellular Load of Acid Organelles
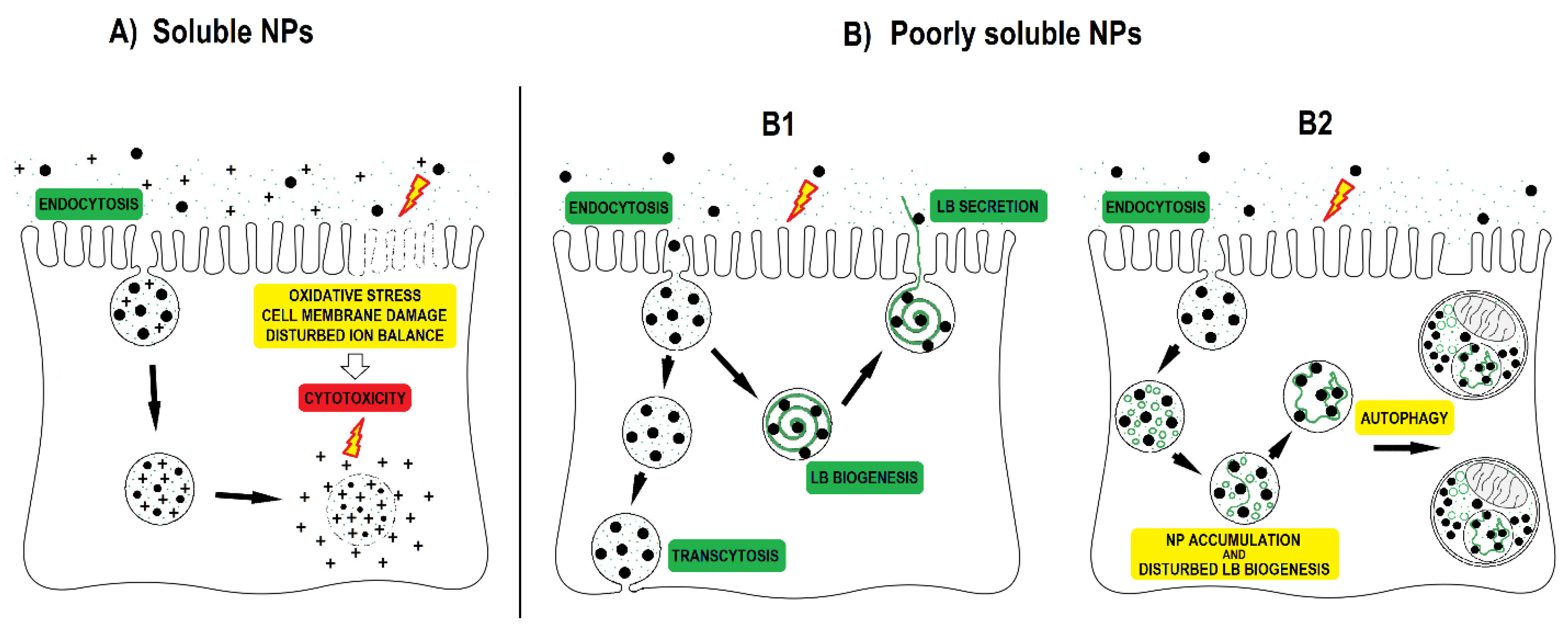
4. Discussion
- Fibrous versus non-fibrous or granular particles;
- Biopersistent versus non-biopersistent materials;
- Materials with high solubility versus low solubility;
- Chemically reactive versus chemically non-reactive materials;
- Materials with high toxicity versus low toxicity.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burden, N.; Aschberger, K.; Chaudhry, Q.; Clift, M.J.D.; Doak, S.H.; Fowler, P.; Johnston, H.; Landsiedel, R.; Rowland, J.; Stone, V. The 3Rs as a framework to support a 21st century approach for nanosafety assessment. Nano Today 2017, 12, 10–13. [Google Scholar] [CrossRef]
- Warheit, D.B. Hazard and risk assessment strategies for nanoparticle exposures: How far have we come in the past 10 years? F1000 Research 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Bevan, R.J.; Kreiling, R.; Levy, L.S.; Warheit, D.B. Toxicity testing of poorly soluble particles, lung overload and lung cancer. Regul. Toxicol. Pharmacol. 2018. [Google Scholar] [CrossRef]
- Arora, S.; Rajwade, J.M.; Paknikar, K.M. Nanotoxicology and in vitro studies: The need of the hour. Toxicol. Appl. Pharmacol. 2012, 258, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Clift, M.J.D.; Gehr, P.; Rothen-Rutishauser, B. Nanotoxicology: A perspective and discussion of whether or not in vitro testing is a valid alternative. Arch. Toxicol. 2011, 85, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Arts, J.H.E.; Hadi, M.; Irfan, M.-A.; Keene, A.M.; Kreiling, R.; Lyon, D.; Maier, M.; Michel, K.; Petry, T.; Sauer, U.G. A decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping). Regul. Toxicol. Pharmacol. 2015, 71, S1–S27. [Google Scholar] [CrossRef]
- Arts, J.H.E.; Hadi, M.; Keene, A.M.; Kreiling, R.; Lyon, D.; Maier, M.; Michel, K.; Petry, T.; Sauer, U.G.; Warheit, D. A critical appraisal of existing concepts for the grouping of nanomaterials. Regul. Toxicol. Pharmacol. 2014, 70, 492–506. [Google Scholar] [CrossRef]
- Oomen, A.G.; Bos, P.M.J.; Fernandes, T.F.; Hund-Rinke, K.; Boraschi, D.; Byrne, H.J.; Aschberger, K.; Gottardo, S.; von der Kammer, F.; Kühnel, D. Concern-driven integrated approaches to nanomaterial testing and assessment–report of the NanoSafety Cluster Working Group 10. Nanotoxicology 2014, 8, 334–348. [Google Scholar] [CrossRef]
- Warheit, D.B.; Brown, S.C. What is the impact of surface modifications and particle size on commercial titanium dioxide particle samples?–A review of in vivo pulmonary and oral toxicity studies–Revised 11-6-2018. Toxicol. Lett. 2019, 302, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Borm, P.J.A.; Driscoll, K.E. The hazards and risks of inhaled poorly soluble particles–where do we stand after 30 years of research? Part. Fibre Toxicol. 2019, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Bakand, S.; Hayes, A. Toxicological considerations, toxicity assessment, and risk management of inhaled nanoparticles. Int. J. Mol. Sci. 2016, 17, 929. [Google Scholar] [CrossRef] [PubMed]
- Mangal, S.; Gao, W.; Li, T.; Zhou, Q.T. Pulmonary delivery of nanoparticle chemotherapy for the treatment of lung cancers: Challenges and opportunities. Acta Pharmacol. Sin. 2017, 38, 782. [Google Scholar] [CrossRef]
- Fytianos, K.; Drasler, B.; Blank, F.; Von Garnier, C.; Seydoux, E.; Rodriguez-Lorenzo, L.; Petri-Fink, A.; Rothen-Rutishauser, B. Current in vitro approaches to assess nanoparticle interactions with lung cells. Nanomedicine 2016, 11, 2457–2469. [Google Scholar] [CrossRef]
- Mansour, H.M.; Rhee, Y.-S.; Wu, X. Nanomedicine in pulmonary delivery. Int. J. Nanomedicine 2009, 4, 299. [Google Scholar] [CrossRef] [PubMed]
- Borm, P.; Cassee, F.R.; Oberdörster, G. Lung particle overload: Old school–new insights? Fibre Toxicol. 2015, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Gupta, N.; Ahsan, F. Particle engineering to enhance or lessen particle uptake by alveolar macrophages and to influence the therapeutic outcome. Eur. J. Pharm. Biopharm. 2015, 89, 163–174. [Google Scholar] [CrossRef]
- Puisney, C.; Baeza-Squiban, A.; Boland, S. Mechanisms of Uptake and Translocation of Nanomaterials in the Lung. Cell. Mol. Toxicol. Nanoparticles 2018, 1048, 21–36. [Google Scholar]
- Whitsett, J.A.; Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 2015, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Kononenko, V.; Erman, A.; Petan, T.; Križaj, I.; Kralj, S.; Makovec, D.; Drobne, D. Harmful at non-cytotoxic concentrations: SiO2-SPIONs affect surfactant metabolism and lamellar body biogenesis in A549 human alveolar epithelial cells. Nanotoxicology 2017, 11, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Olmeda, B.; Martínez-Calle, M.; Pérez-Gil, J. Pulmonary surfactant metabolism in the alveolar airspace: Biogenesis, extracellular conversions, recycling. Ann. Anat.-Anat. Anz. 2017, 209, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gil, J.; Weaver, T.E. Pulmonary surfactant pathophysiology: Current models and open questions. Physiology 2010, 25, 132–141. [Google Scholar] [CrossRef]
- Agassandian, M.; Mallampalli, R.K. Surfactant phospholipid metabolism. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2013, 1831, 612–625. [Google Scholar] [CrossRef]
- Thelen, A.M.; Zoncu, R. Emerging roles for the lysosome in lipid metabolism. Trends Cell Biol. 2017, 27, 833–850. [Google Scholar] [CrossRef] [PubMed]
- Grotz, B.; Geppert, M.; Mills-Goodlet, R.; Hofer, S.; Hofstätter, N.; Asam, C.; Feinle, A.; Kocsis, K.; Berger, T.; Diwald, O. Biologic effects of nanoparticle-allergen conjugates: Time-resolved uptake using an in vitro lung epithelial co-culture model of A549 and THP-1 cells. Environ. Sci. Nano 2018, 5, 2184–2197. [Google Scholar] [CrossRef]
- Shapero, K.; Fenaroli, F.; Lynch, I.; Cottell, D.C.; Salvati, A.; Dawson, K.A. Time and space resolved uptake study of silica nanoparticles by human cells. Mol. Biosyst. 2011, 7, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Shi, T.; Duffin, R.; Albrecht, C.; van Berlo, D.; Höhr, D.; Fubini, B.; Martra, G.; Fenoglio, I.; Borm, P.J.A. Endocytosis, oxidative stress and IL-8 expression in human lung epithelial cells upon treatment with fine and ultrafine TiO2: role of the specific surface area and of surface methylation of the particles. Toxicol. Appl. Pharmacol. 2007, 222, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Moret, F.; Selvestrel, F.; Lubian, E.; Mognato, M.; Celotti, L.; Mancin, F.; Reddi, E. PEGylation of ORMOSIL nanoparticles differently modulates the in vitro toxicity toward human lung cells. Arch. Toxicol. 2015, 89, 607–620. [Google Scholar] [CrossRef]
- Simon-Deckers, A.; Gouget, B.; Mayne-L’Hermite, M.; Herlin-Boime, N.; Reynaud, C.; Carriere, M. In vitro investigation of oxide nanoparticle and carbon nanotube toxicity and intracellular accumulation in A549 human pneumocytes. Toxicology 2008, 253, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Petersen, N.O. Lipid-coated gold nanoparticles promote lamellar body formation in A549 cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2013, 1831, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.A.; Oster, C.G.; Mayer, M.M.; Avery, M.L.; Audus, K.L. Characterization of the A549 cell line as a type II pulmonary epithelial cell model for drug metabolism. Exp. Cell Res. 1998, 243, 359–366. [Google Scholar] [CrossRef]
- Martin, A.; Sarkar, A. Overview on biological implications of metal oxide nanoparticle exposure to human alveolar A549 cell line. Nanotoxicology 2017, 11, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Riebeling, C.; Piret, J.-P.; Trouiller, B.; Nelissen, I.; Saout, C.; Toussaint, O.; Haase, A. A guide to nanosafety testing: Considerations on cytotoxicity testing in different cell models. NanoImpact 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Miller, B.E.; Hook, G.E. Hypertrophy and hyperplasia of alveolar type II cells in response to silica and other pulmonary toxicants. Environ. Health Perspect. 1990, 85, 15–23. [Google Scholar] [PubMed]
- Drasler, B.; Sayre, P.; Steinhaeuser, K.G.; Petri-Fink, A.; Rothen-Rutishauser, B. In vitro approaches to assess the hazard of nanomaterials. NanoImpact 2017, 8, 99–116. [Google Scholar] [CrossRef]
- Kononenko, V.; Drobne, D.; Kononenko, V.; Drobne, D. In Vitro Cytotoxicity Evaluation of the Magnéli Phase Titanium Suboxides (TixO2x−1) on A549 Human Lung Cells. Int. J. Mol. Sci. 2019, 20, 196. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Gliga, A.R.; Calléja, F.M.G.R.; Gonçalves, C.S.A.G.; Wallinder, I.O.; Vrieling, H.; Fadeel, B.; Hendriks, G. Mechanism-based genotoxicity screening of metal oxide nanoparticles using the ToxTracker panel of reporter cell lines. Part. Fibre Toxicol. 2014, 11, 41. [Google Scholar] [CrossRef]
- Romih, T.; Hočevar, S.B.; Kononenko, V.; Drobne, D. The application of bismuth film electrode for measuring Zn(II) under less acidic conditions in the presence of cell culture medium and ZnO nanoparticles. Sens. Actuators B Chem. 2017, 238. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, C. Zn2+ release from zinc and zinc oxide particles in simulated uterine solution. Colloids Surf. B Biointerfaces 2006, 47, 140–145. [Google Scholar] [CrossRef]
- Oh, N.; Park, J.-H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9, 51. [Google Scholar]
- Zhang, S.; Gao, H.; Bao, G. Physical principles of nanoparticle cellular endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef]
- Cho, W.-S.; Duffin, R.; Howie, S.E.M.; Scotton, C.J.; Wallace, W.A.H.; MacNee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn 2+ dissolution inside lysosomes. Part. Fibre Toxicol. 2011, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Kononenko, V.; Repar, N.; Marušič, N.; Drašler, B.; Romih, T.; Hočevar, S.; Drobne, D. Comparative in vitro genotoxicity study of ZnO nanoparticles, ZnO macroparticles and ZnCl2 to MDCK kidney cells: Size matters. Toxicol. Vitr. 2017, 40, 256–263. [Google Scholar] [CrossRef]
- Baber, O.; Jang, M.; Barber, D.; Powers, K. Amorphous silica coatings on magnetic nanoparticles enhance stability and reduce toxicity to in vitro BEAS-2B cells. Inhal. Toxicol. 2011, 23, 532–543. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Mädler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef]
- Wang, F.; Jin, C.; Liang, H.; Tang, Y.; Zhang, H.; Yang, Y. Effects of fullerene C60 nanoparticles on A549 cells. Environ. Toxicol. Pharmacol. 2014, 37, 656–661. [Google Scholar] [CrossRef]
- Horie, M.; Fujita, K.; Kato, H.; Endoh, S.; Nishio, K.; Komaba, L.K.; Nakamura, A.; Miyauchi, A.; Kinugasa, S.; Hagihara, Y. Association of the physical and chemical properties and the cytotoxicity of metal oxide nanoparticles: metal ion release, adsorption ability and specific surface area. Metallomics 2012, 4, 350–360. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, S.-H.; Lee, S.; Lee, D.-K.; Han, Y.; Jeon, S.; Cho, W.-S. Differential Contribution of Constituent Metal Ions to the Cytotoxic Effects of Fast-Dissolving Metal-Oxide Nanoparticles. Front. Pharmacol. 2018, 9, 15. [Google Scholar] [CrossRef]
- Lüllmann-Rauch, R. History and morphology of the lysosome. Lysosomes 2005, 1–16. [Google Scholar]
- Ratoi, M.; Hoet, P.H.M.; Crossley, A.; Dobson, P. Impact of lung surfactant on wettability and cytotoxicity of nanoparticles. RSC Adv. 2014, 4, 20573–20581. [Google Scholar] [CrossRef]
- Oomen, A.G.; Bleeker, E.A.J.; Bos, P.M.J.; van Broekhuizen, F.; Gottardo, S.; Groenewold, M.; Hristozov, D.; Hund-Rinke, K.; Irfan, M.-A.; Marcomini, A. Grouping and read-across approaches for risk assessment of nanomaterials. Int. J. Environ. Res. Public Health 2015, 12, 13415–13434. [Google Scholar] [CrossRef] [PubMed]
- Landvik, N.; Skaug, V.; Mohr, B.; Verbeek, J.; Zienolddiny, S. Criteria for grouping of manufactured nanomaterials to facilitate hazard and risk assessment, a systematic review of expert opinions. Regul. Toxicol. Pharmacol. 2018, 95, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Park, J.H.; Peters, T.M.; Thorne, P.S. Toxicity of copper oxide nanoparticles in lung epithelial cells exposed at the air–liquid interface compared with in vivo assessment. Toxicol. Vitr. 2015, 29, 502–511. [Google Scholar] [CrossRef] [PubMed]
| Nanoparticles | Average Diameter of Nanoparticles [nm] | Average Hydrodynamic Diameter [nm] | Zeta Potential [mV] |
|---|---|---|---|
| ZnO nano | 72 | 85 | −13 |
| ZnO micro | 237 | 113 1 | −17 |
| γ-Fe2O3 | 12 | 108 | −16 |
| γ-Fe2O3+SiO2 | 19 | 118 | −42 |
| γ-Fe2O3+SiO2-COOH | 28 | 128 | −35 |
| γ-Fe2O3+SiO2-NH2 | 30 | 135 | +10 |
| Mix γ-Fe2O3/Fe3O4 | 14 | 110 | −12 |
| Mix γ-Fe2O3/Fe3O4-citrate | 12 | 85 | −25 |
| Mix γ-Fe2O3/Fe3O4-malate | 11 | 90 | −22 |
| C60 | 26 | 185 | −36 |
| Cu | 105 | NA 2 | −17 |
| CuO | 130 | NA 2 | −24 |
| SiO2 | 30 | 40 | −31.5 |
| SiO2-NH2 | 30 | 42 | −20 |
| SiO2-COOH | 30 | 42 | 0 |
| Nanoparticles | Cytotoxicity over 70 % | Cytotoxicity over 50 % | Cytotoxicity over 30 % | Increased Phospholipid Rich Organelles | Increased Acid Organelles |
|---|---|---|---|---|---|
| Cu | + | + | + | − | − |
| CuO | + | + | + | − | − |
| ZnO nano | + | + | + | − | − |
| ZnO micro | − | + | + | − | − |
| Mix γ-Fe2O3/Fe3O4 | − | + | + | − | − |
| Mix γ-Fe2O3/Fe3O4-citrate | − | + | + | − | − |
| Mix γ-Fe2O3/Fe3O4-malate | − | + | + | − | − |
| SiO2 | − | − | + | − | − |
| SiO2-COOH | − | − | + | − | − |
| SiO2-NH2 | − | − | − | − | − |
| C60 | − | − | − | − | − |
| γ -Fe2O3 | − | − | − | − | − |
| γ-Fe2O3+SiO2 | − | − | − | + | + |
| γ-Fe2O3+SiO2-COOH | − | − | − | + | + |
| γ-Fe2O3+SiO2-NH2 | − | − | − | + | + |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kononenko, V.; Warheit, D.B.; Drobne, D. Grouping of Poorly Soluble Low (Cyto)Toxic Particles: Example with 15 Selected Nanoparticles and A549 Human Lung Cells. Nanomaterials 2019, 9, 704. https://doi.org/10.3390/nano9050704
Kononenko V, Warheit DB, Drobne D. Grouping of Poorly Soluble Low (Cyto)Toxic Particles: Example with 15 Selected Nanoparticles and A549 Human Lung Cells. Nanomaterials. 2019; 9(5):704. https://doi.org/10.3390/nano9050704
Chicago/Turabian StyleKononenko, Veno, David B. Warheit, and Damjana Drobne. 2019. "Grouping of Poorly Soluble Low (Cyto)Toxic Particles: Example with 15 Selected Nanoparticles and A549 Human Lung Cells" Nanomaterials 9, no. 5: 704. https://doi.org/10.3390/nano9050704
APA StyleKononenko, V., Warheit, D. B., & Drobne, D. (2019). Grouping of Poorly Soluble Low (Cyto)Toxic Particles: Example with 15 Selected Nanoparticles and A549 Human Lung Cells. Nanomaterials, 9(5), 704. https://doi.org/10.3390/nano9050704






