Immobilization of P. stutzeri on Activated Carbons for Degradation of Hydrocarbons from Oil-in-Saltwater Emulsions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Activated Carbons
2.2.2. Chemical Modification of Activated Carbons
2.2.3. Characterization of Activated Carbons
2.2.4. Immobilization Kinetics
2.2.5. Preparation of Oil-Brine Emulsions (O/W)
2.2.6. Hydrocarbon Degradability Test
2.2.7. Rhamnolipid Quantification
2.2.8. Statistical Analysis
3. Results and Discussion
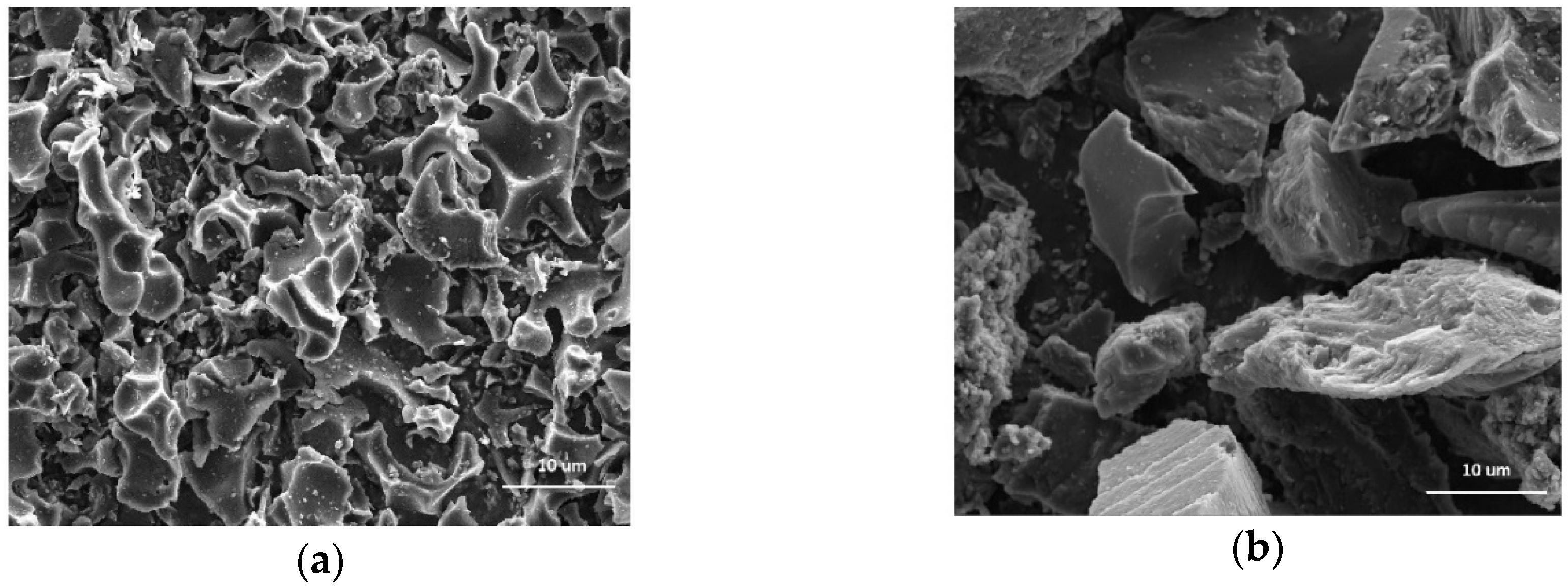
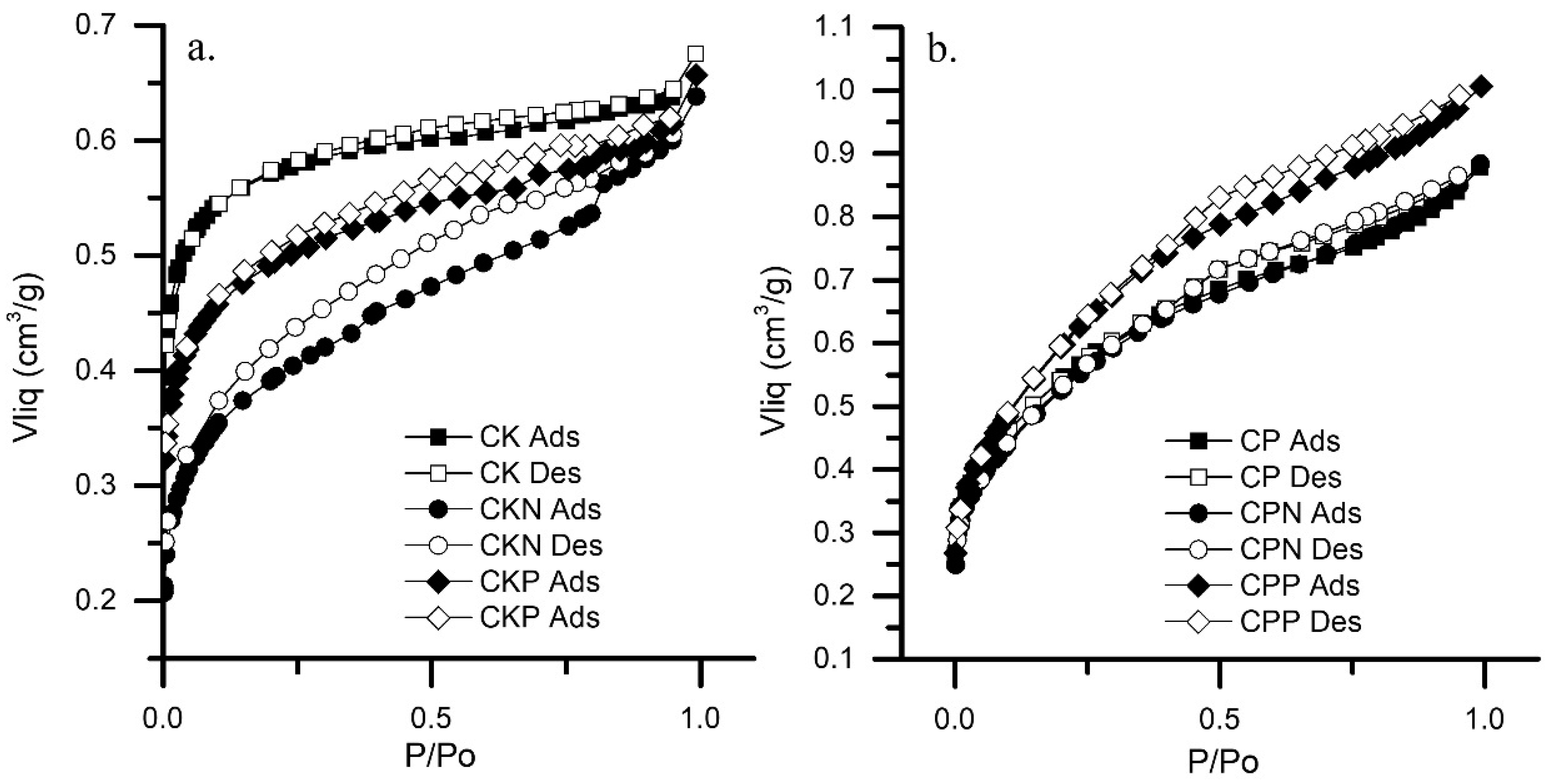
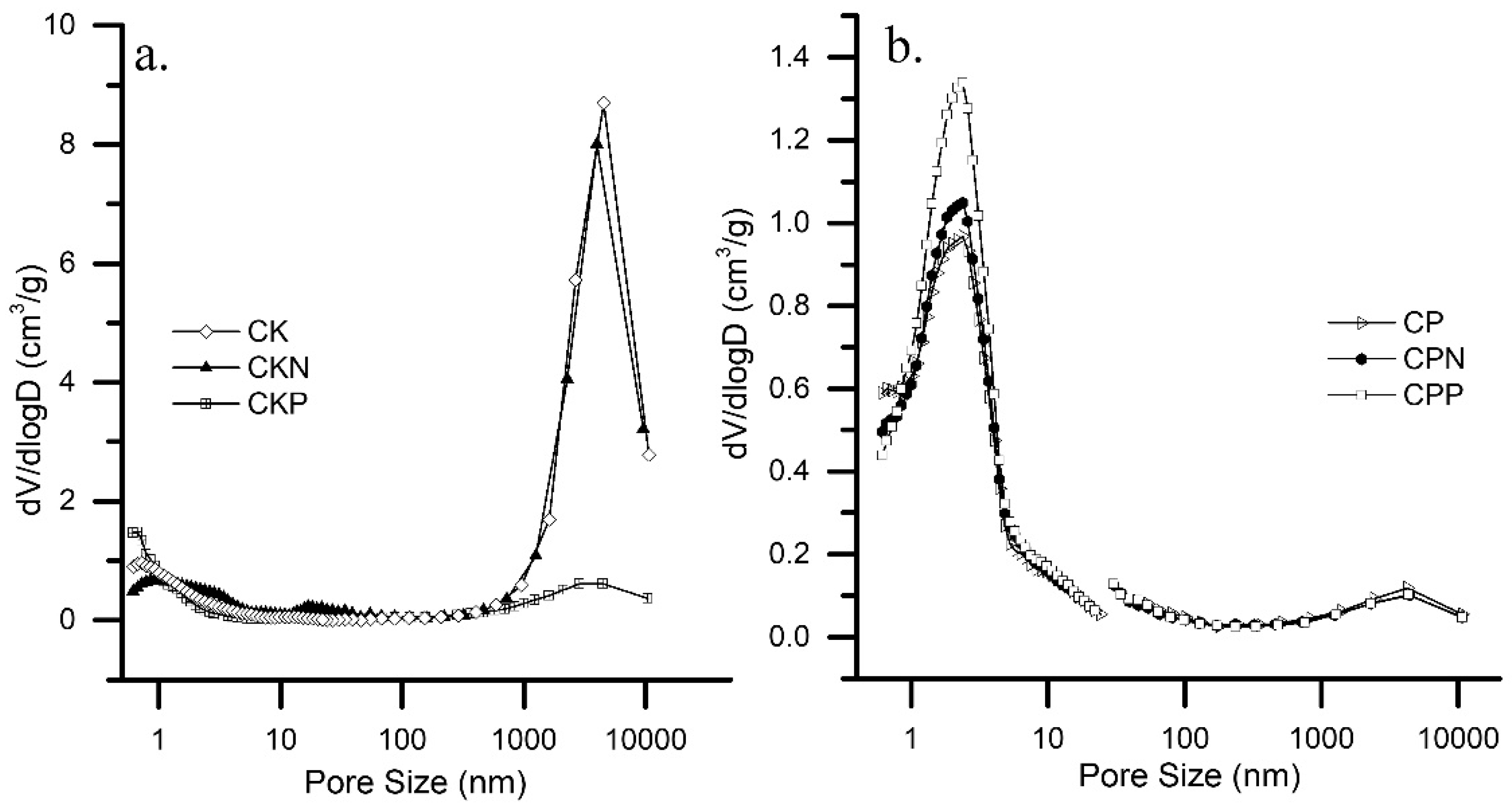
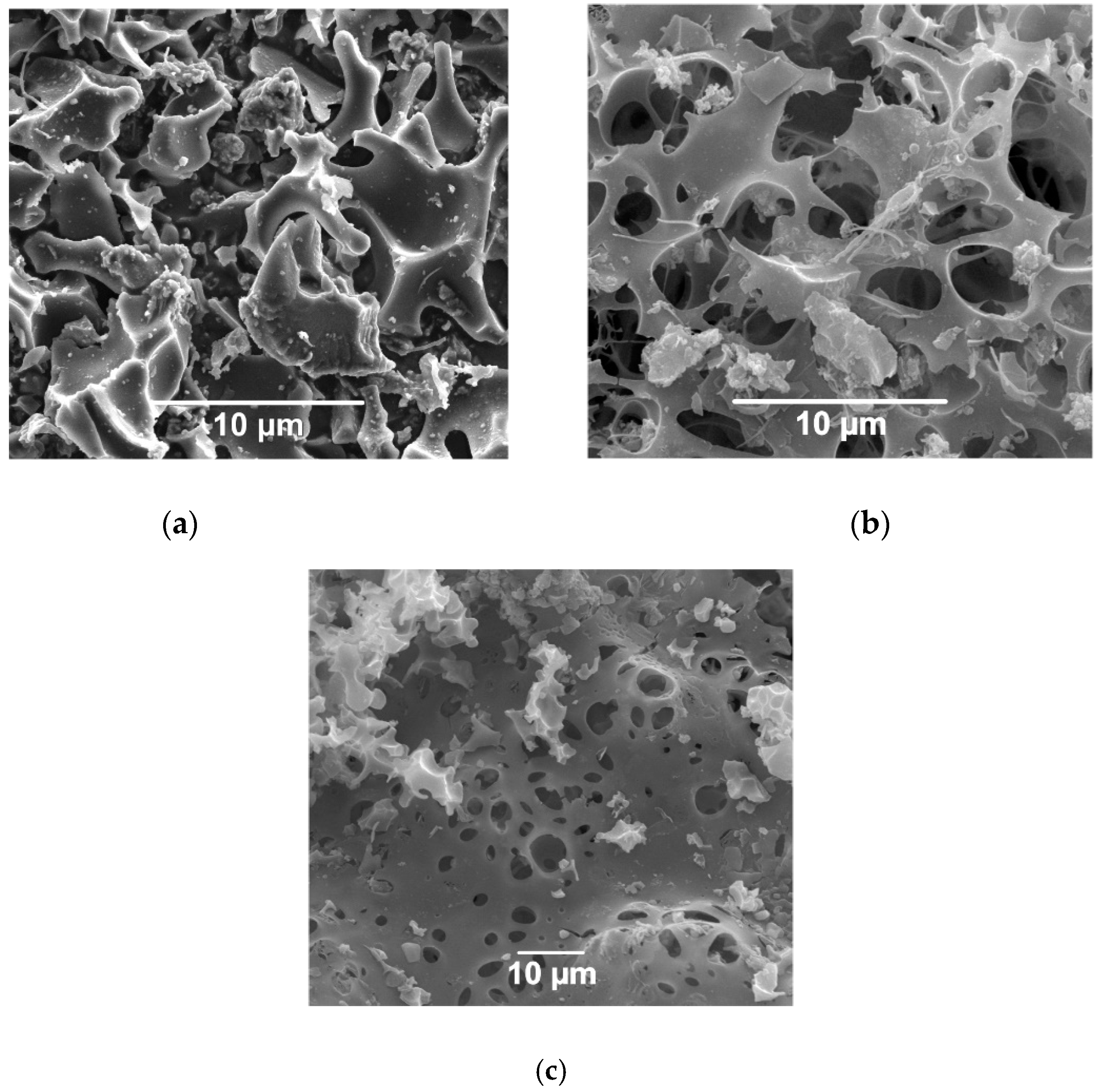
3.1. Characteristics of Activated Carbons
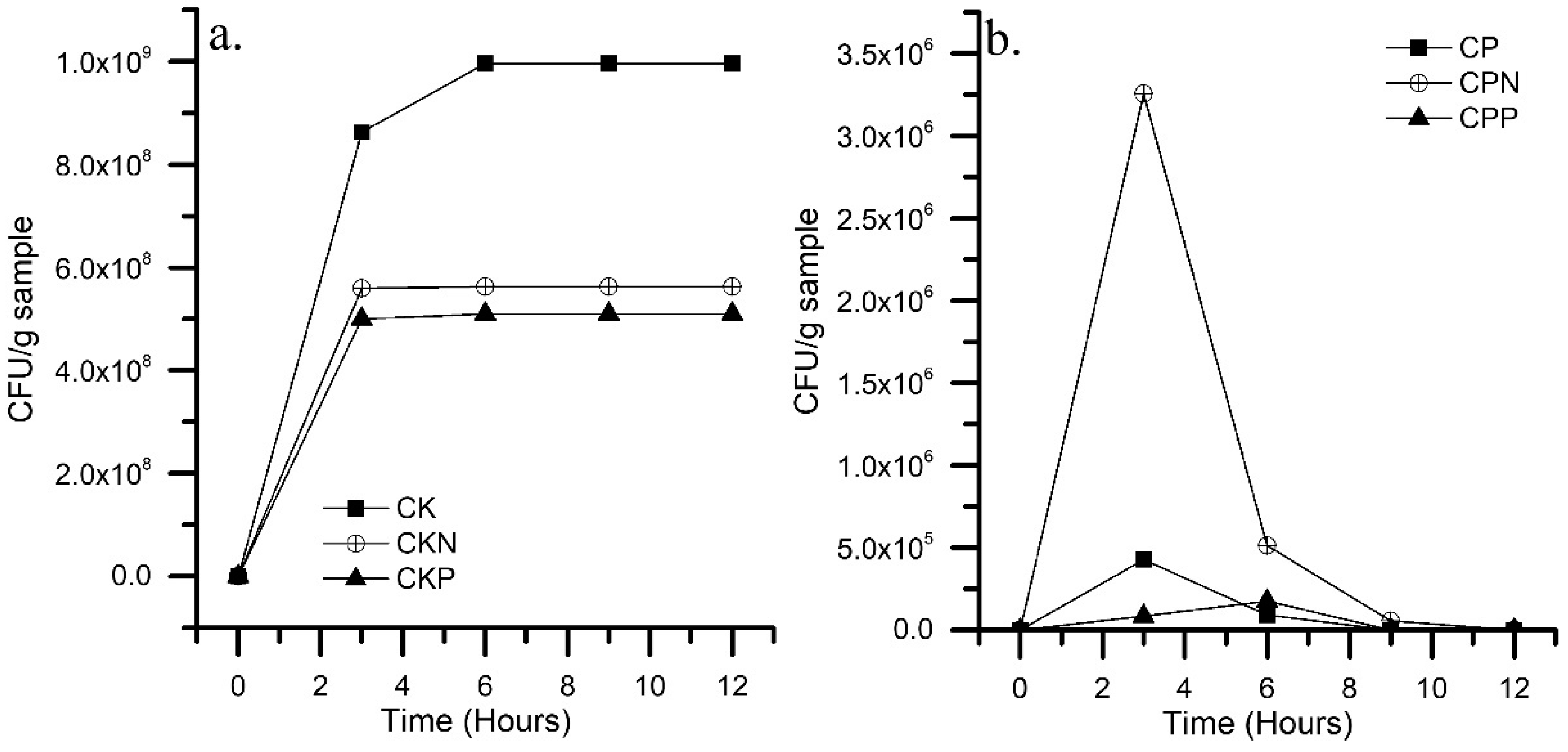
3.2. Immobilization Kinetics
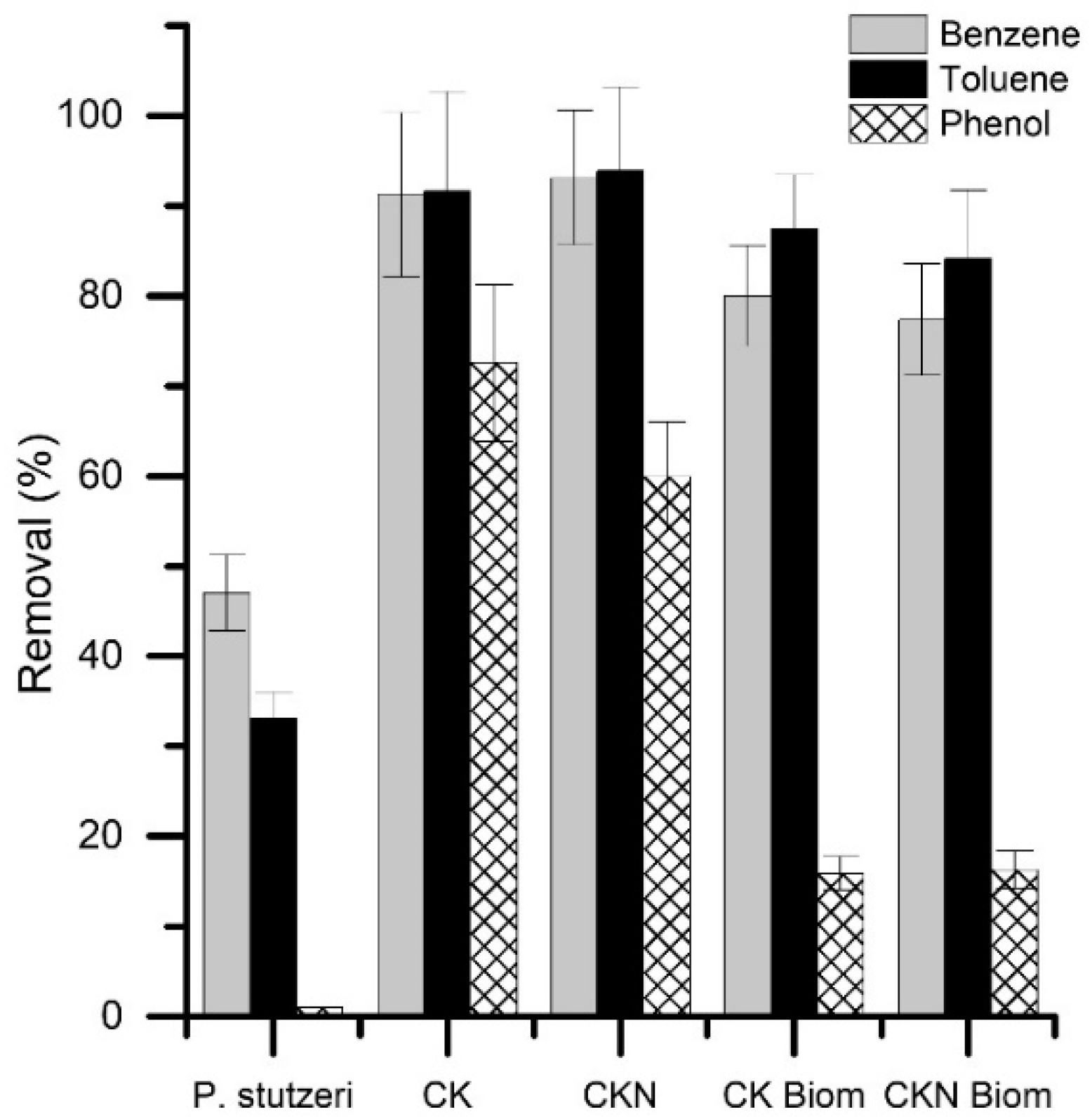
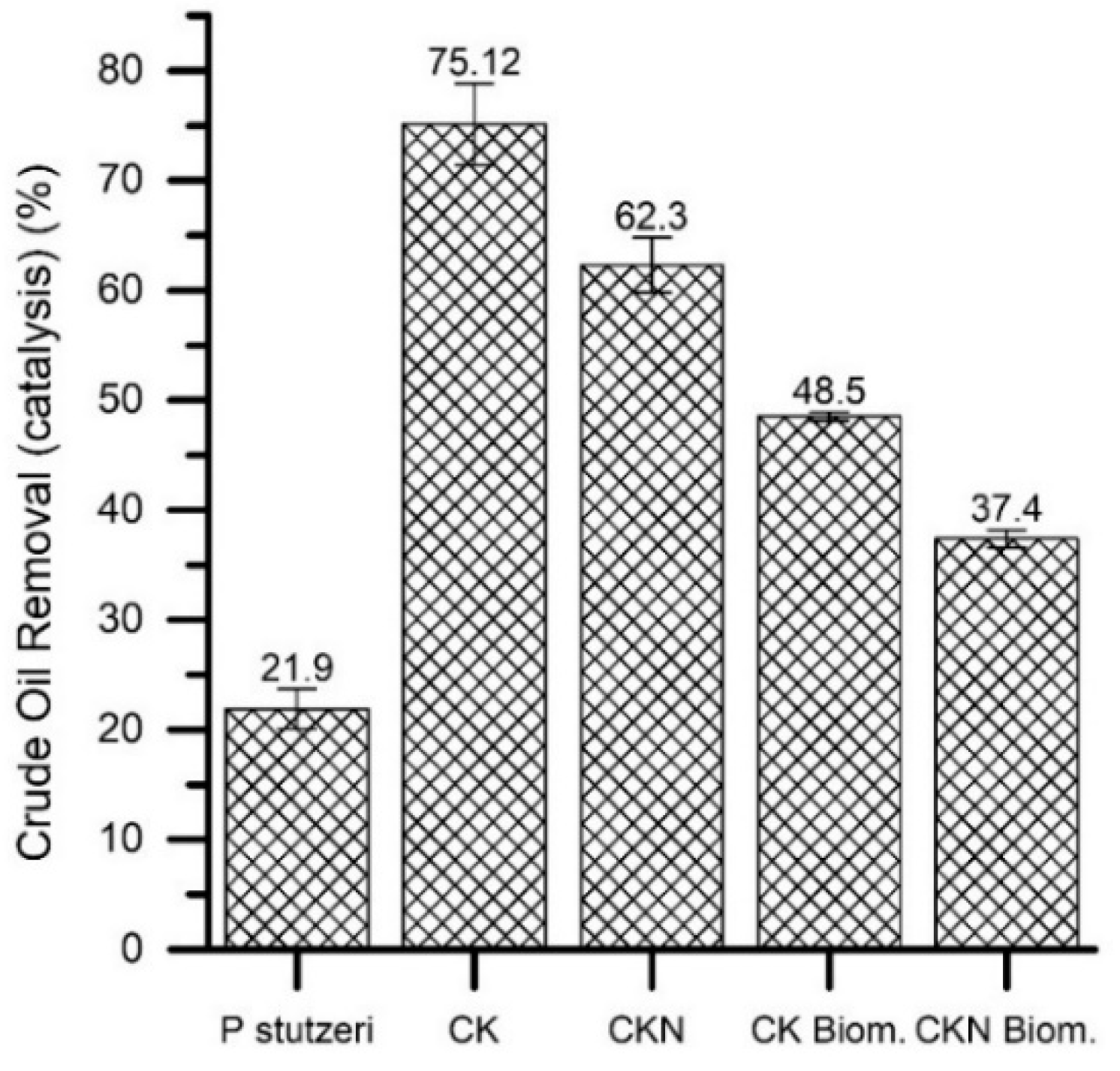
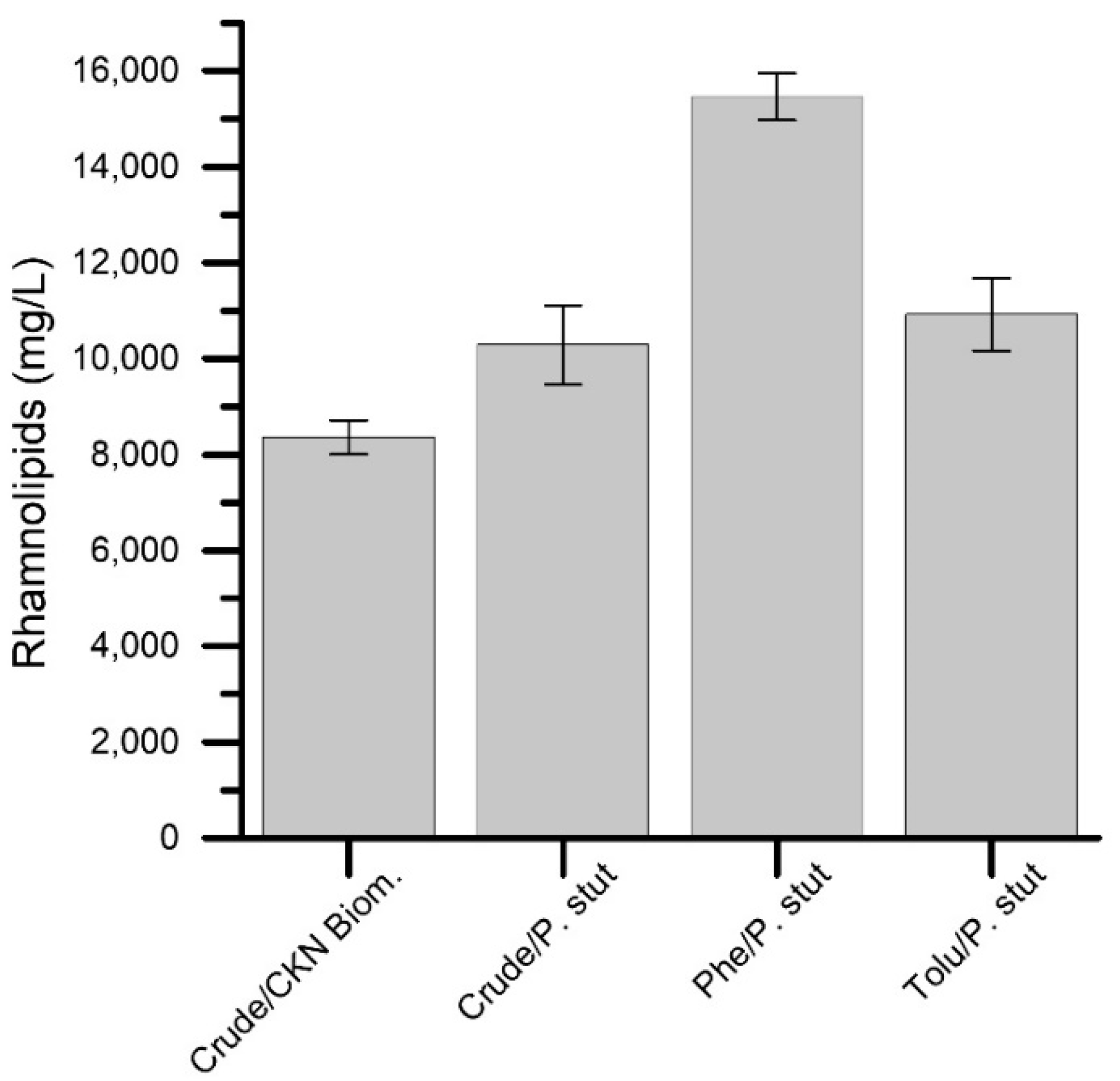
3.3. Hydrocarbon Degradability Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goodale, B.C.; La du, J.; Tilton, S.C.; Sullivan, C.M.; Bisson, W.H.; Waters, K.M.; Tanguay, R.L. Ligand-specific transcriptional mechanisms underlie aryl hydrocarbon receptor-mediated developmental toxicity of oxygenated PAHs. Toxicol. Sci. 2015, 147, 397–411. [Google Scholar] [CrossRef]
- Bao, M.; Wang, L.; Sun, P.; Cao, L.; Zou, J.; Li, Y. Biodegradation of crude oil using an efficient microbial consortium in a simulated marine environment. Mar. Pollut. Bull. 2012, 64, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; López-Linares, F.; Nassar, N.; Carbognani-Arambrri, L.; Pereira-Almao, P. Development of a support for a NiO catalyst for selective adsorption and post-adsorption catalytic steam gasification of thermally converted asphaltenes. Catal. Today 2013, 207, 112–118. [Google Scholar] [CrossRef]
- Castañeda, L.C.; Muñoz, J.A.D.; Ancheyta, J. Combined process schemes for upgrading of heavy petroleum. Fuel 2012, 100, 110–127. [Google Scholar] [CrossRef]
- Wu, H.; Sun, H.; Hong, W.; Mao, L.; Liu, Y. Improvement of Polyamide Thin Film Nanocomposite Membrane Assisted by Tannic Acid–FeIII Functionalized Multiwall Carbon Nanotubes. ACS Appl. Mater. Interfaces 2017, 9, 32255–32263. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, A.; Karimi, M. Numerical Investigation on Multiphase Flow Simulation in a Centrifugal Flotation Cell. Int. J. Coal Prep. Util. 2012, 32, 120–129. [Google Scholar] [CrossRef]
- Marafi, M.; Stanislaus, A.; Absi-Halabi, M. Heavy oil hydrotreating catalyst rejuvenation by leaching of foulant metals with ferric nitrate-organic acid mixed reagents. Appl. Catal. B Environ. 1994, 4, 19–27. [Google Scholar] [CrossRef]
- Duraisamy, T.; Heydari, A.; Henni, A. State of the Art Treatment of Produced Water. Water Treat. 2013. [Google Scholar] [CrossRef]
- Syed, S.; Alhazzaa, M.I.; Asif, M. Treatment of oily water using hydrophobic nano-silica. Chem. Eng. J. 2011, 167, 99–103. [Google Scholar] [CrossRef]
- Ugochukwu, U.C.; Jones, M.D.; Head, I.M.; Manning, D.A.C.; Fialips, C.I. Biodegradation of crude oil saturated fraction supported on clays. Biodegradation 2014, 25, 153–165. [Google Scholar] [CrossRef]
- Ugochukwu, U.C.; Manning, D.A.C.; Fialips, C.I. Microbial degradation of crude oil hydrocarbons on organoclay minerals. J. Environ. Manag. 2014, 144, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Usha, M.S.; Sanjay, M.K.; Gaddad, S.M.; Shivannavar, C.T. Degradation of h-acid by free and immobilized cells of. Braz. J. Microbiol. 2010, 41, 931–945. [Google Scholar] [CrossRef]
- Gentry, T.J.; Rensing, C.; Pepper, I.L. New approaches for bioaugmentation as a remediation technology. Crit. Rev. Environ. Sci. Technol. 2004, 34, 447–494. [Google Scholar] [CrossRef]
- MacNaughton, S.J.; Stephen, J.R.; Venosa, A.D.; Davis, G.A.; Chang, Y.-J.; White, D.C. Microbial Population Changes during Bioremediation of an Experimental Oil Spill. Appl. Environ. Microbiol. 1999, 65, 3566–3574. [Google Scholar] [PubMed]
- El Fantroussi, S.; Agathos, S.N. Is bioaugmentation a feasible strategy for pollutant removal and site remediation? Curr. Opin. Microbiol. 2005, 8, 268–275. [Google Scholar] [CrossRef]
- Biswas, B.; Sarkar, B.; Rusmin, R.; Naidu, R. Bioremediation of PAHs and VOCs: Advances in clay mineral-microbial interaction. Environ. Int. 2015, 85, 168–181. [Google Scholar] [CrossRef]
- Zhou, L.C.; Li, Y.F.; Bai, X.; Zhao, G.H. Use of microorganisms immobilized on composite polyurethane foam to remove Cu(II) from aqueous solution. J. Hazard. Mater. 2009, 167, 1106–1113. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jeong, W.-H.; Karegoudar, T.B.; Kim, C.-K. Stable degradation of benzoate by Klebsiella oxytoca C302 immobilized in alginate and polyurethane. Biotechnol. Bioprocess Eng. 2002, 7, 347–351. [Google Scholar] [CrossRef]
- Angelim, A.L.; Costa, S.P.; Farias, B.C.S.; Aquino, L.F.; Melo, V.M.M. An innovative bioremediation strategy using a bacterial consortium entrapped in chitosan beads. J. Environ. Manag. 2013, 127, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Sekaran, G.; Karthikeyan, S.; Gupta, V.K.; Boopathy, R.; Maharaja, P. Immobilization of Bacillus sp. in mesoporous activated carbon for degradation of sulphonated phenolic compound in wastewater. Mater. Sci. Eng. C 2013, 33, 735–745. [Google Scholar] [CrossRef]
- Valverde-Sarmiento, C.; Espinosa-Iglesias, D.; Bautista-Toledo, M.I.; Álvarez-Merino, M.A.; Maldonado-Hódar, F.J.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. Bacteria supported on carbon films for water denitrification. Chem. Eng. J. 2015, 259, 424–429. [Google Scholar] [CrossRef]
- Ugochukwu, U.C.; Jones, M.D.; Head, I.M.; Manning, D.A.C.; Fialips, C.I. Biodegradation and adsorption of crude oil hydrocarbons supported on “homoionic” montmorillonite clay minerals. Appl. Clay Sci. 2014, 87, 81–86. [Google Scholar] [CrossRef]
- Bailón-García, E.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J. Development of carbon xerogels as alternative Pt-supports for the selective hydrogenation of citral. Catal. Commun. 2015, 58, 64–69. [Google Scholar] [CrossRef]
- Gallegos-Suárez, E.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F. On the micro- and mesoporosity of carbon aerogels and xerogels. The role of the drying conditions during the synthesis processes. Chem. Eng. J. 2012, 181–182, 851–855. [Google Scholar] [CrossRef]
- Zapata-Benabithe, Z.; Carrasco-Marín, F.; De Vicente, J.; Moreno-Castilla, C. Carbon xerogel microspheres and monoliths from resorcinol-formaldehyde mixtures with varying dilution ratios: Preparation, surface characteristics, and electrochemical double-layer capacitances. Langmuir 2013, 29, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Moreno-Castilla, C. On the nature of surface acid sites of chlorinated activated carbons. Carbon N. Y. 2003, 41, 473–478. [Google Scholar] [CrossRef]
- Franco, C.A.; Nassar, N.N.; Cortés, F.B. Removal of oil from oil-in-saltwater emulsions by adsorption onto nano-alumina functionalized with petroleum vacuum residue. J. Colloid Interface Sci. 2014, 433, 58–67. [Google Scholar] [CrossRef]
- Franco Ariza, C.; Martínez, M.; Benjumea, P.; Patiño, E.; Cortés, F. Water Remediation Based on Oil Adsorption Using Nanosilicates Functionalized with a Petroleum Vacuum Residue. Adsorpt. Sci. Technol. 2014, 32, 197–208. [Google Scholar] [CrossRef]
- Juang, R.S.; Tsai, S.Y. Growth kinetics of Pseudomonas putida in the biodegradation of single and mixed phenol and sodium salicylate. Biochem. Eng. J. 2006, 31, 133–140. [Google Scholar] [CrossRef]
- Gałązka, A.; Król, M.; Perzyński, A. The Efficiency of Rhizosphere Bioremediation with Azospirillum sp. and Pseudomonas stutzeri in Soils Freshly Contaminated with PAHs and Diesel Fuel. Pol. J. Environ. Stud. 2012, 21, 345–353. [Google Scholar]
- Elmouwahidi, A.; Zapata-Benabithe, Z.; Carrasco-Marín, F.; Moreno-Castilla, C. Activated carbons from KOH-activation of argan (Argania spinosa) seed shells as supercapacitor electrodes. Bioresour. Technol. 2012, 111, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Biniak, S.; Szymański, G.; Siedlewski, J.; Świa̧tkowski, A. The characterization of activated carbons with oxygen and nitrogen surface groups. Carbon N. Y. 1997, 35, 1799–1810. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; López-Ramón, M.; Carrasco-Marín, F. Changes in surface chemistry of activated carbons by wet oxidation. Carbon N. Y. 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Figueiredo, J.; Pereira, M.F.; Freitas, M.M.; Órfão, J.J. Modification of the surface chemistry of activated carbons. Carbon N. Y. 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Hasegawa, G.; Deguchi, T.; Kanamori, K.; Kobayashi, Y.; Kageyama, H.; Abe, T.; Nakanishi, K. High-Level Doping of Nitrogen, Phosphorus, and Sulfur into Activated Carbon Monoliths and Their Electrochemical Capacitances. Chem. Mater. 2015, 27, 4703–4712. [Google Scholar] [CrossRef]
- Zhu, B.; Qiu, K.; Shang, C.; Guo, Z. Naturally derived porous carbon with selective metal- and/or nitrogen-doping for efficient CO2 capture and oxygen reduction. J. Mater. Chem. A 2015, 3, 5212–5222. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Maldonado-Hódar, F.J.; Pérez-Cadenas, A.F.; Carrasco-Marín, F. Textural and mechanical characteristics of carbon aerogels synthesized by polymerization of resorcinol and formaldehyde using alkali carbonates as basification agents. Phys. Chem. Chem. Phys. 2010, 12, 10365–10372. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Carrasco-Marín, F.; López-Ramón, M.V.; Alvarez-Merino, M.A. Chemical and physical activation of olive-mill waste water to produce activated carbons. Carbon N. Y. 2001, 39, 1415–1420. [Google Scholar] [CrossRef]
- Tourney, J.; Ngwenya, B.T. The role of bacterial extracellular polymeric substances in geomicrobiology. Chem. Geol. 2014, 386, 115–132. [Google Scholar] [CrossRef]
- Feakin, S.J.; Blackburn, E.; Burns, R.G. Inoculation of granular activated carbon in a fixed bed with S-triazine-degrading bacteria as a water treatment process. Water Res. 1995, 29, 819–825. [Google Scholar] [CrossRef]
- Qu, X.; Zhang, Y.; Li, H.; Zheng, S.; Zhu, D. Probing the Specific Sorption Sites on Montmorillonite Using Nitroaromatic Compounds and Hexafluorobenzene. Environ. Sci. Technol. 2011, 45, 2209–2216. [Google Scholar] [CrossRef]
- Zhang, W.; Zhuang, L.; Yuan, Y.; Tong, L.; Tsang, D.C.W. Enhancement of phenanthrene adsorption on a clayey soil and clay minerals by coexisting lead or cadmium. Chemosphere 2011, 83, 302–310. [Google Scholar] [CrossRef]
- Laczi, K.; Kis, A.; Horvath, B.; Maroti, G.; Hegedus, B.; Perei, K.; Rakhely, G. Metabolic responses of Rhodococcus erythropolis PR4 grown on diesel oil and various hydrocarbons. Appl. Microbiol. Biotechnol. 2015, 99, 9745–9759. [Google Scholar] [CrossRef]
- Rapp, P.; Gabriel-Jurgens, L.H.E. Degradation of alkanes and highly chlorinated benzenes, and production of biosurfactants, by a psychrophilic Rhodococcus sp. and genetic characterization of its chlorobenzene dioxygenase. Microbiology 2003, 149, 2879–2890. [Google Scholar] [CrossRef] [PubMed]
- Semple, K.T.; Doick, K.J.; Wick, L.Y.; Harms, H. Microbial interactions with organic contaminants in soil: Definitions, processes and measurement. Environ. Pollut. 2007, 150, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Semple, K.T.; Morriss, A.W.J.; Paton, G.I. Bioavailability of hydrophobic organic contaminants in soils: fundamental concepts and techniques for analysis. Eur. J. Soil Sci. 2003, 54, 809–818. [Google Scholar] [CrossRef]
- Lahlou, M.; Harms, H.; Springael, D.; Ortega-Calvo, J.-J. Influence of Soil Components on the Transport of Polycyclic Aromatic Hydrocarbon-Degrading Bacteria through Saturated Porous Media. Environ. Sci. Technol. 2000, 34, 3649–3656. [Google Scholar] [CrossRef]
- Cheng, Y.; Holman, H.-Y.; Lin, Z. Remediation of Chromium and Uranium Contamination by Microbial Activity. Elements 2012, 8, 107–112. [Google Scholar] [CrossRef]
- Nitschke, M.; Costa, S.G.V.A.O.; Contiero, J. Rhamnolipids and PHAs: Recent reports on Pseudomonas-derived molecules of increasing industrial interest. Process Biochem. 2011, 46, 621–630. [Google Scholar] [CrossRef]
- Wittgens, A.; Tiso, T.; Arndt, T.T.; Wenk, P.; Hemmerich, J.; Müller, C.; Rosenau, F.; Blank, L.M. Growth independent rhamnolipid production from glucose using the non-pathogenic Pseudomonas putida KT2440 Growth independent rhamnolipid production from glucose using the non-pathogenic Pseudomonas putida KT2440. Microb. Cell Fact. 2011, 10, 80. [Google Scholar] [CrossRef] [PubMed]

| Sample | C | O | P |
|---|---|---|---|
| (Mass Fraction %) | (Mass Fraction %) | (Mass Fraction %) | |
| CK | 92.3 | 7.7 | - |
| CP | 85.5 | 7.7 | 6.7 |
| Peak | Binding Energy | Assignment | % Peak | |
|---|---|---|---|---|
| CK | CP | |||
| O1s | 531.3 | C=O/P=O* | 48 | 40 |
| 533.0 | C-O/C-O-P-O/C-PO* | 52 | 60 | |
| C1s | 284.5–284.6 | C=C/C-O-P* | 73 | 72 |
| 285.5–286.4 | C-O | 17 | 17 | |
| 287.4–287.6 | C=O | 4 | 5 | |
| 288.4–288.48 | O=C-OR | 3 | 4 | |
| 290.4–290.8 | 4 | 2 | ||
| P2p | 132.3 | Reduced phosphorus compound C-P (P-1) | - | 39 |
| 133.1 | Pyrophosphate, C-PO3 (P-2) | - | 39 | |
| 134.1 | Polyphosphates and/or phosphates, C–O–PO3 (P-3) | - | 22 | |
| Sample | pHPZC | Bulk Density | SBET | N2-ADSORPTION | MERCURY POROSIMETRY | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vmicro (0.5–2 nm) | Lmicro | Vmeso (2–30 nm) | Lmeso | V0.95 | V2 (6.5–50 nm) | V3 (50–10,000 nm) | Lmacro | ||||
| g·cm−3 | m2·g−1 | cm3·g−1 | nm | cm3·g−1 | nm | cm3·g−1 | cm3·g−1 | cm3·g−1 | nm | ||
| CK | 6.69 | 0.113 | 1395 | 0.496 | 1.48 | 0.119 | 2.20 | 0.638 | 0.054 | 4.906 | 4456 |
| CKN | 8.34 | 0.114 | 879 | 0.312 | 0.95 | 0.286 | 2.17 | 0.600 | 0.114 | 4.420 | 3992 |
| CKP | 4.62 | 0.206 | 1176 | 0.418 | 0.68 | 0.193 | 2.19 | 0.614 | 0.017 | 0.629 | 4533 |
| CP | 2.44 | 0.584 | 1202 | 0.430 | 1,33 | 0.482 | 2.46 | 0.839 | 0.206 | 0.138 | 4251 |
| CPN | 2.96 | 0.546 | 1062 | 0.377 | 1,36 | 0.512 | 2.44 | 0.864 | 0.182 | 0.125 | 4433 |
| CPP | 2.03 | 0.614 | 1229 | 0.437 | 1,40 | 0.609 | 2.44 | 0.971 | 0.199 | 0.123 | 4341 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapata Acosta, K.; Carrasco-Marin, F.; Cortés, F.B.; Franco, C.A.; Lopera, S.H.; Rojano, B.A. Immobilization of P. stutzeri on Activated Carbons for Degradation of Hydrocarbons from Oil-in-Saltwater Emulsions. Nanomaterials 2019, 9, 500. https://doi.org/10.3390/nano9040500
Zapata Acosta K, Carrasco-Marin F, Cortés FB, Franco CA, Lopera SH, Rojano BA. Immobilization of P. stutzeri on Activated Carbons for Degradation of Hydrocarbons from Oil-in-Saltwater Emulsions. Nanomaterials. 2019; 9(4):500. https://doi.org/10.3390/nano9040500
Chicago/Turabian StyleZapata Acosta, Karol, Francisco Carrasco-Marin, Farid B. Cortés, Camilo A. Franco, Sergio H. Lopera, and Benjamín A. Rojano. 2019. "Immobilization of P. stutzeri on Activated Carbons for Degradation of Hydrocarbons from Oil-in-Saltwater Emulsions" Nanomaterials 9, no. 4: 500. https://doi.org/10.3390/nano9040500
APA StyleZapata Acosta, K., Carrasco-Marin, F., Cortés, F. B., Franco, C. A., Lopera, S. H., & Rojano, B. A. (2019). Immobilization of P. stutzeri on Activated Carbons for Degradation of Hydrocarbons from Oil-in-Saltwater Emulsions. Nanomaterials, 9(4), 500. https://doi.org/10.3390/nano9040500








